Abstract
Background
Few data exist on the intergenerational influence of calcium intake during pregnancy on offspring blood pressure.
Methods and Results
As part of the ongoing US prospective cohort study Project Viva, we analyzed 4091 Dinamap blood pressure measurements from 936 six-month-old infants whose mothers had completed food frequency questionnaires during the second trimester of pregnancy. We used mixed models to estimate effects of maternal calcium intake on offspring systolic blood pressure. Mean±SD daily total maternal calcium intake was 1494±523 mg, consisting of 1230±486 mg from foods and 264±191 mg from supplements. Mean±SD 6-month blood pressure was 89.9±12.9 mm Hg. From bottom to top quartile of dietary calcium from foods adjusted for energy intake and measurement conditions, mean infant systolic blood pressures were 91.0, 90.2, 90.9, and 90.2 mm Hg (trend P=0.62). From calcium supplements only, the values were 91.5, 90.2, 90.4, and 88.4 mm Hg (trend P=0.006). After further adjustment for demographic, anthropometric, dietary, social, and economic variables, the decrease in 6-month systolic blood pressure was −3.0 mm Hg (95% CI, −4.9 to −1.1) for each 500-mg increment of maternal supplemental calcium intake during pregnancy. We did not find evidence of effect modification by maternal vitamin D or potassium intake or by infant body mass index. First-trimester calcium intake was not associated with offspring blood pressure.
Conclusions
These observational data suggest that supplementing maternal midgestational calcium intake may lower offspring blood pressure, thus helping to prevent hypertension in the next generation.
Keywords: pregnancy, calcium, blood pressure, pediatrics
Increased calcium intake appears to lower blood pressure levels among children and among pregnant women, especially those with habitually low calcium intake.1–6 Two studies7,8 have suggested that calcium intake during pregnancy is inversely associated with blood pressure in the offspring. This issue is important because, if confirmed, ensuring adequate calcium intake among pregnant women could be a way to prevent hypertension and its sequelae in the next generation. In one of these 2 studies, among 70 mother-infant pairs, McGarvey et al7 reported correlation coefficients of −0.18 and −0.22 between maternal gestational calcium intake and systolic blood pressure at 6 and 12 months of age, respectively. More recently, Belizan et al8 followed up 591 children 5 to 9 years of age whose mothers had taken part in a randomized controlled trial of calcium supplementation started at 20 weeks’ gestation and stopped at delivery. Mean systolic blood pressure was 1.4 mm Hg lower among children of mothers in the intervention group compared with the placebo group, but the 95% CI (−3.2 to 0.5 mm Hg) was consistent with both a small direct effect and the more likely inverse effect. At least one animal model also provides evidence that increased calcium intake during pregnancy causes lower blood pressure in offspring.9
This relative paucity of human and experimental data, coupled with the growing general interest in the effects of maternal diet on offspring cardiovascular risk,10 warrants additional examination of the link between maternal calcium intake and offspring blood pressure. The purpose of this analysis was to examine the associations of calcium intake during pregnancy with systolic blood pressure at 6 months of age among participants in Project Viva, an ongoing US cohort of pregnant women and their children. Because the one previous randomized trial began calcium supplementation in midpregnancy, we elected to focus primarily on calcium intake during the second trimester. In addition to estimating differences between food and supplement sources of calcium, we were interested in the extent to which effects were modified by other maternal dietary factors and by childhood weight status.
Methods
Subjects
Between April 1999 and July 2002, we recruited participants into Project Viva at 8 offices of Harvard Vanguard Medical Associates, a large multispecialty urban/suburban group practice in eastern Massachusetts. At the first study visit, which immediately followed the woman’s initial clinical prenatal visit, we obtained informed consent, administered a brief interview, and provided a take-home self-administered questionnaire that included a validated food frequency questionnaire (FFQ) to assess the woman’s diet in the first trimester.11 At the second study visit at 26 to 28 weeks’ gestation, we again administered a brief interview and provided a questionnaire that included an FFQ covering dietary intake during the second trimester. Within 3 days after delivery, we briefly interviewed the mother in the hospital and performed anthropometric and blood pressure measurements on the infant. At 6 months after birth, we updated covariate information from the mother, and we obtained anthropometric and blood pressure measurements from both mother and child.
