INTRODUCTION AND ANATOMICAL OVERVIEW
It has been known for more than 100 years that neurons present in any cortical area are far from being uniform regarding their morphology and intrinsic and extrinsic connections (Ramon y Cajal, 1893; 1911). This suggests that they are able to interact with each other in a complex fashion. Even if only a fraction of these connections are active in the same time, a network with virtually endless numbers of possible active patterns emerge (Freund and Buzsaki, 1996). Thus, even if we will be able to understand the complete molecular and biophysical properties of a single hippocampal neuron, in order to understand the function of the hippocampal formation, including the dentate gyrus, as a whole, it is indispensable to elucidate both, the intrinsic and extrinsic connections of these cells.
The major cell types and the majority of intrinsic and extrinsic connections of the hippocampal formation, which comprises the cornu ammonis, divided into three subfields, CA1 – CA3 (Lorente de No, 1934) and the dentate gyrus (fascia dentata and hilus) have been well known since the studies of Ramon y Cajal (1893) and Lorente de No (1934) and have been reviewed extensively (e.g., Amaral and Witter, 1989, 1995, Lopes de Silva et al., 1990). It has should be noted, however, that the term hippocampal formation sometimes is used to include the subicular complex and entorhinal cortex (Amaral and Witter, 1995).
The three dimensional position of the hippocampal formation in the brain is rather complex. In the majority of species the hippocampal formation is an elongated structure with its long axis extending in a C-shaped fashion from the septal nuclei of the basal forebrain rostrodorsally, over and behind the diencephalon, to the incipient temporal lobe caudoventrally (sees more details in Amaral and Witter, 1995).
Although there is still a debate about the classification of hippocampal neurons, the simple terms of principal and non-principal neurons is accepted (Freund and Buzsaki, 1996). The hippocampal principal neurons are the pyramidal and granule cells located in the CA1 – CA3 subfield of the ammon's horn and dentate gyrus, respectively. In addition, the mossy cells of the dentate gyrus can be referred to as principal cells, as discussed further below. The granule cells and pyramidal neurons, with their intrahippocampal connections, are the major components of the so- called trisynaptic circuit of the hippocampus, which is unidirectional and glutamatergic. Layer 2 pyramidal cells of the entorhinal cortex project to granule cells (and to some extent, to CA3 pyramidal cells) via the perforant pathway. Granule cells, via their mossy fiber projection, organized in a laminar fashion, terminate on CA3 pyramidal neurons, which send their Schaffer collaterals to the CA1 pyramidal cells. CA1 pyramidal neurons, in turn, project back to the entorhinal cortex, via the subiculum.
As mentioned above, the dentate gyrus is the first stage of the intrahippocampal, excitatory, trisynaptic loop, being the primary target of the majority of entorhinal afferents that terminate in a laminar fashion on granule cell dendrites (see Chapter I) and carry sensory information of multiple modalities about the external world. The principal cells of the dentate gyrus are the granule cells, about 1 million in rats and five million in non-human primates (Claiborne et al., 1986; Seress, 1988) and the mossy cells (Amaral, 1978). The small cell bodies of the granule cells (8-12 μm) form the granule cell layer. Granule cells have two main, radially oriented, spiny dendrites emitting several fine branches, which reach the hippocampal fissure. The axons of the granule cells, the mossy fiber axons, originate from the opposite pole of the soma relative to the dendrites, and enter the dentate hilus, where they give rise to several collaterals (Claiborne et al., 1986). Recurrent collaterals periodically enter the granule cell layer, climb along the cell bodies and dendrites of presumed basket cells, and form synapses (Ribak and Peterson, 1991). The main axonal projection of the granule cells leaves the hilar region and courses through the stratum lucidum of the CA3 subfield, where it forms the giant mossy terminals synapsing on the proximal dendrites of pyramidal cells.
The dentate hilus is located subjacent to the granule cell layer and extends to the border of the dendritic layer of CA3 that is interposed between the upper (suprapyramidal) and lower (infrapyramidal) blades of the dentate gyrus. The principal and most numerous cell type in the hilus is the mossy cell. These neurons are characterized by their densely spiny dendrites and several thorny excrescences on both the cell body and proximal dendritic shafts and their dendrites are mostly confined to the hilus (Amaral, 1978). However, single mossy dendrites were observed penetrating the stratum moleculare (Soltesz and Mody, 1994, Scharfman, 1991,1995b). Axons of mossy cells innervate the inner third of the dentate molecular layer of both the ipsi- and contralateral dentate gyrus and also have collaterals in the hilus (Amaral, 1978, Laurberg and Sorensen, 1981; Ribak et al., 1985; Buckmaster et al., 1996). These intrahilar collaterals of mossy cells terminate on unidentified dendrites in the hilus and on dendrites of hilar interneurons (Frotscher and Zimmer, 1983a, b; Frotscher et al., 1984; Ribak et al., 1985; Buckmaster et al., 1996). Because both, the CA3 pyramidal cells and mossy axons form asymmetric, glutamatergic, excitatory synapses, many authors consider the mossy cells as modified CA3 pyramidal neurons (Soriano and Frotscher, 1994, Scharfman, 1995b, 1999). Therefore, mossy cells are considered as excitatory principal cells that provide long-range ipsilateral and commissural projections into the dentate gyrus (Amaral, 1978).
The electric activity of the aforementioned excitatory signal loop is controlled mainly by different types of local, GABAergic interneurons (for review, see Freund and Buzsaki, 1996) and subcortical and commissural afferents. In this chapter we will outline our recent knowledge regarding the origin and postsynaptic targets in the dentate gyrus of chemically identified subcortical inputs, including afferents originating from the medial septum/diagonal band of Broca (MSDB) GABAergic and cholinergic neurons, dentate projection of neurochemically different types of neurons located in the supramammillary area (SUM), serotonergic fibers from the median raphe (MR), noradrenergic afferents from the pontine nucleus, locus ceruleus, and dopamine axons originating in the ventral tegmental area and that of the commissural system.
1) SEPTO-HIPPOCAMPAL CONNECTIONS
In the 1970s, it had been shown that anterogradely-transported radiolabeled amino acids, injected into the MSDB, could be detected in hippocampal neurons (Rose and Schubert, 1977). Studies in the seventies and eighties, using the combination of retrograde tracer technique and immunostaining for choline acetyltransferase (ChAT) and glutamate decarboxylase (GAD) showed that there were two major populations of MSDB neurons projecting to the hippocampus (for review, see Leranth and Frotscher, 1989). Later, experiments using anterograde labeling with the lectin PHAL revealed two types of septohippocampal fibers. One has large boutons that occur in clusters, whereas the other has small boutons and arborizes more diffusely (Nyakas et al., 1987). Type 1 axons are immunoreactive for GABA and are processes of GABAergic, parvalbumin (PA)-containing (Freund, 1989) neurons located in the midline of the MSDB (Kiss et al., 1990). The type 2 axons are GABA-negative (Freund and Antal, 1988), but are immunoreactive for ChAT, the synthesizing enzyme of acetylcholine (Frotscher and Leranth, 1983, 1986; Leranth and Frotscher, 1987). The parent neurons of these axons are also in the MSDB and are positioned in a way so that they surround the septo-hippocampal GABAergic cells (Kiss et al., 1990).
1.a) MSDB cholinergic innervation of the dentate gyrus
Basic and clinical studies have long recognized the importance of cholinergic mechanisms in cognitive function (Givens et al., 1997), and drugs which increase synaptic acetylcholine levels are currently the most common for the treatment of cognitive deficits associated with disorders such as Alzheimer's disease, albeit, with limited effectiveness (Benzi and Moretti, 1998). The septohippocampal pathway (SH), which originates in the MSDB and shows progressive degeneration in Alzheimer's disease (Whitehouse et al., 1982), has specifically been implicated in cognitive function. Lesions of the fimbria-fornix, which carry SH cholinergic (Lewis and Shute, 1967) fibers to the hippocampal formation, including the dentate gyrus, interfere both with learning and memory tasks and with generation of the theta rhythm in rats (Brito and Brito, 1990). These deficits can be attenuated by grafting acetylcholine-producing cells to the hippocampus (Dunnett et al., 1982; Dickinson-Anson et al., 1998).
Electrophysiological experiments have demonstrated that excitatory, inhibitory, and disinhibitory responses can be recorded as a result of the iontophoretic application of acetylcholine in the dentate gyrus (Wheal and Miller, 1980; Fricke and Prince, 1984). It is not clear, however, from these experiments whether acetylcholine exerts these effects at the level of the primary dendrites, directly onto the perikaryon of principal cells or indirectly, via interneurons (Hounsgard, 1978). Histochemical staining procedures to visualize putative cholinergic neurons and fibers applying acetylcholinesterase (ACHE) reaction were first used in the dentate gyrus in the early 1960's (Storm-Mathisen and Blackstad, 1964). These authors described a laminar pattern of ACHE fiber staining in the dentate gyrus; the most dense band occurring at the interface between the granule cell layer and the molecular layer. Shute and Lewis (1977) modified this ACHE histochemical technique to permit examination of the dentate gyrus, stained for ACHE, using the electron microscope. Their study revealed several neurons histochemically-stained for ACHE, numerous axonal fibers and some synaptic boutons in contact with predominantly dendritic shafts. Biochemical measurements of the levels of ChAT in the dentate gyrus also indicate a supragranular band of intense cholinergic expression (Fonnum, 1970).
The first detailed light and electron microscopic studies using immunostaining for ChAT on the cholinergic innervation of the dentate gyrus were performed in the middle 1980's (Frotscher and Leranth, 1983, 1986; Wainer et al., 1984; Clarke, 1985; Leranth and Frotscher, 1987). The light microscopic analyses revealed a dense plexus of ChAT-immunoreactive fibers in the dentate gyrus forming a supragranular band at the interface between the granule cell layer and molecular layer. Very little staining was in the granule cell layer and only a few fibers were located in the hilar region. In addition, ChAT-immunoreactive neurons could also be observed in the dentate hilus (Clarke, 1985; Frotscher et al., 1986) These ChAT-positive cells are rare and non-principal neurons. They are relatively small with round or ovoid perikarya, which give rise to thin spine-free dendrites and are very similar to ChAT-immunoreactive cells in the neocortex of the same animals but were quite different from cholinergic neurons in the basal forebrain, medial septal nucleus and neostriatum, which were larger and more intensely immunostained. Electron-microscopic analysis of ChAT-containing cells in the hippocampus and fascia dentata revealed that their afferent synaptic contacts are mainly the asymmetric type, and are located on their cell bodies and smooth proximal dendrites. The nuclei of the immunoreactive cells exhibited deep indentations, which are a characteristic of non-pyramidal neurons (Frotscher et al., 1986).
At the electron microscopic level, the vast majority of ChAT-positive synaptic boutons contacts dendritic shafts and forms predominantly symmetric synaptic contacts. In addition, some asymmetric synapses, about 11% of the total number of ChAT synapses (Clarke, 1985), could also be observed on dendritic spines. There is a possibility that axons forming symmetric and asymmetric contacts originate from different population of cholinergic neurons; intrinsic and extrinsic. However, it seems unlikely since, lesions of the septohippocampal pathway cause an almost complete removal of cholinergic markers in the dentate gyrus (Mellgren and Srebro, 1973).
In the dentate gyrus, the majority of the postsynaptic targets of cholinergic boutons are the granule cells and the ChAT-immunoreactive boutons form both axo-dendritic and axo-spine synapses with these neurons. All of the axo-spine synapses observed are asymmetric (Fig. 1). In addition, about 5-10% of all postsynaptic elements of cholinergic axons show ultrastructural features of interneurons. A combined electron microscopic, Golgi impregnation and ChAT immunostaining study has shown symmetric synaptic contacts between ChAT-containing axon terminals and non-spiny, smooth dendritic shafts, characteristic for interneurons (Frotscher and Leranth, 1986; Fig. 2). This 5-10% appears to correspond to the proportion of interneurons in the neuropil, suggesting that the interneurons are contacted in a “quasi-random” fashion. Direct evidence that interneurons are indeed innervated by cholinergic fibers is provided by an electron microscopic double-immunostaining study that showed ChAT-positive axon terminals forming symmetric synaptic contacts with hilar GAD (glutamic acid decarboxylase)- and SOM (somatostatin)-containing neurons (Leranth and Frotscher, 1987).
