Abstract
Embryonic stem (ES) cell lines provide a unique tool for introducing targeted or random genetic alterations through gene replacement, insertional mutagenesis, and gene addition because they offer the possibility for in vitro selection for the desired, but extremely rare, recombinant genotypes. So far only mouse blastocyst embryos are known to have the competence to give rise to such ES cell lines. We recently have established a stable cell line (Mes1) from blastulae of the medakafish (Oryzias latipes) that shows all characteristics of mouse ES cells in vitro. Here, we demonstrate that Mes1 cells also have the competence for chimera formation; 90% of host blastulae transplanted with Mes1 cells developed into chimeric fry. This high frequency was not compromised by cryostorage or DNA transfection of the donor cells. The Mes1 cells contributed to numerous organs derived from all three germ layers and differentiated into various types of functional cells, most readily observable in pigmented chimeras. These features suggest the possibility that Mes1 cells may be a fish equivalent of mouse ES cells and that medaka can be used as another system for the application of the ES cell technology.
Embryonic stem (ES) cell lines directly derived from early embryos (1, 2) offer an in vitro system to study the molecular mechanism underlying the retention of pluripotency of cells and to elucidate the mechanisms of cell commitment, determination, and differentiation during embryogenesis. More importantly, they represent an invaluable tool to identify and isolate novel developmental genes by gene trapping and insertional mutagenesis and to study the functions of known genes in vivo by gene targeting (3–5). The availability of pluripotent ES cells and the ability to produce chimeras from these cells represent the key steps that link genetic manipulations in vitro and phenotypic analysis in vivo.
Stable ES cell lines so far have been limited to the mouse (1, 2), despite numerous attempts in other mammalian (6–11) and nonmammalian species (12). In all of these cases, cultivation of early embryonic cells was possible only for a limited period (7, 12) or their pluripotency only could be maintained partially after extended culture (8, 11). This difficulty has raised concerns that derivation of stable ES lines will remain a unique feature of small rodents and that the ES/knockout technology will be restricted to the mouse.
Small aquarium fish, like the zebrafish and medaka, have attracted considerable attention as a complementary model system for the analysis of vertebrate development (13–15). Their embryos are transparent and easy to observe and manipulate. This accessibility allows the phenotypic analysis of a particular genetic alteration from the earliest developmental stages onward. Therefore, despite the discouraging situation in many other species, attempts have been made toward the derivation of fish ES cell lines. Recently, we and others have established several stable cell lines from medaka blastula embryos (16, 17). In particular, one of these lines, Mes1, has been shown to retain its normal karyotype and pluripotency in vitro (18).
Chimeric fish have been generated by blastula transplantation of noncultured embryonic cells in zebrafish (19), trout (20), and medaka (21). However, no chimeras so far have been produced from long term cultured fish cells. Although the molecular mechanisms underlying the body plan and pattern formation are highly conserved among vertebrates, there are some important morphological and physiological differences in early embryonic development between fish and mammals. One major difference is that teleost fish embryos lack zygotic transcription before the midblastula stage, whereas in mice, zygotic expression starts as early as at the two-cell stage. Fish ES cells in culture, on the other hand, are transcriptionally active. For chimera formation, they are introduced into a transcriptionally inert embryonic environment, in contrast to the mouse situation, in which the transcriptional status in ES cells and the host embryo is comparable. Second, fish blastula cells undergo dramatic changes in shape and size upon in vitro cultivation, resulting in a 30-fold size difference between host and donor cells. Most importantly, blastula cells in the embryo undergo divisions every 30 min, whereas the doubling time of, e.g., Mes1 cells is 48 h (18). Therefore, the ability to produce chimeras from fish cell cultures remained to be determined. Here, we report the efficient production of viable chimeras from Mes1 cells cultivated for more than 60 passages by cell transplantation into blastula recipients. Using genetic labeling, we show that Mes1 cells differentiate into various types of functional cells and contribute during chimeric embryogenesis to numerous organs derived from all three germ layers.
MATERIALS AND METHODS
Cell Culture and Transfection.