Exclusion criteria included multiple gestation (twins, triplets, etc), inability to answer questions in English, plans to move from the area before delivery, and gestational age >22 completed weeks at initial prenatal clinical appointment. Additional details of recruitment and follow-up are presented elsewhere.12
Among 2128 delivered infants in Project Viva, we excluded 462 because of missing second-trimester diet assessment data. Mothers of 232 of the remaining 1666 children did not provide informed consent for examination of the child at 6 months. A further 381 were invited to attend the 6-month examination but did not, chiefly owing to logistical constraints such as availability of transportation.
Among an additional 77, we were not able to obtain blood pressure measurements; in 63 of these 77, the reason was the high, somewhat uncomfortable maximum inflation pressure of the older blood pressure recorder that we used for the first 206 participants at the 6-month examination. Finally, 1 infant had implausible blood pressure values, and 39 had missing covariate data, leaving a sample for analysis of 936 mother-infant pairs with complete data. Human subjects committees of Harvard Pilgrim Health Care, Brigham and Women’s Hospital, and Beth Israel Deaconess Medical Center approved the study protocols.
Measurements
Details of the names, sources, and types of variables used in Project Viva are given elsewhere.12 Briefly, at the 2 visits during pregnancy, in addition to assessment, we collected demographic, social, economic, and health information. From prenatal outpatient medical records, we obtained serial maternal weights and blood pressure measurements, and we obtained gestational dating measurements from ultrasound examinations. At both newborn and 6-month time points, we measured infant length with a research standard length board and blood pressure data with a Dinamap (Critikon, Inc) Pro 100 (or, before February 21, 2001, model 8100) automated oscillometric recorder. For each of 5 measurements taken 1 minute apart, we also recorded infant position (supine or in bouncy seat), extremity used, cuff size, infant state (quiet sleep, active sleep, quiet awake, crying), time of day, and ambient temperature. At the 6-month visit, we measured the mother’s blood pressure, weight, and height, and we queried the mother about infant feeding practices in the first 6 months of life.
At both the early- and later-pregnancy visits, we evaluated the maternal diet assessment with a semiquantitative FFQ, which was modified for use in pregnancy from the extensively validated FFQ used in the Nurses’ Health Study and other large cohort studies and further biochemically calibrated for use in pregnancy.11 The time referent for the first-trimester FFQ was “during this pregnancy,” ie, from the date of the last menstrual period until the assessment. To assess vitamin and supplement intake periconceptionally and during the first trimester, we administered a separate interview. The FFQ administered later in pregnancy reflected intakes during the second trimester; the time referent was “the past 3 months.” For this instrument, we queried vitamins/supplements as part of the self-completed FFQ. To calculate intake of calcium and other nutrients from foods, we used the Harvard nutrient database used for the Nurses’ Health Study and other large cohort studies.13
Data Analysis
Our main outcome was systolic blood pressure at 6 months of age, which we measured in each infant up to 5 times on a single occasion. We chose systolic over diastolic pressure because it is a better predictor of later outcomes and is more accurately measured with the instrument we used.14,15 In total, 606 infants had 5 measurements, 173 had 4, 84 had 3, 44 had 2, and 29 had 1, for a total of 4091 measurements. Although the first measurement is generally higher than the second through fifth measurement, including it in analyses tends to improve precision when absolute levels are not as important as differences.16
To assess associations between predictors and systolic blood pressure, we used mixed models that incorporate each of the up to 5 blood pressure measurements from each infant as repeated outcome measures.17 An advantage of this modeling approach, compared with using the average of available measures for each child as the outcome, is that individuals with more measurements and less variability among those measurements get more weight than those with fewer measurements and/or more variability.
We assessed confounding by examining the association of predictors of interest with blood pressure before and after adding covariates to the model. All models, including the “unadjusted” model, included adjustment for energy intake and for the blood pressure measurement conditions of cuff size, infant position, appendage used, machine model, measurement sequence number, infant state, and clinic site. Ambient temperature did not add information, so we did not include it in the models. We used the nutrient residuals method to control for energy intake.18 We ran separate models for calcium from foods only and calcium from supplements only. To ensure approximate linearity, we first ran these unadjusted models by examining infant blood pressure by quartile of maternal calcium intake. In further multivariate models, we calculated linear regression effect estimates and 95% CIs for a 500-mg increment of elemental calcium intake, which was close to the difference in mean intakes between top and bottom quartiles of supplemental calcium (424 mg). We used SAS version 8.02 (SAS Institute) for all analyses.