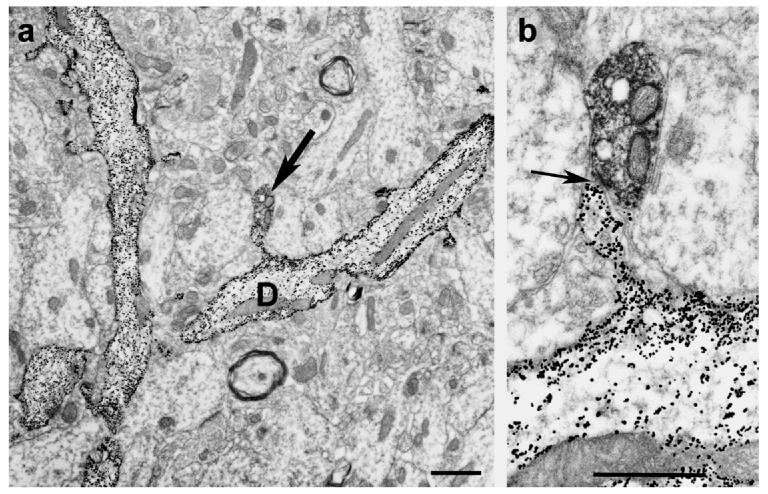
Fig. 1.
Low (Panel a) and high power (Panel b) electron micrographs (received from Dr. Michael Frotscher) show the result of a combined Golgi impregnation and ChAT immunostaining experiment. On Panel a, Golgi-impregnated (gold-toned) spiny granule cell dendrites are seen. Arrow on the same panel points at a ChAT immunoreactive bouton contacting the spine head of the dendrite (D). Panel b shows the asymmetric synaptic contact (arrow) between the two profiles.
Bar scales= 1 μm.
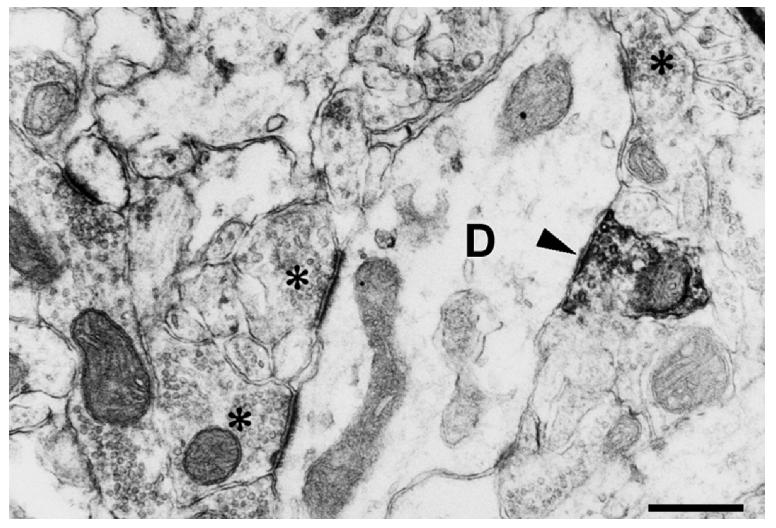
Fig. 2.
Electron micrograph taken from the molecular layer of the dentate gyrus immunostained for ChAT. A ChAT-immunoreactive axon terminal forms asymmetric synaptic contact with a non-spiny dendritic shaft (D). The lack of dendritic spines indicate that this dendrite is the process of an interneuron. Asterisks label axon terminals forming asymmetric synapses with the same dendrite.
Bar scale= 1 μm.
In addition to the cholinergic innervation of dentate granule cells and interneurons, MSDB cholinergic cells innervate mossy cells densely. A correlated light and electron microscopic double-immunostaining study has demonstrated numerous axo-somatic synaptic contacts between ChAT-containing axon terminals and calcitonin gene-related peptide (CGRP)-immunoreactive neurons in the dentate hilar area (Deller et al., 1999). CGRP is an accepted marker for mossy cells (Freund et al. 1997). Since mossy cells project for a long distance along the longitudinal axis of the hippocampus (Ribak et al., 1985;), the cholinergic innervation of these neurons could modulate granule cell excitability throughout large portions of the dentate gyrus.
1.b) MSDB GABAergic innervation of the dentate gyrus
It has been shown that when the perforant path is stimulated with a brief test pulse, it evokes excitatory postsynaptic potentials (EPSPs) in the granule cell dendrites, they could be recorded collectively in the dentate hilus as a positive-going field potential. At greater stimulus intensities, a negative-going component is superimposed on the positive-going field potential, as the result of the synchronous activation of many granule cells. This component is referred to as a population spike (PS). When a conditioning pulse is applied to the medial septum just prior to a perforant path test pulse, the EPSP remains unchanged, but the PS is greater than that expected from the sum of the potentials evoked by either stimulus alone (Alvarez-Leefmans and Gardner-Medwin, 1975; Fantie and Goddard, 1982). Thus, the septohippocampal input appears to facilitate the discharge of granule cells. An interesting and unexpected finding of these studies was that stimulation of the medial septum alone, at a site which produced the facilitation of the PS in the dentate gyrus, did not necessarily evoke a field potential in the dentate gyrus. This finding, together with the failure of medial septal stimulation to affect the perforant path-evoked field potential, upon which the PS is superimposed suggests that the facilitation does not result from summation of excitatory input to the granule cells. Two mechanisms that have been proposed by Buzsaki (1984) to account for these effects are: 1) medial septal input acts directly on granule cells to facilitate activation (Robinzon and Racine, 1984), and 2) the medial septum acts indirectly, via the inhibitory interneurons responsible for feed-forward and feed-back inhibition (Anderson and Eccles, 1962; Buzsaki and Eidelburg, 1982; Kandel et al., 1961; Mosko et al., 1973).
Anatomical studies of the septo-dentate termination patterns show that the innervation of the supragranular and molecular layers are considerably less dense than the innervation of the dentate hilus and subgranular layer (Chandler and Crutcher, 1983; Lynch et al., 1973). Although MSDB fibers in the supragranular and molecular layers are likely to terminate on granule cells, fibers in the subgranular and hilar region appear to terminate on cells having the morphological characteristics of interneurons (Chandler and Crutcher, 1983; Rose and Schubert, 1977). If SH fibers form excitatory connections onto inhibitory interneurons, their activation might synchronize granule cell responses, resulting in a larger population spike from a subsequent perforant path input (Buzsaki, 1984). Alternatively, if they form inhibitory connections onto the interneurons, septal activation might reduce both tonic and feed-forward inhibition of the granule cells, allowing a larger number of these neurons to be activated by the perforant path input, again resulting in a larger population spike. To determine the validity of these hypotheses, Bilkey and Goddard (1985) performed a very elegant study. They have shown that a conditioning pulse to the medial septum, although eliciting no field potential of its own, facilitated the granule cell population spike evoked by perforant path stimulation and infusion into the dentate hilus of a GABAAreceptor antagonist, picrotoxin, blocked the facilitation. This observation suggests that the facilitatory effect of MSDB stimulation is mediated through an inhibitory connection from the MSDB onto inhibitory interneurons in the dentate gyrus, and that this connection may utilize the neurotransmitter GABA.
Indeed, it has been demonstrated in a series of concomitant morphological studies that SH (including the septo-dentate) GABAergic terminals always terminate on GABAergic interneurons, in the rat (Freund and Antal., 1988; Gulyas et al., 1990) and monkey hippocampus (Gulyas et al., 1991). Experiments using double immunostaining for PHAL and markers for different subsets of interneurons have revealed that all examined subpopulations of hippocampal GABAergic interneurons, including those co-expressing parvalbumin, calbindin, SOM, neuropeptide Y, cholecystokinin, and vasoactive intestinal polypeptide, receive input from GABAergic septohippocampal afferents (Freund and Antal, 1988; Gulyas et al., 1990; Miettinen and Freund, 1992a,b; Acsady et al., 1993). The typical innervation pattern is multiple, “climbing fiber-like” contacts on the soma, and proximal and distal dendrites of the postsynaptic interneurons (Fig. 3). All these contacts have proved to be symmetrical synapses. The area where most of the interneurons appear to receive septal GABAergic input is the CA3 subfield, particularly strata oriens and pyramidale (Rose and Schubert, 1977; Nyakas et al., 1987; Freund and Antal, 1988; Gaykema et al., 1990). The dentate hilus is also heavily innervated, but in the CA1 region, a relatively smaller proportion of the interneurons receive multiple synaptic inputs. No systematic differences have been observed in the termination pattern of septal afferents between the dorsal and ventral hippocampus.
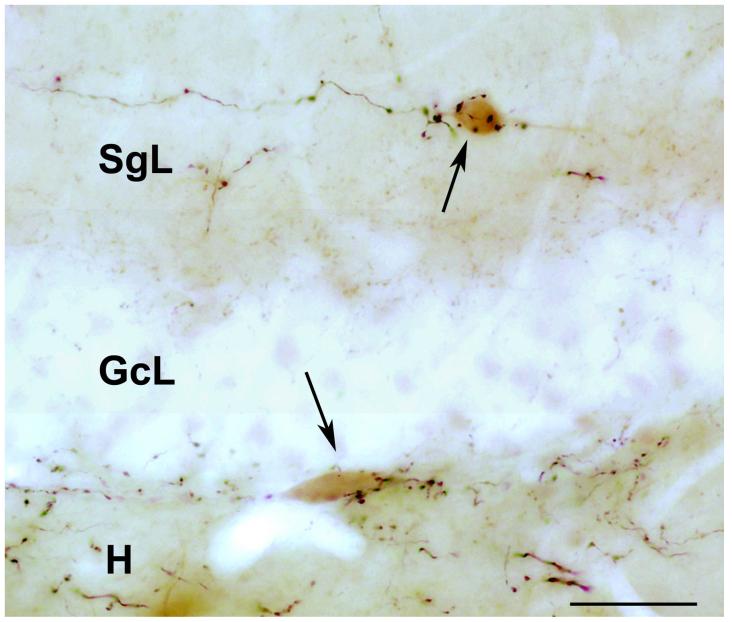
Fig. 3.
Light micrograph (kindly provided by Dr. Attila Gulyas) shows the result of a combined anterograde tracing and calretinin immunostaining study, in the dentate gyrus. The anterograde tracer, biotinylated dextran amine (BDA) was injected into the medial septum diagonal band. Large boutons of BDA-containing axons form multiple, basket-like, putative synaptic contacts with the soma of calretinin-immunoreactive (labeled with a brown diaminobenzidine reaction product) neurons. One of these cells (arrows) located in the supragranular layer (SgL) the other is at the border between the granule cell layer (GcL) and dentate hilar area (H).
Bar scale= 50 μm.
