Abstract
The polarity of biological mediums controls a host of physiological processes such as digestion, signaling, transportation, metabolism, and excretion. With the recent widespread use of near-infrared (NIR) fluorescent dyes for biological imaging of cells and living organisms, reporting medium polarity with these dyes would provide invaluable functional information in addition to conventional optical imaging parameters. Here, we report a new approach to determine polarities of macro- and microsystems for in vitro and potential in vivo applications using NIR polymethine molecular probes. Unlike the poor solvatochromic response of NIR dyes in solvents with diverse polarity, their fluorescence lifetimes are highly sensitive, increasing by a factor of up to 8 on moving from polar to nonpolar mediums. We also established a correlation between fluorescence lifetime and solvent orientation polarizability and developed a lifetime polarity index for determining the polarity of complex systems, including micelles and albumin binding sites. Because of the importance of medium polarity in molecular, cellular, and biochemical processes and the significance of reduced autofluorescence and deep tissue penetration of light in the NIR region, the findings reported herein represent an important advance toward using NIR molecular probes to measure the polarity of complex biological systems in vitro and in vivo.
INTRODUCTION
Biological microenvironments and mediums are complex solvation systems described by diverse parameters including pH, viscosity, solutes concentration, and temperature. A host of physiological processes such as digestion, signaling, transportation, metabolism, and excretion is controlled by solvation. Although water is the major solvent in biological systems, the presence of a myriad of solute molecules at high concentrations effectively changes the polarity, especially local polarity within proteins, vesicles, cells, and tissues. As a result, deviations from the intricate balance between hydrophilicity and hydrophobicity might lead to fatal illnesses. For example, sudden alteration in hydrophilicity of amyloids have been linked to Alzheimer's (1) and prion (2) diseases, and mutations in genes encoding lung surfactants are associated with acute respiratory failure (3). Monitoring such changes in vivo is essential for early diagnosis of diseases and prevention of serious complications.
Current methods for studying polarities are mostly based on the use of dyes whose molecular spectral profiles are significantly affected by polarity of the medium. Molecular probes with significant changes in their infrared (IR) and Raman vibrations (4), NMR (5), and electron spin resonance (6) shifts, ultraviolet/visible/near-infrared (NIR) absorption, and fluorescence (7,8) have been developed. The latter class of compounds, known as solvatochromic dyes, has found widespread applications in many areas of chemistry, materials science, and biology. Solvatochromic dye-mediated polarity measurements (8) have been used to determine the polarity of the dye environment. Thus, they can report on the expression, location, interactions, conformational changes, and activities of target proteins or other biological molecules (9–12). Direct attachment of these dyes to proteins of interest is a useful method to study conformational changes through molecular interactions that provide hydrophobic pockets for the dye (13–15). However, most of the solvatochromic dyes are photoactive in the visible wavelengths, limiting the translation of the in vitro studies into in vivo applications. Recent studies have shown that excitation of molecules with low-energy NIR light between 700- and 900-nm wavelengths improves detection sensitivity, minimizes cell damage and autofluorescence, extends the spectral window for monitoring polarity with multiple dyes, and allows light to penetrate much deeper in tissue than visible light (16).
Recently, many NIR probes have been synthesized based on variations of the polymethine scaffold. Some of the dyes, such as indocyanine green (ICG), have received approval by the U.S. Food and Drug Administration for use in humans because they are safe and possess favorable optical properties. These properties have allowed their use as fluorescent probes in many biological applications, including in vivo molecular imaging (17,18). Unfortunately, most NIR polymethine dyes exhibit poor solvatochromic properties compared with common visible solvatochromic dyes such as naphthylamines (19) and merocyanines (20). In addition to their broad absorption and emission spectral profiles and small phase shifts in solvents of diverse polarity, biological inhomogeneity further complicates analysis of NIR solvatochromism because of overlapping steady-state spectra. Thus, practical application of NIR solvatochromism to determine milieu polarity is challenging.
In this study, we report the use of changes in the fluorescence lifetime properties of NIR dyes to determine the polarity of solvent and biological media. The method harnesses the strengths of NIR optical methods, the insensitivity of fluorescence lifetime to dye concentration, and the large change in lifetime without perturbing significantly the absorption and emission spectral profiles of the dyes to report the polarity of solvents and biological microenvironments. Because all changes occur within the same NIR excitation and detection window, a simple source-detector system will be adequate for the measurements, which further eliminates errors arising from differences in wavelength-dependent light-tissue interactions.
MATERIALS AND METHODS
Sample preparation
Cypate, LS-277, and LS-288 were synthesized as described previously (21,22). Bacteriochlorophyll from Rhodopseudomonas sphaeroides was purchased from Frontier Scientific (Logan, UT). Bovine serum albumin (BSA) fraction V Aldrich (Milwaukee, WI) was dissolved in 0.01 M buffer made from phosphate buffered saline (PBS). Tween-80 (10% ethanol solution), ICG, IR-820, ICG and 3,3-diethylthiatricarbocyanine iodine (DTTCI), methanol (liquid chromatography/mass spectrometer grade), ethanol (anhydrous), acetone (histological grade), DMSO (American Chemical Society (A.C.S.) grade), methylene chloride (A.C.S. grade), and chloroform (A.C.S. grade) were purchased from commercial sources. Solvents were not degassed.