Results
Of the 936 women, 25% classified themselves as racial/ethnic minorities (Table 1). Reflective of a generally employed and insured managed-care population, few women had less than a high school education or had annual household incomes below $20 000. Compared with mothers of infants in the entire cohort, participants in this analysis had higher educational status (37% versus 29% completed more than a college degree) and were more often white (75% versus 66%) but were similar in household income, marital status, age, and body mass index.
TABLE 1.
Participant Characteristics
| Maternal Characteristics | Mean (SD) or n (%) |
|---|---|
| Daily calcium intake, mg, mean (SD) | |
| Second trimester | |
| Total | 1494 (523) |
| From foods only | 1230 (486) |
| From supplements | 264 (191) |
| First trimester | |
| Total | 1330 (516) |
| From foods only | 1128 (461) |
| From supplements | 203 (242) |
| Daily intake of other nutrients, second trimester, mean (SD) | |
| Potassium, mg | 3363 (1101) |
| Magnesium, mg | 350 (115) |
| Vitamin D, IU | 604 (193) |
| Daily energy intake, kcal, mean (SD) | |
| Second trimester | 2113 (613) |
| First trimester | 2043 (634) |
| Other maternal factors, mean (SD) | |
| Age at enrollment, y | 32.5 (4.7) |
| Prepregnancy body mass index, kg/m2 | 24.4 (5.1) |
| Third-trimester systolic blood pressure, mm Hg | 111.3 (8.1) |
| Race/ethnicity, n (%) | |
| White | 698 (75) |
| Black or African American | 101 (11) |
| Hispanic or Latina | 48 (5) |
| Asian | 50 (5) |
| Other | 39 (4) |
| Highest grade level completed, n (%) | |
| Less than high school or high school diploma | 58 (6) |
| Some college/technical school | 190 (20) |
| College graduate | 343 (37) |
| Postgraduate degree | 345 (37) |
| Marital status, n (%) | |
| Married or cohabitating | 886 (95) |
| Divorced/separated/never married/other | 50 (5) |
| Previous pregnancies, n (%) | |
| 0 | 308 (33) |
| ≥1 | 628 (67) |
| Household income, n (%) | |
| ≤$20 000 | 19 (2) |
| $20 001–$40 000 | 71 (8) |
| $40 001–$70 000 | 207 (22) |
| >70 000 | 587 (63) |
| Don’t know | 19 (2) |
| Missing | 33 (4) |
| Infant characteristics | |
| Sex, n (%) | |
| Girl | 470 (50) |
| Boy | 466 (50) |
| Newborn period, n (%) | |
| Birth weight, g | 3510 (531) |
| Infant feeding at 6 months, n (%) | |
| Breast only | 241 (26) |
| Mixed | 241 (26) |
| Weaned | 369 (39) |
| Formula only | 85 (9) |
| At 6-mo visit, mean (SD) | |
| Age, mo | 6.5 (0.7) |
| Length, cm | 66.7 (2.7) |
| Weight, kg | 8.1 (1.0) |
| Body mass index, kg/m2 | 18.2 (1.8) |
| Systolic blood pressure, mm Hg | 89.9 (12.9) |
Data are from 936 mother-infant pairs participating in Project Viva.
Mean second-trimester maternal calcium intake was 1494 mg/d, including contributions from both foods and calcium supplements (Table 1). Approximately 94% of the women were taking supplements, chiefly in the form prenatal vitamin preparations, from which the mean daily calcium intake was 195 mg. Approximately 29% of subjects were taking additional calcium supplements, primarily in the form of calcium-containing antacids, with a mean calcium intake of 281 mg. From foods only, the mean calcium intake was 1230 mg. First-trimester calcium intake was similar, a mean of 1330 mg from foods and 203 mg from supplements. First- and second-trimester total energy intakes were 2043 and 2113 kcal, respectively (Table 1).
