Summary
Adoptive transfer experiments with relatively large input numbers (~106) of TCR-transgenic T-cells are widely used to model endogenous T-cell responses to infection or immunization. Here, we show that input numbers of naïve TCR-tg T cells sufficient to squelch the endogenous response to the same epitope substantially alter the kinetics, proliferative expansion, phenotype and efficiency of memory generation by the TCR-tg T cells in response to infection. Thus, responses from non-physiologic input numbers of TCR-tg T cells fail to accurately mimic the endogenous T cell response. Importantly, seeding as few as ~10–50 TCR-tg T-cells, which constitute only a fraction of the endogenous repertoire, allowed vigorous proliferation and analysis of TCR-tg cells after infection in a scenario representing normal physiology for any individual TCR. These data strongly suggest that modeling the endogenous T-cell response with TCR-tg cells will require every effort to approximate the endogenous precursor frequency.
Introduction
Memory CD8 T cells are generated after infections with intracellular pathogens and provide enhanced protection against reinfection, thus these cells are an important goal of vaccination (Seder and Ahmed, 2003). However, effective vaccine design is hampered by our incomplete understanding of the factors controlling the generation of memory T cells (Badovinac and Harty, 2006; Kaech et al., 2002b). Most estimates suggest that the repertoire of naïve CD8 T cells capable of responding to any specific Ag ranges from 10–1000 cells per mouse (Blattman et al., 2002; Bousso et al., 1998; Casrouge et al., 2000; Kedzierska et al., 2006; Pewe et al., 2004). These naive T cells are activated in secondary lymphoid tissue when they encounter mature Ag-expressing dendritic cells (DC) (Heath and Carbone, 2001). Activated Ag-specific CD8 T cells then undergo robust proliferation and differentiation to an effector (Te) population that disseminates throughout the body to combat the infection (Busch et al., 1998; Butz and Bevan, 1998; Harty et al., 2000; Murali-Krishna et al., 1998). In addition to acquiring the ability to kill and produce cytokines, Te cells undergo characteristic changes in expression of surface molecules that regulate T cell biology (Badovinac and Harty, 2006; Kaech et al., 2002b).
Next CD8 T cells undergo programmed contraction in numbers, which occurs irrespective of the duration of infection or Ag-display (Badovinac et al., 2002). The remaining Ag-specific CD8 T cells, generally representing 5–10% the peak number, form the initial memory pool. Under conditions where the pathogen is cleared, memory CD8 T cell populations can remain remarkably stable in number(Badovinac and Harty, 2006; Kaech et al., 2002b). As might be predicted, cells that initially survive contraction express a similar phenotype to Te, with the exception that the fraction of CD127hi cells increases and grB levels rapidly decrease. The absence of CD62L and CCR7 expression on these populations places them in the “effector memory”(Tem) subset, which cannot enter lymph nodes but are able to enter tertiary tissues (Masopust et al., 2001; Sallusto et al., 2004). With additional time, memory CD8 T cells undergo further changes in phenotype and function including upregulation lymph node homing receptors (Harrington et al., 2000; Kaech et al., 2003; Sallusto et al., 2004; Schluns et al., 2000; Wherry and Ahmed, 2004), which permit these “central memory” T cells (Tcm)(Sallusto et al., 2004) to re-enter lymph nodes and undergo robust expansion in response to secondary infection (Roberts et al., 2005; Wherry et al., 2003).
Many of the surface proteins that distinguish naïve, effector and memory T cell populations have defined roles that support their use as surrogate markers for functionally distinct subsets. In addition, whereas some pathogens elicit robust endogenous CD8 T cell responses, readily detectable by MHC class I tetramers, Ag-stimulated intracellular cytokine staining (ICS) or ELISPOT, endogenous responses are not always detectable for some pathogens or protein Ag, where dominant epitopes may not be known. To overcome this limitation, T-cell receptor-transgenic (TCR-tg) cells have been transferred to naïve mice to increase, in a controlled fashion, the number of naïve Ag-specific precursors in the recipient host. This extremely powerful approach allows tracking of marked TCR-tg T cells, comparison of WT and knockout TCR-tg T cells in the same host and the generation of very large numbers of Ag-specific effector and memory TCR-tg populations. In fact, pathogens like L. monocytogenes(Foulds et al., 2002), VSV(Kim et al., 1998) and others have been manipulated to express Ags such as ovalbumin, for which defined epitopes and TCR-tg T cells (OT-1, OT-11 and DO.11.10) exist.
Based on the initial description of this approach (Kearney et al., 1994), most studies have employed adoptive transfer of ~106 naïve TCR-tg cells. While this number is 1,000–10,000-fold higher than estimates of the naïve Ag-specific repertoire, the behavior of the transferred TCR-tg T cells after immunization or infection is widely believed to accurately model the endogenous T cell response. However, recent studies have questioned this assumption, showing that high TCR-tg precursor frequencies alter specific CD8 T cell behavior (Hataye et al., 2006; Kemp et al., 2004; Marzo et al., 2005). Whether high TCR-tg T cell precursor frequencies cause a more global alteration in T cell behavior has not been determined. Here, we directly addressed the impact of TCR-tg precursor frequency on the numerical and phenotypic changes associated with expansion, contraction and memory CD8 T cell generation after infection. The results indicate that initial TCR-tg precursor frequency dictates critical aspects of the CD8 T cell response to infection. Strikingly, only when the input number of TCR-tg T cells approximated the endogenous frequency did the transferred cells exhibit the kinetic and phenotypic/functional properties associated with the corresponding endogenous CD8 T cell response. Thus, the assumption that responses from non-physiologic numbers of TCR-tg T cells accurately mimic the endogenous response is incorrect. Importantly, we document that TCR-tg T cells seeded at a fraction of the endogenous precursor frequency can be readily studied during the expansion and memory phases after infection. These data strongly suggest that studies with TCR-tg T cells should make every effort to approximate the endogenous precursor frequency.
Results
An apparent “ceiling” in the effector CD8 T cell response to infection
A previous report, based on an adoptive transfer system with varying input numbers of OT-1 TCR-tg T cells and DC vaccination, suggested that a “ceiling” exists with respect to expansion in numbers of Te (Kemp et al., 2004). We recently showed that DC vaccination, in the absence of inflammation, accelerates the acquisition of memory properties by responding T cells when compared to infection (Badovinac et al., 2005). The current studies initiated with the hypothesis that DC immunization caused the ceiling in CD8 T cell responses and that such a ceiling may not occur after infection, where very large endogenous CD8 T cell responses can be observed (Butz and Bevan, 1998; Murali-Krishna et al., 1998).
To address this notion, we transferred graded numbers (5 × 101 to 5 × 105) of Thy1.1 OT-1 cells from naïve donors into C57BL/6 (B6, Thy1.2) recipients, allowing us to study the OT-1 and endogenous Ova-specific CD8 T cell responses in the same mice. TCR-tg recipients and control B6 mice were infected with an actA-deficient strain of L. monocytogenes expressing ovalbumin (attenuated (Att) LM-Ova) that contains the Ova257 epitope recognized by OT-1 cells. We evaluated all groups 7 days post infection (p.i.), the peak of the endogenous Ova-specific CD8 T cell response against Att LM-Ova (Foulds et al., 2002), (Figure 1A). Control mice mounted a vigorous endogenous Ova-specific responses in the spleen, representing ~21% of CD8 T cells (Figure 1B) and ~5 × 106 CD8 T cells/spleen (Figure 1C). Mice seeded with intermediate (5 × 103–4) or high (5 × 105) OT-1 cells exhibited increased Ova-specific CD8 T cells compared to controls, however, consistent with the earlier study (Kemp et al., 2004), these groups all contained similar numbers of OT-1 TCR-tg cells in the spleen at d7 p.i. This did not result from incomplete recruitment (Kaech and Ahmed, 2001) since the responding OT-1 cells were CD44hi at d7, and, in separate experiments, all OT-1 cells in the highest input number group had completely diluted CFSE after Att LM-Ova infection (data not shown). These data suggest that increasing the precursor frequency of OT-1 TCR tg T cells limits the efficiency of Te generation on a per input cell basis.
Figure 1. Magnitude of CD8 T cell expansion at d7 p.i. does not correlate with input numbers of OT-1 cells.