For spectroscopy study in homogenous solutions, dyes were dissolved in 150 μL of DMSO as stock solutions. To prevent inner effect in fluorescent measurement, aliquots (10 μL) were diluted with the solvent of interest until the absorbance was ≤0.15. In some cases, the samples were dissolved at higher concentrations in methylene chloride and chloroform to obtain a measurable signal for fluorescence lifetime measurements. For optical measurement in heterogeneous media, dyes were dissolved in DMSO at 50–100 μM concentration. In dye-albumin experiments, 5 μL of DMSO solution of a dye was added to the solution of BSA (50 mg) dissolved in 1 mL of 0.01 M PBS buffer. In dye-micelle measurements, Tween-80 solution in ethanol (10 μL) was added to 5 μL of DMSO solution of a dye and the mixture was diluted with 1 mL of 0.01 M PBS buffer. All mixtures were vortexed for 1 min. The aliquots from the solutions were diluted with PBS buffer to adjust the absorbance to 0.1 to prevent an inner filter effect in monitoring the steady-state and lifetime fluorescence measurements.
Steady-state spectra
The steady-state absorption and fluorescence spectra were recorded on a spectrophotometer and spectrofluorometer, respectively. For uniformity, molar absorptivities were calculated from the standard curves at 720 nm and at the wavelengths of the maximum absorption of each dye in methanol. Fluorescent quantum yield was measured at 720 nm with slit widths of 5 nm for both excitation and emission. The fluorescence quantum yield of ICG in DMSO (Ψ = 0.12) (23) was used as a reference standard. All measurements were conducted at room temperature.
Fluorescence lifetime measurement
Fluorescence lifetime was measured using a time-correlated single photon counting (TCSPC) technique (Horiba, Kyoto, Japan) with excitation source NanoLed at 773 nm (Horiba) and impulse repetition rate of 1 MHz at 90° to a R928P detector (Hamamatsu Photonics, Hamamatsu City, Japan). The detector was set to 820 nm with a 20 nm band pass. The electrical signal was amplified by a TB-02 pulse amplifier (Horiba), and the amplified signal was fed to the constant fraction discriminator (CFD) (Philips, Eindhoven, The Netherlands). The first detected photon represented the start signal by the time-to-amplitude converter (TAC), and the excitation pulse triggered the stop signal. The multichannel analyzer (MCA) recorded repetitive start-stop signals from the TAC and generated a histogram of photons as a function of time-calibrated channels (6.88 ps/channel) until the peak signal reached 10,000 counts. The lifetime was recorded on a 50 ns scale. The instrument response function was obtained by using Rayleigh scatter of Ludox-40 (Sigma-Aldrich, St. Louis, MO) (0.03% in Millipore quality water) in a quartz cuvette at 773 nm emission. DAS6 v6.1 decay analysis software (Horiba) was used for lifetime calculations. The goodness of fit was judged by χ2 values and Durbin-Watson parameters and visual observations of fitted line, residuals, and autocorrelation function.
RESULTS
Solvatochromic properties of NIR polymethine dyes are weak
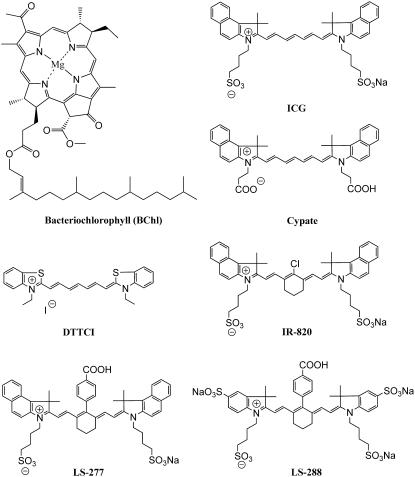
Some symmetrical polymethine dyes and bacteriochlorophyll (Fig. 1) were examined to establish their solvent-dependent optical properties. The solvent polarity was defined in terms of solvent orientation polarizability (24) derived as a combination of dielectric constant and refraction index (Eq. 1). Solvents (water, methanol, ethanol, acetone, dimethylsulfoxide (DMSO), methylene chloride, and chloroform) were chosen to cover a wide range of solvent orientation polarizabilities from polar (0.320—water) to nonpolar (0.148—chloroform):
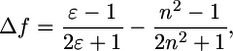 |
(1) |
where ɛ is the solvent dielectric constant and n is the solvent refractive index.
FIGURE 1.
Structures of NIR fluorescent probes used in this study.
