Abstract
The molecular mechanism of how insects recognize intruding microorganisms and parasites and distinguish them from own body structures is not well known. We explored evolutionary adaptations in an insect parasitoid host interaction to identify components that interfere with the recognition of foreign objects and cellular encapsulation. Because some parasitoids provide protection for the developing wasp in the absence of an overt suppression of the insect host defense, we analyzed the surface of eggs and symbiotic viruses for protective properties. Here we report on the molecular cloning of a 32-kDa protein (Crp32) that is one of the major protective components. It is produced in the calyx cells of the female wasp ovaries and attached to the surface of the egg and other particles including polydnaviruses. The recombinant protein confers protection to coated objects in a cellular encapsulation assay suggesting that a layer of Crp32 may prevent cellular encapsulation reactions by a local inactivation of the host defense system.
Keywords: immune recognition, encapsulation, calyx fluid, Cotesia rubecula, Pieris rapae
It is well known that insects react quickly to foreign objects that are introduced into the hemolymph forming capsules that include hemocytes and extracellular hemolymph components (1). However, we are still ignorant about the molecular process of invertebrate immune recognition. Because insects lack specific receptors that are generated by somatic mechanisms during ontogeny of each individual organism, recognition of pathogens is believed to involve molecular patterns rather than particular structures (2). Although intracellular regulatory processes of immune functions are highly conserved, even between vertebrate and invertebrate species (3), some insect recognition molecules that potentially identify foreign objects, like lectins (e.g., ref. 4) or hemolin (5, 6), are not conserved and may not be present in all insects. This could be an indication that individual insect species acquire subsets of recognition molecules when adapting to specific environmental conditions and its respective microorganisms and parasites.
The process of recognition raises the question of how insects distinguish self from nonself. In the open circulatory system, the surface of basement membranes separating tissues against the hemolymph is of great importance to preclude encapsulation of own tissues (7). The molecular significance of this observation is not clear, but protective properties are also apparent in encapsulation assays, where extracellular matrix components from vertebrates are used to prevent attachment and spreading of insect cells (8). During insect development, the capacity to protect tissues at the lining against the own defense system appears to be added to the basement membrane by hemocytes (9). This suggests that the immune system in insects involves two elements, a recognition process using patterns of external features from potential pathogens and the protection of own tissues and structures at the hemolymph lining.
To gain access to insect proteins that interfere with extracellular recognition processes, we decided to explore genetic adaptations in parasitoid host interactions to identify components that provide egg protection against the host defense. These insect interactions impose extreme selection pressures on both organisms and are ideal experimental systems to isolate wasp components that specifically interfere with host immune functions. Components that protect the wasp egg are usually part of maternal secretions that can be isolated from the female oviducts.
In Cotesia rubecula the developing embryos and larvae are protected against the host, Pieris rapae, defense reactions by a combination of two different mechanisms involving the evasion of the host defense (10) and the suppression of the cellular capacity to mount an encapsulation reaction by a polydnavirus (CrV)-encoded protein (11, 12). Both mechanisms are essential for the completion of wasp growth inside the caterpillar. Because the inactivation of hemocytes by the virus-coded suppressor is not observed until a few hours following parasitization, a surface coating of egg depositions by maternal secretions must be effective to protect the egg, and possibly the symbiotic viruses, against the immediate attack by host hemocytes.
Assuming that viruses and eggs are protected by a similar mechanism involving maternal secretions, we raised antibodies against purified polydnaviruses and tested the egg surface for cross-reacting proteins from the calyx fluid. Subsequent experiments showed that two proteins on the egg surface cross-reacted with the anti-virus antibodies, and that the protective properties on the egg surface can be masked with anti-virus antibodies leading to an encapsulation of the injected egg inside the caterpillar (10). To analyze the protective surface properties at the molecular level we decided to clone the major cross-reacting protein component on the surface of the egg depositions. Here, we describe a 32-kDa protein that is produced in the calyx cells of the wasp and attached to the surface of the egg on its passage from the ovary into the oviduct. The protein has properties that can be used to coat biotic and abiotic objects against the defense reactions of insects.