1.c) Interactions between the septohippocampal cholinergic and GABAergic systems
In order to better understand the function of the septohippocampal projection system that contains a cholinergic and a GABAergic component, the anatomical and functional interaction between these two systems must be briefly discussed, at the level of the MSDB
It has to be noted that intrinsic cholinergic mechanisms operating within the MSDB are also critical for learning and memory. Infusion of muscarinic agonists directly into the MSDB elicit continuous hippocampal theta rhythm (Monmaur and Breton, 1991; Lawson and Bland, 1993) and facilitate learning and memory-related behaviors both in young (Givens and Olton, 1990) and aged rats (Markowska et al., 1995). Also, intraseptal infusions of muscarinic agonists can also alleviate systemic scopolamine-induced amnesia, suggesting that the MSDB is a critical locus for the mnemonic effects of muscarinic drugs (Givens and Olton, 1995). In general, it is assumed that improvements in MSDB-related learning and memory tasks occur as a result of an increase in hippocampal acetylcholine (ACh) release (Monmaur and Breton, 1991; Givens and Olton, 1994, 1995; Apartis et al., 1998; Bland and Oddie, 1998; Dickinson-Anson et al., 1998). As such, it has been presumed that the memory-enhancing effects of intraseptal administration of muscarinic agonists occurs due to increased firing of MSDB cholinergic neurons (Markowska et al., 1995; Givens and Sarter, 1997). These assumptions have been based on earlier studies that reported a muscarinic receptor-mediated increase in firing of MSDB neurons (Dutar et al., 1983; Lamour et al., 1984), which lead to the hypothesis that Ach, via muscarinic receptors, has a positive feed-back effect on MSDB cholinergic neurons (Dutar et al., 1983; Lamour et al., 1984; see rev in, Wu et al., 2004). However, a recent combined electrophysiological and morphological study (Wu et al., 2004), using a novel fluorescent labeling technique to selectively visualize live septohippocampal cholinergic neurons has demonstrated that administration of muscarinic agonists to the MSDB do not excite septohippocampal cholinergic neurons, instead they inhibit a subpopulation of them. In contrast, septo-hippocampal GABAergic neurons (visualized by retrograde tracing and parvalbumin immunostaining techniques) are profoundly excited by muscarine administration into the MSDB. Thus the cognition enhancing effects of muscarinic drugs in the MSDB cannot be attributed to an increase in hippocampal ACh release. Instead, disinhibitory mechanisms (Freund and Antal, 1988), due to increased impulse flow in the septo-hippocampal GABAergic pathway, may underlie the cognition-enhancing effects of muscarinic agonists. In support of this view is the morphological observation that axon collaterals of the septohippocampal cholinergic neurons heavily innervate MSDB septo-hippocampal GABAergic neurons (Leranth and Frotscher, 1989; Fig. 4). These connections could be involved in the aforementioned process.
Fig. 4.
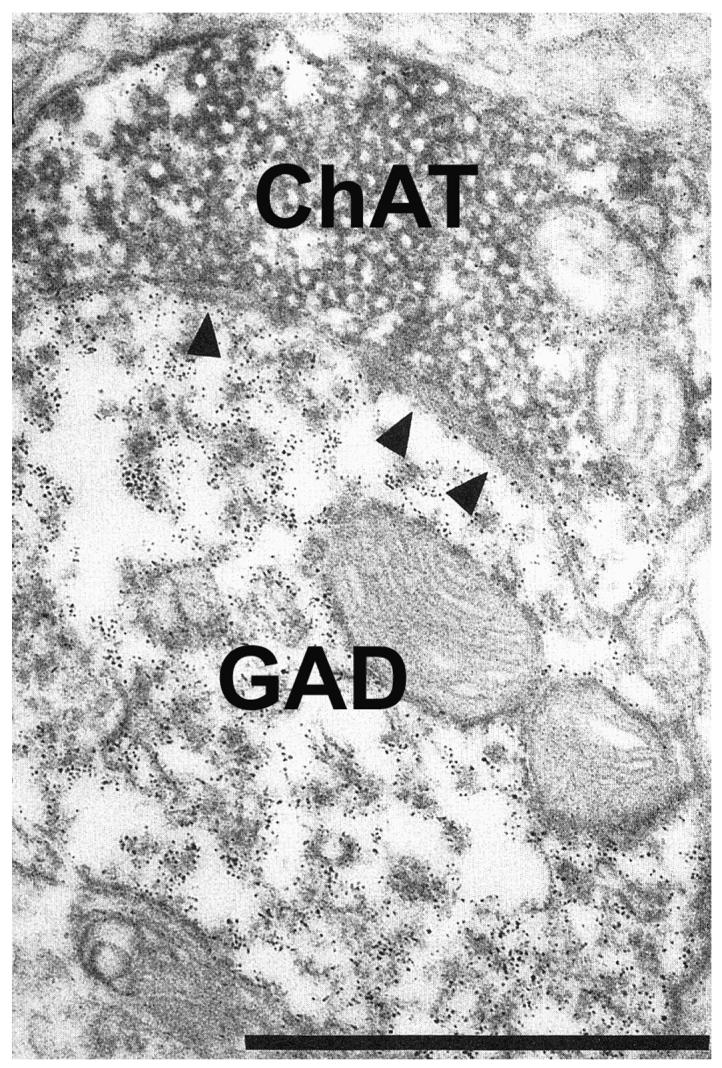
Electron micrograph shows the result of a double immunostaining experiment for choline acetyltransferase (ChAT) and glutamic acid decarboxylase (GAD), in the rat medial septum diagonal band of Broca. Immunoreactivity for ChAT and GAD was visualized with two contrasting immunomarker, diaminobenzidine reaction and ferritin labeling, respectively. A ChAT-immunoreactive bouton form asymmetric synaptic contact (arrowheads) with a GAD immunoreactive dendrite.
Bar scale= 1 μm.
2)SUPRAMAMILLO-DENTATE CONNECTIONS
It has been shown that prestimulation of supramammillary (SUM) neurons significantly enhances perforant path-elicited population spikes in the fascia dentata (Mizumori et al., 1989), an effect that could be mimicked by glutamate injections to the lateral supramammillary area (Carre and Harley, 1991). Moreover, Dahl and Winson (1986) speculated about a neuronal control mechanism, a “gate,” mediated by supramammillary afferents that would facilitate information flow in the rat dentate gyrus, in a behavior-dependent manner.
Since the mid-1970's, it has been known that a projection exists between the SUM and hippocampus in the rat (Segal and Landis, 1974). Later, Amaral and Cowan (1980) showed that horseradish peroxidase (HRP) injected into monkey hippocampus also results in labeled cells in the SUM. Furthermore, it was noted that more cells ipsilateral to the site of injection were labeled than cells in the contralateral SUM and a large number of SUM efferents terminate in the dentate gyrus since, local application of the retrograde tracer, Evans blue to the upper blade of the dentate gyrus produced labeled cell bodies in the SUM (Harley et al. 1983). Labeling was observed throughout the rostrocaudal aspect of the SUM. Therefore, Amaral and Cowan (1980) and Harley et al. (1983) postulated that afferents from the SUM to the hippocampus involve at least as many cells in the SUM as the septal neurons, which give rise to the septohippocampal projection. The specific pathway containing SUM efferents to the hippocampus remains debatable. The medial forebrain bundle (Haglund et al., 1984) and fornix (Veazey et al. 1982) have been suggested as likely candidates. More specifically, Veazey et al. (1982) have postulated that the medial forebrain bundle carries SUM efferents to septal nuclei, whereas the fornix carries projection from the SUM to the hippocampus. At the level of dorsal hippocampus, labeled fibers have been described in the fimbria (Haglund et al., 1982) or the subcallosal fornix (Wiss et al., 1979).
Regarding the neurochemical nature of the SUM-hippocampal pathway, studies were able to demonstrate that calretinin-immunoreactive axon terminals in the inner molecular layer of the dentate gyrus (and in the pyramidal layer of CA2) originate in the SUM. Colocalization studies provided evidence that these large projecting neurons contained both calretinin and substance P (SP) but lacked GABA(Nitsch and Leranth, 1993). This observation indicates that the nature of the hypothalamo-hippocampal projection to the dentate is likely to be excitatory. In fact, SP-containing, long projection systems have been shown to exert excitatory actions (Nicoll et al., 1980). Conversely, in the rat, the functional properties of the hypothalamo-hippocampal afferent system have been reported to be at least partially inhibitory (Segal, 1979). The study by Segal (1979) raises a question of how can the physiological action on hippocampal principal neurons of a putatively excitatory pathway become inhibitory. A possible explanation for this is that the supramammillo-hippocampal afferents, in addition to terminating on principal neurons (Magloczky et al., 1994), innervate hippocampal GABAergic interneurons.
Indeed, in a study dealing with the innervation of the primate hippocampal formation, thick and varicose SP-immunoreactive axons, forming basket-like structures were identified adjacent to the granule cell layer and in the hilar area of the dentate gyrus and in the molecular layer of the middle portion of CA3 has been observed (Nitsch and Leranth, 1994). The location of these basket-like structures outside the principal cell layers indicates that they contact hippocampal non-principal neurons. Furthermore, these SP-containing axons disappear after fimbria-fornix transection, indicating that they are of extrinsic origin (Nitsch and Leranth, 1994). A subsequent study on non-human primates (Leranth and Nitsch, 1994), applying correlated light and electron microscopic immunocytochemical, double-labeling technique for SP and parvalbumin and SP and calbindin and subsequent postembedding GABA-immunostaining revealed that this supramammillo-hippocampal afferent system establishes multiple, exclusively asymmetric synapses with three specific subpopulations of nonpyramidal cells: (1) a small portion of parvalbumin-containing basket cells located in or adjacent to the granule cell layer of the dentate gyrus (Fig. 5), which, therefore, inhibit only a subpopulation of granule cells; (2) some of the calbindin-immunoreactive neurons located in the hilar area and in the granule cell layer (Fig. 6); and (3) calbindin-positive cells occurring exclusively in the stratum moleculare of the middle portion of the CA3 subfield. Postembedding immunostaining for GABA revealed that the aforementioned calbindin-containing cells in area CA3 are GABAergic inhibitory neurons. The results of this study indicate that supramammillary afferents, a portion of which is glutamatergic (Kiss et al., 2000) can effectively filter the information flow at different points along the trisynaptic circuit in the monkey hippocampal formation. Dentate granule cells, which are only stimulated by SUM afferents, will transfer excitatory signals differently than those that are controlled by a feed-forward inhibitory mechanism initiated by these fibers (see Fig. 10, in Leranth and Nitsch, 1994).
Fig. 5.
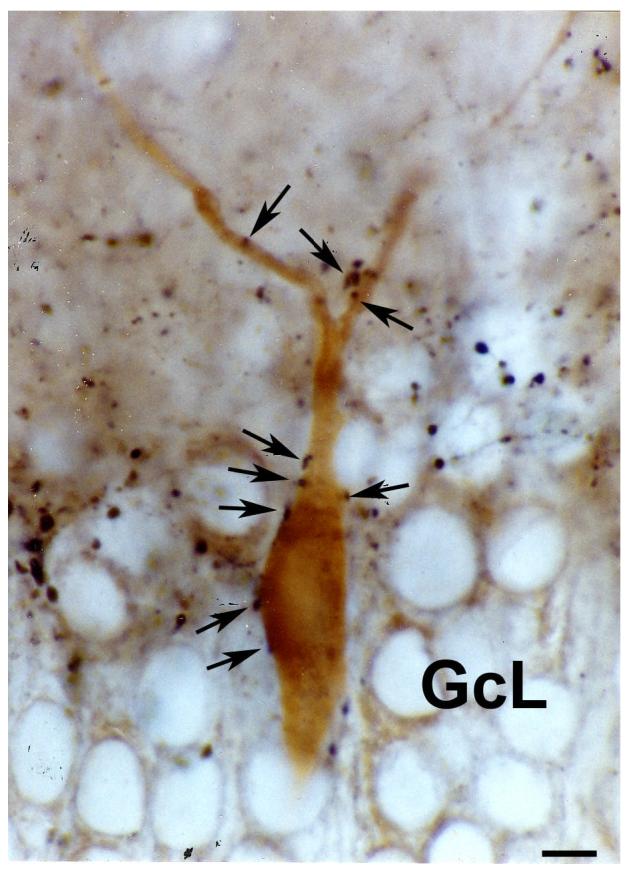
Light micrograph taken from a double immunostained vibratome section of the monkey dentate gyrus. Immunoreactivity for substance P was labeled with a dark-blue Ni-diaminobenzidine reaction, while immunostaining for parvalbumin was visualized by a brown diaminobenzidine reaction. The soma and dendrites of the parvalbumin-containing cell embedded into the granule cell layer (GcL) is contacted by several substance P-immunoreactive axon terminals (arrows).
Bar scale= 10 μm.