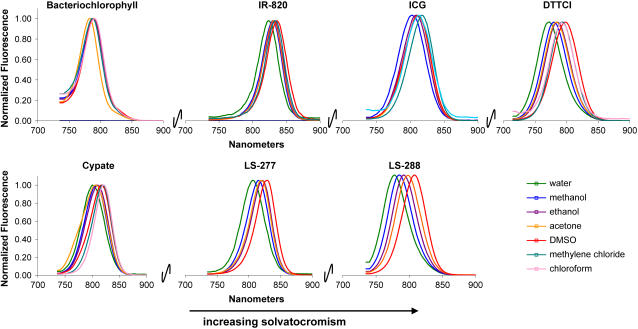
In a typical experiment, a dye was dissolved in a small amount of DMSO and diluted with the solvent of interest. The dyes used in the study are highly soluble in solvents with high polarity (except water) and much less soluble in nonpolar solvents. Normalized solvent-dependent absorption and fluorescence (Fig. 2) spectra of the dyes in various solvents demonstrate that the polymethine dyes exhibit hypsochromic shifts with increasing solvent polarity, whereas bacteriochlorophyll did not show a noticeable change in the solvents. For polymethine dyes, small spectral displacements were observed in the absorption (10–40 nm) and emission (8–30 nm) spectra, with largest shifts occurring in solvents with low orientation polarizability. We also observed that solvent polarity affected molar absorptivity and fluorescence quantum yield and, hence, the overall fluorescence intensity, defined here as the product of molar absorptivity and fluorescence quantum yield. In general, the molecular probes showed relatively higher fluorescent intensity in methanol, ethanol, acetone, and DMSO than in nonpolar solvents and water.
FIGURE 2.
Normalized emission spectra of polymethine dyes and bacteriochlorophyll at 720 nm (700 nm for DTTCI).
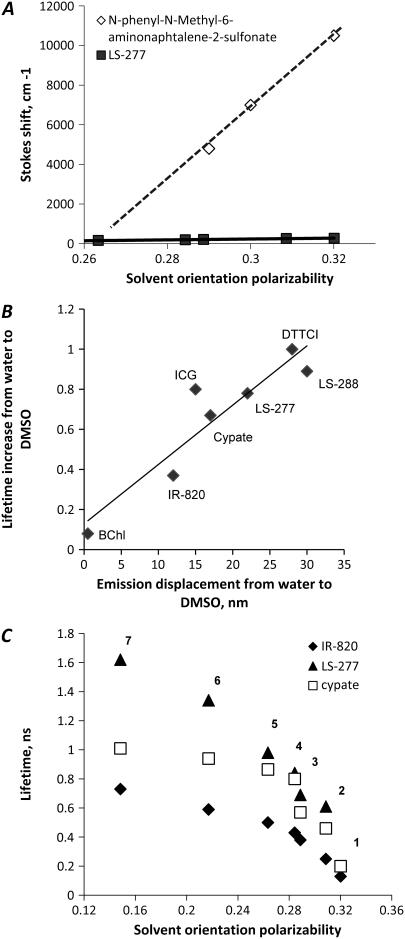
To evaluate the steady-state sensitivity of the fluorescent dyes, a Lippert-Magada plot was constructed (Fig. 3 A), where the energy difference expressed in wavenumbers (cm−1) between the ground and excited states (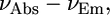 Stokes shift) is plotted against solvent orientation polarizability Δf derived from Eq. 1. The first part of the equation reflects reorientation of the solvent molecules and redistribution of the electrons in solvent molecules during the fluorescence lifetime, and the second part accounts for only reorganization of electrons. Thus, solvent orientation polarizability represents an orientation effect of solvent molecules decoupled from their electronic perturbations. For a typical solvatochromic dye, the dependence of a Stokes shift on Δf is linear in the absence of solvent-specific interactions, where the slope of the plot reflects solvatochromic sensitivity of a fluorophore. According to the Lippert equation (24), Stokes shift is proportional to the square of the change in dipole moments between the first excited and ground states (2). The most sensitive solvatochromic compounds are those with the steepest slope and therefore the largest change in the dipole moment. For ICG, LS-288, cypate, DTTCI, and bacteriochlorophyll, the slopes are nearly zero, indicating insignificant change in the dipole moments and therefore low sensitivity to solvent polarity. DTTCI has the largest slope of the molecular probes studied, albeit much smaller than the slopes of commonly used visible solvent polarity probes. Fig. 3 A illustrates such a difference by comparing the Lippert-Magada plots of N-phenyl-N-methyl-6-aminonaphtalene-2-sulfonate (25) and LS-277. Clearly, the steady-state sensitivity of NIR dyes to solvent polarity is poor, limiting their suitability for this application:
Stokes shift) is plotted against solvent orientation polarizability Δf derived from Eq. 1. The first part of the equation reflects reorientation of the solvent molecules and redistribution of the electrons in solvent molecules during the fluorescence lifetime, and the second part accounts for only reorganization of electrons. Thus, solvent orientation polarizability represents an orientation effect of solvent molecules decoupled from their electronic perturbations. For a typical solvatochromic dye, the dependence of a Stokes shift on Δf is linear in the absence of solvent-specific interactions, where the slope of the plot reflects solvatochromic sensitivity of a fluorophore. According to the Lippert equation (24), Stokes shift is proportional to the square of the change in dipole moments between the first excited and ground states (2). The most sensitive solvatochromic compounds are those with the steepest slope and therefore the largest change in the dipole moment. For ICG, LS-288, cypate, DTTCI, and bacteriochlorophyll, the slopes are nearly zero, indicating insignificant change in the dipole moments and therefore low sensitivity to solvent polarity. DTTCI has the largest slope of the molecular probes studied, albeit much smaller than the slopes of commonly used visible solvent polarity probes. Fig. 3 A illustrates such a difference by comparing the Lippert-Magada plots of N-phenyl-N-methyl-6-aminonaphtalene-2-sulfonate (25) and LS-277. Clearly, the steady-state sensitivity of NIR dyes to solvent polarity is poor, limiting their suitability for this application:
 |
(2) |
where h is the Planck constant, c is the speed of light, νAbs and νEm are the wave numbers of absorption and emission, respectively (cm−1), a is the Onsanger radius, ɛ is a solvent dielectric constant and n is a solvent refractive index, 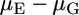 is the difference between dipole moment in the ground and excited state, and C is a constant.