MATERIALS AND METHODS
Protein and Nucleic Acid Analysis.
C. rubecula eggs collected from parasitized P. rapae caterpillars or from wasp ovaries were incubated in 1% SDS for 5 min. The supernatant containing the dissolved proteins from the eggs surface was collected.
Northern and Southern blot analyses were performed as described (13) by using [32P]-labeled cDNA coding for Crp32 as a probe under stringent conditions (65°C). Total RNA was extracted from wasps and ovaries as described (14) and used for Northern blot analysis and cDNA library construction. Western blot analysis was carried out as described (13) by using an antiserum made in a rabbit against purified CrVs (10). The bound antibodies were visualized by using a goat anti-rabbit alkaline phosphatase-conjugated antibody.
Peptide Microsequencing.
Acrylamide gel pieces containing the band corresponding to the 32-kDa protein were excised from a preparative SDS/10% polyacrylamide gel containing samples of virus-free calyx fluid (10). The protein was eluted by homogenizing the gel slices in 50 mM Tris⋅HCl and 0.1% SDS, pH 8.0, and incubated overnight at 4°C. The gel matrix was removed by centrifugation. The protein was then precipitated from solution by slowly adding ice-cold ethanol to a final concentration of 80% and centrifuging for 30 min at 13,000 × g. The precipitate was dissolved in 50 μl of 50 mM Tris⋅HCl and 0.1% SDS, reduced by adding 2-mercaptoethanol to a concentration of 4 mM, and heated for 45 min at 60°C. Reduced cysteine residues were alkylated by adding iodoacetamide in water to a final concentration of 50 mM and incubating for 15 min in the dark. To obtain optimal conditions for trypsin digestion, 50 μl of a solution containing 50 mM Tris⋅HCl (pH 7.2), 20% dimethylformamide, and 4 mM CaCl2 was added to the protein solution. Trypsin in water was added to obtain a protease to protein ratio of 1:20 and the digestion was carried out at 37°C for up to 44 h and stopped by boiling for 5 min. The sample was lyophilized, resuspended in 3 M guanidine hydrochloride, and incubated for 30 min at room temperature before centrifugation at 5,000 × g for 10 min. HPLC was carried out by loading 50 μl of the sample onto a HPLC column (Vydac, Hesperia, CA) and selected peptide fractions were collected and sequenced on a protein sequencer (Hewlett–Packard G1000A).
Immunoscreening and Bacterial Expression.
A cDNA expression library was constructed from female C. rubecula wasps by synthesizing cDNA molecules from poly(A)+ RNA isolated from the wasps and ligating into lambda gt11 arms (Promega). The primary titer was 1.5 × 1011 plaque forming units. Following isopropyl-β-d-thiogalactoside (IPTG) induction 6 × 105 plaques were screened with an antiserum raised against CrVs (1:5,000 dilution) (10). The secondary antibody (alkaline phosphatase-conjugated anti-rabbit Ig, Pierce) was used at a dilution of 1:5,000. Plaques were screened as described (15). Phage DNA was isolated from phage lysates by using a kit for lambda DNA preparation (Qiagen, Chatsworth, CA) according to manufacturer’s instructions. Out of five clones that showed labeling in the primary screen, three contained Crp32 sequences (see below).
Lysogens of positive plaques were produced (Promega, manufacturer’s instructions) and induced by 10 mM IPTG and analyzed for the protein production by Western blot analysis by using the anti-CrV-antiserum as a probe.
Elution of Antibodies from Nitrocellulose Blots.