Mes cell lines were derived from blastula-stage embryos of medaka strain HB32C and were maintained under feeder-free culture conditions in ESM3 medium (17, 18). One of these, Mes1, was reinitiated at passages 20–60 from frozen stocks and used for transplantation. Before transplantation, the cells were passaged at least twice in ESM4 medium. The ESM4 medium was modified from the ESM3 medium. ESM4 medium contains 1 mM phenylthiourea (Sigma), higher concentrations of medaka embryo extract (1 embryo/ml), and human basic fibroblast growth factor (10 ng/ml; Tebu, Frankfurt) and lacks human leukemia inhibitory factor.
pCMVgfp is a construct expressing the cDNA for the human codon-optimized, red-shifted mutant green fluorescent protein (hGFP-S65T) from the human cytomegalovirus early enhancer/promoter (CMV). It was derived by removal of a 2-kb BamHI–BamHI fragment containing the neomycin resistance gene from pRc/CMV/GFP, which was constructed by cloning the hGFP-S65T sequence as a HindIII–XbaI fragment from phGFP-S65T (CLONTECH) between the HindIII and XbaI sites in pRc/CMV (Invitrogen). For genetic labeling, Mes1 donor cells were transfected (22) with pCMVgfp and, 2 days later, checked for transfection efficiency by flow cytometry and used for transplantation.
Cell Transplantation.
Single cells were obtained by trypsinization, rinsed, and resuspended in cell transplantation medium (TM: 100 mM NaCl/5 mM KCl/5 mM Hepes, pH 7.1) for microinjection within 2 h at room temperature. Outbred albino medaka strains (i1 and i3) (23, 24) were used as the host. Embryos were collected shortly after fertilization and dechorionated as described (17, 21). For transplantation, they were arranged in a single row on V-shaped 1.5% agarose ramps in Ringer’s solution (25) in 6-cm dishes covered with balanced salt saline (21) containing 1% polyethylene glycol. Microinjection of cells into blastulae was performed by using a self-built cell transplantator system mounted on a Leitz micromanipulator. Transplantation needles were made from 1-mm borosilicate glass capillaries (Clark Electromedical Instruments, Pangbourne, England) with a vertical pipette puller (Bachofer, Reutlingen, Germany). A fine forceps was used to clip the tips of the needles to an opening of 20–30 μm in diameter. The opening was beveled on a capillary sharpener (Bachofer). The needle was filled with TM and connected to the transplantator containing light mineral oil (Sigma). The cell suspension was pipetted onto a flat surface and sucked into the needle. Between 50 and 100 cells were injected into the deep cell layer of each midblastula recipient. Single blastomeres were dispersed (17) and transplanted as described for Mes1 cells, except that the injection needle had a larger opening of 50–60 μm in diameter. The injected embryos were incubated in balanced salt solution–polyethylene glycol mixture at 18°C for the first 2 days and then at 26°C until hatching at day 10. Dechorionated, noninjected control embryos from wild-type and albino strains were reared under the same conditions.
DNA Isolation and PCR.
DNA was isolated from single embryos (25) or adult fish (26). PCR primers TyrA (5′-AAGGAGTGCTGTCCAGTGTGG) and TyrC (5′-TGTGCCTGTGGTGATGACGTA) correspond to positions 421 → 441 and 769 ← 789, respectively, of the medaka tyrosinase cDNA (27); TyrB (5′-GGGGGAGTAATTCAGGGTAGA) corresponds to the very 3′ terminal sequence of the insert interrupting exon 1 of the tyrosinase gene (28). PCR was run for 35 cycles (94°C for 30 s; 58°C for 30 s, and 72°C for 1 min) in a volume of 25 μl containing 50 ng of DNA and 5 pM of each of TyrA and TyrC (set 1) or TyrB and TyrC (set 2). Ten microliters of PCR products from each set was mixed and separated on agarose gels. The intensity of PCR bands was determined by densitometry on an Enhanced Analysis System (Herolab).