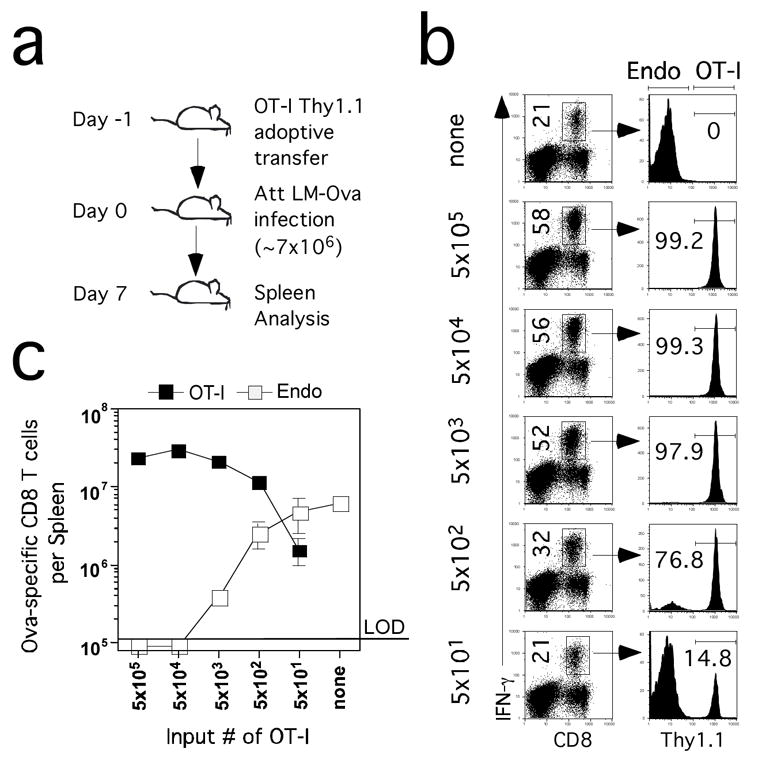
(A) Naïve B6 Thy1.2 mice received the indicated numbers of naïve Thy1.1 OT-1 cells and were infected with Att LM-Ova (~7×106 CFU/mouse) (B) Ova257-specific CD8 T cells detected by ICS for IFN-γ on day 7 p.i. in representative mice seeded with the indicated numbers of OT-1 cells. Numbers in the left column represent frequencies of all Ova257-specific CD8 T cells in the spleen. Numbers in the right column represent the frequencies of Thy1.1+ OT-1 cells among all Ova-specific IFN-γ producing cells. (C) Total numbers (mean +/− SD) of Thy1.1+ (OT-1) and Thy1.1− (endogenous – Endo) Ova257-specific CD8+ T cells per spleen (n=2–3 mice per group). LOD – limit of detection.
Consistent with other studies where TCR-tg T cells are used as models of intraclonal competition (Kedl et al., 2002), mice containing intermediate or high numbers of OT-1 cells either completely (5 × 104–5 input groups) or substantially (5 × 103 input group) inhibited the endogenous Ova-specific response (Figure 1B,C). In contrast, mice containing 5 × 102 OT-1 cells had a robust endogenous response to Ova, consisting of several million Ag-specific CD8 T cells/spleen at d7 p.i., despite the presence of >107 OT-1 cells/spleen at the same time point (Figure 1C). Mice that received 50 OT-1 cells had an endogenous Ova-specific CD8 T cell response that was similar to the control mice. Interestingly, the OT-1 response in mice seeded with 50 input cells reached >106 TCR-tg cells/spleen at d7. This is a 20,000-fold expansion in numbers (~14 divisions per input cell) if all of the transferred OT-1 contributed to the response and all the cells remained in the spleen. However, previous studies suggest that at least as many Ag-specific CD8 T cells distribute throughout the body after infection (Masopust et al., 2001). In addition, most studies suggest a 10–30% “take” of transferred TCR-tg cells (Badovinac et al., 2003; Blattman et al., 2002). Together, these factors increase the potential fold-expansion to as much as 400,000 or ~19 divisions per input OT-1 cell in mice seeded with 50 OT-1. In contrast, OT-1 cells in mice seeded with 5 × 105 TCR-tg T cells only underwent a 40 to 400-fold increase based on the same calculations, suggesting that high initial precursor frequencies limit the expansion in numbers of Te.
Initial precursor frequency alters the phenotype of CD8 T cells at day 7 p.i
Endogenous Ova-specific Te at d7 p.i. display characteristic effector phenotype and function, where the majority of the cells are CD127lo, CD62Llo, CD43(1B11)hi and express detectable intracellular grB with about 50% of the cells producing TNF and the majority failing to produce IL-2 (Figure 2A,B). Strikingly, OT-1 cells at d7 p.i. in mice seeded with 5 × 103 or more TCR-tg cells exhibited a substantially different pattern of expression for each of these molecules. These OT-1 cells were uniformly CD127hi, grBlo and most cells produced IL-2 and TNF after stimulation. In addition, most OT-1 cells at d7 p.i. in mice seeded with 5 × 105 TCR-tg cells, a standard dose adoptive transfer studies, were also CD62Lhi. In contrast, OT-1 cells in mice that were seeded with 50 or 500 TCR-tg cells had a surface phenotype and functional attributes at d7 p.i. that were similar to endogenous Ova-specific Te. These data clearly indicate that initial precursor frequencies that are high enough to squelch the endogenous CD8 T cell response have a dramatic effect not only on the fold-expansion but also the phenotype and function of the responding TCR-tg T cells.
Figure 2. Initial precursor frequency alters the functional and phenotypic characteristics of CD8 T cells early after infection.
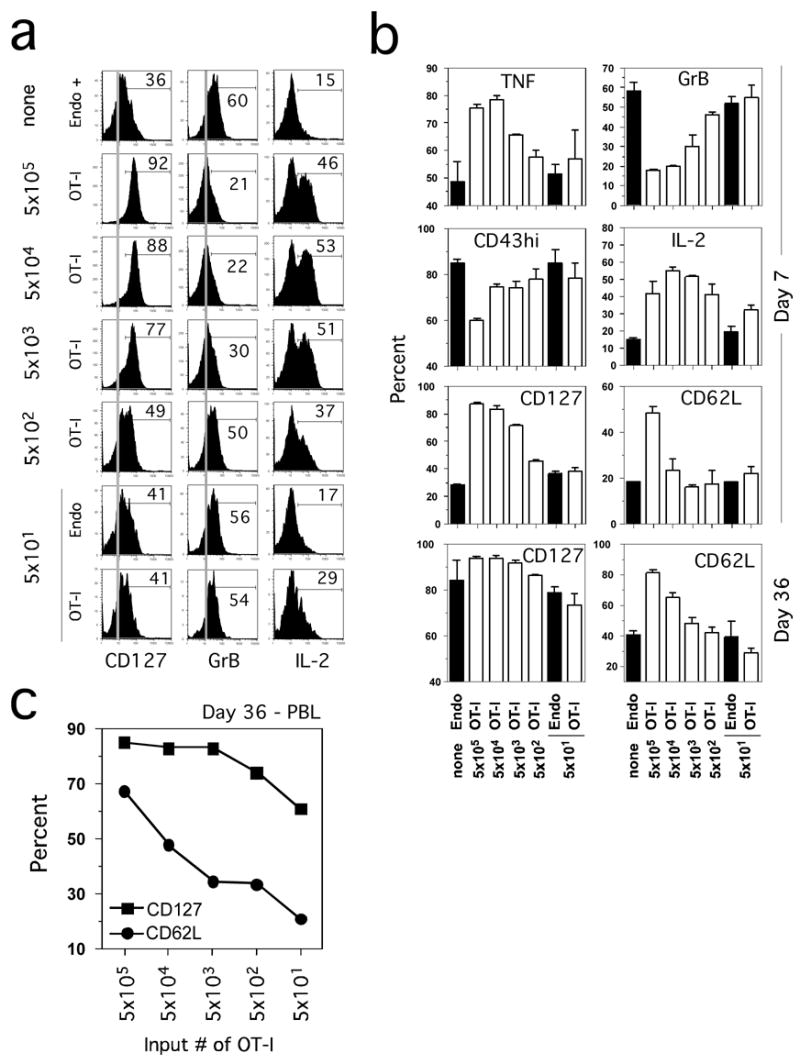
(A) Purified naïve OT-1 Thy1.1 cells at the indicated numbers were transferred into B6 Thy1.2 mice and one day later the recipient mice were immunized with Att LM-Ova. Phenotypic (CD127) and functional (grB and IL-2) status of Ova257-specific CD8 T cells from representative mice on day 7 p.i. Grey lines represent the isotype control staining that was indistinguishable between groups. Percent of Thy1.1+ (OT-1) and/or Thy1.1− (Endo) Ova257-specific CD8 T cells that expressed the indicated molecules or produced IL-2 after Ag stimulation is indicated. (B) Percent (mean + SD for 2–3 mice per group) of Ova257-specific CD8 T cells expressing TNF, grB, CD43 (1B11)hi, IL-2, CD127, and CD62L at day 7 and day 36 (CD127 and CD62L) post challenge. (C) Expression of CD127 and CD62L on gated OT-1 Thy1.1+ CD8 T cells detected in the pooled blood samples from the indicated groups at d36 p.i.