To obtain antibodies that are directed against Crp32 lacking possible posttranslational modifications, antibodies were eluted from preparative Western blots, containing the recombinant protein from lysogens, as previously described (16). Briefly, the desired bands were excised from the blot after staining for alkaline phosphatase activity and transferred to a tube containing PBS (138 mM NaCl/2.7 mM KCl/1.47 mM KH2 PO4/7.3 mM Na2HPO4, pH 7.6). Antibodies were eluted from the fragments by a quick series of three washes for 30 sec each time in elution buffer [50 mM glycine⋅HCl, pH 2.3, 50 mM NaCl/0.5% (vol/vol) Tween 20/100 μg/ml BSA). The elutes were neutralized immediately by adding Na2PO4 to a final concentration of 50 mM.
cDNA Cloning and Sequencing.
Lambda DNA isolated from selected recombinant phages was digested with KpnI and SacI restriction enzymes closest to the EcoRI sites flanking the insert and ligated into the same sites in a pBluescript plasmid vector (pBluescript SK+, Stratagene). The insert was sequenced by termination reaction in an automative sequencer (Applied Biosystems Sequencer) using λgt11 specific forward and reverse primers (Promega) and six primers that were designed on the basis of the sequence data from the DNA insert.
Protein Expression in Bacteria.
The cDNA fragment coding for Crp32 was amplified by PCR by using two primers binding to the 5′-end (5′-GCGCGGTACCCATGGATAAGAAGATAATA-3′) and 3′-end (5′-GCGCGGTACCTGGCCTTTTTTTGGCAGTCC-3′) of the Crp32-coding region (primer sequences are underlined). The primers contained overhangs that could be used for ligation with KpnI restriction sites. The amplified product was isolated from a low melting agarose gel (Promega) and digested with KpnI. The fragment was cloned into the KpnI site in pQE31 (Qiagen) under a lac promoter and transformed into Escherichia coli (M15). The production of the protein containing an additional 6× histidine-tag residues was induced by 0.1 mM IPTG for 2 h at 37°C. The fusion protein that was found to be soluble in the cytoplasm of the bacteria was captured by using Ni resin beads (Qiagen) following sonication in sonication buffer (50 mM sodium phosphate, pH 7.8/300 mM NaCl) and eluted with 110 mM imidazole after an initial removal of bacterial proteins with 65 mM imidazole.
In Vitro and In Vivo Encapsulation Assay.
One day prior to the assay, several wells in a microtiter plate were seeded with Sf21 cells (Spodoptera frugiperda cell culture). Cells were removed from the wells and replaced by fresh Grace’s insect culture medium (GIBCO/BRL) saturated with phenylthiouria. This leaves a layer of secreted proteins with an apparent effect on adhesion and degranulation of hemocytes that are in contact with the plastic surface. Two 4th instar P. rapae caterpillars were bled directly in each well to which resin beads were added. Ni-NTB beads (Qiagen) were covered with recombinant Crp32 and with bacterial proteins by incubating the beads with the soluble fraction of the bacteria containing the plasmid (pQE31-Crp32) and bacteria containing the vector pQE31. To mask Crp32-covered beads with antibodies, the beads were incubated with anti-CrV antiserum (1:500) that specifically recognizes the Crp32 protein for 2 h at 4°C and washed three times before adding to hemocyte monolayers.
Beads covered with Crp32 and an unrelated plant virus protein (grapewine leafrole-associated closterovirus type 1, GLRaV-1, coat protein) expressed in the same bacterial expression system, were injected into 4th instar P. rapae caterpillars and dissected 24 h following injection.
RESULTS
Calyx Fluid Proteins on the Virus and Egg Surface.
When parasitoid eggs were incubated with the antiserum against CrVs and injected into naive caterpillars, they were encapsulated by host hemocytes (10). To explore whether virus-related proteins are attached to the parasitoid egg, eluted proteins from the egg surface were analyzed on a Western blot using the anti-CrV antiserum as a probe. In addition to the 65-kDa protein (Crp65) that was previously identified in the calyx fluid and on CrVs, a 32-kDa protein (Crp32) was strongly recognized by the antiserum (Fig. 1A). Because wasp eggs and symbiotic polydnaviruses appear to be both protected against the host defense by a surface coat, we suspected that the two proteins found on both objects are probably involved in a protective function.