RESULTS
The Mes1 line was established from the wild-type pigmented HB32C strain. To investigate whether pigmentation is a useful marker to monitor chimera production in the particular donor and host strain combinations available for this study, albino recipients were transplanted with 10–50 blastula-derived, noncultured cells of the donor strain. Seventy percent of the host embryos developed to chimeras showing variegated black pigmentation. Similar results were obtained with blastula-derived, short term cultured cells (3–9 days) (data not shown).
Eight transplantation experiments were performed with Mes1 cells from various passages (27–66). In each of these experiments, pigmented chimeras were obtained. Altogether, 551 embryos were injected, and 263 survived through the pigmentation stage. Fifteen embryos developed one to many wild-type pigment cells. The overall frequency for pigmented chimeras was 6% (Table 1). The melanocytes in these chimeras were found on the head (four cases), inside the head (two cases), on the trunk (two cases), in the eye (two cases), and on the yolk sac (five cases) (Fig. 1). Continuous examination of these chimeras at various developmental stages revealed that these Mes1-derived cells underwent active proliferation and differentiation in vivo (Fig. 1 C and D).
Table 1.
Production of pigmented chimeras from MES1 cells
| Experiment | Passage- days of culture | Embryos injected, n | Embryos scored, n | Pigmented embryos, n |
|---|---|---|---|---|
| 1. | 27–205 | 78 | 34 | 1 |
| 2. | 28–225 | 96 | 33 | 1 |
| 3. | 31–234 | 69 | 60 | 3 |
| 4. | 37–310 | 100 | 58 | 5 |
| 5. | 36–252 | 57 | 19 | 1 |
| 6. | 40–320 | 44 | 15 | 1 |
| 7. | 66–396 | 43 | 7 | 1 |
| 8. | 66–397 | 64 | 37 | 2 |
| Total | 551 | 263 (48%)* | 15 (6%)† |
Proportion of embryos injected that were alive at the time of scoring for chimerism by pigmentation beginning at day 3: number scored/number injected × 100.
Proportion of embryos scored for pigmentation that showed wild-type pigment cells: number pigmented/number scored × 100.
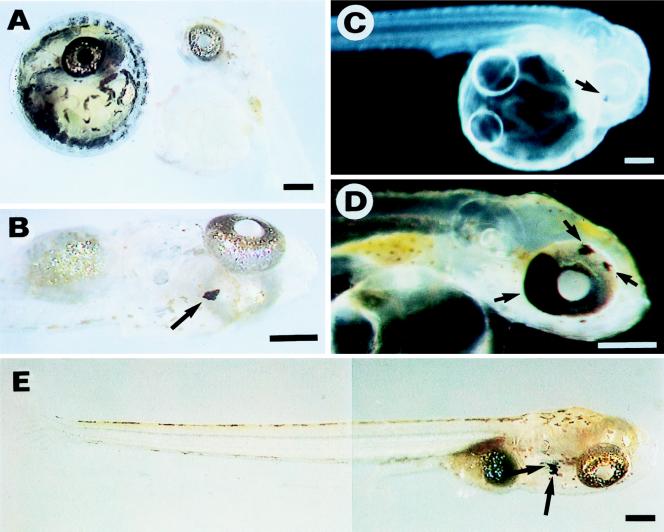
Figure 1.
Pigmented chimeras obtained from transplanted Mes1 cells. (A) Embryos (at day 7) of the donor (Left) and host (Right) strains. The donor, but not host, strain, shows dark pigmentation in the eye, head, trunk, and yolk sac. (B–E) Pigmented chimeras from Mes1 cells transplanted at different passages. Transplantation of cells at passage 31 (234 days of culture) (B), at passage 40 (320 days) (C and D), and at passage 36 (252 days) (E). Arrows indicate Mes1 cell-derived wild-type melanocytes in the chimeras. (B) A chimera showing a melanocyte on the head. (C and D) A chimera at different developmental stages. At day 4 (C), only a single small pigmented area is evident in the retina. By day 10 (D), expansion of this pigmented area to approximately one-third of the whole retina is paralleled by the appearance of two other pigmented areas, indicating proliferation and differentiation of Mes1 cells. (E) Pigmented chimeric fry showing melanocytes inside the head in the opercular region. (Bars = 200 μm.)