is the difference between dipole moment in the ground and excited state, and C is a constant.
FIGURE 3.
(A) Lippert-Magada plots of N-phenyl-N-methyl-6-aminonaphtalene-2-sulfonate (25; reproduced from that reference) and LS-277. (B) Change in lifetime versus spectral shift in emission between DMSO and water (DMSO and methanol for bacteriochlorophyll). (C) Lifetime of cyanine probes versus solvent orientation polarizability. (1) Water, (2) methanol, (3), ethanol, (4) acetone, (5) DMSO, (6) methylene chloride, and (7), chloroform.
Fluorescence lifetimes of NIR polymethine dyes are highly sensitive polarity reporters
In contrast to the small steady-state spectral response, solvent polarity has a much larger impact on fluorescence lifetime of NIR polymethine dyes. As shown in Fig. 3 B, there is a general trend between the sensitivity of fluorescence lifetime properties and steady-state spectral shifts of the dyes dissolved in DMSO and water (DMSO and methanol for bacteriochlorophyll). Generally, probes with larger steady-state emission shifts exhibit a larger change in their lifetimes. For example, bacteriochlorophyll is insensitive to solvent polarity and, consequently, shows no lifetime change in different solvents. In contrast, DTCCI and LS-288, which experienced relatively larger shift of their emission maxima (28–30 nm) from water to DMSO, also had a threefold lifetime increase.
Plotting lifetimes against solvent polarizability (Δf, Eq. 3) revealed a curvilinear increase in the lifetime for most of the dyes as solvent polarity decreases (see Fig. 3 C for representative examples). For example, the fluorescence lifetime of LS-277 increased by a factor of 8 from water to chloroform. Analysis of experimental data with Prism statistical software (GraphPad Software, San Diego, CA) gave the empirical Eq. 4. Rearrangement of Eq. 3 to Eq. 4 provided the expression for determining the polarity of solvent systems from measured fluorescence lifetime. In this equation, Δf0 is the value corresponding to the imaginary solvent in which the probe's lifetime is equal to zero (intersection between the x axis and extrapolated curve) and corresponded to 0.33 for most of the dyes; τv is a hypothetical lifetime in a vacuum determined by extrapolating the data to the y axis, and K is the dye-specific lifetime-solvatochromic coefficient:
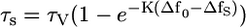 |
(3) |
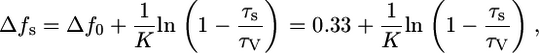 |
(4) |
where τs is the lifetime in unknown solvent system, τv is the lifetime of a probe in vacuum, Δf0 is the solvent orientation polarizability in hypothetical solvent where the lifetime is equal to 0, and K is the dye-specific lifetime-solvatochromic coefficient.
Both parameters τv and K for the polymethine dyes derived from experimental data provide an index for estimating polarities of more complex systems (Table 1).
TABLE 1.
Lifetime solvatochromic parameters derived from fitting experimental data to Eq. 3
| Entry | τv ns | K |
|---|---|---|
| IR-820 | 0.74 ± 0.03 | 21.0 ± 2.0 |
| Cypate | 1.03 ± 0.07 | 26.8 ± 4.5 |
| ICG | 1.11 ± 0.11 | 27.5 ± 6.7 |
| LS-277 | 1.72 ± 0.14 | 12.9 ± 2.6 |
| LS-288 | 1.72 ± 0.1 | 20.1 ± 3.0 |
| DTTCI | 1.75 ± 0.13 | 35 ± 6.2 |
Fluorescence lifetime reports the inner polarity of micelles and albumin
Having established the relationship between solvent polarity and fluorescence lifetime, we measured the polarity inside a micelle formed by Tween-80, commonly used as a solubilizing agent for in vivo imaging. Tween-80 undergoes rapid esterase-sensitive breakdown in plasma and its content can bind rapidly to plasma proteins such as albumin. Therefore, by measuring the polarity of the dye's microenvironment, it is possible to determine the micelle degradation and dye transfer to albumin in vivo. From the limited pool of dyes, we identified three groups of probes with respect to their lifetime sensitivity to solvent change: insensitive (bacteriochlorophyll), moderately sensitive (ICG, cypate, IR-820), and highly sensitive (LS-277, LS-288, and DTTCI). A representative dye from each group was used to evaluate the polarities of heterogeneous systems such as inner cavities in micelles and binding sites in albumin. Both micelles and albumin have been used often as models for steady-state solvatochromic fluorescence lifetime studies.