Figure 1.
(A) SDS/PAGE analysis of protein extracts from C. rubecula (eg). Proteins eluted from ovary-dissected eggs, (cf) virus-free calyx fluid, (v) purified polydnaviruses, and (ly) lysate from recombinant phages containing the cDNA coding for Crp32. The corresponding Western blot of the gel is shown in right in which an antiserum against purified CrVs was used as a probe. A 65- and a 32-kDa protein are visible in eg, cf, and v fractions. Note the similarity in size between the recombinant protein and Crp32 from ovarian secretions. Molecular weight markers are indicated in kDa. (B) Northern blot analysis of RNA extracted from ovaries (ov) and the remaining carcass (car) of C. rubecula female wasps. Similar amounts of RNA were loaded for each sample (2 μg). The samples were probed with two cDNA clones (A and C) obtained from screening the C. rubecula cDNA library. Both probes hybridized to a 1.5 RNA fragment in the ovary sample. (MW) Molecular mass markers.
Molecular Cloning of Crp32 coding cDNA.
Two independent approaches for the isolation and identification of the Crp32 coding DNA were explored. Firstly, we sequenced proteolytic peptides from a piece of acrylamide gel that contained the putative Crp32 band. This was possible because a Coomassie blue-stained band was readily identified as the Crp32 protein on Western blots and appeared to be separated from other protein bands on the gel. Five peptide sequences were obtained comprising a total of 54 amino acids (see Fig. 4).
Figure 4.
DNA nucleotide sequence and deduced amino acid sequence of the cDNA fragment coding for Crp32 of C. rubecula. The hydrophobic region at the N terminus, representing a putative transmembrane region is boxed in gray and the cytoplasmic tail in blank. Amino acid sequences obtained from microsequencing of the protein are underlined. Two cDNA clones obtained from screening C. rubecula cDNA library were found to differ in the amino acid at position −1 with respect to the initiation codon. The termination site at the end of the ORF is indicated by a dot. A putative hyaluronan binding motif is underlined by a dotted line.
In a second approach we constructed a bacterial expression library from cDNA fragments of C. rubecula female wasps and screened the recombinant phages for fusion proteins by using the anti-CrV antiserum as a probe. From three positive clones, two had identical insert size. The DNA inserts of the two independent clones were used as a probe in a Northern blot comprising RNA isolated from the wasp ovaries. Both probes strongly recognized a 1.5-kb RNA fragment (Fig. 1B), indicating that they are derived from the same gene. Under these exposure conditions, no hybridization signal was detected in the RNA fraction isolated from the wasp carcasses devoid of ovaries (Fig. 1B). This suggests that the isolated gene is highly expressed in the ovaries.
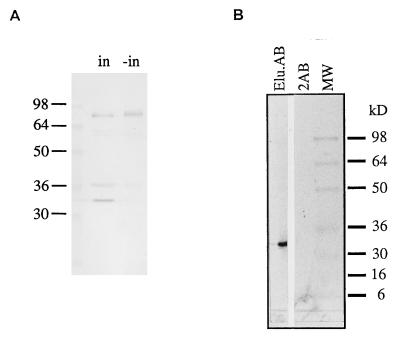
To compare the recombinant and native protein, a fusion protein was produced by inducing a lambda lysogen containing the DNA-insert. The proteins were analyzed on a Western blot using anti-CrV antiserum as a probe. A 32-kDa protein was only found in the induced fraction (Fig. 2A) similar in size to the protein on the egg surface (Fig. 1A, lysate). To exclude antibodies from the anti-CrV antiserum that recognize possible glycosylation modifications of Crp32, we eluted antibodies from a preparative Western blot containing the fusion protein. These specific antibodies were used as a probe on a Western blot comprising protein extracts from the wasp ovaries. Under these conditions only a 32-kDa protein was recognized (Fig. 2B) indicating that the protein is produced mainly in the ovaries.