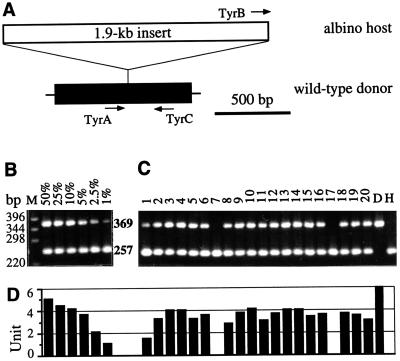
The melanin-containing pigment cells are the end product of a single one of many cell lineages and thus comprise only a minority of the cells in a developing embryo. Therefore, monitoring pigmentation could lead to an underestimation of chimera frequency. To determine more precisely the chimera frequency and degree of chimerism, an assay was devised to identify donor cells in the host embryos by PCR. The albino strain i1 is homozygous for a deficient tyrosinase gene that carries an 1.9-kb insert within its first exon (28). Accordingly, three PCR primers were designed: TyrA and TyrC define a 369-bp fragment specific for the wild-type donor strain, and TyrB and TyrC generate a fragment of 257 bp unique to the albino strain (Fig. 2A). With this assay, it was possible to detect at least 1% contribution of donor-derived DNA in the albino background as determined by serial dilutions of donor in host DNA (Fig. 2B). Of hatchlings and fry, 18 of 20 (8–10 days after transplantation) randomly sampled from the third experiment (Table 1) showed the donor-specific band (Fig. 2C). The contribution of Mes1-derived DNA per chimera was estimated to range roughly from 2 to 10% (Fig. 2D).
Figure 2.
PCR detection of Mes1-derived chimeras. (A) Schematic structure of the tyrosinase gene in the wild-type Mes1 donor (HB32C) and albino host strains (i1). Only the first exon (black box) of the gene and the 1.9-kb insert (open box) interrupting the exon are shown. PCR primers are represented by arrows. TyrA and TyrC define a fragment of 369 bp specific to the donor strain, whereas TyrB and TyrC give rise to a 257-bp fragment unique to the host strain. (B) Sensitivity of the PCR assay. Lane M, 1-kb marker (GIBCO), with sizes shown in base pairs. Numbers in percentages indicate proportions of donor strain-derived DNA diluted with that of the host strain. The 369-bp, donor-specific band is detectable if the donor DNA represents at least 1% of the input DNA. (C) Screening of Mes1-injected embryos from the third transplantation experiment (Table 1). The 369-bp, donor-specific band is evident in 18 of 20 hatchlings and fry from Mes1-transplanted embryos (lanes 1–20) and in the donor (lane D) but not the host (lane H) strain. All host embryos display the 257-bp band. Lanes 1–20, pigmented (lanes 1 and 2) and nonpigmented (lanes 3–20) fry (lanes 1–11) and hatchlings (lanes 12–20). The second pigmented chimeric fry (lane 2) is shown in Fig. 1E. (D) Graphs of intensities of the 369-bp, donor-specific band in the samples shown in B and C. The intensity of the 369-bp band in the 1% lane (B) is equivalent to 1 arbitrary unit.