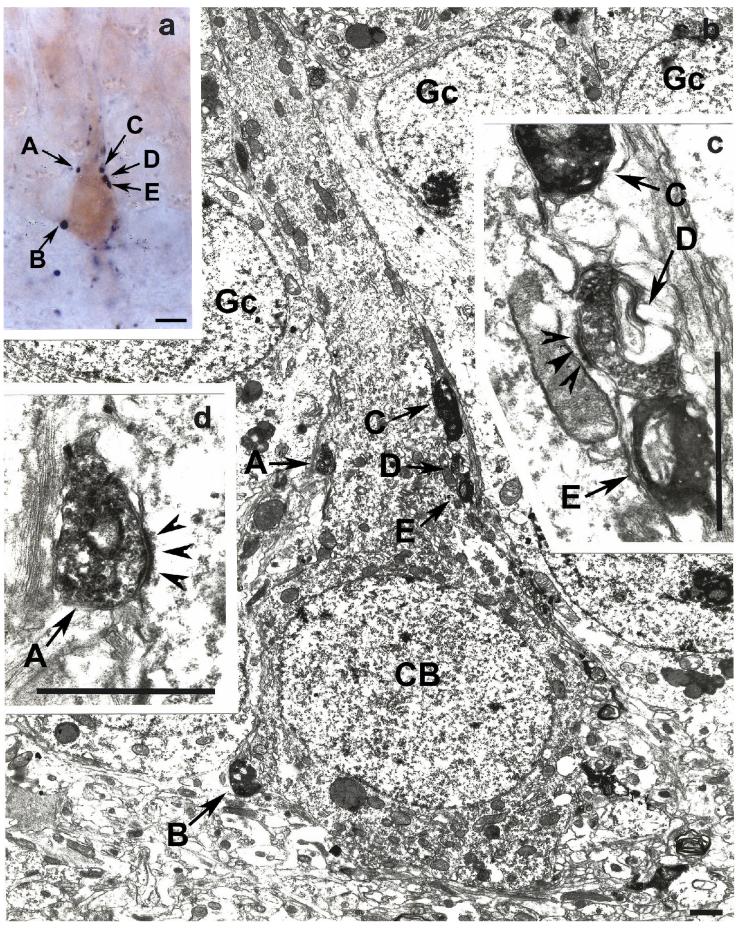
Fig. 6.
Light (Panel a) and electron micrographs (Panels b, c, d) depicted from the monkey dentate gyrus demonstrate the result of a correlated light and electron microscopic double immunostaining for substance P (labeled by a dark-blue blue Ni-diaminobenzidine reaction) and calbindin (brown diaminobenzidine chromogen). Panel a shows a calbindin-immunoreactive neuron embedded into the granule cell layer forming putative synaptic contacts (A – D) with substance P-containing axon terminals. Electron microscopic analysis of ultrathin sections cut from the same area shows that boutons A and D form robust asymmetric synaptic contacts (arrowheads on panels c and d) with the soma of this calbindin-containing cell (CB on panel b). Gc- granule cell.
Bar scales= panel a, 10 μm; panels b – c, 1 μm.
3) CATECHOLAMINERGIC BRAINSTEM-DENTATE CONNECTIONS
3.a) Serotonergic afferents
The serotonergic raphe-hippocampal pathway has a powerful effect on hippocampal electric activity, depression-associated synaptic plasticity, and cognitive behavior. Electrophysiological and pharmacological studies have shown that the typical effect of serotonin on hippocampal neurons is a hyperpolarization evoked by an increase in K+ conductance, but depolarization and reduction of afterhyperpolarization were also reported (for review, see Freund et al., 1990).
The serotonergic innervation of the hippocampus originates largely from the median raphe (MR) and a less numerous projection arises from the dorsal raphe (for review, see Tork 1990) and includes two types of fibers (Kosofsky and Molliver, 1987). In the dentate gyrus, the most numerous are the thin axons with small evenly distributed varicosities that are present in the subgranular area. The other fiber type has larger boutons that form clusters along the hilar border of the stratum granulosum and contact secondary dendritic branches of two cell types: GABAergic, pyramidal-shaped neurons located subjacent to the granule cell layer, and fusiform cells in the hilus. Both types of these GABAergic cells seem to contain calbindin, but none of them are immunoreactive for parvalbumin (Freund et al., 1990; Halasy et al., 1992).
Based on these morphological data, it has been suggested that the two types of serotonergic axons have different mechanisms of action in the hippocampus. Axons with small varicosities (originating in the dorsal raphe; Kosofsky and Molliver, 1987) release serotonin at non-synaptic sites, diffusely, and target cells having 5-HT1-2 receptors, to exert a slow, tonic, G-proteinmediated action. The other type of serotonergic axons with large boutons (originating in the medial raphe; Kosofsky and Molliver, 1987) always form synaptic contacts with GABAergic interneurons that have 5-HT3 receptors (Halasy et al., 1992). Thus, stimulation of medial raphe serotonin neurons could result in fast excitation of these GABAergic cells and an enhanced GABAA receptor-mediated inhibition of granule cells, a mechanism described in the CA1 area (Roppert and Guy, 1991).
An important question is whether there is convergence of the MSDB and MR hippocampal inputs on the same hippocampal interneurons. A very elegant morphological study of Miettinen and Freund (1992a) has demonstrated that parvalbumin-containing interneurons are innervated by MSDB GABAergic afferents, but are avoided by axons originating in the medial raphe. On the other hand, calbindin- and, to a smaller extent, cholecystokinin-containing interneurons are targets for both pathways. In some cases the same individual calbindin- or cholecystokinin-containing neurons received multiple contacts from afferents of both MSDB and medial raphe origin. Thus, these observations indicate that different subcortical nuclei modulate largely different inhibitory circuits. However, considering the occasional convergence of the two subcortical nuclei not only onto the same type, but even onto the same individual interneurons, the authors proposed that a particular inhibitory function, most probably feed-forward inhibition in the distal dendritic region, is under the control of both pathways.
For the sake of the readers it has to be noted that median raphe serotonin system could also effect the hippocampus via an indirect route. The median raphe serotonergic neurons heavily innervate the MSDB (Leranth and Vertes, 1999) and exert a robust stimulatory effect on parvalbumin-containing septohippocampal GABAergic neurons (Alreja, 1996). These GABAergic septal cells, in turn, selectively innervate hippocampal basket and chandelier cells (Freund and Antal, 1988), which are known to have powerful inhibitory effects on the output sector (soma and axon hillock) of principal neurons (Freund and Antal, 1988). Thus, serotonergic stimulation of the septohippocampal GABA system results in a disinhibition of principal cells. The situation is more complex, because a population of median raphe serotonergic neurons project to both the hippocampus and MSDB (e.g., McKenna and Vertes, 2001).
3.b) Noradrenergic and dopaminergic afferents to the dentate gyrus
Noradrenergic fibers in the hippocampus originate from the locus ceruleus. Noradrenaline in the dentate gyrus promotes and permits long-term perforant path potentiation. Furthermore, phasic locus ceruleus activation produces a delayed protein synthesis-dependent long-term potentiation of synaptic plasticity, suggesting a selective role in long-term memory and increases in the perforant path-evoked population spike (see Chapter X). Potentiation of the perforant path population spike by noradrenaline could be the result of increased granule cell excitability or reduced inhibition from interneurons of granule cells, or both. However, unit recording in the dentate gyrus shows that exogenous noradrenaline inhibits granule cells and excites presumed inhibitory interneurons (Brown et al., 2005). On the other hand, activation of α2- or β-adrenoceptors excites both the interneurons and granule cells (Brown et al., 2005). Increased inhibitory interneuron activity and inhibition of granule cells is inconsistent with the enhanced granule cell responsiveness and enhanced plasticity in the dentate gyrus reported with population recording. Thus, a critical question concerns the action of noradrenaline on the dentate gyrus interneurons.
In the hippocampal formation, noradrenergic innervation is particularly dense in areas receiving mossy fiber inputs, including the hilus of the dentate gyrus and stratum lucidum of the CA3 (e.g., Moudy et al., 1993). In these two areas, the majority of noradrenergic varicosities do not make conventional synaptic contacts. However, those that form synapses terminate on dendritic shafts and somata of GABAergic interneurons forming symmetric membrane specializations (Frotscher and Leranth, 1988; Milner and Bacon, 1989). It should be noted, however, that asymmetric synaptic contacts between tyrosine hydroxylase-containing (presumably noradrenergic) axon terminals and dendritic spines were also observed, in the stratum lucidum of CA3 (Frotscher and Leranth, 1988). symmetric (presumably inhibitory) synapses support earlier physiological studies suggesting that noradrenaline disinhibits hippocampal pyramidal neurons by decreasing the excitability of GABAergic interneurons (e.g., Madison and Nicoll, 1988).
In contrast to the very dense noradrenergic innervation of the dentate gyrus, this structure receives only a minor and diffusely distributed dopaminergic projection that arises mainly from the ventral tegmental area (Swanson, 1982). In spite of the well-established effects of dopamine in hippocampus related mnemonic functions, little is known about the mechanisms of these effects, which are likely to reside within the hippocampus. Most of our knowledge derives from receptor studies, characterizing the role of D1 – D5 receptors. The results of these studies indicate that in the dentate gyrus, via the aforementioned dopamine receptor subtypes, dopamine is involved in depotentiation and serves to maintain synaptic facilitation in recently potentiated pathways. The net effect helps consolidate information storage (e.g. in, Manahan-Voughan and Kulla, 2003).
4) COMMISSURAL CONNECTIONS OF THE DENTATE GYRUS
The majority of commissural fibers occupy the inner molecular layer of the dentate gyrus (e.g., Blackstad 1956). However, some commissural fibers were described that do not follow the “classical” pattern of fiber lamination and terminate in the outer molecular layer (Deller, 1998; Deller et al., 1995, 1996). The main postsynaptic targets of the commissural projection are the granule cell dendrites (e.g., Frotscher and Zimmer, 1983b; Seress and Ribak 1984), but they also innervate interneurons (Frotscher and Zimmer, 1983a; Seress and Ribak, 1984), which were shown to contain GAD (Frotscher et al., 1984). These interneurons do not represent a homogeneous cell population since, they could be distinguished by their different neuropeptide content, e.g., vasoactive intestinal polypeptide (Leranth and Frotscher, 1983), neuropeptide Y (Deller and Leranth 1990), and parvalbumin (Deller et al. 1994). This arrangement, that commissural fibers terminate on both principal and inhibitory interneurons in the dentate gyrus, represents the anatomical basis of the feed-forward inhibitory mechanism elicited by stimulation of the commissural system (Buzsaki and Eidelberg, 1981; Buzsaki, 1984).
Retrograde tracing experiments revealed that the majority of the cells of origin of the commissural system located in the hilus of the fascia dentata (e.g., Hjorth-Simonsen and Laurberg 1977; Berger et al., 1980) and several groups of neurons have been identified that contribute to this fiber system. The majority of commissural fibers are axon collaterals of the glutamate-containing mossy cells that are also considered as associational/commissural neurons (Ribak et al. 1985; Scharfman and Schwartzkroin 1988; Scharfman 1992; Soriano and Frotscher 1994; Scharfman 1995). The second major population of cells projecting to the contralateral hippocampus includs different types of GABAergic interneurons. The first hint of a possible contribution of GABAergic interneurons to the commissural projection was published by Seress and Ribak (1983). They showed that in the hilar area, more than 60% of neurons were GAD positive, whereas about 80% of hilar neurons project commissurally, suggesting that at least some of the hilar projection must be GABAergic. Later, this proposition was verified by experiments using combination of retrograde tracing and GAD immunochemistry, as well as anterograde labeling and degeneration (Ribak et al., 1986). The GABAergic commissural projection is not homologous. Different populations of commissurally projecting GABAergic cells co-express SOM (Zimmer et al. 1983; Leranth and Frotscher 1987), neuropeptide Y (Deller and Leranth 1990), parvalbumin (Goodman and Sloviter 1992), or other markers (Leranth and Frotscher 1987; Sloviter and Nilaver 1987). However, not all neurons of a given cell type have a commissural collateral. While most, if not all mossy cells appear to project bilaterally (e.g., Frotscher et al. 1991; Scharfman 1992), only 4–5% of the SOM-(Zimmer et al. 1983) and only 2% of the NPY-containing neurons (Deller and Leranth 1990) project to the contralateral dentate gyrus. In addition to the aforementioned neurons, commissurally projecting CA3c pyramidal neurons were also described (Gottlieb and Cowan 1973; Laurberg 1979; Voneida et al. 1981). These CA3c pyramids appear to have commissural collaterals, which arborize in the contralateral hilus, similar to their ipsilateral collaterals (Ishizuka et al. 1990; Li et al. 1994).