Figure 2.
(A) Western blot analysis of recombinant proteins from IPTG-induced (in) and -uninduced (−in) phages isolated from screening a C. rubecula expression cDNA library and probed with anti-CrV antiserum. A 32-kDa protein is only visible in the induced sample. (B) Protein extracts from C. rubecula ovaries probed with antibodies eluted from Crp32 protein produced by recombinant phages (Elu.AB) and secondary antibodies as a control (2AB). A 32-kDa protein was recognized by the specific antibody.
Because polydnaviruses are produced in an ovarian gland, we wanted to elucidate whether the gene that codes for Crp32 is part of the circularized virus genome. DNA from purified viruses and genomic DNA from male wasps, where circularized virus molecules are absent, was tested on a Southern blot using the cDNA fragment coding for the protein as a probe. No significant hybridization signal was detected in the viral DNA (Fig. 3), compared with signals from similar blots tested with a virus-specific probe (11). However, the probe reacted with the genomic DNA from male wasps (Fig. 3). This indicates that the Crp32 production in ovarian cells is not derived from a circularized virus genome but a wasp gene that may be unrelated to polydnaviruses.
Figure 3.
Southern blot analysis of genomic DNA isolated from CrVs (v) and C. rubecula male wasps digested with restriction enzymes EcoRI and HindIII. The membrane was probed with [32P]-labeled cDNA fragment coding for Crp32. The absence of a significant signal in the virus DNA indicates that the gene is not part of circularized CrV genome. The corresponding agarose gel micrograph is shown at left.
Deduced Protein Sequence.
The DNA sequence of the large cDNA fragment contained an ORF of 765 bp with a deduced protein of 253 amino acids (Fig. 4). A methionine codon at the beginning of the ORF was identified as a possible initiation codon (17). Sequence analysis of the second, slightly smaller cDNA fragment revealed that another transcript of the gene exists, with an adenine instead of cytosine at position −1 (Fig. 4). The remaining nucleotide sequences of the two cDNA fragments are identical.
The deduced protein sequence includes the peptide sequences obtained from microsequencing of the tryptic peptide fragments from the protein (Fig. 4). Because the molecular identity of the protein coding DNA and corresponding peptides was established by two independent approaches, this indicates that the cloned DNA codes for the Crp32. The calculated molecular mass for the deduced protein sequence is ≈27.5 kDa compared with the estimated size of 32 kDa from its migration in SDS/PAGE.
The deduced protein sequence contains a transmembrane domain at the N terminus without a consensus cleavage site (18) indicating that the protein might not be released from the cell surface (Fig. 4). The protein also contains several domains, where positively charged amino acids and lipophilic amino acids are spaced in such a way to provide an amphipathic α-helical structure for the protein. Using the data bank at National Centre for Biological Information (Bethesda, MD), the deduced protein sequence for Crp32 was compared with other proteins but did not show any significant similarity to existing protein sequences.
Crp32-Like Proteins.
To explore whether Crp32 is restricted to wasps, we analyzed protein extracts from a nonparasitized caterpillar for antigenic similarities to Crp32. When specific antibodies against recombinant Crp32 were tested on Western blots a slightly larger protein (34 kDa) in P. rapae hemocytes was found to cross-react (Fig. 5B). Although the degree of protein similarity remains to be confirmed by cloning Crp32 homologs in other species, this observation could suggest that Crp32 is a member of a family of conserved insect proteins.
Figure 5.