The results of our study are summarized in Table 2. Bacteriochlorophyll, which is generally nonfluorescent in water, became highly fluorescent when exposed to Tween-80 without significant change in lifetime (2.2 ns). The fluorescence increase is due to an increase in fluorescence quantum yield of the dye in a hydrophobic environment and does not reflect solvatochromism. Because its lifetime does not correlate with solvent polarity, this dye is not suitable for use as a lifetime polarity reporter. Moderately sensitive cypate and highly sensitive DTTCI showed lifetimes of 0.47 ns and 1.16 ns, respectively. Using Eq. 4, the calculated orientation polarizability of inner micelle is 0.30, which lies between methanol and ethanol. Interestingly, the independent results with these dyes are in excellent agreement with each other. By using a multiexponential decay model to assess the existence of other fluorescent states, we determined that the micelle environment is quite homogeneous. In both cases, >97% of the fluorescent signal belonged to a single fluorescent state.
TABLE 2.
Evaluation orientation polarizabilities and polarities of the inner part of Tween-80 micelle and the binding sites of bovine serum albumin
| System | NIR probe | Sites | Lifetime, ns (Corrected fractional contributions [fi]) | Calculated orientation polarizability, Δf | Polarity |
|---|---|---|---|---|---|
| Tween-80 | Bacteriochlorophyll | Inner micelle | 2.2 | Unknown | Unknown |
| Cypate | Inner micelle | 0.50 (98%) | 0.305 | Methanol-ethanol | |
| DTTCI | Inner micelle | 1.16 (97%) | 0.299 | Methanol-ethanol | |
| Bovine serum albumin | Bacteriochlorophyll | n/a | No fluorescence | n/a | n/a |
| Cypate | Binding site | 0.47 (82%) | 0.307 | Methanol-ethanol | |
| Binding site | 0.97 (17%) | 0.222 | DMSO-methylene chloride | ||
| DTCCI | Binding site | 1.14 (97%) | 0.300 | Methanol-ethanol |
In another example, we assessed the polarity of binding sites in BSA. Albumin is the major component of serum blood proteins and participates in transporting fatty acids, drugs, and other small molecules to tissues. The protein has a number of binding sites with different geometry and polarities (26,27). The fluorescence lifetime perturbations of the fluorophores at these binding sites were evaluated by a three-exponential model of bound fluorescent dyes (28,29). We incubated the NIR dyes in the presence of BSA in PBS buffer. In the experiments, we kept the concentration of the dye low to avoid potential saturations of one of the binding sites. The measured decay of cypate fluorescence gave two major fractional contributions, fa = 60% and fb = 37%, revealing two binding sites for cypate, whereas DTTCI fluorescence decays indicate only one major contributing site (f = 97%). Bacteriochlorophyll did not show significant fluorescence to measure its lifetime, apparently due to weak binding to albumin and quenching by water. The apparent polarity of one of the binding sites calculated using Eq. 4 showed solvent orientation polarizability Δf = 0.30, corresponding to methanol-ethanol solution and describing a relatively hydrophilic environment. The other binding site is much more hydrophobic, with orientation polarizability Δf = 0.22, which is between DMSO and methylene chloride.
Fractional contribution reflects the fractions of fluorescence steady-state spectra for each component and does not represent the real stoichiometry. For quantitative analysis, it would be useful to know the distribution of the dye in a complex medium. In this study, we assumed that optical properties of the dyes in microenvironments do not significantly vary from properties of the dyes dissolved in solvents with similar orientation polarizability. Thus, the distribution could be calculated if the relative fluorescence intensity and fractional contributions are known using Eq. 5. The corrected fractional contributions [fa and fb] for cypate were found to be 82% and ∼17% for the hydrophilic and hydrophobic sites, respectively. For DTTCI, the corrected and noncorrected numbers are the same (97%):
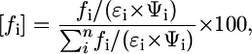 |
(5) |
where [fi] is the corrected fractional contribution, fi is the fractional component percentage obtained from decay analysis, n is the number of fractional components, ɛi is molar absorptivity, and Ψi is the fluorescence quantum yield.
DISCUSSION
NIR polymethine dyes have recently gained special attention in cellular and tissue imaging because they are biocompatible and NIR light between 700 and 900 nm can penetrate much deeper in tissues with concomitant reduction in tissue autofluorescence. Environmental changes for these dyes are typically manifested by an increase or decrease in their fluorescence intensity. Tracking changes in fluorescence intensity in complex systems is difficult without using ratiometric methods and because of the need to know the concentration of the dye contributing to the detected emission. Other NIR dye properties such as molar absorptivity and relative fluorescence efficiency index are also sensitive to polarity and may be used as solvent polarity parameters. However, in this study, the measured fluorescence intensity changes do not appear to correlate with solvent polarity parameters. This ambiguity is best illustrated by the higher fluorescence intensity of cypate in ethanol than in the less polar acetone but lower in the less polar DMSO. Ideally, ratiometric imaging with NIR solvatochromic dyes would overcome some of these problems, but the dyes exhibit small spectral shifts in solvents with different polarities. Therefore, the NIR polymethine dyes are poor solvent polarity probes in complex media.