Based on the existence of a GABAergic commissural projection, Freund and Buzsaki (1996) have suggested that there is a component of direct inhibition in the feed-forward inhibitory response evoked in the dentate gyrus by commissural stimulation (Buzsaki and Eidelberg, 1981; Buzsaki, 1984).
5) GLUTAMATERGIC INNERVATION OF THE DENTATE GYRUS
During the last several decades, visualizing neurons that utilize glutamate as a neurotransmitter has proven to be a difficulty due to the lack of specific glutamatergic markers. As a result, one of the most influential and significant neuronal systems has remained grossly underinvestigated. At the turn of the millennium, two independent research groups discovered that a protein that has previously been suggested to mediate the Na+-dependent uptake of inorganic phosphate across the plasma membrane, also transports glutamate into synaptic vesicles (Bellocchio et al., 2000; Takamori et al., 2000). This protein, originally called brain-specific Na+-dependent inorganic phosphate contransporter (BNPI) (Ni et al., 1994), became the first of what are now called vesicular glutamate transporters (VGLUT1), and revolutionized research about the central glutamatergic system. Shortly after this groundbreaking discovery, a second (VGLUT2) and a third (VGLUT3) vesicular glutamate transporter has also been identified and cloned (Aihara et al., 2000; Bai et al., 2001; Fremeau et al., 2002; Gras et al., 2002; Schafer et al., 2002; Takamori et al., 2002). Since then, a vast amount of data has been collected that has led to a better understanding of the brain glutamatergic circuitry, including that in the dentate gyrus.
5.a) BNPI/VGLUT1
Distribution of BNPI/VGLUT1 containing fibers and terminals in the rat dentate gyrus has been investigated by several groups (Bellocchio et al., 1998; Fremeau et al., 2001; Kaneko and Fujiyama, 2002; Kaneko et al., 2002). VGLUT1 fiber density is moderate to intense in most regions of the dentate gyrus except the granule cell layer (Kaneko et al., 2002). A more detailed description has come from Bellocchio and colleagues (Bellocchio et al., 1998). The outer two-thirds of the molecular layer label more strongly for BNPI than does the inner one-third. Further examination of the CA3 region under higher magnification revealed coarse granular labeling at the periphery of the pyramidal cell layer, strongly suggestive of mossy fiber synapses. Electron microscopic, immunoperoxidase labeling in stratum lucidum of CA3 showed prominent reaction product in large axon terminals having the morphological characteristics of mossy fiber boutons. In the hilar area of the dentate gyrus, where mossy fiber collaterals also terminate, a few large terminals similar to those in the CA3 region contain BNPI immunoreactivity. Many smaller terminals in the dentate gyrus that form asymmetric (Gray type I) synapses with dendritic spines are also labeled for BNPI. Symmetric synapses do not contain detectable BNPI, supporting a specific role for the protein in excitatory transmission. In addition, many terminals forming asymmetric synapses do not contain detectable BNPI, indicating expression only in a subset of excitatory synapses (Bellocchio et al., 1998).
The distribution of BNPI containing boutons in the stratum moleculare suggests that they represent mainly perforant path inputs from the entorhinal cortex. Indeed, hybridization with the BNPI probe resulted in a strong hybridization signal in neuron-enriched regions of the entorhinal cortex (Ni et al., 1995). On the other hand, the BNPI positivity of mossy fiber-like terminals in the hilus and CA3 suggests that they originate from granule cells. Similar to cell bodies elsewhere in the brain, the somata of dentate gyrus granule cells show no detectable BNPI immunoreactivity (Bellocchio et al., 1998). By contrast, a strong BNPI hybridization signal has been observed in the pyramidal neurons of the hippocampus and granule cells of the dentate gyrus, suggesting that these cells synthesize the protein and then transport it to their terminals (Ni et al., 1994; Ni et al., 1995; Fremeau et al., 2001; Herzog et al., 2001). In conclusion, BNPI/VGLUT1 appears to be the primary glutamatergic marker of principal neurons, including dentate granule cells (Varoqui et al., 2002).
5.b) DNPI/VGLUT2
Prior to its identification as VGLUT2 (Aihara et al., 2000), this protein has been known as differentiation-associated Na+-dependent inorganic phosphate cotransporter (DNPI), having similar functions as BNPI (Hisano et al., 2000). In a rather comprehensive study, VGLUT2-positive fibers and terminals in the rat hippocampus have been characterized, and their possible sources of origin defined (Halasy et al., 2004). The highest density of VGLUT2-immunoreactive boutons has been observed in the inner molecular layer, while only scattered VGLUT2 positive fibers have been detected in the other areas of the dentate gyrus, such as the stratum moleculare and the hilus. In general, VGLUT2-immunoreactive boutons establish exclusively asymmetric synapses. Analysis of VGLUT2-positive synaptic boutons revealed that the majority of postsynaptic targets in the dentate gyrus are dendritic spines, followed by dendritic shafts and granule cell somata (Halasy et al., 2004). These findings are consistent with the results of previous studies in the rat (Fremeau et al., 2001; Kaneko and Fujiyama, 2002; Kaneko et al., 2002; Varoqui et al., 2002).
Because only low levels of VGLUT2 mRNA expression have been observed within the hippocampus of rats (Hisano et al., 2000; Fremeau et al., 2001; Herzog et al., 2001), and the granule cell layer of the dentate gyrus shows no signal for DNPI by in situ hybridization (Fremeau et al., 2001), dentate VGLUT2 positive boutons are likely to be of extrahippocampal origin. It has to be noted, however, that VGLUT2 mRNA in mice is concentrated in pyramidal neurons of the hippocampus (Bai et al., 2001), indicating the potential for considerable species differences in the hippocampal expression of VGLUT2. In rats, Fremeau and colleagues suggest a hypothalamic origin for the supragranular VGLUT2-positive fiber cluster because cells in the hypothalamus that project to this layer strongly express DNPI mRNA (Fremeau et al., 2001). In addition to the hypothalamus, VGLUT2 has been found in most septal neurons (Hajszan et al., 2004), and several of these neurons are also positive for GABAergic or cholinergic markers (Danik et al., 2005). However, deafferentation studies (fimbria-fornix transection and entorhinal cortex ablation) led to no significant differences in either the density or distribution pattern of VGLUT2-positive boutons in the dentate gyrus (Halasy et al., 2004), suggesting that the majority of dental VGLUT2 boutons are of intrahippocampal origin. Indeed, light microscopic observation of the hippocampus of colchicine-treated rats revealed a large number of VGLUT2-immunoreactive cell bodies. In the dentate gyrus, the hilar mossy cells represent the most heavily labeled population, while a small proportion of granule cells in the subgranular zone are also VGLUT2-positive (Halasy et al., 2004). It is well known that mossy cells in the dentate gyrus project to the inner molecular layer (Frotscher et al., 1991). Thus, VGLUT2-containing hilar mossy cells may be the source of the VGLUT2 fiber network in the dentate gyrus.
5.c) VGLUT3
VGLUT3 is the only known vesicular glutamate transporter that began its ‘career’ without previous history as inorganic phosphate cotransporter. It has been identified in a direct search for additional VGLUT molecules, and cloned (Fremeau et al., 2002; Schafer et al., 2002; Takamori et al., 2002). VGLUT3-immunoreactive terminals surround the granule cell layer of the rat dentate gyrus. VGLUT3 labeling is intense in the molecular layer, corresponding to the proximal part of the granule cell dendrites, and along the hilar border with the granule cell layer, corresponding to the zone where granule cell axons emerge. A dense VGLUT3 network also surrounds the soma of granule cells (Fremeau et al., 2002; Gras et al., 2002; Herzog et al., 2004). A similar distribution pattern has been observed in mice (Schafer et al., 2002). In rats, immunoparticles for VGLUT3 accumulate over vesicle clusters in terminals making classical asymmetrical synapses in the hippocampus (Gras et al., 2002). However, a large number of VGLUT3-positive terminals also form symmetrical synapses (Fremeau et al., 2002; Gras et al., 2002). Further, the labeled terminals make contact with the shaft of proximal dendrites, a location characteristic of inhibitory synapses. However, only a subset of symmetric synapses in the hippocampus appears to stain for VGLUT3 (Fremeau et al., 2002).
Regarding the source of dentate VGLUT3 fibers, one possibility is an intrahippocampal origin. Although moderate levels of VGLUT3 mRNA expression have been observed in the principal cells of pyramidal and dentate granule cell layers in the rat (Fremeau et al., 2002), the distribution of VGLUT3-immunoreactive fibers resembles that observed with markers of GABA terminals. Indeed, the VGLUT3 gene is expressed in scattered interneurons in the hilus of the dentate gyrus (Fremeau et al., 2002; Gras et al., 2002; Herzog et al., 2004). A similar distribution pattern of VGLUT3 mRNA has been demonstrated in mice (Schafer et al., 2002). Immunoreactivity for VGLUT3 is undetectable in pyramidal and dentate granule cells (Fremeau et al., 2002; Somogyi et al., 2004). More importantly, a partial colocalization of VGLUT3 and the GABA marker glutamic acid decarboxylase (GAD) has been observed in the hilar interneurons of the dentate gyrus by means of both in situ hybridization (Herzog et al., 2004) and immunohistochemistry (Fremeau et al., 2002). In addition, colocalization of VGLUT3 with vesicular inhibitory amino acid transporter, a marker of GABAergic neurons and boutons revealed numerous double-labeled nerve endings in the perisomatic terminals that contact the soma of granule cells (Herzog et al., 2004). More specifically, Somogyi and colleagues (Somogyi et al., 2004) have shown that all VGLUT3-positive somata are immunoreactive for cholecystokinin, a marker of a subpopulation of basket cells, and none express markers for other interneuron types in the hippocampus. Boutons expressing VGLUT3, cholecystokinin and GAD are most abundant in the cell layers of the hippocampus (Somogyi et al., 2004).
Another source of dentate VGLUT3 fibers may be the subcortical neurons, such as the septohippocampal neurons, the mesolimbic dopaminergic and raphe serotonergic cells. However, the data obtained so far are controversial. In the rat, the substantia nigra pars compacta and ventral tegmental area contain moderate levels of VGLUT3 mRNA, suggesting expression by dopamine neurons (Fremeau et al., 2002). By contrast, no VGLUT3 mRNA expression has been detected in the substantia nigra by Herzog and colleagues (Herzog et al., 2004), and lack of VGLUT3 expressing neurons has also observed in the septum and the vertical limb of the diagonal band in the same study. Furthermore, a very high density of VGLUT3 mRNA has been found in the dorsal and median raphe nuclei (Fremeau et al., 2002; Herzog et al., 2004). All of the serotonin transporter-positive neurons also express VGLUT3 in the dorsal and median raphe. Interestingly, numerous neurons from the dorsal raphe express VGLUT3 but no serotonin transporter (Gras et al., 2002). However, double-staining for VGLUT3 and the catecholamine marker tyrosine hydroxylase or the plasma membrane serotonin transporter revealed no colocalization in the axons of the dentate gyrus (Fremeau et al., 2002). On the other hand, Somogyi and colleagues (Somogyi et al., 2004) have found a population of VGLUT3 boutons in the hippocampus that are negative for GAD but are labeled for vesicular monoamine transporter type 2 (VMAT2), plasmalemmal serotonin transporter or serotonin, but no colocalization has been found in terminals containing VGLUT3 and vesicular acetylcholine transporter. In mice, the highest VGLUT3 mRNA expression levels and highest density of VGLUT3 mRNA-expressing cells have been observed in the raphe nuclei. In contrast to its mRNA, VGLUT3 immunoreactivity is undetectable in the cell bodies of raphe nuclei in vivo (Schafer et al., 2002). In the hippocampus, VGLUT3 immunoreactivity is absent from cholinergic synapses. Confocal double-immunofluorescence revealed the presence of VGLUT3 in a subpopulation of both thick and thin VMAT2-positive varicose fibers in the hippocampus, as well as the absence of VGLUT3 from tyrosine hydroxylase positive terminals. Thus, the hippocampal VGLUT3/VMAT2 system most likely stems from the dorsal and median raphe nuclei and represents a novel neuronal projection subsystem encoding both glutamatergic and serotonergic neurotransmission in the mouse (Schafer et al., 2002).