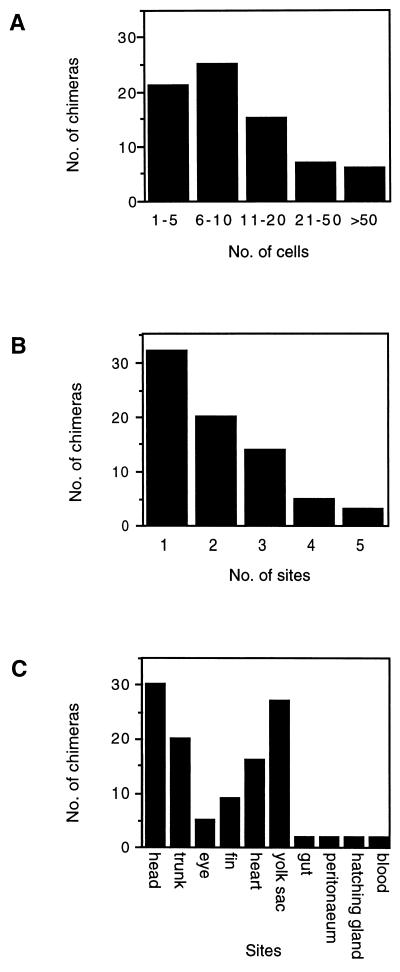
To address whether Mes1 cells are able to contribute also to other cell lineages in addition to the pigment cell lineage, we derived Mes1 cell transfectants transiently expressing GFP from the CMV promoter. This promoter is strong and shows no tissue-specific restriction of reporter expression in medaka (25). After transfection using a modified calcium phosphate precipitation procedure (22), 7% of Mes1 cells expressed GFP before transplantation as determined by flow cytometry. GFP-transfected cultures were used for injection into blastulae and resultant embryos were scored for GFP-expressing cells by fluorescent microscopy. Of hatchlings and fry, 74 of 78 (10 days after transplantation) that developed from 86 injected embryos were GFP-positive. Thus, injection of only 3–7 GFP-positive cells (7% of 50–100 cells) was sufficient to give a 95% colonization rate. The number of GFP-expressing cells varied from 1 to >50 per chimera (Fig. 3A). Such cells were found in 1–5 different compartments (Fig. 3B). They were distributed into a wide variety of tissues and organs including the embryonic integument, internal organs, and extraembryonic structures (e.g., yolk sac) (Fig. 3C). Similar results also were obtained in another series of experiments, where a LacZ expression construct was used for transfecting Mes1 cells.
Figure 3.
GFP-expressing Mes1 cells in 10-day-old chimeras. (A) Number of GFP-expressing Mes1 cells per chimera. (B) Number of distribution sites of GFP-expressing Mes1 cells per chimera. (C) Sites of GFP-expressing Mes1 cells in chimeras. “Head” includes the brain, gill operculum, jaws, otic vesicle; “trunk” includes the tail, spinal cord, notocord, and somites; “eye” means the retina and lens; and “heart” includes the atrium, ventricle, ventral body wall, aorta, and conus arteriosus.
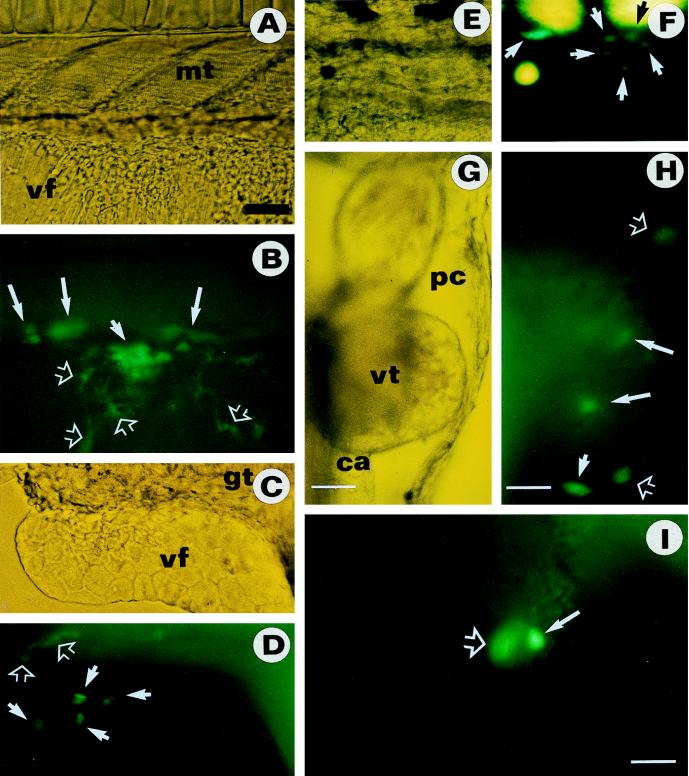
The size and morphology of GFP-expressing cells varied considerably depending on the tissues or organs. Evidence that Mes1 cells developed to terminally differentiated cells from not only the pigment cell lineage but also from other cell lineages came from chimeras having GFP-positive cells in the fin and heart. In the fin, individual large, flat epithelial cells could be identified, and GFP-positive donor cells were indistinguishable from recipient cells in morphology (Fig. 4 C and D). In the heart, the GFP-expressing donor cells were found in the atrium, ventricle, and associated structures (Fig. 4 G, H, and I). These elongated cells contracted rhythmically. They are assumed to represent differentiated heart muscle cells.