Mean gestational age at birth was 39.6 weeks. Approximately 6% of infants were born before 37 completed weeks’ gestation, and about 2% were born at ≥42 weeks’ gestation. Mean birth weight was 3510 g (Table 1), and the correlation between maternal calcium intake and birth weight was minimal (Pearson’s r=0.02 for supplemental calcium). Mean systolic blood pressure at 6 months of age, 89.9 mm Hg, was comparable to estimates from other studies of children of this age.19–22
In unadjusted analysis that controlled only for blood pressure measurement conditions and energy intake, total maternal consumption of calcium in the second trimester of pregnancy was modestly inversely associated with infant systolic blood pressure level, with a difference between top and bottom quartiles of intake of −2.3 mm Hg (trend across quartiles P=0.06). However, this finding masked 2 different associations: No association was evident for calcium from foods only (−0.8 mm Hg; trend P=0.62), and there was a strong inverse association with calcium supplements (−3.1 mm Hg; trend P=0.006) (Figure). We did not observe effects on offspring blood pressure of calcium intake in the first trimester from either foods (−0.1 mm Hg; trend P=0.75) or supplements (−1.3 mm Hg; trend P=0.37).
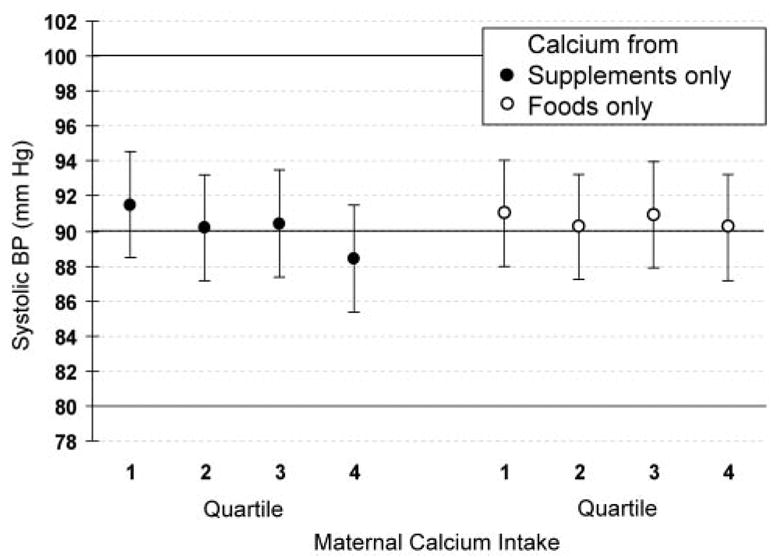
Systolic blood pressure at 6 months of age by quartile of maternal calcium intake in second trimester of pregnancy adjusted for blood pressure measurement conditions and energy intake. Data are from 936 mother-infant pairs participating in Project Viva. Values are mean±SE. For trend across quartiles, P=0.62 for food sources of calcium, P=0.006 for supplemental sources.
Further multivariate models confirmed these observations, showing little confounding by demographic, socioeconomic, or anthropometric variables (Table 2). For calcium from supplements, the unadjusted model estimated a decrease of −3.4 mm Hg (95% CI, −5.3 to −1.4) for each 500-mg increment of ingested calcium. The addition of sociodemographic variables, maternal body mass index, late-gestation blood pressure, and infant body mass index resulted in slight attenuation of the estimate to −3.0 mm Hg (95% CI, −4.9 to −1.1). Finally, addition to the model of birth weight, which could be construed as an intermediate in the causal pathway instead of a true confounder, did not change the estimate (Table 2). In a separate analysis including dietary calcium from foods only, the fully adjusted estimate was −0.04 mm Hg (95% CI, −1.1 to 1.0) for each 500-mg increment in daily intake (Table 2).
TABLE 2.
Association of Maternal Second-Trimester Calcium Intake With Offspring Systolic Blood Pressure at 6 Months of Age
| Estimate (95% CI)
|
|||
|---|---|---|---|
| Model | Covariates | Calcium From Foods Only | Calcium From Supplements Only |
| 1 | Crude (adjusted for energy + blood pressure measurement conditions only)* | −0.3 (−1.4–0.7) | −3.4 (−5.3–−1.4) |
| 2 | Model 1 + sociodemographic factors, maternal BMI, and blood pressure† | −0.1 (−1.1–1.0) | −3.1 (−5.0–−1.2) |
| 3 | Model 2 + infant BMI at 6 months | −0.02 (−1.1–1.0) | −3.0 (−4.9–−1.1) |
| 4 | Model 3 + birth weight | −0.04 (−1.1–1.0) | −3.0 (−4.9–−1.1) |
BMI indicates body mass index. Data are from multivariate mixed regression models among 936 mother-infant pairs participating in Project Viva. Regression estimate is change in infant blood pressure (mm Hg) for each 500-mg increment in maternal calcium intake.
Measurement conditions were infant state, extremity, cuff size, body position, measurement sequence number, and clinic site.