We also determined if input frequency affected CD127 and CD62L expression on memory OT-1 cells in the spleen (Figure 2B). More than 90% of splenic OT-1 memory CD8 T cells at d36 expressed CD127 in mice seeded with 5 × 103 or more naive OT-1. In contrast, ~75–80% of the endogenous Ova-specific or the OT-1 T cells from mice seeded at 50 TCR-tg cells expressed CD127. In addition, a substantially higher proportion of CD62Lhi cells were observed in the progeny of high input numbers of OT-1 cells compared to the slow acquisition of CD62L in the progeny of low input numbers of OT-1. These latter data are consistent with a recent report suggesting that initial precursor frequency controlled lineage commitment of Tem and Tcm (Marzo et al., 2005).
Sampling human immune responses is limited to analysis of blood lymphocytes. To determine if blood T cells would exhibit similar surface marker expression as the spleen cells, we analyzed the impact of input TCR-tg numbers on the phenotype (CD127 and CD62L) of OT-1 in the blood at d36 p.i. (Figure 2C). The same trend of CD127 (expressed by most cells, but clearly lowest at in mice that initially received 50 OT-1) and CD62L expression was observed in blood memory OT-1 cells. Thus, T cells in the blood are representative of T cells in the secondary lymphoid organs. In addition, these results show that it is possible to study memory OT-1 cells derived from very low input numbers (~50) of naïve TCR-tg cells.
Input precursor frequency dictates the kinetics and efficiency of the CD8 T cell response
The results suggested that intermediate-high initial precursor frequencies have a dramatic effect on the phenotype of CD8 T cells examined at the normal peak of expansion of the endogenous response. Importantly, expression of many markers by Ag-specific CD8 T cells is dynamic with respect to time after stimulation. To limit animal numbers and also to track the OT-1 response over time in individual hosts, we transferred a range of naïve OT-1 cells (5 × 101–5 × 105) and obtained blood samples on d3-7 and at several later time points after Att LM-Ova infection. OT-1 TCR-tg T cell responses to infection were detectable in the blood of all groups of mice even those that received only 50 OT-1 (Figure 3A). Additionally, the pattern of expansion, contraction and generation of memory OT-1 cells was similar in all groups and coordinate between individuals within groups (Figure 3B).
Figure 3. Input CD8 T cell precursor frequency dictates the kinetics and efficiency of the CD8 T cell response.
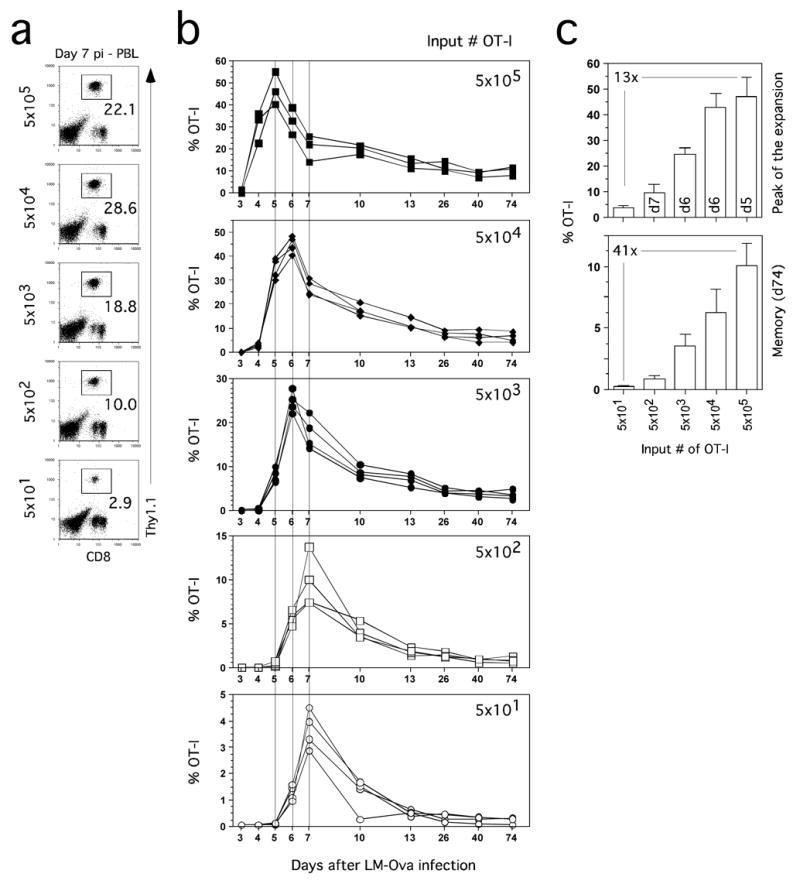
Purified naïve OT-1 Thy1.1 cells at the indicated numbers were transferred into B6 Thy1.2 mice and one day later mice were immunized with Att LM-Ova. Expansion of OT-1 Thy1.1 cells was followed in the blood at indicated days p.i.. (A) Detection of OT-1 cells in the blood of representative mice on day 7 p.i. Numbers represent the frequency of OT-1 cells among PBL. (B) Kinetic analysis of OT-1 response in the blood. Responses of individual mice in each group are shown. (C) Frequency of OT-1 cells detected at the peak (top, day indicated inside bars) of expansion or at a memory (bottom, day 74 p.i.) time point. Numbers inside panels indicate the fold-difference in frequency of OT-1 cells between the highest and lowest input groups.
However, these data also show that initial precursor frequency substantially impacts the kinetics and magnitude of the OT-1 response. For example, input number directly controlled the day when peak frequencies of OT-1 cells were detected in the blood (Figure 3B). High input OT-1 numbers (5 × 105) resulted in a peak at d5 p.i., intermediate input numbers (5 × 103–4) resulted in a peak at d6 p.i., whereas low input numbers (5 × 10 1–2) resulted in a peak at d7 p.i. Importantly, OT-1 cells from the high and intermediate input groups had undergone substantial contraction by d7 p.i. In part, this may explain the apparent ceiling in the OT-1 response observed by analysis of the spleen only at d7 p.i. (Figure 1). Thus, evaluation only of the d7 time point in mice that received a high input number of OT-1, a standard approach in the field, could be misleading with regard to comparison to the endogenous CD8 T cell response.
In addition, the magnitude of the peak in the blood was related to the input number of OT-1 (Figure 3B). However, this relationship was not apparent when data only from the standard peak (d7 p.i.) time point were analyzed (Figure 3A, B). More strikingly, the relationship between input numbers and magnitude of the peak OT-1 response was not proportional. Mice seeded with 5 × 105 naïve OT-1 had only 13-fold higher peak frequency of OT-1 cells than mice receiving 5 × 101 naïve OT-1 (Figure 3C), despite a 10,000-fold difference in input TCR-tg T cells. These data clearly indicate that high initial precursor frequencies limit the expansion of TCR-tg T cells.
Relatively stable numbers of memory OT-1 were detected from d13 to d74 in all groups (Figure 3B). However, increasing the input number of OT-1 cells by 10,000 fold (from 5 × 101 to 5 × 105) only resulted in ~40-fold increase in memory cell frequency at d74 p.i. (Figure 3C). Thus, increasing input numbers also does not result in a proportional gain in memory CD8 T cells. Similar data were recently reported for CD4 T cell responses, where it was concluded that intraclonal competiton may regulate memory T cell numbers (Hataye et al., 2006). From these data we conclude that the efficiency of memory CD8 T cell generation decreases as the input number of TCR-tg T cells increases. Together, these studies also reveal the power, reproducibility and sensitivity to kinetic analysis of TCR-tg responses in the blood of mice containing titrated input numbers of naïve precursors.