Figure 4.
Chimeric fry from Mes1 cells transiently expressing GFP. (A, C, E, and G) Bright-field micrographs. (B, D, F, H, and I) Dark-field fluorescent micrographs. (A and B) GFP-expressing Mes1 cells in the myotome (mt; large arrows) and the ventral fin (vt) as clustered (small arrow) and single (hollow arrows) cells. (C and D) GFP-expressing Mes1 cells in the ventral fin (vf; arrows) and the gut (gt; hollow arrows). Note the distinct epithelial phenotype of GFP-expressing cells that are morphologically indistinguishable from surrounding recipient cells. The gut is recognizable by its background autofluorescence. (E and F) GFP-expressing Mes1 cells in the trunk (arrows). GFP-positive cells show green fluorescence and can be distinguished from large, yellow autofluorescent recipient pigment cells. (G and H) GFP-expressing Mes1 cells in the ventral body wall surrounding the pericard (pc; hollow arrows), the ventricle (vt; large arrows), and conus arteriosus (ca; small arrow) of the heart. (I) GFP-expressing Mes1 cells in the atrium (solid arrow) and ventricle (hollow arrow) of the heart. The anterior is to the right, the dorsal side is up. [Bar = 50 μm (A–F and I) and 10 μm (G and H).]
DISCUSSION
This study demonstrates that, in fish, despite a number of dramatic physiological differences between long term cultured blastula-derived cells and developing embryos, it is possible to generate viable chimeras at a high frequency by using the Mes1 cell line in medaka. The present findings also reveal two prominent properties of Mes1 cells. First, these cells retain their in vivo pluripotency because they are able to survive, proliferate, and contribute to many different cell lineages and differentiate into functional cell types (e.g., pigment cells, cardiac muscle cells, and fin epithelial cells) during chimeric embryogenesis. Second, they maintain this pluripotency after cryostorage and genetic manipulations in vitro because thereafter their ability to participate in host embryogenesis was not restricted. Thus, Mes1 cells appear to be a fish equivalent of murine ES cells (1, 2).
To date, in vertebrates other than the mouse, pigmented chimeras from cultured cells have been reported in chicken (12) and zebrafish (29). In chicken, however, these cells had been cultured only for few passages before transplantation into recipients. In zebrafish, pigmented embryos have been obtained from blastula-derived cells that had been cultured for only 2 days before being used for transplantation. In the latter case, those cell cultures consisted of a significant portion of well differentiated melanocytes. Therefore, it is not clear whether the melanocytes appearing in the albino host embryos resulted from in vitro-differentiated melanocytes or from their committed precursors already present in the cell culture injected. In our experiments, Mes1 cells during long term cultivation under the conditions described did not develop a single melanocyte. Thus, the pigmented fry from transplanted albino blastulae are definite chimeras, with their pigmented cells being the descendants of in vivo differentiated derivatives of pluripotent Mes1 cells.
In the present study, chimerism was analyzed by three lines of evidence. Black pigment cells of the ES cell donor strain in albino recipients demonstrated the functional contribution of donor cells to the pigment lineage. Melanin pigmentation is therefore a useful marker for the documentation of single Mes1 cells in that specific lineage in living chimeras. This method revealed a chimera frequency of 6%. Because the pigment cell lineage represents only a minor fraction of embryonic tissues, the chimera frequency judged by pigmentation is expected to be an underestimation. Indeed, a significantly higher chimera frequency was obtained by PCR. This method is sensitive enough to detect chimeras harboring as low as 1% of donor cells in any tissue. It revealed a chimera frequency of 90%. Furthermore, it allows for a quantitative estimation of the proportional contribution from donor cells to chimeras. Genetic labeling of donor cells by transfection with a GFP expression construct detected a chimera frequency of up to 95%. It proved the most powerful approach to analyze differentiation of Mes1 cells in vivo. Living embryos can be examined continuously, and even a single GFP-expressing donor cell provides a signal sufficient for microscopic observation. Using this approach, we were able to show proliferation and differentiation of transplanted Mes1 cells and their contribution to all major organs in the chimeras.