Protein extracts from recombinant bacteria, P. rapae and C. rubecula analyzed on Western blots. (A) Crp32 production by recombinant bacteria. IPTG-induced (in) and -uninduced (un) recombinant bacteria containing pQE31-Crp32 plasmid; a 34-kDa fusion protein was recognized by anti-CrV antiserum in induced bacteria, the size difference to the calyx protein (cf), is due to additional histidine-tag peptide sequences in the bacterial fusion protein. (B) Antigenic similarity to a protein from nonparasitized caterpillars. Protein extracts from hemocytes isolated from naive P. rapae caterpillars (Hc) by using eluted antibodies from a preparative Western blot containing the recombinant Crp32 from bacteria (elu). No cross-reaction was observed with the cell-free hemolymph (Hl) using the specific antibody and the secondary antibody (2nd).
Protective Properties of Crp32.
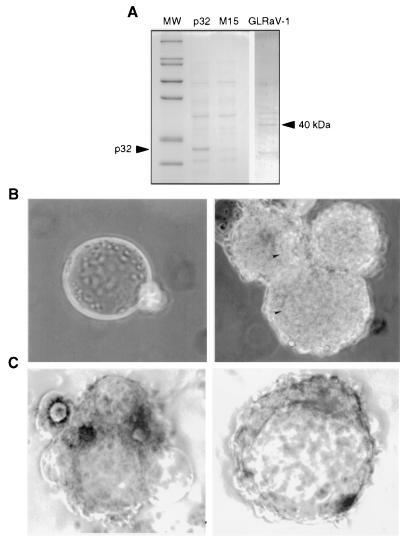
To investigate the involvement of Crp32 in conferring protection to foreign objects intruding the open circulatory system of insects, we tested resin beads covered with a bacterial fusion protein in an in vitro encapsulation assay. The cDNA coding for Crp32 was inserted into a plasmid vector under the control of a lacZ promoter to produce a fusion protein containing a histidine-tag peptide that was used to bind the protein to Ni-resin. The fusion protein was found to be 34 kDa (Fig. 5A), slightly larger than the native protein due to histidine residues from the vector. The Crp32-coated beads contain the 34-kDa fusion protein in addition to a number of bacterial proteins with fortuitous Ni-binding properties, which are similar in control and Crp32-coated beads (Fig. 5A).
When the Crp32-coated beads (Fig. 6A) were incubated with isolated P. rapae hemocytes and examined 24 h later no evidence of encapsulation was observed (Fig. 6B), whereas control beads devoid of Crp32 were encapsulated. In our hands, the encapsulation rate observed in control beads is more than half and is somewhat influenced by the ratio of hemocyte numbers and beads added to the assay. Under these conditions no signs of encapsulation were observed on Crp32-covered beads. Moreover, when the Crp32-covered beads were incubated with antibodies that specifically recognize the protein and added to hemocytes encapsulation was observed (Fig. 6C). In in vivo experiments in which the Crp32-covered beads were injected into P. rapae caterpillars, the beads were protected and no encapsulation was observed (data not shown). However, when beads were covered with an unrelated recombinant plant virus protein (Fig. 6A) and injected into caterpillars, encapsulation occurred (Fig. 6C). This suggests that recombinant Crp32 is capable of protecting abiotic objects against the cellular encapsulation reaction and is probably one of the functional components involved in passive protection against the host immune responses.
Figure 6.
(A) SDS/PAGE analysis of surface proteins from Ni-resin beads incubated with lysate from bacteria containing the p32-coding DNA inserted into the expression plasmid (p32), beads incubated with bacterial lysate from the vector bacteria (M15) and beads incubated with bacterial lysate containing an unrelated plant virus protein (GLRaV-1). Expression conditions were chosen to produce identical amounts of fusion proteins. Under these conditions bacterial proteins that have fortuitous Ni-binding properties are found on all bead surfaces, with an additional 34-kDa protein present on beads treated with lysates from p32-expressing bacteria (arrowhead) and an additional 40-kDa protein from the grapewine leafrole-associated closterovirus type 1 coat protein (arrowhead). (MW) Molecular mass markers from the top: 200, 116.3, 97.4, 66.3, 55.4, 36.5, 31, 21.5, 14.4, and 6 kDa. (B) In vitro and in vivo encapsulation results. Beads coated with proteins from bacteria containing a p32-expressing plasmid (Left) and beads covered with proteins from bacteria containing the expression vector (Right), were incubated with P. rapae hemocytes. In p32-covered beads no signs of hemocyte attachment was detected, whereas control beads were encapsulated (arrowheads). (C) Beads covered with p32 and masked with anti-CrV antiserum were incubated with P. rapae hemocytes (Left). In vivo encapsulation assay using beads covered with an unrelated plant virus protein (GLRaV-1 coat protein, see A) were injected into caterpillars and dissected 24 h later (Right). Control beads covered with recombinant p32 were not encapsulated under these conditions (not shown).