Maternal race/ethnicity, education, number of previous pregnancies, marital status, prepregnancy body mass index and third-trimester systolic blood pressure, and infant age and sex.
Addition to the model of maternal intakes of potassium, magnesium, and/or vitamin D or of breastfeeding status at 6 months of age did not materially change the estimates for supplemental calcium, nor did any of these nutrients predict the blood pressure outcome (data not shown). Given previous work suggesting effect modification by offspring body mass index,8 we also examined the associations of maternal supplemental calcium intake with 6-month blood pressure within quartiles of infant body mass index. In the fully adjusted multivariate models, the estimates from bottom to top body mass index quartile were 0.8, −4.4, −3.9, and −4.4 mm Hg for a 500-mg increment in supplemental calcium intake. Although these findings may imply a calcium effect limited to the top 3 quartiles of infant body mass index, the P value for a multiplicative interaction term with a continuous term for calcium intake was 0.20, arguing against substantial effect modification. We also found no effect modification by intake of potassium or vitamin D. For potassium intake above the median (3204 mg), the association between supplemental calcium and offspring blood pressure was −3.2 mm Hg; for below the median, the estimate was −3.1 mm Hg. For strata defined by intake of vitamin D (median, 610 IU), analogous estimates were −3.9 and −3.0 mm Hg.
Because most supplemental calcium was derived from prescribed prenatal vitamins, we also considered the possibility that the effects we observed from supplemental calcium were the result of constituents of these multiple vitamin preparations other than the calcium itself. In this analysis, we compared the 6-month systolic blood pressure level among infants of mothers in 3 groups: the 55 who took no supplements, the 608 whose only supplement was a prenatal vitamin preparation, and the 266 who took both prenatal vitamins and additional calcium supplements. Too few (n=7) took additional calcium but no prenatal vitamin preparation to be included in this analysis. Compared with nontakers, the covariate-adjusted blood pressure level among takers of only prenatal vitamins was −1.7 mm Hg (95% CI, −4.8 to 1.4) lower compared with −4.0 mm Hg (95% CI, −7.3 to −0.7) lower among takers of both types of supplements.
Discussion
The paradigm of fetal origins of cardiovascular disease posits that perturbations to the in utero environment have long-lasting influence on cardiovascular risk.10 One type of perturbation is alteration of maternal diet, because maternal diet is one part of the “supply line” that provides nutrients to the fetus.23,24 Maternal intake of calcium is of particular interest, especially as it relates to blood pressure, a major cardiovascular risk factor.
In this prospective cohort study of pregnant women and their infant offspring, we found that maternal calcium intake during the second trimester of pregnancy was inversely associated with systolic blood pressure level at 6 months of age. This association, however, was limited to a strong association with supplemental calcium, with no evidence for an association with calcium derived from food sources. This finding confirms and extends data from an experimental animal model9 and a randomized controlled trial among humans,8 both of which suggested an effect of maternal midgestational calcium supplementation on lowering offspring blood pressure. Although randomized studies tend to minimize confounding of unmeasured and measured covariates, advantages of our observational study included the ability to examine food and supplemental sources of calcium, to assess first- and second-trimester intakes, and to control for variation in postnatal factors. In addition, there was little evidence for confounding by social, economic, or anthropometric factors in our analyses. One other observational study also found an inverse association between calcium intake during pregnancy and both systolic and diastolic blood pressures at 12 and 6 months of age.7 In that study, however, it was not clear how much of the ingested calcium was from foods compared with supplements; the diet assessment was conducted postpartum; and the time referent for calcium intake was the entire pregnancy, not a specific trimester.
The association of blood pressure with supplemental calcium but not with calcium from foods may be due to differential physiological effects. One small study of non-pregnant women showed effects on parathyroid hormone levels in response to ingested calcium that were stronger with a calcium salt supplement than with milk, sesame seeds, or spinach but not with cheese.25 Another small study among young adults demonstrated that bioavailability of calcium from supplements was at least as high as from yogurt and higher than from liquid milk.26 Large observational studies of both men and women have noted differential effects of supplemental and food calcium on a different end point, kidney stones in adults.27,28 In those studies, calcium from foods appeared to be protective, whereas supplemental calcium was unrelated to or slightly increased the risk of nephrolithiasis. The mechanism underlying that difference is not clear but may be related to absorption or renal handling of oxalate, a frequent constituent of kidney stones.29 Such a mechanism, however, is probably not a factor in the association of calcium intake with blood pressure regulation, which more likely is related to effects on parathyroid hormone or vitamin D metabolism.30 In contrast to studies showing different effects of supplemental compared with food-derived calcium, at least 2 others have demonstrated similar absorption or bioavailability of calcium from calcium carbonate and from a variety of food sources,31,32 although those 2 studies involved much older participants. Also, a meta-analysis of randomized trials of increasing calcium intake among non-pregnant adults showed that the small reductions in blood pressure were not different between dietary and nondietary calcium sources.33 In any case, the lack of published physiological data among pregnant women allows only speculative comments about these issues.