Compared with steady-state measurements, fluorescence lifetime of some NIR dyes is highly sensitive to their immediate environment. Its insensitivity to the dye concentration minimizes the effects of concentration artifacts and photobleaching (30). Because it is less affected by light scattering (31,32), excitation intensity, or sample turbidity (33), fluorescence lifetime-dependent measurement of polarity can be effective in simple or heterogeneous systems such as solvents or cells and tissues.
We have shown that the fluorescence lifetime and spectral shifts of the NIR dyes follow similar trends with respect to solvent polarity (Fig. 3 B). However, two distinct mechanisms are involved. In steady-state solvatochromism, absolute change of dipole moment value is the major reason for spectral shifts, whereas conformational stability of the excited molecule is the most critical determinant in fluorescence lifetime measurement. The theoretical basis of solvatochromism has been studied in great detail, and a number of approaches from low level valence theory to highly sophisticated quantum mechanics calculations have been applied (34,35). Strong solvatochromism is caused by a large change in permanent dipole moments Δμ = μ* − μg upon excitation (8), which requires a certain level of asymmetry in molecular structure and the presence of electron-donor and electron-acceptor groups, typically heteroatoms, in the same molecule. In the ground state, the molecules are in the neutral state, with both ends uncharged. For example, the N-phenyl-N-methyl-6-aminonaphtalene-2-sulfonate described above, structurally similar Dapoxyls, merocyanine dyes (36), and other solvatochromic compounds belong to this group. Upon excitation, the large alteration in dipole moments occurs via major redistribution of charges on heteroatoms, causing significant displacement of spectral bands.
A priori, symmetrical polymethine dyes should not exhibit solvatochromism because the ground and excited states' resonance structures are identical. However, weak solvatochromism in symmetrical NIR polymethine dye was observed, reflecting the change in dipole moment attributed to the loss of symmetry center in the excited state due to local perturbations of charges in the polymethine chain. Currently, there is no consensus on the causes of the observed perturbation in the symmetry of the molecules. Ischenko et al. (37) rationalized the loss of symmetry upon excitation via nucleophilic solvation of positively charged centers along the polymethine chain by negatively charged ends of solvent dipoles, whereas Momicchioli et al. (38) explained asymmetrization through spatial separation between the highest occupied molecular orbital and lowest unoccupied molecular orbital in the twisted excited state resulting in a weak charge-transfer complex. Irrespective of the pathway, the net effect results in poor solvatochromism and points to the need for a new method to measure solvent polarity.
Although the fluorescence lifetime of polymethine dyes is highly sensitive to the polarity of the medium, it is less studied than solvatochromism. Fluorescence lifetime directly reflects conformational stability of the excited state. Solvent molecules might stabilize or destabilize the excited state in a variety of ways: hydrogen bonding, solvent molecules reorientation, etc. Considering that the NIR absorption bands in polymethine dyes arise from electronic transition involving the π electrons along the polymethine chain, the cation in the ground state of the polymethine dyes resonates between two limiting canonical structures. Thus, all the bonds along the methine chain are practically equivalent, with a bond order of 1.5, similar to aromatics. Based on some NMR and crystallography studies, the ground state of the molecules are predominantly in an all-trans configuration (39,40). In the excited state, the bond order lowers significantly, in contrast to the aromatic analogs. For that reason, conjugated aromatics have a much longer lifetime than polymethine chain-like dyes (e.g., the fluorescence lifetimes of aromatic fluorescein, pyrromethene, and 1-aminonaphtalene-4-sulfonic acid are 4.1 ns, 5.6 ns, and 11.5 ns, respectively).
Excitation rearranges electrons and reduces the electron density in the double bonds along the polymethine chain, facilitating vibrational rotations and allowing the molecule to twist during its lifetime. The twisted conformation probably undergoes trans-cis isomerization leading to nonradiative decay and short lifetime of the excited state. As in any unstable conformation, the stability of the excited state and therefore the lifetime becomes exceedingly sensitive to the local environment, including solvent polarity. The polar medium facilitates trans-cis transformation, effectively shortening the lifetime, whereas the nonpolar environment stabilizes the excited state, thereby increasing the fluorescence lifetime. Thus, the fluorescence lifetime becomes a highly sensitive and an attractive method for probing the polarity of solvents and complex biological systems.