DISCUSSION
Experimental evidence presented here indicates that parasitoid eggs are protected against hemocyte attack by a 32-kDa (Crp32) protein. The recombinant protein attached to Ni-resin beads precludes hemocytes in vivo and in vitro from attaching to the surface, thus protecting the beads from being encapsulated. Control beads, lacking the Crp32 protein, or those covered with an unrelated protein were encapsulated. Similarly, when Crp32-coated beads were masked with the antibodies against the protein, encapsulation occurred, confirming previous observations that C. rubecula eggs injected into caterpillars are protected from the host encapsulation reaction, whereas the eggs masked with antibodies are encapsulated (10). The molecular mechanism of Crp32-mediated protection is not known. The protein could prevent hemocytes from recognizing the coated beads as a foreign object or, alternatively preclude encapsulation by a local inactivation of attacking hemocytes. The surface properties of the beads used in the encapsulation assay suggest a local inactivation of hemocytes on Crp32-coated beads, because control and Crp32-coated beads are both covered with bacterial proteins that have fortuitous Ni-binding properties (Fig. 6A), suggesting that Crp32 is interspersed among bacterial proteins rather than completely covering the beads.
The deduced protein sequence is not related to any known protein sequence in protein data banks. In addition to amphipathic α-helical domains, the protein sequence contains a common hyaluronan binding motif (19) (Fig. 4). Hyaluronan is a glycosaminoglycan containing disaccharide units of N-acetyl glucosamine and glucuronic acid (20) that is present in the extracellular matrix and on cell surfaces, affecting cell behavior such as adhesion, motility, and growth (20). The functional significance of these structural features of the protein are not clear.
A protective coating of egg depositions has also been described in other host-parasitoid interactions. A fibrous layer was described on Cardiochiles nigriceps (Hym: Braconidae) eggs that originates from the wasp ovaries (21). This layer was implied in passive evasion of the parasitoid eggs against encapsulation by Heliothis virescens hemocytes. A more recent observation (22) indicates the involvement of a protein (p50) on the polydnavirus envelope in the protection of Cotesia kariyai (Hym: Braconidae) eggs from the cellular immune reactions of the host, Pseudoletia separata. Glass capillaries precoated with p50 were not encapsulated compared with the noncoated control. Antigenic similarities of the protein with hemolymph components were assumed to be responsible for a molecular disguise (22). Although no sequence homology exists among Crp32 and the available N-terminal peptide sequences of p50, the two proteins may have a similar function.
Whether Crp32 is a viral protein or unrelated to symbiotic polydnaviruses is not clear. Although the protein expression correlates with polydnavirus gene expression in the wasp, the absence of Crp32-protein coding DNA from virus particles suggests that the gene is not part of the circularized polydnavirus genome. However, the gene may be part of proviral DNA not included in the amplified polydnavirus genome. Further studies are required to decide whether the protein is a virus envelope protein or a nonviral protein secreted by calyx cells into the lumen, where it is attached to virus particles and the surface of eggs on its passage from the ovaries into the oviduct.
The observation that a cross-reactive protein of 34 kDa in size is found in hemocytes of nonparasitized P. rapae caterpillars suggests that similar molecules may also exist in lepidopteran species involving physiological functions that are probably independent of parasitoid-host interactions. In this context, the tissue-specific over-expression of Crp32 in the calyx fluid of the wasp may be an adaptation to the endoparasitic lifestyle, allowing the egg surface to be covered by the protein.