Another possibility for the apparent protective effect of supplemental calcium is that it is not the calcium at all. Approximately two thirds of supplemental calcium in our study was derived from the use of multivitamins or prescribed prenatal vitamins as opposed to specific calcium supplements. Thus, some other constituent of the vitamin preparations could be the active moiety. We found, however, that infants of mothers who took both multiple vitamin preparations and additional calcium supplements had lower blood pressure levels than did infants whose mothers took prenatal vitamins alone. Furthermore, we did not find other potentially active nutrients, including potassium, magnesium, and vitamin D, to be important predictors or confounders. A further possibility is that some unmeasured characteristic of those who take vitamin could explain the results. But adjustment for an assortment of sociodemographic factors did not materially change the magnitude of observed effects. Thus, we conclude that the observed effect of supplemental calcium is likely the result of the calcium itself.
The study of Belizan et al8 suggested a stronger effect of calcium supplementation on blood pressure of children with higher body mass index. In contrast to their findings, we did not observe a monotonic increase in effect estimates as body mass index increased, although our findings raise the possibility that the blood pressure–lowering effect of calcium may be restricted to the top 3 quartiles of offspring body mass index.
Most studies of maternal diet and offspring cardiovascular risk have relied on poor proxies for maternal diet such as birth weight,10 population experiences of malnutrition,34,35 crude summary nutrient measures,36,37 or retrospective diet histories.7 Other studies of childhood blood pressure with prospectively collected maternal diet information have not reported data on calcium intake.38 In contrast, this study incorporated validated FFQs administered prospectively near the end of the first and second trimesters of pregnancy. Other strengths of this study included multiple measurements of blood pressure on each infant and extensive covariate information.
The relatively high socioeconomic position of our participants could limit generalizability. Loss to follow-up of participants from birth to 6 months produced a sample for analysis with relatively fewer participants from minority racial/ethnic groups and lower socioeconomic strata. Although 6 months is a relatively early age to measure blood pressure outcomes, blood pressure tracking throughout childhood starts at this age,19,39,40 and blood pressure–lowering effects of at least one intervention in the first 6 months of life, sodium restriction, appear to be long-lasting.41,42 Nevertheless, follow-up of this cohort to later ages will add useful information to the question of enduring effects of maternal calcium on offspring blood pressure.
In conclusion, in this observational study, higher maternal intake of calcium from supplements in midpregnancy was associated with lower blood pressure in the offspring. These findings support the importance of pregnant women consuming adequate amounts of calcium. Although several studies of pregnant women in developed countries, including this one, show that mean intakes of calcium exceed recommended standards,43–45 a sizable minority of such women do not meet these recommendations. Moreover, a large proportion of pregnant women in at least one developing country appear to be calcium deficient.4 Previous studies show that ensuring sufficient calcium intake during pregnancy prevents adverse hypertensive outcomes of pregnancy,6 and our findings suggest that it may also lower the risk of offspring hypertension.
Acknowledgments
This work was supported by grants from the US National Institutes of Health (HD-34568, HL-64925, HL-68041) and by Harvard Medical School and the Harvard Pilgrim Health Care Foundation.
Footnotes
Presented in part at the 42nd Meeting of the Council on Epidemiology and Prevention, American Heart Association, Miami, Fla, March 5–8, 2003, and published in abstract form (Circulation. 2003;107:e7001).