6) CONCLUSION
One common feature of the extrinsic dentate afferent systems is that they originate from a relatively small number of neurons. However, the majority of these afferents are able to exert a powerful control over the electric activity of the hippocampus. In some of the afferent systems, this efficacy is due to the fact that the majority of the extrinsic afferents terminate on a relatively small, but specific populations of neurons. These target cells include different type of GABAergic interneurons, which, in turn have the ability to control a large number of principal cells (Buzsaki, 1984; Freund and Buzsaki, 1996). For example, The MSDB GABAergic neurons seem to innervate only the GABAergic chandelier and basket cells that have a great influence (disinhibition) on the output sector, soma and axon hillock, of granule cells (Freund and Antal, 1988). Serotonergic fibers originating in the MR, also selectively innervate a specific population of calbindin-containing GABA cells that terminate on the dendritic shafts of principal cells. Thus, in contrast to the disinhibitory function of the MSDB-hippocampal GABA system, activation of the MR-hippocampal serotonergic pathway could result in inhibition of the input of principal neurons (Freund et al., 1990). The termination pattern of SUM afferents seems also be very specific. The overwhelming majority of fibers originating in the SUM form robust asymmetric synaptic contacts with the primary dendrites of granule cells (Magloczky et al., 1994; Leranth and Nitsch, 1994; Nitsch and Leranth, 1996) and, in addition, terminate on specifically positioned GABA interneurons, in a way that they could effectively filter/contrast the signal flow in the trisynaptic circuit (Leranth and Nitsch, 1994).
The functional importance of these three major extrahippocampal afferent systems is highlighted by recent observations related to the mnemonic functions of the hippocampus that are associated with synaptoplastic effects of gonadal hormones. Local estrogen administration into the MSDB (Lam and Leranth, 2003), MR (Prange-Kiel et al., 2004), and SUM (Leranth and Shanabrough, 2001) of ovariectomized rats all resulted in a robust increase in the density of spine synapses in the hippocampus. In contrast, transection of the fimbria fornix, which contains the hippocampal projection of these subcortical structures, prevents (Leranth et al., 2000) the known effects (Gould et al., 1990).
Acknowledgements
This work was supported by NIH grants MH060858 and NS402644 to C.L. and MH074021 to T.H., as well as by a Hungarian National Office for Research and Technology grant RET-08/04.
7) LITERATURE CITED
- Aihara Y, Mashima H, Onda H, Hisano S, Kasuya H, Hori T, Yamada S, Tomura H, Yamada Y, Inoue I, Kojima I, Takeda J. Molecular cloning of a novel brain-type Na(+)-dependent inorganic phosphate cotransporter. J. Neurochem. 2000;74(6):2622–2625. doi: 10.1046/j.1471-4159.2000.0742622.x. [DOI] [PubMed] [Google Scholar]
- Alreja M. Excitatory actions of serotonin on GABAergic neurons of the medial septum diagonal band of Broca. Synapse. 1966;22:15–27. doi: 10.1002/(SICI)1098-2396(199601)22:1<15::AID-SYN2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. J. Camp. Neurol. 1980;189:573–591. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. 2nd ed Academic Press; New York: 1995. pp. 443–494. [Google Scholar]
- Anderson P, Eccles JC. Inhibitory phasing of neuronal discharge. Nature (London) 1962;196:645–647. doi: 10.1038/196645a0. [DOI] [PubMed] [Google Scholar]
- Apartis E, Poindessous-Jazat FR, Lamour YA, Bassant MH. Loss of rhythmically bursting neurons in rat medial septum following selective lesion of septohippocampal cholinergic system. J Neurophysiol. 1998;79:1633–1642. doi: 10.1152/jn.1998.79.4.1633. [DOI] [PubMed] [Google Scholar]
- Bai L, Xu H, Collins JF, Ghishan FK. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J. Biol. Chem. 2001;276(39):36764–36769. doi: 10.1074/jbc.M104578200. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RTJ, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289(5481):957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J. Neurosci. 1998;18(21):8648–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzi G, Moretti A. Is there a rationale for the use of acetylcholinesterase inhibitors in the therapy of Alzheimer's disease? Europ J Pharmacol. 1998;346:1–13. doi: 10.1016/s0014-2999(98)00093-4. [DOI] [PubMed] [Google Scholar]
- Berger TW, Semple-Rowland S, Basset J. Hippocampal polymorph neurons are the cells of origin for ipsilateral association and commissural afferents to the dentate gyrus. Brain Res. 1980;215:329–336. doi: 10.1016/0006-8993(81)90512-6. [DOI] [PubMed] [Google Scholar]
- Blackstad TW. Commissural connections of the hippocampal region of the rat, with special reference to their mode of termination. J Comp Neurol. 1956;105:417–537. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- Bland BH, Oddie SD. Anatomical, electrophysiological and pharmacological studies of ascending brainstem hippocampal synchronizing pathways. Neurosci Biobehav Rev. 1998;22:259–273. doi: 10.1016/s0149-7634(97)00013-4. [DOI] [PubMed] [Google Scholar]
- Brito GN, Brito LS. Septohippocampal system and the prelimbic sector of frontal cortex: a neuropsychological battery analysis in the rat. Behav Brain Res. 1990;36:127–46. doi: 10.1016/0166-4328(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Brown RA, Walling SG, Milway JS, Harley CW. Locus ceruleus activation suppresses feedforward interneurons and reduces beta-gamma electroencephalogram frequencies while it enhances theta frequencies in rat dentate gyrus. J Neurosci. 2005;25:1985–1991. doi: 10.1523/JNEUROSCI.4307-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Wentzel HJ, Kunkel DD, Schwartzkroin PA. Axon arbors and synaptic connections of hippocampal mossy cells in the rat in vivo. J Comp Neurol. 1996;366:270–292. doi: 10.1002/(sici)1096-9861(19960304)366:2<270::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Feed-forward inhibition in the hippocampal formation. Progr Neurobiol. 1984;22:131–153. doi: 10.1016/0301-0082(84)90023-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Czeh G. Commissural and perforant path interactions in the rat hippocampus. Field potentials and unitary activity. Exp Brain Res. 1981;43:429–38. doi: 10.1007/BF00238387. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Eidelburg E. Commissural projection to the dentate gyrus of the rat: evidence for feed-forward inhibition. Brain Res. 1981;230:346–350. doi: 10.1016/0006-8993(81)90413-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Eidelburg E. Direct afferent excitation and long-term potentiation of hippocampal interneurons. J. Neurophysiol. 1982;48:597–607. doi: 10.1152/jn.1982.48.3.597. [DOI] [PubMed] [Google Scholar]
- Carre GP, Harley CW. Population spike facilitation in the dentate gyrus following glutamate administration to the lateral supramammillary nucleus. Brain Res. 1991;568:307–310. doi: 10.1016/0006-8993(91)91415-w. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Clarke DJ. Cholinergic innervation of the rat dentate gyrus: an immunocytochemical and electron microscopical study. Brain Res. 1985;360:349–354. doi: 10.1016/0006-8993(85)91253-3. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Felix D, McLennan H. GABA and hippocampal inhibition. Br J Pharmacol. 1970;40:88t–883. doi: 10.1111/j.1476-5381.1970.tb10663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D, Winson J. Influence of neurons of the parafascicular region on neuronal transmission from perforant path through dentate gyrus. Brain Res. 1986;377:211–219. doi: 10.1016/0006-8993(86)90888-7. [DOI] [PubMed] [Google Scholar]
- Danik M, Cassoly E, Manseau F, Sotty F, Mouginot D, Williams S. Frequent coexpression of the vesicular glutamate transporter 1 and 2 genes, as well as coexpression with genes for choline acetyltransferase or glutamic acid decarboxylase in neurons of rat brain. J. Neurosci. Res. 2005;81(4):506–521. doi: 10.1002/jnr.20500. [DOI] [PubMed] [Google Scholar]
- Deller T. The anatomical organization of the rat fascia dentata: new aspects of laminar organization as revealed by anterograde tracing with phaseolus vulgaris-leucoagglutinin. Anat Embryol. 1998;197:89–103. doi: 10.1007/s004290050122. [DOI] [PubMed] [Google Scholar]
- Deller T, Katona I, Cozzari C, Frotscher M, Freund TF. Cholinergic innervation of mossy cells in the rat fascia dentata. Hippocampus. 1999;9:314–320. doi: 10.1002/(SICI)1098-1063(1999)9:3<314::AID-HIPO10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Deller T, Leranth C. Synaptic connections of NPY-immunoreactive neurons in the rat hilar area. J Comp Neurol. 1990;300:433–447. doi: 10.1002/cne.903000312. [DOI] [PubMed] [Google Scholar]
- Deller T, Martinez A, Nitsch R, Frotscher M. A novel entorhinal projection to the rat dentate gyrus: direct innervation of proximal dendrites and granule cell bodies of granule cells and GABAergic neurons. J Neurosci. 1996;16:3322–3333. doi: 10.1523/JNEUROSCI.16-10-03322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Nitsch R, Frotscher M. Associational and commissural afferents of parvalbumin-immunoreactive neurons in the rat hippocampus: a combined immunocytochemical and PHAL study. J Comp Neurol. 1994;350:612–622. doi: 10.1002/cne.903500408. [DOI] [PubMed] [Google Scholar]
- Deller T, Nitsch R, Frotscher M. Phaseolus vulgaris- Leucoagglutinin (PHAL) tracing of commissural fibers to the rat fascia dentata: evidence for a previously unknown commissural projection to the outer molecular layer. J Comp Neurol. 1995;352:55–68. doi: 10.1002/cne.903520105. [DOI] [PubMed] [Google Scholar]
- Deller T, Nitsch R, Frotscher M. Heterogeneity of the commissural projection to the rat dentate gyrus: a Phaseolus vulgaris- Leucoagglutinin tracing study. Neuroscience. 1996;75:111–121. doi: 10.1016/0306-4522(96)00255-2. [DOI] [PubMed] [Google Scholar]
- Dickinson-Anson H, Aubert I, Gage FH, Fisher LJ. Hippocampal grafts of acetylcholine-producing cells are sufficient to improve behavioral performance following a unilateral fimbria-fornix lesion. Neuroscience. 1998;84:771–81. doi: 10.1016/s0306-4522(97)00543-5. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Low WC, Iversen SD, Stenevi U, Bjorklund A. Septal transplants restore maze learning in rats with fornix-fimbria lesions. Brain Res. 1982;251:335–348. doi: 10.1016/0006-8993(82)90751-x. [DOI] [PubMed] [Google Scholar]
- Dutar P, Lamour Y, Jobert A. Acetylcholine excites identified septo-hippocampal neurons in the rat. Neurosci Lett. 1983;43:43–47. doi: 10.1016/0304-3940(83)90126-x. [DOI] [PubMed] [Google Scholar]