Predictive power of surface phenotype is lost at high input number
Current data suggest that CD127 expression identifies an effector population enriched in memory CD8 T cell precursors, i.e.those cells that survive contraction (Huster et al., 2004; Kaech et al., 2003). We addressed the relationship between CD127 expression, input OT-1 number and contraction by analyzing blood samples obtained from mice seeded with different input numbers of OT-1 cells prior to infection with Att LM-Ova (Figure 4). Representative profiles for CD127 and CD62L on blood OT-1 cells at d7 p.i. are shown in Figure 4A. In the low input group, most OT-1 cells exhibited an effector phenotype and were CD127lo and CD62Llo. In contrast, most OT-1 cells at d7 p.i. in the high number input group were CD127hi and CD62Lhi, a phenotype usually associated with Tcm. This increased CD127 and CD62L expression could result from the fact that the OT-1 response in the high input group peaked at d5. However, kinetic analysis of CD127 and CD62L expression revealed that input numbers had a substantial impact on the fraction of OT-1 cells expressing these proteins (Figure 4B). For example, mice that received 5 × 103 or more OT-1 already exhibited a high proportion of CD127 expressing cells on the first day they could be detected (d3-5). The fraction of OT-1 cells expressing CD127 rapidly increased in these mice, exceeding 65% positive by d7 p.i. and reaching stable frequencies of 80–90% positive by d13 pi. This is much faster than reported for endogenous CD8 T cell responses after infection (Badovinac et al., 2004; Huster et al., 2004; Kaech et al., 2003). In contrast, OT-1 cells responding in mice seeded with low input TCR-tg numbers (5 × 101 or 5 × 102) had substantially reduced early CD127 expression and exhibited a relatively slow increase in the fraction of CD127 expressing cells, thus mimicking the endogenous response.
Figure 4. Precursor frequency controls the degree and kinetics of CD127 and CD62L modulation in responding CD8 T cells.
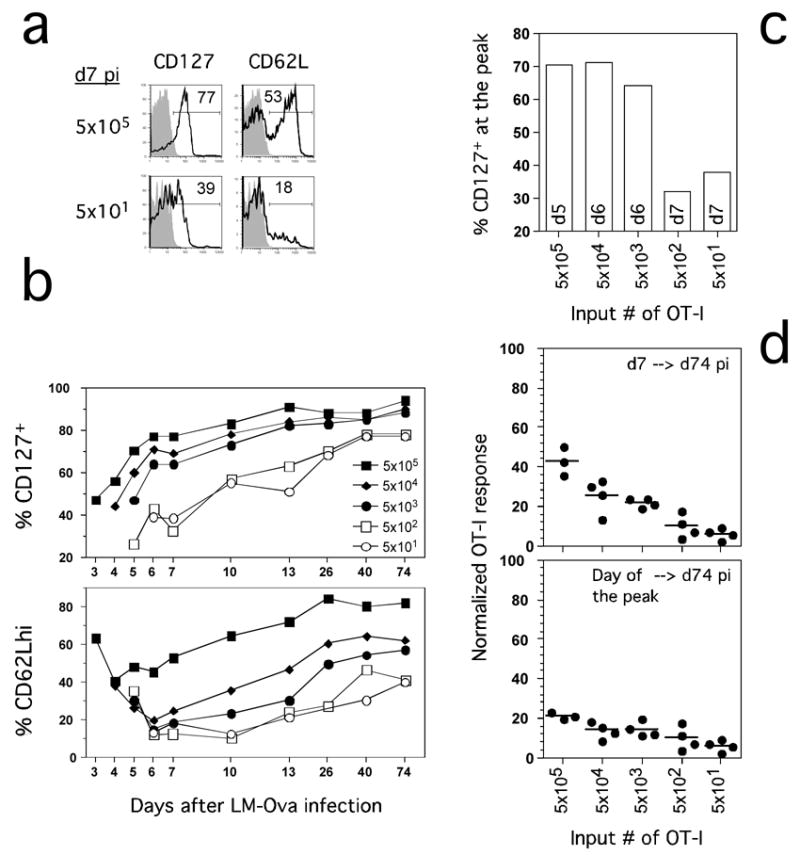
(A) Representative CD127 and CD62L expression on OT-1 cells from the blood at day 7 p.i.. Shaded histograms represent isotype control staining. Numbers represent frequency of cells that are positive for indicated markers. (B) Kinetic analysis of CD127 and CD62L expression on OT-1 cells at indicated days p.i.. Pooled PBL from each group were analyzed. (C) Expression of CD127 on OT-1 cells from the indicated groups of mice at the peak of the response (day indicated inside the bars). (D) Degree of contraction of OT-1 cells at day 74 p.i. is normalized from frequencies of OT-1 cells in the blood (calculated as 100%) at day 7 (upper panel) or day of the peak of the response (lower panel). Individual mice are shown.
Naïve T cells express CD62L, allowing entry into lymph nodes. Activated CD8 T cells downregulate CD62L, and effector populations are usually CD62Llo. The fraction of memory cells expressing CD62L increases with time and this marker, in conjunction with CCR7 and the capacity to produce IL-2 have been used to differentiate Tcm from Tem (Sallusto et al., 2004). Importantly, the lineage relationships between Tem and Tcm based on CD62L expression have been evaluated recently with TCR-tg adoptive transfer models. While initial studies suggested a linear differentiation pathway where Tem gave rise to Tcm (Wherry et al., 2003), this conclusion has been questioned by studies that suggested stable Tem and Tcm populations in mice containing low input numbers of TCR-tg T cells (Marzo et al., 2005). Consistent with this latter study, CD62L expression on OT-1 cells responding to infection was also dictated by input cell numbers, albeit with a different pattern than CD127. In mice that received the highest TCR-tg numbers (5 × 105), CD62L expression only decreased on ~60% of the responding OT-1 cells by d4, and began to increase one day later. More than 70% of OT-1 cells in this group expressed CD62L by d13, again much faster than observed with endogenous responses to infection (Wherry et al., 2003). In contrast, CD62L expression was decreased substantially at d6-7 in all other groups, however, input TCR-tg T cell number dictated the rate at which the OT-1 cells recovered CD62L expression, with the most delayed recovery exhibited by the mice containing the lowest (5 × 101 and 5 × 102) OT-1 input numbers. Together, these studies show that precursor frequency controls the fold-expansion and both the degree and kinetics of CD127 and CD62L modulation in responding T cells.
Whether the groups were compared at d7p.i., the peak of the endogenous response (Figure 4B), or at the real peak of the expansion in each group (Figure 4C), most (>65%) OT-1 cells were CD127hi in mice seeded with 5 × 103 or more input TCR-tg cells. When contraction of OT-1 cells at d74 was normalized to d7 p.i. in all groups, the fraction of cells surviving to memory was highest at high and intermediate input number, consistent with the increased expression of CD127 on these populations (Figure 4D, top). However, these data are misleading, since normalization based on the true peak of expansion in each group resulted in only a modest increase in the percentage of cells surviving in the high and intermediate input groups (Figure 4D, bottom). Thus, CD127 expression of responding OT-1 cells in mice seeded with high or intermediate numbers of TCR-tg cells does not identify populations enriched in memory precursors. These studies clearly indicate that expression of CD127 does not, in itself, protect responding T cells from contraction. More importantly, these data reveal that surface markers such as CD127 are not reliable predictors of T cell subset behavior, when derived from high input numbers of TCR-tg cells. Finally, these results were generalizable and not solely a consequence of infecting mice with a high dose of Att LM-Ova because mice seeded with non-physiologic numbers of TCR-tg cells exhibited similar alterations in response kinetics and the phenotype of the responding OT-1 cells after infection with 1) lower doses of Att LM-Ova, 2) virulent LM-Ova (~0.1 LD50), or 3) vaccinia virus expressing the Ova257 epitope (data not shown).