References
- 1.Gillman MW, Oliveria SA, Moore LL, et al. Inverse association of dietary calcium with systolic blood pressure in young children. JAMA. 1992;267:2340–2343. [PubMed] [Google Scholar]
- 2.Gillman MW, Hood MY, Moore LL, et al. Effect of calcium supplementation on blood pressure in children. J Pediatr. 1995;127:186–192. doi: 10.1016/s0022-3476(95)70293-8. [DOI] [PubMed] [Google Scholar]
- 3.Dwyer JH, Dwyer KM, Scribner RA, et al. Dietary calcium, calcium supplementation, and blood pressure in African American adolescents. Am J Clin Nutr. 1998;68:648–655. doi: 10.1093/ajcn/68.3.648. [DOI] [PubMed] [Google Scholar]
- 4.Belizan JM, Villar J, Gonzales L, et al. Calcium supplementation to prevent hypertensive disorders of pregnancy. N Engl J Med. 1991;325:1399–1405. doi: 10.1056/NEJM199111143252002. [DOI] [PubMed] [Google Scholar]
- 5.Villar J, Repke J, Belizan J, et al. Calcium supplementation reduces blood pressure during pregnancy: results of a randomized controlled clinical trial. Obstet Gynecol. 1987;70:317–322. [PubMed] [Google Scholar]
- 6.Atallah AN, Hofmeyr GJ, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. :CD001059. doi: 10.1002/14651858.CD001059. [DOI] [PubMed] [Google Scholar]
- 7.McGarvey ST, Zinner SH, Willett WC, et al. Maternal prenatal dietary potassium, calcium, magnesium, and infant blood pressure. Hypertension. 1991;17:218–224. doi: 10.1161/01.hyp.17.2.218. [DOI] [PubMed] [Google Scholar]
- 8.Belizan JM, Villar J, Bergel E, et al. Long term effect of calcium supplementation during pregnancy on the blood pressure of offspring: follow up of a randomised controlled trial. BMJ. 1997;315:281–285. doi: 10.1136/bmj.315.7103.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergel E, Belizan JM. A deficient maternal calcium intake during pregnancy increases blood pressure of the offspring in adult rats. Br J Obstet Gynaecol. 2002;109:540–545. [PubMed] [Google Scholar]
- 10.Barker DJP. Mothers, Babies, and Disease in Later Life. 2. London, UK: Harcourt Brace & Co, Ltd; 1998. [Google Scholar]
- 11.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, et al. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. doi: 10.1016/j.annepidem.2004.03.001. In press. [DOI] [PubMed] [Google Scholar]
- 12.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, et al. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 15.Whincup PH, Bruce NG, Cook DG, et al. The Dinamap 1846SX automated blood pressure recorder: comparison with the Hawksley random zero sphygmomanometer under field conditions. J Epidemiol Community Health. 1992;46:164–169. doi: 10.1136/jech.46.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;92:1049–1057. doi: 10.1161/01.cir.92.4.1049. [DOI] [PubMed] [Google Scholar]
- 17.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 18.Willett W. Nutritional Epidemiology. 2. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 19.Zinner SH, Rosner B, Oh W, et al. Significance of blood pressure in infancy: familial aggregation and predictive effect on later blood pressure. Hypertension. 1985;7:411–416. [PubMed] [Google Scholar]
- 20.Schachter J, Kuller LH, Perfetti C. Blood pressure during the first two years of life. Am J Epidemiol. 1982;116:29–41. doi: 10.1093/oxfordjournals.aje.a113400. [DOI] [PubMed] [Google Scholar]
- 21.de Swiet M, Fayers P, Shinebourne EA. Value of repeated blood pressure measurements in children: the Brompton study. BMJ. 1980;280:1567–1569. doi: 10.1136/bmj.280.6231.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerra A, Monteiro C, Breitenfeld L, et al. Genetic and environmental factors regulating blood pressure in childhood: prospective study from 0 to 3 years. J Hum Hypertens. 1997;11:233–238. doi: 10.1038/sj.jhh.1000415. [DOI] [PubMed] [Google Scholar]
- 23.Gillman MW. Epidemiological challenges in studying the fetal origins of adult chronic disease. Int J Epidemiol. 2002;31:294–299. [PubMed] [Google Scholar]
- 24.Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30:15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Karkkainen MU, Wiersma JW, Lamberg-Allardt CJ. Postprandial parathyroid hormone response to four calcium-rich foodstuffs. Am J Clin Nutr. 1997;65:1726–1730. doi: 10.1093/ajcn/65.6.1726. [DOI] [PubMed] [Google Scholar]