- Fantie BD, Goddard GV. Septal modulation of the population spike in the fascia dentata produced by perforant path stimulation in the rat. Brain Res. 1982;252:227–237. doi: 10.1016/0006-8993(82)90390-0. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Topographical and subcellular localization of choline acetyltransferase in rat hippocampal formation. J Neurochem. 1970;17:1029–1037. doi: 10.1111/j.1471-4159.1970.tb02256.x. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci USA. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. GABAergic septohippocampal neurons contain parvalbumin. Brain Res. 1989;478:375–381. doi: 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyas AI, Acsady L, Gorcs T, Toth K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proc Natl Acad Sci USA. 1990;87:8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Hajos N, Acsady L, Gorcs TJ, Katona I. Mossy cells of the rat dentate gyrus are immunoreactive for calcitonin gene-related peptide (CGRP) Eur J Neurosci. 1997;9:1815–1830. doi: 10.1111/j.1460-9568.1997.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Fricke RA, Prince DA. Electrophysiology of dentate gyrus granule cells. J Neurophysiol. 1984;51:195–209. doi: 10.1152/jn.1984.51.2.195. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. The cholinergic innervation of the rat fascia dentata: identification of target structures on granule cells by combining choline acetyltransferase immunocytochemistry and Golgi impregnation. J Comp Neurol. 1986;243:58–70. doi: 10.1002/cne.902430106. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Catecholaminergic innervation of pyramidal and GABAergic nonpyramidal neurons in the rat hippocampus. Double label immunostaining with antibodies against tyrosine hydroxylase and glutamate decarboxylase. Histochemistry. 1988;88:3 13–319. doi: 10.1007/BF00570289. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C, Lubbers K, Oertel WH. Commissural afferents innervate glutamate decarboxylase immunoreactive nonpyramidal neurons in the guinea pig hippocampus. Neurosci Lett. 1984;46:137–143. doi: 10.1016/0304-3940(84)90431-2. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Schlander M, Leranth C. Cholinergic neurons in the hippocampus. A combined light- and electron-microscopic immunocytochemical study in the rat. Cell Tissue Res. 1986;246:293–301. doi: 10.1007/BF00215891. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Seress L, Schwerdtfeger WK, Buhl E. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J Comp Neurol. 1991;312:145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Zimmer J. Commissural fibers terminate on nonpyramidal neurons in the guinea pig hippocampus-a combined Golgi/EM degeneration study. Brain Res. 1983a;265:289–293. doi: 10.1016/0006-8993(83)90344-x. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Zimmer J. Lesion-induced mossy fibers to the inner molecular layer of the rat fascia dentata: identification of postsynaptic granule cells by the Golgi-EM technique. J Comp Neurol. 1983b;336:170–173. doi: 10.1002/cne.902150306. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Luiten PG, Nyakas C, Traber J. Cortical projection patterns of the medial septum-diagonal band complex. J Comp Neurol. 1990;293:103–124. doi: 10.1002/cne.902930109. [DOI] [PubMed] [Google Scholar]
- Givens BS, Olton DS. Cholinergic and GABAergic modulation of medial septal area: effect on working memory. Behav Neurosci. 1990;104:849–55. doi: 10.1037//0735-7044.104.6.849. [DOI] [PubMed] [Google Scholar]
- Givens BS, Olton DS. Local modulation of basal forebrain: effects on working and reference memory. J Neurosci. 1994;14:3578–3587. doi: 10.1523/JNEUROSCI.14-06-03578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens BS, Olton DS. Bidirectional modulation of scopolamine-induced working memory impairments by muscarinic activation of the medial septal area. Neurobiol Learn Mem. 1995;63:269–76. doi: 10.1006/nlme.1995.1031. [DOI] [PubMed] [Google Scholar]
- Givens B, Sarter M. Modulation of cognitive processes by transsynaptic activation of the basal forebrain. Behav Brain Res. 1997;84:1–22. doi: 10.1016/s0166-4328(96)00146-5. [DOI] [PubMed] [Google Scholar]
- Goodman JH, Sloviter RS. Evidence for commissurally projecting parvalbumin-immunoreactive basket cells in the dentate gyrus of the rat. Hippocampus. 1992;2:13–22. doi: 10.1002/hipo.450020103. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DI, Cowan WM. Autoradiographic studies of the commissural and ipsilateral association connections of the hippocampus and dentate gyrus of the rat. I. The commissural connections. J Comp Neurol. 1973;149:393–422. doi: 10.1002/cne.901490402. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Gorcs TJ, Freund TF. Innervation of different peptide-containing neurons in the hippocampus by GABAergic septal afferents. Neuroscience. 1990;37:31–44. doi: 10.1016/0306-4522(90)90189-b. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Seress L, Toth K, Acsady L, Antal M, Freund TF. Septal GABAergic neurons innervate inhibitory interneurons in the hippocampus of the macaque monkey. Neuroscience. 1991;41:381–390. doi: 10.1016/0306-4522(91)90334-k. [DOI] [PubMed] [Google Scholar]
- Haglund L, Swanson LW, Hler C. The projection of the supramammillary nucleus to the hippocampal formation: an immunohistochemical and anterograde transport study with the lectin PHA-L in the rat. J Comp Neurol. 1984;229:17l–185. doi: 10.1002/cne.902290204. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Alreja M, Leranth C. Intrinsic vesicular glutamate transporter 2-immunoreactive input to septohippocampal parvalbumin-containing neurons: novel glutamatergic local circuit cells. Hippocampus. 2004;14:499–509. doi: 10.1002/hipo.10195. [DOI] [PubMed] [Google Scholar]
- Halasy K, Hajszan T, Kovacs EG, Lam TT, Leranth C. Distribution and origin of vesicular glutamate transporter 2-immunoreactive fibers in the rat hippocampus. Hippocampus. 2004;14:908–918. doi: 10.1002/hipo.20006. [DOI] [PubMed] [Google Scholar]
- Halasy K, Miettinen R, Szabat E, Freaund TF. GABAergic interneurons are the major postsynaptic targets of median raphe afferents in the rat dentate gyrus of the rat hippocampus. Eur J Neurosci. 1992;5:411–429. doi: 10.1111/j.1460-9568.1992.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Halasy K, Somogyi P. Subdivisions in the multiple GABAergic innervation of granule cells in the dentate gyrus of the rat hippocampus. Eur J Neurosci. 1993;5:411–429. doi: 10.1111/j.1460-9568.1993.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Harley CW, Lacaille J-C, Galway M. Hypothalamic afferents to the dorsal dentate gyrus contain acetylcholinesterase. Brain Res. 1983;270:335–339. doi: 10.1016/0006-8993(83)90609-1. [DOI] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J. Neurosci. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisano S, Hoshi K, Ikeda Y, Maruyama D, Kanemoto M, Ichijo H, Kojima I, Takeda J, Nogami H. Regional expression of a gene encoding a neuron-specific Na(+)-dependent inorganic phosphate cotransporter (DNPI) in the rat forebrain. Brain Res. Mol. Brain Res. 2000;83:34–43. doi: 10.1016/s0169-328x(00)00194-7. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A, Laurberg S. Commissural connections of the fascia dentata in the rat. J Comp Neurol. 1977;174:591–606. doi: 10.1002/cne.901740404. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J. Presynaptic inhibitory action of acetylcholine in area CA1 of the hippocampus. Exp Neurol. 1978;62:787–797. doi: 10.1016/0014-4886(78)90284-4. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Spencer WA, Brinley FJ. Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42:243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J. Comp. Neurol. 2002;444(1):39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kiss J, Patel AJ, Freund TF. Distribution of septohippocampal neurons containing parvalbumin or choline acetyltransferase in the rat brain. J Comp Neurol. 1990;298:362–372. doi: 10.1002/cne.902980308. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME. The serotonergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and medial raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- Lâm TT, Leranth C. Role of the medial septum diagonal band of Broca cholinergic neurons in estrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. European J Neuroscience. 2003;17:1997–2005. doi: 10.1046/j.1460-9568.2003.02637.x. [DOI] [PubMed] [Google Scholar]
- Lamour Y, Dutar P, Jobert A. Septo-hippocampal and other medial septum diagonal band neurons: electrophysiological and pharmacological properties. Brain Res. 1984;309:227–239. doi: 10.1016/0006-8993(84)90588-2. [DOI] [PubMed] [Google Scholar]
- Laurberg S. Commissural and intrinsic connections of the rat hippocampus. J Comp Neurol. 1979;184:685–708. doi: 10.1002/cne.901840405. [DOI] [PubMed] [Google Scholar]
- Laurberg S, Sorensen KE. Associational and commissural collaterals of neurons in the hippocampal formation (hilus fasciae dentatae and subfield CA3) Brain Res. 1981;212:287–300. doi: 10.1016/0006-8993(81)90463-7. [DOI] [PubMed] [Google Scholar]
- Lawson VH, Bland BH. The role of the septohippocampal pathway in the regulation of hippocampal field activity and behavior: analysis by the intraseptal microinfusion of carbachol, atropine, and procaine. Exp Neurol. 1993;120:132–144. doi: 10.1006/exnr.1993.1047. [DOI] [PubMed] [Google Scholar]
- Leranth C, Frotscher M. Commissural afferents to the rat hippocampus terminate on vasoactive intestinal polypeptide-like immunoreactive nonpyramidal neurons: An EM immunocytochemical degeneration study. Brain Res. 1983;276:357–36. doi: 10.1016/0006-8993(83)90747-3. [DOI] [PubMed] [Google Scholar]
- Leranth C, Frotscher M. Cholinergic innervation of hippocampal GAD- and somatostatin-immunoreactive commissural neurons. J Comp Neurol. 1987;261:33–47. doi: 10.1002/cne.902610104. [DOI] [PubMed] [Google Scholar]
- Leranth C, Frotscher M. The organization of the septal region in the rat brain: Cholinergic-GABAergic interconnections and the termination of hippocampo-septal fibers. J Comp Neurol. 1989;289:304–314. doi: 10.1002/cne.902890210. [DOI] [PubMed] [Google Scholar]
- Leranth C, Nitsch R. Morphological evidence that hypothalamic substance P-containing afferents are capable of filtering the signal flow in the monkey hippocampal formation. J Neurosci. 1994;14:4079–4094. doi: 10.1523/JNEUROSCI.14-07-04079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M. Supramammillary area mediates subcortical estrogenic action on hippocampal synaptic plasticity. Exp Neurology. 2001;167:445–450. doi: 10.1006/exnr.2000.7585. [DOI] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Horvath TL. Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience. 2000;101:349–356. doi: 10.1016/s0306-4522(00)00369-9. [DOI] [PubMed] [Google Scholar]
- Leranth C, Vertes RP. Median raphe serotonergic innervation of medial septum/diagonal band of Broca (MSDB) parvalbumin-containing neurons: possible involvement of the MSDB in the desynchronization of hippocampal EEG. J Comp Neurol. 1999;410:586–598. [PubMed] [Google Scholar]
- Lewis PR, Shute CCD. The cholinergic limbic system: projections to hippocampal formation, medial cortex nuclei of the ascending cholinergic reticular system and the subfornical organ and supra-optic crest. Brain. 1967;90:521–537. doi: 10.1093/brain/90.3.521. [DOI] [PubMed] [Google Scholar]
- Li X-G, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Lopes de Silva FH, Witter MP, Boeijinga PH, Lohman AHM. Anatomic organization and physiology of the limbic cortex. Physiol Rev. 1990;70:453–511. doi: 10.1152/physrev.1990.70.2.453. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. Studies on the structure of the cerebral cortex II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- Lynch G, Rose G, Gall C. Anatomical and functional aspects of the septohippocampal projections. In: Elliot K, Whelan J, editors. Functions of the Hippocampal System. Elsevier; Amsterdam: 1978. pp. 5–24. [Google Scholar]
- Madison DV, Nicoll RA. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain Res. 1988;442:131–138. doi: 10.1016/0006-8993(88)91440-0. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Acsady L, Freund TF. Principal cells are the postsynaptic targets of supramammillary afferents in the hippocampus of the rat. Hippocampus. 1994;4:322–334. doi: 10.1002/hipo.450040316. [DOI] [PubMed] [Google Scholar]