During transplantation, 50–100 Mes1 donor cells were injected into each recipient embryo. At the time of injection, 7% of Mes1 donor cells were GFP-positive. If injected Mes1 cells were not proliferative, then chimeras would be expected to have only 3–7 GFP-positive cells. That some of the chimeras contained >50 GFP-positive cells indicates a eightfold increase in cell number and thus the active proliferation of Mes1 cells during embryogenesis. Indeed, Mes1 cell proliferation was clearly visible in those chimeras in which the pigmented area expanded continuously.
In mice, the successful production of chimeras is depending not only on the pluripotency of ES cells but also on the genetic compatibility between the ES cell and host strains (30). The availability of numerous different medaka strains will make it possible to determine whether this is also true in fish and to identify the most suitable combinations of donor–host strains. In fish, there are some important morphological and physiological differences between ES cells and host embryos. Although such differences, as shown here, do not prevent the generation of chimeric embryos, they may influence the frequency of chimeras and the degree of chimerism. Thus, it can be anticipated that optimization of transplantation conditions after testing a plethora of parameters (e.g., adjusting host and donor cell cycles and testing donor–host strains) will further improve the efficacy of chimera production and the contribution of fish ES cells to every cell lineage including the germ line.
The ability of Mes1 cells to differentiate into various functional cell types and their wide distribution into all major organ systems derived from all three germ layers suggest that they are not restricted in their potential to contribute to particular cell lineages. As the first long term cultured ES cell line established from a nonmurine vertebrate species that gives rise to somatic chimeras with high efficacy, the Mes1 line provides a system to study many aspects of cell differentiation in vitro and in chimeric embryos in fish as a lower vertebrate system. Its pluripotency and normal karyotype make this cell line a potent source for the production of genetically manipulated cell culture-derived animals by cell transplantation or nuclear transplantation.
Acknowledgments
We thank Dr. Y. Hyodo-Taguchi, Chiba, Japan, for kindly supplying founder fish for our medaka stocks, Dr. K. Friedrich for pRc/CMV/GFP, Dr. A. Simm for the cytometric analysis, and J. Deng for breeding fish. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 465), the Commission of the European Community (BIO2-CT-93-0430), and the Fonds der Chemischen Industrie to M.S.
ABBREVIATIONS
- ES
embryonic stem
- GFP
green fluorescent protein
- Mes
medaka ES
- ESM
ES cell medium
- CMV
cytomegalovirus
References
- 1.Evans M J, Kaufman M H. Nature (London) 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin G R. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyner A L. BioEssays. 1991;13:649–656. doi: 10.1002/bies.950131206. [DOI] [PubMed] [Google Scholar]
- 4.Pascoe W, Kemler R, Wood S A. Biochim Biophys Acta. 1991;1114:209–221. doi: 10.1016/0304-419x(92)90016-r. [DOI] [PubMed] [Google Scholar]
- 5.Doetschman T, Williams P, Maeda N. Dev Biol. 1988;127:224–227. doi: 10.1016/0012-1606(88)90204-7. [DOI] [PubMed] [Google Scholar]