TCR-tg T cells can be studied as a fraction of the endogenous response
The repertoire of naïve T cells specific for any particular Ag consists of a relatively small number of cells, most of which express unique TCR (Blattman et al., 2002; Bousso et al., 1998; Casrouge et al., 2000; Kedzierska et al., 2006; Pewe et al., 2004). Ideally, TCR-tg T cells would be studied as one specificity, present at one or a few cells, amongst a diverse repertoire responding to infection to mimic the endogenous response. In addition to the physiologic relevance of this low-number adoptive transfer approach, the ability to obtain sufficient numbers of TCR-tg T cells from the blood of naïve donor mice allows a single TCR-tg mouse to serve as donor for multiple experiments, thus maximizing resourses. We sought to determine the minimum number of transferred OT-1 cells that would yield detectable and reproducible expansion in the blood and spleen after LM-Ova infection (Figure 5A). We obtained 200 μl (~1 × 106 PBL) of blood from a naïve OT-1 donor and determined that 17.5% of total PBMC were TCR-tg based on Vβ5 and CD8 co-expression (Figure 5B). We then transferred PBL, containing ~350, ~70, ~14 or ~3 OT-1 cells into recipient mice (Figure 5B). Readily detectable OT-1 responses were observed in the blood at d6 p.i. with Att LM-Ova of all mice that received ~14 or more OT-1 cells prior to infection (Figure 5C, D). Remarkably, at least one of four mice that received ~3 naïve OT-1 made a detectable response in the blood. Despite the potential for variability after injecting such small numbers of OT-1 cells, the responses within each group were surprisingly consistent and input dose responsive based on the percent of OT-1 of total PBL (Figure 5C) or as a fraction of CD8 blood cells (Figure 5D). In addition, the massive expansion of the low numbers of transferred OT-1 cells after infection was not a result of the somewhat variable, but usually small fraction of CD44hi cells found in naïve donors (Supplemental Figure 1). Thus, the immune system is remarkably efficient in identifying, activating and vigorously expanding even extremely low numbers of naïve TCR-tg T cells and the CD8 T cell response to infection can be modeled in the blood with input numbers of TCR-tg T cells representing a fraction of the endogenous repertoire.
Figure 5. OT-I TCR-tg T cells at low input numbers can be followed in vivo and behave as an endogenous CD8 T cells after infection.
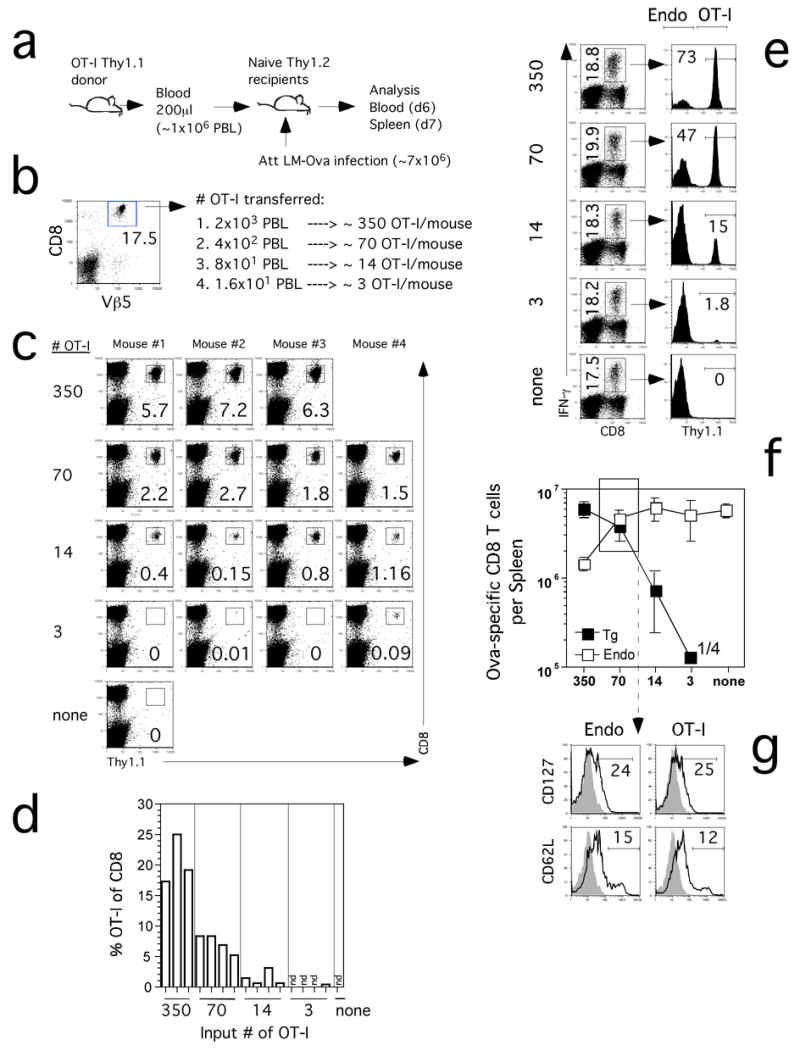
(A) Blood from an OT-1 Thy1.1 mouse was used as a source of naïve TCR-tg cells that were transferred into naïve B6 Thy1.2 mice one day before Att LM-Ova infection. (B) Frequency of OT-1 (Vβ5+ CD8+) in the blood of the donor mouse used for calculation of OT-I numbers transferred. (C) Detection of OT-1 cells in the blood of the recipient mice on day 6 p.i. Numbers represent the frequency of OT-1 cells among PBL. Individual mice are shown. (D) Frequency of OT-1 cells out of CD8 cells in the blood. (E) Ova257-specific CD8 T cells detected in the spleen by ICS for IFN-γ on day 7 p.i. in representative mice that received the indicated numbers of OT-1 cells. Numbers in the left column represent frequencies of all Ova257-specific CD8 T cells in the spleen. Numbers in the right column represent the frequencies of Thy1.1+ OT-1 CD8 T cells among all IFN-γ producing cells. (F) Total numbers (mean +/− SD) of Thy1.1+ (OT-1) and Thy1.1− (endogenous – Endo) Ova257-specific CD8 T cells per spleen (n=3–4 mice per group). Numbers inside the panel indicate that 1 out of 4 analyzed mice had detectable OT-1 response in the lowest input group. (G) Representative profiles of CD127 and CD62L expression on gated endogenous (Endo) Ova257-specific or OT-1 CD8 T cells in the spleen at day 7 p.i. Profiles are from mice that received 70 OT-1 cells, indicated by the box in panel F. Shaded histograms represent isotype control staining. Numbers represent the frequency of Ova257-specific CD8 T cells that are positive for CD127 or CD62L.
Functional analysis of endogenous and OT-1 responses in the spleen at d7 p.i. yielded similar results, again, all mice that received ~14 or more TCR-tg cells had a detectable OT-1 response (Figure 5E, F). This analysis also confirmed the OT-1 response from the one mouse that received ~3 OT-1. There are several additional important points from this experiment. First, injection of ~350 or less OT-1 cells had minimal impact on the overall magnitude of the Ova-specific CD8 T cell response compared to control mice that did not receive OT-1 cells (Figure 5E, F). Second, mice that received ~70 TCR-tg cells had equivalent endogenous Ova-specific and OT-1 responses in the spleen at d7 p.i. (Figure 5F). Importantly, mice seeded with only 50 OT-1 had ~1 × 105 memory OT-1 cells in the spleen at d36 p.i. (data not shown), thus, memory can be generated and studied with input numbers of TCR-tg T cells below the endogenous precursor frequency. Interestingly, mice seeded with ~14 OT-1 cells yielded a TCR-tg CD8 T cell response representing 15% of the total Ova-specific response at d7 p.i. In these mice, the ~14 OT-1 cells underwent at minimum 50,000-fold expansion (~16 division) or 500,000-fold expansion if corrected for take (~21 divisions) to 7 × 105 cells/spleen. These data provide strong evidence that TCR-tg adoptive transfer studies can be successfully undertaken when input number of naïve TCR-tg T cells represent only a fraction of the endogenous precursors and thus approach a true representation of T cell physiology. Of equal importance, the phenotype of the TCR-tg cells at d7 p.i. in mice receiving ~70 OT-1 was superimposable for CD127 and CD62L expression with the endogenous Ova-specific CD8 T cells in the same mice (Figure 5F). Together, these data strongly argue that TCR-tg T cells behave like the endogenous response only when present at or below the endogenous precursor frequency.
Initial precursor frequency affects the P14 TCR-tg response to infection
One important question is whether the response of other TCR-tg T cells is also dictated by initial precursor frequency or if this phenomenon is limited to OT-1 cells. To address this question, we used P14 mice that are transgenic for a TCR specific for the LCMV GP33-41 epitope (Ashton-Rickardt et al., 1994). Initially, we transferred either 4 × 105 or 4 × 103 splenic P14 Thy1.1 cells into B6 recipients one day prior to infection with LCMV and subsequent analysis in the blood (Figure 6A). Similar to the OT-1 model, mice that initially had high input numbers of P14 cells exhibited a peak in the blood at d6 p.i. and contracted by d8, whereas the P14 cells in mice seeded with 100-fold fewer TCR-tg cells continued to increase until d8 p.i. (Figure 6B). Although not as dramatic as the OT-1 model, P14 cells at d8 p.i. in mice seeded with 4 × 105 input TCR-tg cells displayed a higher fraction of CD127 and CD62L positive cells than P14 cells that arose from 4 × 103 input TCR-tg T cells (Figure 6C) Thus, initial precursor frequency also alters the kinetics and phenotype of P14 TCR-tg T cells responding to LCMV infection.