In summary, we have shown that the fluorescence lifetime of NIR polymethine dyes are highly sensitive to the polarity of solvents and biological mediums. We found that the fluorescence decay of polymethine dyes is monoexponential in a wide variety of solvents, except where they are not completely soluble or they form aggregates. A fluorescence lifetime-based polarity index was also developed for predicting the polarity of complex systems. This method overcomes the poor solvatochromism of these biocompatible NIR dyes and provides a strategy to translate in vitro studies into in vivo. In developing this method, we used primarily the solvent orientation polarizability to determine polarity. Because solvent polarity is a complex phenomenon encompassing other factors that could affect fluorescence lifetime, a more robust equation that integrates these additional factors such as viscosity, osmolality, and pH may be needed to account for potential deviations from standard polarity measurements.
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Supplementary Material
Acknowledgments
We thank Dr. Yunpeng Ye for preparing cypate.
This research was supported in part by National Institutes of Health grants R01 CA109754, R01 EB1430, 1 U01 HL080729, and U54 CA119342.
Editor: Gerard Marriott.
References
- 1.Gordon, D. J., J. J. Balbach, R. Tycko, and S. C. Meredith. 2004. Increasing the amphiphilicity of an amyloidogenic peptide changes the beta-sheet structure in the fibrils from antiparallel to parallel. Biophys. J. 86:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA. 95:13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitsett, J. A., and T. E. Weaver. 2002. Hydrophobic surfactant proteins in lung function and disease. N. Engl. J. Med. 347:2141–2148. [DOI] [PubMed] [Google Scholar]
- 4.Orti, E., P. M. Viruela, R. Viruela, F. Effenberger, V. Hernandez, and J. T. Lopez Navarrete. 2005. Raman and theoretical study of the solvent effects on the sizable intramolecular charge transfer in the push-pull 5-(dimethylamino)-5′-nitro-2,2′-bithiophene. J. Phys. Chem. A. 109:8724–8731. [DOI] [PubMed] [Google Scholar]
- 5.Danielson, M. A., and J. J. Falke. 1996. Use of 19F NMR to probe protein structure and conformational changes. Annu. Rev. Biophys. Biomol. Struct. 25:163–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinhoff, H. J. 2004. Inter- and intra-molecular distances determined by EPR spectroscopy and site-directed spin labeling reveal protein-protein and protein-oligonucleotide interaction. Biol. Chem. 385:913–920. [DOI] [PubMed] [Google Scholar]
- 7.Nigam, S., and S. Rutan. 2001. Principles and applications of solvatochromism. Appl. Spectrosc. 55:362a–370a. [Google Scholar]
- 8.Reichardt, C. 1994. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 94:2319–2358. [Google Scholar]
- 9.Cerione, R. A. 1994. Fluorescence assays for G-protein interactions. Methods Enzymol. 237:409–423. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano, K. A., and D. L. Taylor. 1998. Fluorescent-protein biosensors: new tools for drug discovery. Trends Biotechnol. 16:135–140. [DOI] [PubMed] [Google Scholar]
- 11.Toutchkine, A., V. Kraynov, and K. Hahn. 2003. Solvent-sensitive dyes to report protein conformational changes in living cells. J. Am. Chem. Soc. 125:4132–4145. [DOI] [PubMed] [Google Scholar]
- 12.Chirico, G., S. Bettati, A. Mozzarelli, Y. Chen, J. D. Muller, and E. Gratton. 2001. Molecular heterogeneity of O-acetylserine sulfhydrylase by two-photon excited fluorescence fluctuation spectroscopy. Biophys. J. 80:1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gidalevitz, T., C. Biswas, H. Ding, D. Schneidman-Duhovny, H. J. Wolfson, F. Stevens, S. Radford, and Y. Argon. 2004. Identification of the N-terminal peptide binding site of glucose-regulated protein 94. J. Biol. Chem. 279:16543–16552. [DOI] [PubMed] [Google Scholar]
- 14.Gochin, M., R. Savage, S. Hinckley, and L. Cai. 2006. A fluorescence assay for rapid detection of ligand binding affinity to HIV-1 gp41. Biol. Chem. 387:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prusakiewicz, J. J., A. S. Felts, B. S. Mackenzie, and L. J. Marnett. 2004. Molecular basis of the time-dependent inhibition of cyclooxygenases by indomethacin. Biochemistry. 43:15439–15445. [DOI] [PubMed] [Google Scholar]
- 16.Anderson, R. R., and J. A. Parrish. 1981. The optics of human skin. J. Invest. Dermatol. 77:13–19. [DOI] [PubMed] [Google Scholar]
- 17.Achilefu, S. 2004. Lighting up tumors with receptor-specific optical molecular probes. Technol. Cancer Res. Treat. 3:393–409. [DOI] [PubMed] [Google Scholar]
- 18.Ntziachristos, V., A. G. Yodh, M. Schnall, and B. Chance. 2000. Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement. Proc. Natl. Acad. Sci. USA. 97:2767–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seliskar, C. J., and L. Brand. 1971. Solvent dependence of the luminescence of N-arylaminonaphthalenesulfonates. Science. 171:799–800. [DOI] [PubMed] [Google Scholar]
- 20.Lu, Z., S. J. Lord, H. Wang, W. E. Moerner, and R. J. Twieg. 2006. Long-wavelength analogue of PRODAN: synthesis and properties of anthradan, a fluorophore with a 2,6-donor-acceptor anthracene structure. J. Org. Chem. 71:9651–9657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achilefu, S., R. B. Dorshow, J. E. Bugaj, and R. Rajagopalan. 2000. Novel receptor-targeted fluorescent contrast agents for in vivo tumor imaging. Invest. Radiol. 35:479–485. [DOI] [PubMed] [Google Scholar]
- 22.Lee, H., J. C. Mason, and S. Achilefu. 2006. Heptamethine cyanine dyes with a robust C-C bond at the central position of the chromophore. J. Org. Chem. 71:7862–7865. [DOI] [PubMed] [Google Scholar]
- 23.Benson, R. C., and H. A. Kues. 1978. Fluorescence properties of indocyanine green as related to angiography. Phys. Med. Biol. 23:159–163. [DOI] [PubMed] [Google Scholar]
- 24.Lakowicz, J. R. 2006. Principles of Fluorescence Spectroscopy. Springer, New York.