- 26.Talbot JR, Guardo P, Seccia S, et al. Calcium bioavailability and parathyroid hormone acute changes after oral intake of dairy and nondairy products in healthy volunteers. Osteoporos Int. 1999;10:137–142. doi: 10.1007/s001980050208. [DOI] [PubMed] [Google Scholar]
- 27.Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Curhan GC, Willett WC, Rimm EB, et al. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328:833–838. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- 29.Coe FL, Parks JH, Favus MJ. Diet and calcium: the end of an era? Ann Intern Med. 1997;126:553–555. doi: 10.7326/0003-4819-126-7-199704010-00010. [DOI] [PubMed] [Google Scholar]
- 30.Zemel MB. Calcium modulation of hypertension and obesity: mechanisms and implications. J Am Coll Nutr. 2001;20:428S–435S. doi: 10.1080/07315724.2001.10719180. [DOI] [PubMed] [Google Scholar]
- 31.Martini L, Wood RJ. Relative bioavailability of calcium-rich dietary sources in the elderly. Am J Clin Nutr. 2002;76:1345–1350. doi: 10.1093/ajcn/76.6.1345. [DOI] [PubMed] [Google Scholar]
- 32.Recker RR, Bammi A, Barger-Lux MJ, et al. Calcium absorbability from milk products, an imitation milk, and calcium carbonate. Am J Clin Nutr. 1988;47:93–95. doi: 10.1093/ajcn/47.1.93. [DOI] [PubMed] [Google Scholar]
- 33.Griffith LE, Guyatt GH, Cook RJ, et al. The influence of dietary and nondietary calcium supplementation on blood pressure: an updated meta-analysis of randomized controlled trials. Am J Hypertens. 1999;12:84–92. doi: 10.1016/s0895-7061(98)00224-6. [DOI] [PubMed] [Google Scholar]
- 34.Ravelli G, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 35.Roseboom TJ, van der Meulen JH, van Montfrans GA, et al. Maternal nutrition during gestation and blood pressure in later life. J Hypertens. 2001;19:29–34. doi: 10.1097/00004872-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Campbell DM, Hall MH, Barker DJP, et al. Diet in pregnancy and the offspring’s blood pressure 40 years later. Br J Obstet Gynaecol. 1996;103:273–280. doi: 10.1111/j.1471-0528.1996.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 37.Shiell AW, Campbell-Brown M, Haselden S, et al. High-meat, low-carbohydrate diet in pregnancy: relation to adult blood pressure in the offspring. Hypertension. 2001;38:1282–1288. doi: 10.1161/hy1101.095332. [DOI] [PubMed] [Google Scholar]
- 38.Adair LS, Kuzawa CW, Borja J. Maternal energy stores and diet composition during pregnancy program adolescent blood pressure. Circulation. 2001;104:1034–1039. doi: 10.1161/hc3401.095037. [DOI] [PubMed] [Google Scholar]
- 39.Fuentes RM, Notkola IL, Shemeikka S, et al. Tracking of systolic blood pressure during childhood: a 15-year follow-up population-based family study in eastern Finland. J Hypertens. 2002;20:195–202. doi: 10.1097/00004872-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Schachter J, Kuller LH, Perfetti C. Blood pressure during the first five years of life: relation to ethnic group (black or white) and to parental hypertension. Am J Epidemiol. 1984;119:541–543. doi: 10.1093/oxfordjournals.aje.a113771. [DOI] [PubMed] [Google Scholar]
- 41.Dahl LK, Knudsen KD, Heine MA, et al. Effects of chronic excess salt ingestion: modification of experimental hypertension in the rat by variations in the diet. Circ Res. 1968;22:11–18. doi: 10.1161/01.res.22.1.11. [DOI] [PubMed] [Google Scholar]
- 42.Geleijnse JM, Hofman A, Witteman JCM, et al. Long-term effects of neonatal sodium restriction on blood pressure. Hypertension. 1996;29:913–917. doi: 10.1161/01.hyp.29.4.913. [DOI] [PubMed] [Google Scholar]
- 43.Levine RJ, Hauth JC, Curet LB, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337:69–76. doi: 10.1056/NEJM199707103370201. [DOI] [PubMed] [Google Scholar]
- 44.Rogers I, Emmett P. Diet during pregnancy in a population of pregnant women in South West England: ALSPAC Study Team: Avon Longitudinal Study of Pregnancy and Childhood. Eur J Clin Nutr. 1998;52:246–250. doi: 10.1038/sj.ejcn.1600543. [DOI] [PubMed] [Google Scholar]
- 45.National Academy of Sciences. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, DC: National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]