- Manahan-Voughan D, Kulla A. Regulation of depotentiation and long-term potentiation in the dentate gyrus of freely moving rats by dopamine D2-like receptors. Cerebral Cortex. 2003;13:123–135. doi: 10.1093/cercor/13.2.123. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Olton DS, Givens B. Cholinergic manipulations in the medial septal area: age-related effects on working memory and hippocampal electrophysiology. J Neurosci. 1995;15:2063–2073. doi: 10.1523/JNEUROSCI.15-03-02063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Collateral projections from the median raphe nucleus to the medial septum and hippocampus. Brain Res Bull. 2001;54:619–630. doi: 10.1016/s0361-9230(01)00465-8. [DOI] [PubMed] [Google Scholar]
- Mellgren SI, Srebro B. Changes in acetylcholinesterase and distribution of degenerating fibres in the hippocampal region after septal lesion in the rat. Brain Research. 1973;52:19–35. doi: 10.1016/0006-8993(73)90648-3. [DOI] [PubMed] [Google Scholar]
- Miettinen R, Freund TF. Convergence and segregation of septal and median raphe inputs onto different subsets of hippocampal inhibitory interneurons. Brain Res. 1992a;594:263–272. doi: 10.1016/0006-8993(92)91133-y. [DOI] [PubMed] [Google Scholar]
- Miettinen R, Freund TF. Neuropeptide-Y-containing interneurons in the hippocampus receive synaptic input from median raphe and GABAergic septal afferents. Neuropeptides. 1992b;22:185–1 93. doi: 10.1016/0143-4179(92)90161-o. [DOI] [PubMed] [Google Scholar]
- Milner TA, Bacon CE. GABAergic neurons in the rat hippocampal formation: ultrastructure and synaptic relationships with catecholaminergic terminals. J Neurosci. 1989;10:3410–27. doi: 10.1523/JNEUROSCI.09-10-03410.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJY, McNaughton BL, Barnes CA. A comparison of supramammillary and medial septal influences on hippocampal field potentials and single-unit activity. J Neurophysiol. 1989;61:15–31. doi: 10.1152/jn.1989.61.1.15. [DOI] [PubMed] [Google Scholar]
- Monmaur P, Breton P. Elicitation of hippocampal theta by intraseptal carbachol injection in freely moving rats. Brain Res. 1991;544:150–155. doi: 10.1016/0006-8993(91)90898-6. [DOI] [PubMed] [Google Scholar]
- Mosko S, Lynch G, Cotman CW. The distribution of septal projections to the hippocampus in the rat. J. Comp. Neurol. 1973;52:163–174. doi: 10.1002/cne.901520204. [DOI] [PubMed] [Google Scholar]
- Moudy AM, Kunkel DD, Schwartzkroin PA. Development of dopamine-beta-hydroxylase-positive fber innervation of the rat hippocampus. Synapse. 1993;15:307–318. doi: 10.1002/syn.890150407. [DOI] [PubMed] [Google Scholar]
- Ni B, Rosteck PRJ, Nadi NS, Paul SM. Cloning and expression, of a cDNA encoding a brain-specific Na(+)-dependent inorganic phosphate cotransporter. Proc. Natl. Acad. Sci. U. S. A. 1994;91(12):5607–5611. doi: 10.1073/pnas.91.12.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni B, Wu X, Yan GM, Wang J, Paul SM. Regional expression and cellular localization of the Na(+)-dependent inorganic phosphate cotransporter of rat brain. J. Neurosci. 1995;15(8):5789–5799. doi: 10.1523/JNEUROSCI.15-08-05789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schenker C, Leeman SE. Substance P as a transmitter candidate. Ann Rev Neurosci. 1980;2:227–268. doi: 10.1146/annurev.ne.03.030180.001303. [DOI] [PubMed] [Google Scholar]
- Nitsch R, Leranth C. Calretinin immunoreactivity in the monkey hippocampal formation. II: Intrinsic GABAergic and hypothalamic non-GABAergic systems. An experimental tracing and coexistence study. Neuroscience. 1993;55:797–812. doi: 10.1016/0306-4522(93)90442-i. [DOI] [PubMed] [Google Scholar]
- Nitsch R, Leranth C. Calretinin immunoreactivity in the monkey hippocampal formation. II: Intrinsic GABAergic and hypothalamic non-GABAergic systems. An experimental tracing and coexistence study. Neuroscience. 1993;55:797–812. doi: 10.1016/0306-4522(93)90442-i. [DOI] [PubMed] [Google Scholar]
- Nyakas C, Luiten PGM, Spencer DG, Traber J. Detailed projection patterns of septa1 and diagonal band efferents to the hippocampus in the rat with emphasis on innervation of CAI and dentate gyrus. Brain Res Bull. 1987;18:533–545. doi: 10.1016/0361-9230(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Rune GM, Leranth C. Median raphe mediates estrogenic effects to the hippocampus in female rats. European J Neuroscience. 2004;19:309–317. doi: 10.1111/j.0953-816x.2003.03124.x. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Estructura del asfa de Ammon y fascia dentata. Ann SOC Esp Hist Nat. 1893:22. [Google Scholar]
- Ramon y Cajal S. Histologie de systeme nerveux de I'Homme et des vertebres tomme II. Maloine; Paris: 1911. [Google Scholar]
- Ribak CE, Peterson GM. Intragranular mossy fibers in rats and gerbils form synapses with the somata and proximal dendrites of basket cells in the dentate gyrus. Hippocampus. 1991;1:355–364. doi: 10.1002/hipo.450010403. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Amaral DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Peterson GM, Serooggy KB, Fallon JH, Schmued LC. A GABAergic inhibitory component within the hippocampal commissural pathway. J Neursci. 1986;6:3492–3498. doi: 10.1523/JNEUROSCI.06-12-03492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GB, Racine RJ. Interactions between septodentate and perforant-path inputs to the dentate gyrus. Soc. Neurosci. Abstr. 1984;10:79. [Google Scholar]
- Roppert N, Guy N. Serotonin facilitates GABAergic transmission in the CA1 region of the rat hippocampus in vitro. J Physiol (London) 1991;441:121–136. doi: 10.1113/jphysiol.1991.sp018742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, Schubert P. Release and transfer of [3H]adenosine derivatives in the cholinergic septa1 system. Brain Res. 1977;121:353–357. doi: 10.1016/0006-8993(77)90158-5. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J. Biol. Chem. 2002;277(52):50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Ribak CE, Gall CM, Mody I, editors. Differentiation of rat dentate neurons by morphology and electrophysiology in hippocampal slices: granule cells, spiny hilar cells and aspiny ‘fast-spiking’ cells. Epilepsy Res Suppl. 1992;7:93–109. The dentate gyrus and its role in seizures. [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. J Neurophysiol. 1995;74:179–194. doi: 10.1152/jn.1995.74.1.179. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. The role of nonprincipal cells in dentate gyrus excitability and its relevance to animal models of epilepsy and temporal lobe epilepsy. Adv Neurol. 1999;79:805–820. [PubMed] [Google Scholar]
- Scharfman HE, Schwartzkroin PA. Electrophysiology of morphologically identified mossy cells of the dentate hilus recorded in guinea pig hippocampal slices. J Neurosci. 1988;8:3812–3821. doi: 10.1523/JNEUROSCI.08-10-03812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Buckmaster PS, Strowbridge BW, Kunkel DD, Owens J, Jr., Pokorny J. Possible mechanisms of seizure-related cell damage in the dentate hilus. Epilepsy Res. 1996;12:317–324. [PubMed] [Google Scholar]
- Segal M, Landis S. Afferents to the hippocampus of the rat studied with the method of retrograde transport of horseradish peroxidase. Brain Res. 1974;78:I–15. doi: 10.1016/0006-8993(74)90349-7. [DOI] [PubMed] [Google Scholar]
- Segal M. A potent inhibitory monosynaptic hypothalamo-hippocampal connection. Brain Res. 1979;162:137–141. doi: 10.1016/0006-8993(79)90762-5. [DOI] [PubMed] [Google Scholar]
- Seress L. Interspecies comparison of the hippocampal formation shows increased emphasis on the regio superior in the Ammon's horn of the human brain. J Hirnforsch. 1988;29:335–340. [PubMed] [Google Scholar]
- Seress L, Ribak CE. Direct commissural connections to basket cells of the hippocampal dentate gyrus: anatomical evidence for feed-forward inhibition. J Neurocytol. 1984;13:215–225. doi: 10.1007/BF01148116. [DOI] [PubMed] [Google Scholar]
- Shute CCD, Lewis PR. Electron microscopy of cholinergic terminals and acetylcholinesterase-containing neurons in the hippocampal formation of the rat. Z. Zellforsch. Mikrosk. Anat. 1966;69:334–343. doi: 10.1007/BF00406286. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Nilaver G. Immunocytochemical localization of GABA-, cholecystokinin, vasoactive intestinal polypeptide, and somatostatin-like immunoreactivity in the area dentata and hippocampus of the rat. J Comp Neurol. 1987;256:42–60. doi: 10.1002/cne.902560105. [DOI] [PubMed] [Google Scholar]
- Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, Shigemoto R, Watanabe M, Somogyi P. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur. J. Neurosci. 2004;19:552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Mody I. Patch-clamp recordings reveal powerful GABAergic inhibition in dentate hilar neurons. J Neurosci. 1994;14:2365–2376. doi: 10.1523/JNEUROSCI.14-04-02365.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano E, Frotscher M. Mossy cells of the rat fascia dentata are glutamate-immunoreactive. Hippocampus. 1994;4:65–70. doi: 10.1002/hipo.450040108. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Blackstad TW. Cholinesterase in the hippocampal region. Distribution and relation to architectonics and afferent systems. Acta Anat. 1964;56:216–253. [PubMed] [Google Scholar]
- Swanson LW. The projection of the ventral tegmental area and adjacent regions: A combined fluorescent and retrograde tracer and immunofluorescence study in the rat. Brain Res Bul. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takamori S, Malherbe P, Broger C, Jahn R. Molecular cloning and functional characterization of human vesicular glutamate transporter 3. Embo. Rep. 2002;3(8):798–803. doi: 10.1093/embo-reports/kvf159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tork I. Anatomy of the serotonergic system. Ann NY Acad Sci USA. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RB, Amaral DG, Cowan WM. The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). II. Efferent connections. J Comp Neurol. 1982;207:135–l56. doi: 10.1002/cne.902070204. [DOI] [PubMed] [Google Scholar]
- Voneida TJ, Vardaris RM, Fish SE, Reiheld CT. The origin of the hippocampal commissure in the rat. Anat Rec. 1981;201:91–103. doi: 10.1002/ar.1092010112. [DOI] [PubMed] [Google Scholar]
- Wainer BH, Bolam JP, Freund TF, Henderson Z, Totterdell S, Smith AD. Cholinergic synapses in the rat brain: a correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Res. 1984;308:69–76. doi: 10.1016/0006-8993(84)90918-1. [DOI] [PubMed] [Google Scholar]
- Wheal HV, Miller JJ. Pharmacological identification of acetylcholine and glutamate excitatory systems in the dentate gyrus of the rat. Brain Res. 1980;182:145–155. doi: 10.1016/0006-8993(80)90837-9. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wiss JM, Swanson LW, Cowan WM. Evidence for an input to the molecular layer and the stratum granulosum of the dentate gyrus from the supramammillary region of the hypothalamus. Anat Embrvol. 1979;156:165–176. doi: 10.1007/BF00300012. [DOI] [PubMed] [Google Scholar]
- Wu M, Hajszan T, Xu C, Leranth C, Alreja M. Group I metabotropic glutamate receptor activation produces a direct excitation of identified septohippocampal cholinergic neurons. J Neurophysiology. 2004;92:1216–1225. doi: 10.1152/jn.00180.2004. [DOI] [PubMed] [Google Scholar]
- Zimmer J, Laurberg S, Sunde N. Neuroanatomical aspects of normal and transplanted hippocampal tissue. In: Seifert W, editor. Neurobiology of the hippocampus. Academic Press; New York: 1983. pp. 39–64. [Google Scholar]