Abstract
Direct syntheses of 7,8-dihydroxycalamenene and mansonone C were achieved. The cis-stereochemistry required for the synthesis of 7,8-dihydroxycalamenene was introduced by an intramolecular cyclization directed by allylic strain.
Allylic 1,3-strain has been used in acyclic systems to direct the introduction of new stereogenic centers.1 Notable examples include the work of Kishi,2 Adam,3 and Giese.4 We are not aware of any application of allylic strain to control the relative stereochemistry in disubstituted tetralins. Tetralins such as 1, 3, and 4 have attracted considerable synthetic attention (Figure 1).5 7,8-Dihydroxycalamenene (1a) exhibits useful anti-infective activity.6 Hydroxycalamenene (1b) was isolated from Hypericum elodeoides.7 Mansonone C (2), extracted from the heartwood of Mansonia altissima,8 was found to possess promising antifungal, larvicidal, and anti-oxidant properties.9 Schmalz recorded a synthesis of 1a using arenechromium complexes to introduce the relative stereo-chemistry.10
Figure 1.
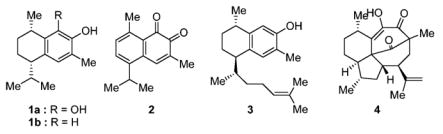
Tetralin-derived products and mansonone C.
Many synthetic approaches to these compounds begin with natural products such as menthone wherein the relative stereochemistry has already been established.11
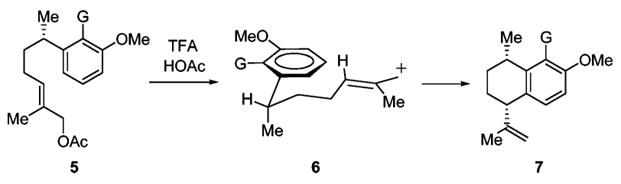
Several researchers have noted that attempts to install stereochemistry by epimerization or by cyclization onto the aromatic ring have led to mixtures.12,13 We report herein that a system such as 5, wherein allylic strain between G and the methyl group forces the methyl group to be axial as the six-membered ring is being formed, affords exclusively the cis-stereoisomer 7 (Scheme 1).
Scheme 1.
In order to evaluate the directing effect, we first synthesized allylic acetate 8 (Scheme 2). Allylic acetate 8 was synthesized starting from 6-methyl-5-hepten-2-one and the anion of 1,4-dimethoxybenzene. Dehydroxylation of the resulting benzylic alcohol using Li/NH3 followed by allylic oxidation by the method of Sharpless14 yielded an allylic alcohol which, upon acetylation, afforded 8. Surprisingly, cyclization of allylic acetate 8 using the conditions of Ma and Zheng15 afforded tetralin 10 and naphthalene 11 in 51% and 37% yields, respectively. Compound 9, the expected product, was not isolated.
Scheme 2.
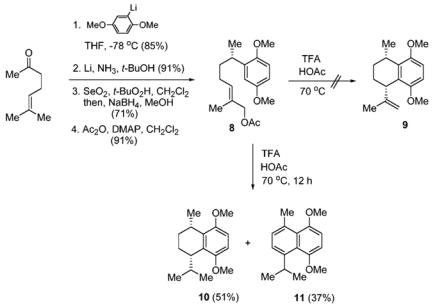
We believe that compounds 10 and 11 result from a novel cation-mediated disproportionation reaction. Comparison of the NMR spectrum of 10 with that of the literature compound13 showed that the cis-stereoisomer was exclusively formed.
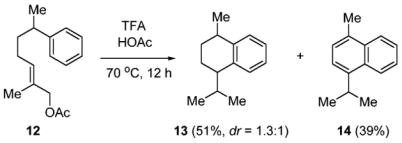
In support of this allylic strain assisted stereoselective cyclization, we have found that cyclization of allylic acetate 12, which does not contain the directing group G, affords tetralin 13 as a 1.3:1 mixture of diastereomers (Scheme 3).
Scheme 3.