Acknowledgments
We thank Peter Hoj for sharing the method of protein microsequencing and the biotechnology group at the Waite Campus for help with the peptide separation using the microbore column. We also thank Markus Beck for help with the bacterial expression of Crp32 and Claudia Fazeli for providing GLRaV-1 recombinant coat protein. This project was supported by a grant from the Australian Research Council to O. Schmidt.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviation: IPTG, isopropyl β-d-thiogalactoside.
Data desposition: The sequence reported in this paper has been deposited in the Genbank database (accession no. AF050670).
References
- 1.Ratcliffe N A. In: Cellular Defense Responses of Insects: Unresolved Problems. Beckage N E, Thompson S N, Federici B A, editors. Vol. 1. San Diego: Academic; 1993. pp. 269–304. [Google Scholar]
- 2.Medzhitov R, Janeway C A., Jr Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr Nature (London) 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 4.Haq S, Kubo T, Kurata S, Kobayashi A, Natori S. J Biol Chem. 1996;271:20213–20218. doi: 10.1074/jbc.271.33.20213. [DOI] [PubMed] [Google Scholar]
- 5.Sun S-C, Linström I, Boman H G, Faye I, Schmidt O. Science. 1990;250:1729–1732. doi: 10.1126/science.2270488. [DOI] [PubMed] [Google Scholar]
- 6.Ladendorff N E, Kanost M R. Arch Insect Biochem Physiol. 1991;18:285–300. doi: 10.1002/arch.940180410. [DOI] [PubMed] [Google Scholar]
- 7.Rizki R M, Rizki T M. In: Surface Changes on Hemocytes during Encapsulation in Drosophila melanogaster. Gupta A P, editor. New York: Wiley; 1986. [Google Scholar]
- 8.Pech L L, Trudean D, Strand M R. J Insect Physiol. 1995;41:801–807. [Google Scholar]
- 9.Fessler J H, Fessler L I. Annu Rev Cell Biol. 1989;5:309–339. doi: 10.1146/annurev.cb.05.110189.001521. [DOI] [PubMed] [Google Scholar]
- 10.Asgari S, Schmidt O. J Insect Physiol. 1994;40:789–795. [Google Scholar]
- 11.Asgari S, Hellers M, Schmidt O. J Gen Virol. 1996;77:2653–2662. doi: 10.1099/0022-1317-77-10-2653. [DOI] [PubMed] [Google Scholar]
- 12.Asgari S, Schmidt O, Theopold U. J Gen Virol. 1997;78:3061–3070. doi: 10.1099/0022-1317-78-11-3061. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 14.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Theopold U, Pintér M, Daffre S, Tryselius Y, Friedrich P, Nässel D R, Hultmark D. Mol Cell Biol. 1995;15:824–834. doi: 10.1128/mcb.15.2.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith D E, Fisher A P. J Cell Biol. 1984;99:20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavener R C, Ray S C. Nucleic Acid Res. 1991;19:3185–3192. doi: 10.1093/nar/19.12.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakai K, Kanehisa M. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang B, Yang B L, Savani R C, Turley E A. EMBO J. 1994;13:286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson R M, Venot A, Bevilacqua M P, Linhardt R J, Stamenkovic I. Annu Rev Cell Dev Biol. 1995;11:601–631. doi: 10.1146/annurev.cb.11.110195.003125. [DOI] [PubMed] [Google Scholar]
- 21.Davies D H, Vinson S B. J Insect Physiol. 1986;32:1003–1010. [Google Scholar]
- 22.Hayakawa Y, Yazaki K. Eur J Biochem. 1997;246:820–826. doi: 10.1111/j.1432-1033.1997.00820.x. [DOI] [PubMed] [Google Scholar]