Figure 6. Initial precursor frequency alters the kinetics and phenotype of P14 TCR-tg T cells responding to LCMV.
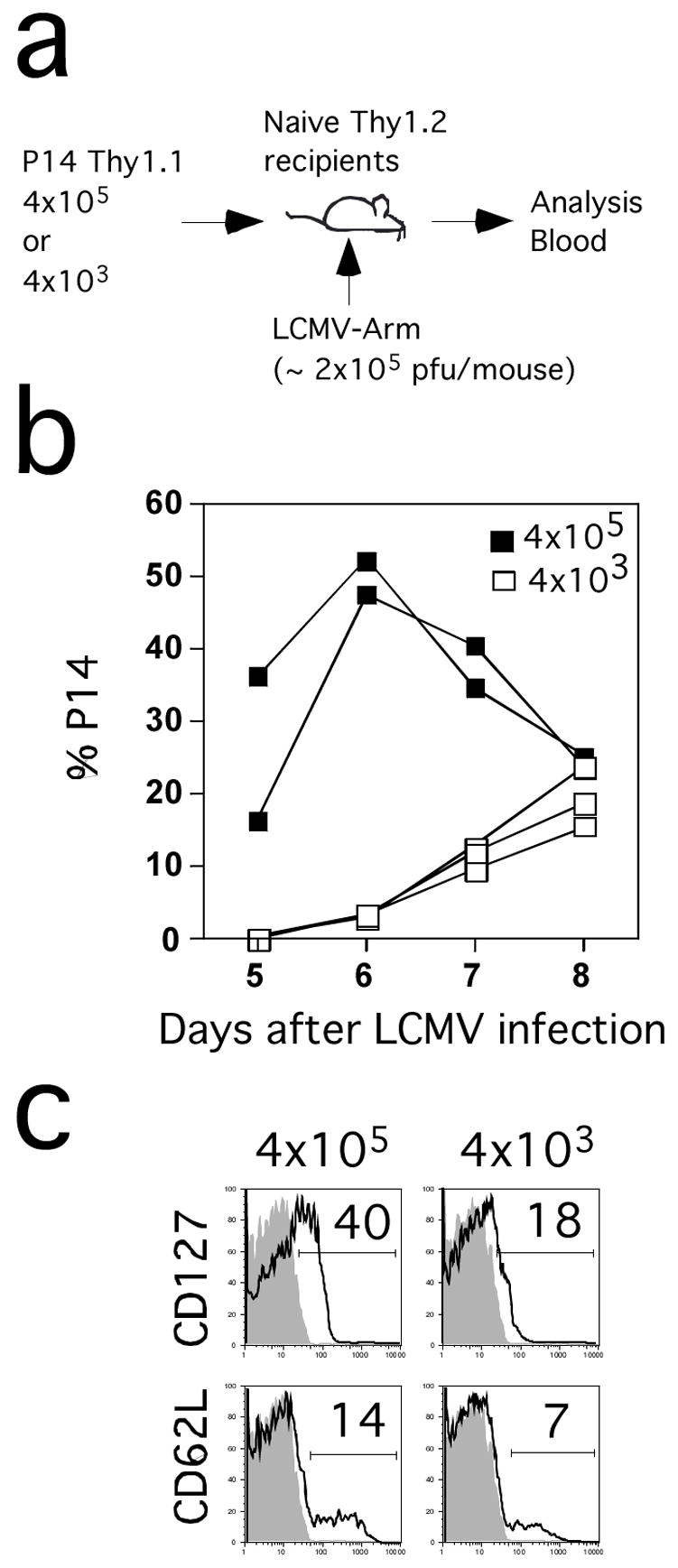
(A) Purified naïve P14 Thy1.1 cells at the indicated numbers were transferred into B6 Thy1.2 mice and one day later the recipient mice were infected with LCMV. Expansion of P14 Thy1.1+ cells was followed in the blood at indicated days p.i.. (B) Kinetic analysis of P14 responses in the blood. Responses of individual mice from each group are shown. (C) CD127 and CD62L expression on gated P14 cells from pooled blood samples from each group at day 8 p.i. Shaded histograms represent isotype control staining. Numbers represent the frequency of P14 cells that are positive for CD127 or CD62L.
Previous studies used a titration approach with P14 cells (corrected for “take”) to suggest that the endogenous repertoire of GP33-specific CD8 T cells was in the range of 50–100 cells/spleen (Blattman et al., 2002). A major assumption in this approach is that a single TCR can be used to model a diverse repertoire. A subsequent study estimated TCR repertoire diversity based on sequencing a subset of GP33-specific TCR and suggested that B6 mice may contain 1000–1500 precursors (Pewe et al., 2004). Importantly, evaluation of the P14 response in the blood at d8 after LCMV infection of mice seeded with ~6–6,000 input TCR-tg cells (Figure 7A) demonstrated detectable responses in all mice that received ~6 (~ 2 TCR-tg cells/recipient with a ”take” of 30%) or more P14 cells (Figure 7B). Again, the response between groups was dependent on the dose of input cells and very reproducible within groups (Figure 7B, C). As opposed to the OT-1 model where 5 × 103 input cells largely eliminated the endogenous response, a robust endogenous GP33-specific CD8 T cell response could be detected even in mice initially seeded with 6,000 P14 cells (Figure 7D, E). Importantly, the phenotype of the endogenous GP33-specific CD8 T cells and P14 cells in the spleen with respect to CD127 and CD62L expression was essentially identical with an input number of 6,000 P14 cells (Figure 7F). Thus, compared to OT-1 and LM-Ova infection higher numbers of P14 T cells can be transferred to mimic the endogenous response to LCMV infection. These data indicate that the precise numbers of TCR-tg T cells that can mimic the endogenous response must be empirically determined for each model. However, the results from both model systems demonstrate that adoptive transfer of high numbers of TCR-tg T cells, the standard practice in the field, will substantially alter the kinetics, magnitude of expansion and phenotype of the responding T cell populations.
Figure 7. P14 TCR-tg cells at low input numbers mimic the endogenous CD8 T cell response after LCMV infection.