- 6.Notarianni, E., Galli, C., Laurie, S., Moor, R. M. & Evans, M. J. (1991) J. Reprod. Fertil. 43, Suppl., 255–260. [PubMed]
- 7.Sukoyan M A, Golubitsa A N, Zhelezova A I, Shilov A G, Vatolin S Y, Maximovsky L P, Andereeva L E, McWhir J, Pack S D, Bayborodin S I, et al. Mol Reprod Dev. 1992;33:418–431. doi: 10.1002/mrd.1080330408. [DOI] [PubMed] [Google Scholar]
- 8.Sims M, First N L. Proc Natl Acad Sci USA. 1993;90:6143–6147. doi: 10.1073/pnas.91.13.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iannaccone P M, Taborn G U, Garton R L, Caplice M D, Brenin D R. Dev Biol. 1994;163:288–292. doi: 10.1006/dbio.1994.1146. [DOI] [PubMed] [Google Scholar]
- 10.Thomson J A, Kalishman J, Golos T G, Durning M, Harris C P, Becker R A, Hearn J P. Proc Natl Acad Sci USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell K H S, McWhir J, Ritchie W A, Wilmut I. Nature (London) 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- 12.Pain B, Clark M E, Shen M, Nakazawa H, Sakurai M, Samarut J, Etches R J. Development. 1996;122:2339–2348. doi: 10.1242/dev.122.8.2339. [DOI] [PubMed] [Google Scholar]
- 13.Powers D A. Science. 1989;246:352–358. doi: 10.1126/science.2678474. [DOI] [PubMed] [Google Scholar]
- 14.Shima A, Shimada A. Proc Natl Acad Sci USA. 1991;88:2545–2549. doi: 10.1073/pnas.88.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozato K, Wakamatsu Y. Dev Growth Differ. 1994;36:437–443. doi: 10.1111/j.1440-169X.1994.00437.x. [DOI] [PubMed] [Google Scholar]
- 16.Wakamatsu Y, Ozato K, Sasado T. Mol Mar Biol Biotechnol. 1994;3:185–191. [PubMed] [Google Scholar]
- 17.Hong Y, Schartl M. Mol Mar Biol Biotechnol. 1996;5:93–104. [PubMed] [Google Scholar]
- 18.Hong Y, Winkler C, Schartl M. Mech Dev. 1996;60:33–44. doi: 10.1016/s0925-4773(96)00596-5. [DOI] [PubMed] [Google Scholar]
- 19.Lin S, Long W, Chen J, Hopkins N. Proc Natl Acad Sci USA. 1992;89:4519–4523. doi: 10.1073/pnas.89.10.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson E, Cloud J G. Proc Natl Acad Sci USA. 1992;89:9425–9428. doi: 10.1073/pnas.89.20.9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakamatsu Y, Ozato K, Hashimoto H, Kinoshita M, Sakaguchi M, Iwamatsu T, Hyodo-Taguchi Y, Tomita H. Mol Mar Biol Biotechnol. 1993;2:325–332. [Google Scholar]
- 22.Hong Y, Winkler C, Brem G, Schartl M. Aquaculture. 1993;111:215–226. [Google Scholar]
- 23.Hyodo-Taguchi Y, Egami N. Zool Sci. 1985;2:305–316. [Google Scholar]
- 24.Hyodo-Taguchi Y, Winkler C, Kurihara Y, Schartl A, Schartl M. Mech Dev. 1997;68:27–35. doi: 10.1016/s0925-4773(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 25.Winkler C, Vielkind J R, Schartl M. Mol Gen Genet. 1991;226:129–140. doi: 10.1007/BF00273596. [DOI] [PubMed] [Google Scholar]
- 26.Schartl M, Wilde B, Schlupp I, Parzefall J. Evolution. 1995;49:827–835. doi: 10.1111/j.1558-5646.1995.tb02319.x. [DOI] [PubMed] [Google Scholar]
- 27.Inagaki H, Bessho Y, Koga A, Hori H. Gene. 1994;150:319–324. doi: 10.1016/0378-1119(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 28.Koga A, Suzuki M, Inagaki H, Bessho Y, Hori H. Nature (London) 1996;383:30. doi: 10.1038/383030a0. [DOI] [PubMed] [Google Scholar]
- 29.Bradford C S, Sun L, Barnes D W. Mol Mar Biol Biotechnol. 1994;3:78–86. [PubMed] [Google Scholar]
- 30.Kawase E, Suemori H, Takahashi N, Okazaki K, Hashimoto K, Nakatsuji N. Int J Dev Biol. 1994;38:385–390. [PubMed] [Google Scholar]