- 25.Seliskar, C. J., and L. Brand. 1971. Electronic spectra of 2-aminonaphthalene-6-sulfonate and related molecules. II. Effects of solvent medium on the absorption and fluorescence spectra. J. Am. Chem. Soc. 93:5414–5420. [Google Scholar]
- 26.Sudlow, G., D. J. Birkett, and D. N. Wade. 1976. Further characterization of specific drug binding sites on human serum albumin. Mol. Pharmacol. 12:1052–1061. [PubMed] [Google Scholar]
- 27.Curry, S., H. Mandelkow, P. Brick, and N. Franks. 1998. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat. Struct. Biol. 5:827–835. [DOI] [PubMed] [Google Scholar]
- 28.Abugo, O. O., R. Nair, and J. R. Lakowicz. 2000. Fluorescence properties of rhodamine 800 in whole blood and plasma. Anal. Biochem. 279:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Togashi, D. M., and A. G. Ryder. 2006. Time-resolved fluorescence studies on bovine serum albumin denaturation process. J. Fluoresc. 16:153–160. [DOI] [PubMed] [Google Scholar]
- 30.Lichtman, J. W., and J. A. Conchello. 2005. Fluorescence microscopy. Nat. Methods. 2:910–919. [DOI] [PubMed] [Google Scholar]
- 31.Kuwana, E., and E. M. Sevick-Muraca. 2002. Fluorescence lifetime spectroscopy in multiply scattering media with dyes exhibiting multiexponential decay kinetics. Biophys. J. 83:1165–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerussi, A. E., J. S. Maier, S. Fantini, M. A. Franceschini, W. W. Mantulin, and E. Gratton. 1997. Experimental verification of a theory for the time-resolved fluorescence spectroscopy of thick tissues. Appl. Opt. 36:116–124. [DOI] [PubMed] [Google Scholar]
- 33.Ntziachristos, V., and R. Weissleder. 2002. Charge-coupled-device based scanner for tomography of fluorescent near-infrared probes in turbid media. Med. Phys. 29:803–809. [DOI] [PubMed] [Google Scholar]
- 34.Bertolino, C. A., A. M. Ferrari, C. Barolo, G. Viscardi, G. Caputo, and S. Coluccia. 2006. Solvent effect on indocyanine dyes: a computational approach. Chem. Phys. 330:52–59. [Google Scholar]
- 35.Cramer, C. J., G. R. Famini, and A. H. Lowrey. 1993. Use of calculated quantum chemical properties as surrogates for solvatochromic parameters in structure-activity relationships. Acc. Chem. Res. 26:599–605. [Google Scholar]
- 36.Diwu, Z., Y. X. Lu, C. L. Zhang, D. H. Klaubert, and R. P. Haugland. 1997. Fluorescent molecular probes. 2. The synthesis, spectral properties and use of fluorescent solvatochromic Dapoxyl(TM) dyes. Photochem. Photobiol. 66:424–431. [Google Scholar]
- 37.Ishchenko, A. A., V. A. Svidro, and N. A. Derevyanko. 1989. Solvatofluorochromy of cationic cyanine dyes. Dyes Pigments. 10:85–96. [Google Scholar]
- 38.Momicchioli, F., A. S. Tatikolov, D. Vanossi, and G. Ponterini. 2004. Electronic structure and photochemistry of squaraine dyes: basic theoretical analysis and direct detection of the photoisomer of a symmetrical squarylium cyanine. Photochem. Photobiol. Sci. 3:396–402. [DOI] [PubMed] [Google Scholar]
- 39.Kim, S. H., S. K. Han, J. J. Kim, W. T. Lim, N. H. Heo, and K. N. Koh. 1998. Crystal structure of a photoconductive dithiosquarylium dye: 2,4-bis(1,3,3-trimethyl-1-indolinylidenemethyl)cyclobutenediylium-1,3-dithiolate. Dyes Pigments. 39:259–266. [Google Scholar]
- 40.Benniston, A. C., A. Harriman, and K. S. Gulliya. 1994. Photophysical properties of merocyanine 540 derivatives. J. Chem. Soc. Faraday Trans. 90:953–961. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.