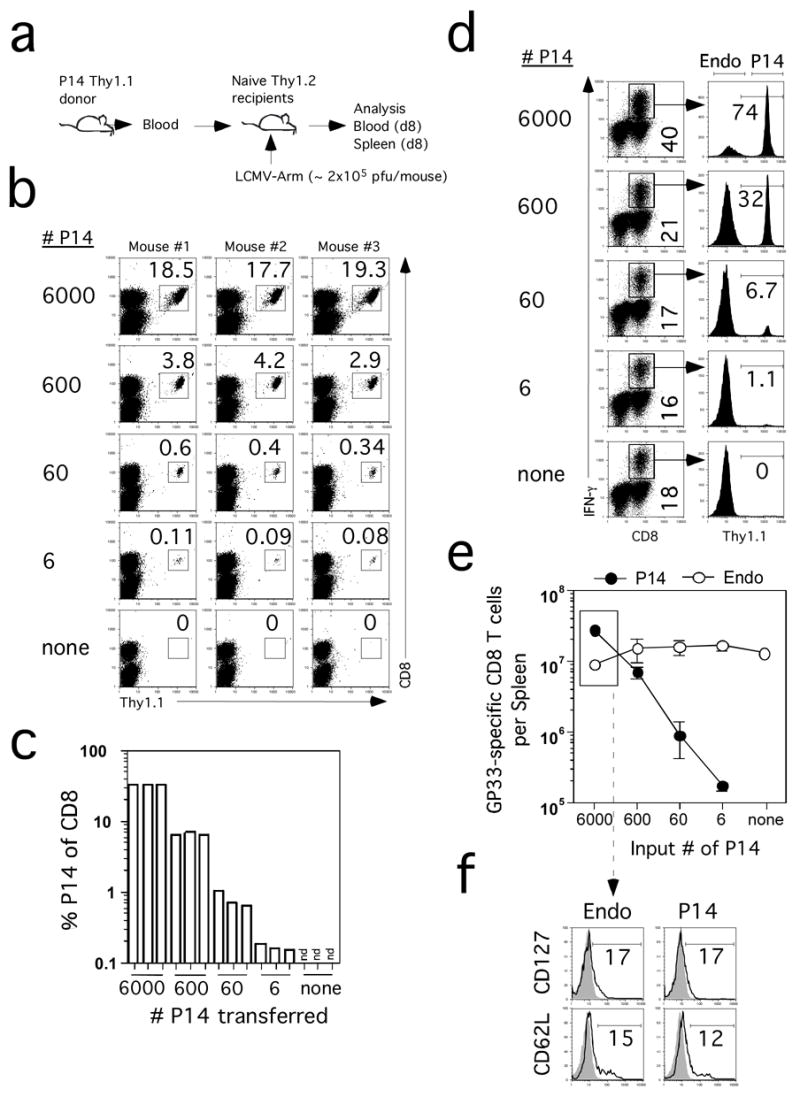
(A) Blood from a P14 Thy1.1+ mouse was used as the source of the indicated numbers of naïve TCR-tg cells, which were transferred into naïve B6 Thy1.2 mice one day before LCMV infection. (B) Detection of P14 cells in the blood of recipient mice on day 8 p.i. Numbers represent the frequency of P14 cells among PBL. Individual mice are shown. (C) Frequency of P14 TCR-tg T cells of all CD8+ cells in the blood. (D) GP33-specific splenic CD8+ T cells detected by ICS for IFN-γ on day 7 p.i. in representative mice that received the indicated numbers of P14 cells before infection. Numbers in the left column represent frequencies of all GP33-specific CD8+ T cells in the spleen. Numbers in the right column represent the frequencies of Thy1.1+ P14 CD8+ T cells among all IFN-γ producing cells. (E) Total numbers (mean +/− SD) of Thy1.1+ (P14) and Thy1.1− (endogenous – Endo) GP33-41-specific CD8+ T cells per spleen (n=3 mice per group). (F) Representative profiles of CD127 and CD62L expression on gated endogenous (Endo) GP33-specific or P14 CD8+ T cells in the spleen at day 8 p.i.. Profiles are from mice that received 6000 P14 cells, indicated by the box in panel E. Shaded histograms represent isotype control staining. Numbers represent the frequency of GP33-specific CD8+ T cells that are positive for CD127 or CD62L.
Discussion
The use of TCR-tg T cells in adoptive transfer assays has been widely employed to study the T cell response to infection or vaccination. In most studies, representing more than a thousand publications to date, 106 or more TCR-tg T cells are transferred into naïve mice prior to initiating infection or immunization. Despite the fact that these mice now contain 1,000–10,000-fold higher Ag-specific naïve precursors than the endogenous repertoire, the response of these high numbers of TCR-tg T cells has been widely assumed to accurately mimic the endogenous T cell response to infection. It is only recently that this notion has been challenged by experiments showing that precursor frequency can affect specific aspects of the T cell response (Hataye et al., 2006; Kemp et al., 2004; Marzo et al., 2005). Here, we show that most facets of the CD8 T cell response, including kinetics, proliferation, surface molecule expression, effector function and the efficiency of memory generation are substantially altered when the initial number of TCR-tg T cells is sufficiently high to inhibit the endogenous CD8 T cell response to the same Ag. Given that the T cell response evolved to initiate from rare precursor cells, these results may not be surprising. However, the data suggest that the use of TCR-tg T cells to model the endogenous CD8 T cell response may only be reliable under conditions where these cells represent only a fraction of the endogenous repertoire.
Our data indicate that the behavior (kinetics, proliferative expansion, phenotype, function) of CD8 T cells is progressively altered from the endogenous response as the number of naïve TCR-tg T cells transferred increases. However, comparison of the OT-1 and P14 TCR-tg systems, stimulated by Att LM-Ova or LCMV respectively, suggest that the precise number of naïve TCR-tg needed to mimic the endogenous response will depend on the model system. For example, transfer of 5 × 103 OT-1 resulted substantially inhibited the endogenous Ova-specific CD8 T cell response, accelerated the peak and compromised the proliferative responses of the OT-1 cells and resulted in the abnormally fast downregulation of grB and accelerated acquisition of memory phenotype and cytokine profiles compared to the endogenous response or lower input numbers of OT-1. In contrast, mice that received 4–6 × 103 P14 cells still mounted a robust endogenous CD8 T cell response, the P14 cells proliferated extensively and exhibited a normal d8 peak and also had CD127 and CD62L expression that was super-imposable to the endogenous GP33-specific response. Thus, a ”one size fits all” approach to determining how many TCR-tg T cells to use in mimicking the endogenous CD8 T cell response is not likely to be successful.
What variables control the number of naïve TCR-tg T cells that both permit and behave with similar characteristics to an endogenous response? Activated OT-1 cells exhibit a relatively high functional avidity compared to the endogenous Ova-specific response (VPB and JTH, unpublished). In contrast, P14 TCR-tg T cells may fall within the normal spectrum of the endogenous GP33-specific CD8 T cell response (Blattman et al., 2002). Thus, specific characteristics (affinity, avidity) of the TCR may be relevant. The ability to study the response of low numbers (~10–50 input cells) of TCR-tg T cells in the context of an endogenous response, as shown here for both OT-1 and P14 cells, is the best way to approximate the normal contribution of any specific TCR to an immune response while still allowing the experimental manipulations (adoptive transfer, comparison of gene deficient and WT TCR-tg T cells in the same host) that provide such powerful tools for comparative analysis of T cell function. Similarly, the infection or vaccination model may be an important variable in determining the appropriate number of TCR-tg T cells to mimic the endogenous response. LCMV infection stimulates an extremely robust endogenous CD8 T cell response to the GP33 epitope. This may be due to a relatively high number of naïve precursors specific for this epitope (Pewe et al., 2004). An elevated endogenous precursor frequency may increase the number of TCR-tg cells needed to out compete the endogenous response. In total, our data suggest that adoptive transfer of TCR-tg numbers that permit a concurrent and substantial endogenous response will also permit the TCR-tg T cells to behave as the endogenous response. However, this number must be empirically determined for each model system by careful titration experiments and concurrent evaluation of the endogenous T cells response to the same Ag.
If we accept that TCR-tg T cells only mimic the endogenous response when initially present at numbers that approximate the endogenous repertoire, what can be learned from experiments with high input numbers of TCR-tg T cells? While our results strongly suggest that the default choice to use “standard” high numbers (~106) of input numbers of TCR-tg T cells will invariably mislead investigators, we believe that careful titration experiments can still inform the field with respect to the behavior of T cells. One of the strongest correlations we observed was between high input TCR-tg T cell numbers, altered kinetics with reduced proliferative expansion after infection and the atypical modulation of surface phenotype. For example, robust expansion consisting of 16–19 divisions from the endogenous CD8 T cell repertoire or low numbers of input TCR-tg T cells was associated with a peak at d7-8 p.i. and the manifestation of an effector phenotype (CD127lo, CD62Llo, CD43 (1B11)hi and function, TNFint, IL-2lo) in most IFN-γ producing OT-1 T cells. Expression of each of these markers was essentially the opposite in OT-1 TCR-tg cells that were seeded at high (5 × 105) or intermediate (5 ×103–4) numbers prior to infection. The OT-1 cells seeded at high or intermediate input numbers underwent greatly reduced expansion in numbers (200–4000-fold, or 8–12 divisions) after LM-Ova infection. These data suggest that the molecular processes imprinting the normal regulation of surface molecules may critically depend on events that occur during the terminal 4–6 divisions that take place from low input numbers of TCR-tg cells. This notion is consistent with recently described differences in conversion from Tem to Tcm depending on whether the starting number of TCR-tg T cells was relatively high or in the range of the endogenous response (Marzo et al., 2005). Careful evaluation of the molecular regulation of these gene products under conditions of low and high input TCR-tg T cells will likely provide an important tool to address the role of division history in regulating the phenotype of CD8 T cell populations.