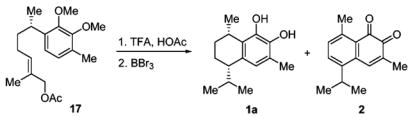
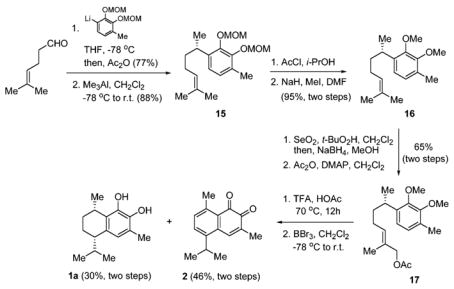
With the stereochemistry of 10 established, we began the synthesis of 1a by the reaction of 5-methyl-4-hexen-1-al16 with the anion of 1,2-bis(methoxymethoxy)-3-methylbenzene (Scheme 4). The resulting alkoxide was in situ acetylated by the addition of acetic anhydride. The displacement of the acetate to the corresponding methyl group was achieved using Me3Al to afford 15. In order to enhance the stability for the acid-mediated cyclization, MOM protecting groups were converted to the more stable methyl ether 16. It was then oxidized and acetylated by the same methods used to generate 8. Cyclization of 17 using trifluoroacetic acid in acetic acid at 70 °C for 12 h followed by deprotection by the use of BBr3 generated 7,8-dihydroxycalamenene (1a) in two steps (30% yield). Interestingly enough, mansonone C (2) was also obtained from the same reaction in two steps (46% yield). The direct synthesis of 7,8-dihydroxycalamenene (1a) demonstrates the advantage of employing the concept of allylic strain in organic synthesis.
Scheme 4.
Acknowledgments
We thank the National Institutes of Health (Grant No. P01 ES12020) and the Office of Dietary Supplements for partial financial support through the Center for Research on Botanical Dietary Supplements at Iowa State University.
Footnotes
Supporting Information Available: Experimental procedures, characterization data, and NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hoffmann RW. Chem Rev. 1989;89:1841. [Google Scholar]; Johnson F. Chem Rev. 1968;68:375. [Google Scholar]
- 2.Johnson MR, Nakata T, Kishi Y. Tetrahedron Lett. 1979:4343. [Google Scholar]
- 3.Adam W, Wirth T. Acc Chem Res. 1999;32:703. [Google Scholar]
- 4.Giese B, Bulliard M, Zeitz HG. Synlett. 1991:425. [Google Scholar]
- 5.Erogorgiaene (3): Rodriguez AD, Ramirez C. J Nat Prod. 2001;64:100. doi: 10.1021/np000196g.Elisapterosin B (4): Rodriguez AD, Ramirez C, Rodriguez II, Barnes CL. J Org Chem. 2000;65:1390. doi: 10.1021/jo9914869.
- 6.Wahyouno S, Hoffmann JJ, Bates RB, McLaughlin SP. Phytochemistry. 1991;30:2175. [Google Scholar]
- 7.Mathela DK, Mathela CS, Dev V. J Indian Chem Soc. 1984;61:792. [Google Scholar]
- 8.Marini Bettòlo GB, Casinovi CG, Galeffi C. Tetrahedron Lett. 1965:4857. [Google Scholar]
- 9.Tiew P, Ioset JR, Kokpol U, Chavasiri W, Hostettmann K. Phytother Res. 2003;17:190. doi: 10.1002/ptr.1260. [DOI] [PubMed] [Google Scholar]
- 10.Schmalz HG, Hollander J, Arnold M, Duerner G. Tetrahedron Lett. 1993;34:6259. [Google Scholar]
- 11.Serra S, Fuganti C. Tetrahedron Lett. 2005;46:4769. [Google Scholar]
- 12.Ramaiah P, Rao AS. Synth Commun. 1989;19:931. [Google Scholar]
- 13.Beckwith PLM, Ghisalberti EL, Jefferies PR, Raston CL, White AH. Aust J Chem. 1988;41:351. [Google Scholar]
- 14.Umbreit MA, Sharpless KB. J Am Chem Soc. 1977;99:5526. [Google Scholar]
- 15.Ma S, Zhang J. Tetrahedron. 2003;59:6273. [Google Scholar]
- 16.Kraus GA, Kim J. Org Lett. 2004;6:3115. doi: 10.1021/ol048847d. [DOI] [PubMed] [Google Scholar]