In addition, the titration experiments and ensuing modulations in proliferation and phenotype of the responding TCR-tg T cells allow us to address several outstanding issues in T cell biology. Much recent effort has been devoted to identifying memory CD8 T cell precursors (those cells that will survive contraction) at the peak of the effector stage of the response to infection (Badovinac and Harty, 2006; Kaech et al., 2002a; Kaech et al., 2003; Madakamutil et al., 2004; Wong et al., 2004). For example, CD127 expression at this time point was suggested to define a population enriched for memory precursors (Huster et al., 2004; Kaech et al., 2003). Our titration experiments reveal that the degree of CD8 T cell contraction was largely unaffected by the input numbers of TCR-tg T cells when the actual peak of the response was identified by kinetic analyses. This occurred despite the fact that the vast majority of OT-1 T cells (60–70%) were CD127 positive at the respective peak of the expansion phase in mice that were seeded with 5 × 103 or more TCR-tg T cells. These data support previous studies suggesting that CD127 expression itself was insufficient to protect T cells from contraction (Badovinac et al., 2005; Lacombe et al., 2005) and underscore the problem in using phenotypic markers, that may be meaningful in discriminating T cell populations that derive from endogenous numbers of precursors, to characterize T cells that are generated from non-physiologic precursor frequencies. In addition, our data dispute one prevailing model in the literature-that T cell populations that undergo less proliferation will generate higher memory (undergo less contraction). Indeed, a 10,000-fold increase in input OT-1 numbers only resulted in a 40-fold increase in memory OT-1 cells after infection. Thus, decreased proliferation and the associated accelerated acquisition of memory phenotype observed with non-physiologic input numbers of TCR-tg T cells actually results in less efficient generation of memory T cell numbers. This finding is not unique to CD8 T cells as it has recently been reported for CD4 T cell memory generation (Hataye et al., 2006). Thus, we feel that carefully designed TCR-tg titration experiments and comparisons with the endogenous T cell response can address the role of proliferation in progression of T cells through the effector to memory stages of the immune response.
In summary, our results provide strong evidence that adoptive transfer of high numbers (~106) of TCR-tg T cells, the standard approach in the field for the last decade, cannot be used to accurately mimic the endogenous CD8 T cell response to infection. In some instances, such as the OT-1 model of LM-Ova infection, transfer of as few as 5 ×103 TCR-tg cells still results in an abnormal response, while this number appears appropriate for studies of the P14 cells responding to LCMV infection. Thus, every strategy to use TCR-tg T cells to mimic the endogenous response should start with a careful titration of input precursor numbers. Our results may have particular importance for the emerging areas of multi-photon imaging to evaluate early events in the immune response, where most investigators use adoptive transfer of high numbers of labeled TCR-tg T cells and Ag-laden DC to more readily visualize interactions. Given the aberrant T cell responses observed after infection of mice with high input numbers of TCR-tg T cells, it seems plausible that early interactions of these cells may also be affected by high precursor numbers. Thus, we strongly suggest that every effort be made to work toward minimizing the number of TCR-tg T cells evaluated in any experiment where understanding the endogenous T cell response is the goal. As shown here, it is readily possible to detect and characterize effector and memory CD8 T cell responses derived from TCR-tg cells seeded at numbers representing only a fraction of the endogenous repertoire. After all, this situation represents true physiology and will likely present the most accurate platform for applications of TCR-tg T cells towards modeling the endogenous T cell response to infection or vaccination.
Experimental Procedures
Mice, Bacteria and Virus infection
C57BL/6 (B6, Thy1.2) mice were from the National Cancer Institute (Frederick, MD). OT-1(Ova257-specific) and P14 (GP33-specific) transgenic Thy1.1 mice were described (Ashton-Rickardt et al., 1994; Hogquist et al., 1994). Pathogen-infected mice were housed at appropriate biosafety conditions. Mice were used at 6–10 weeks of age. Animal experiments were performed under approved Institutional Animal Care and Use Committee protocols. Att LM-Ova was grown, injected i.v. and quantified as described (Badovinac et al., 2005; Foulds et al., 2002). The Armstrong strain of LCMV (2×105 pfu/mouse i.p.) was used as described (Badovinac et al., 2002).
Adoptive-transfer experiments
Thy1.1 OT-I and P14 cells were purified from the spleens of naïve donors by negative selection to enrich for CD8 cells (StemSep, Vancouver) and were transferred at the indicated numbers into naïve B6 Thy1.2 mice followed one day later by infection with either Att LM-Ova or LCMV. In some experiments TCR-tg T cells were obtained from the blood of naïve donors. The actual number of cells transferred was verified by co-staining for CD8 and β-chain of the transgenic TCR.
Antibodies and peptides
Antibodies of the indicated specificity with appropriate fluorochromes were used: IFN-γ (clone XMG1.2, eBioscience), CD8 (53–6.7, Pharmingen), Thy1.1 (OX-7, Pharmingen), TNF (MP6-XT22, eBioscience), CD127 (A7R34, eBioscience), CD62L (MEL-14, eBioscience), IL-2 (JES6-5H4, Pharmingen), anti-human Granzyme B (Caltag), and isotype controls IgG2a, IgG2b (eBR2a, KLH/Gb-1-2, eBioscience) and mouse IgG1 (Caltag). Ova257-264, and GP33-41 peptides were described(Hogquist et al., 1994; Murali-Krishna et al., 1998).
Quantification of Ag-specific CD8 T cells
The magnitude of the epitope-specific CD8 T cell response was determined either by intracellular IFN-γ staining (ICS) as described(Badovinac et al., 2002), and/or by staining for Thy1.1 marker exclusively expressed on transferred TCR-tg cells. For determination of TCR-tg T cells in the blood, small samples (~20μl) were obtained from tail tip snips at the indicated days p.i. and were processed for individual determination of Thy1.1 CD8+ TCR-tg cells or pooled within groups for additional phenotypic analysis. The number of TCR-Tg T cells (Thy1.1+ CD8+) is presented as frequency of PBL or of CD8+ cells.
Supplementary Material
Acknowledgments
We thank R. Podyminogin for technical assistance, S. Perlman and S. Varga for critical review of the manuscript. Supported by grants from the NIH (J.T.H.) and American Cancer Society (V.P.B. and J.S.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher HP, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8 T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8 T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8 T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8 T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. CD8 T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousso P, Casrouge A, Altman JD, Haury M, Kanellopoulos J, Abastado JP, Kourilsky P. Individual variations in the murine T cell response to a specific peptide reflect variability in naive repertoires. Immunity. 1998;9:169–178. doi: 10.1016/s1074-7613(00)80599-3. [DOI] [PubMed] [Google Scholar]
- Busch DH, Pilip IM, Vijh S, Pamer EG. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8 T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J Exp Med. 2000;191:1241–1246. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Tvinnereim AR, White DW. CD8 T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4 T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19 doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8 memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Ahmed R. Memory CD8 T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002a;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002b;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Kedl RM, Schaefer BC, Kappler JW, Marrack P. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat Immunol. 2002;3:27–32. doi: 10.1038/ni742. [DOI] [PubMed] [Google Scholar]
- Kedzierska K, Day EB, Pi J, Heard SB, Doherty PC, Turner SJ, Perlman S. Quantification of repertoire diversity of influenza-specific epitopes with predominant public or private TCR usage. J Immunol. 2006;177:6705–6712. doi: 10.4049/jimmunol.177.10.6705. [DOI] [PubMed] [Google Scholar]
- Kemp RA, Powell TJ, Dwyer DW, Dutton RW. Cutting edge: regulation of CD8 T cell effector population size. J Immunol. 2004;173:2923–2927. doi: 10.4049/jimmunol.173.5.2923. [DOI] [PubMed] [Google Scholar]
- Kim SK, Reed DS, Olson S, Schnell MJ, Rose JK, Morton PA, Lefrancois L. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc Natl Acad Sci U S A. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8 memory T lymphocyte precursors following peptide immunization. J Immunol. 2005;175:4400–4407. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- Madakamutil LT, Christen U, Lena CJ, Wang-Zhu Y, Attinger A, Sundarrajan M, Ellmeier W, von Herrath MG, Jensen P, Littman DR, Cheroutre H. CD8αα-mediated survival and differentiation of CD8 memory T cell precursors. Science. 2004;304:590–593. doi: 10.1126/science.1092316. [DOI] [PubMed] [Google Scholar]
- Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8 T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Pewe LL, Netland JM, Heard SB, Perlman S. Very diverse CD8 T cell clonotypic responses after virus infections. J Immunol. 2004;172:3151–3156. doi: 10.4049/jimmunol.172.5.3151. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Seder RA, Ahmed R. Similarities and differences in CD4 and CD8 effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Wong P, Lara-Tejero M, Ploss A, Leiner I, Pamer EG. Rapid development of T cell memory. J Immunol. 2004;172:7239–7245. doi: 10.4049/jimmunol.172.12.7239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


