Abstract
Background:
Butorphanol is an opioid analgesic with partial agonist actions at μ- and κ-opioid receptors (MOR and KOR). Previous studies have demonstrated that both MOR antagonists and KOR agonists are effective in alleviating intrathecal morphine–induced itch in primates. The aim of the study was to investigate the effectiveness of butorphanol as an antipruritic and to elucidate the receptor mechanisms underlying butorphanol's antipruritic effect in primates.
Methods:
Adult rhesus monkeys were used in the behavioral assays for measuring itch/scratching and analgesia. The dose–response curves of butorphanol were studied using selective MOR and KOR antagonists. In addition, the effect of butorphanol as an antipruritic was studied on subcutaneous and intrathecal morphine–induced itch and analgesia. KOR-selective antagonists were further used to compare the degrees of MOR and KOR activation underlying the antipruritic effect of butorphanol.
Results:
Butorphanol alone produced analgesia with slight itch responses, and both effects were blocked by a MOR antagonist, clocinnamox (0.1 mg/kg). In contrast, a KOR antagonist, 5′ -guanidinylnaltrindole (1 mg/kg), increased butorphanol-elicited itch. Systemic butorphanol (0.0032– 0.032 mg/kg) dose-dependently attenuated systemic or intrathecal morphine–induced itch. In addition, butorphanol either potentiated or maintained morphine-induced analgesia without producing sedation. KOR-selective antagonists, 5′-guanidinylnaltrindole (1 mg/kg) and nor-binaltorphimine (3.2 mg/kg), only partially reversed the antipruritic effect of butorphanol with different durations of KOR antagonism.
Conclusions:
Butorphanol is effective in attenuating systemic or spinal morphine–induced itch without reducing morphine analgesia. This study provides functional evidence that both partial MOR and KOR agonist actions contribute to the effectiveness of butorphanol as an antipruritic in primates.
SPINAL administration of opioids is one of the most frequent analgesic methods in humans.1,2 However, the most common side effect of spinal morphine administration is itch/pruritus, which sometimes is severe. This lessens the value of spinal opioids for pain relief and is treated by different drugs with variable success.3,4 Recent studies have demonstrated that the same μ-opioid receptors (MOR) mediate both intrathecal morphine–induced analgesia and itch/scratching responses in primates, making it difficult to separate these effects.5,6 Therefore, MOR antagonists such as naloxone, although they are effective antipruritics, are not useful because they reverse the opioid analgesia in patients.7-9 Following a different tactic, it has been suggested that activation of κ-opioid receptors (KOR) can attenuate intrathecal morphine–induced itch/scratching responses without disrupting intrathecal morphine analgesia in primates.10 KOR agonists can also inhibit itch/scratching elicited by various pruritogenic agents in rodents.11,12 More importantly, a recent clinical trial showed that a novel KOR agonist, nalfurafine, is effective in treating patients suffering from uremic pruritus.13 These findings support the therapeutic potential of KOR agonists as antipruritics.
Butorphanol is an opioid analgesic with partial agonist actions at both MOR and KOR; it displays high affinity for both MOR and KOR and shows low to medium efficacy in activating receptors in cell lines expressing MOR or KOR.14,15 Several clinical studies have shown that butorphanol is effective in alleviating opioid-induced itch.16-18 It is important to study further the relative roles of MOR and KOR in producing butorphanol's analgesic and antipruritic effect in primates.
Therefore, using the pharmacologic approach, the aim of this study was to investigate the receptor mechanisms underlying the behavioral effects of butorphanol in monkeys. First, dose–response curves for systemic butorphanol-induced itch and analgesia were determined in the presence and absence of selective MOR and KOR antagonists. Second, the effectiveness of butorphanol as an antipruritic was evaluated in both subcutaneous and intrathecal morphine–induced itch and analgesia. Third, KOR-selective antagonists were used to compare the degrees of KOR and MOR actions underlying the anti-scratching effect of butorphanol.
Materials and Methods
Subjects
Twelve adult intact male and female rhesus monkeys (Macaca mulatta) with body weights ranging between 7.6 and 13.8 kg were used. Six monkeys (three males and three females) participated in the dose–response and antagonist studies of butorphanol, and in the effects of butorphanol on subcutaneous morphine–induced itch and analgesia. The remaining six monkeys (three males and three females) participated in the studies using intrathecal morphine. The monkeys were housed individually with free access to water and were fed approximately 25–30 biscuits (Purina Monkey Chow; Ralston Purina, St. Louis, MO) and fresh fruit daily. No monkey had exposure to any opioid receptor agonist or antagonist for 1 month before the current study. The monkeys were housed in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. The studies were conducted in accordance with the University Committee on the Use and Care of Animals at the University of Michigan (Ann Arbor, Michigan) and the Guide for the Care and Use of Laboratory Animals (Washington, D.C., National Academy Press, revised 1996).
Drugs
Butorphanol tartrate, nor-binaltorphimine (nor-BNI) 2HCl (Sigma-Aldrich, St. Louis, MO), morphine sulfate (Mallinckrodt, St. Louis, MO), and 5′-guanidinylnaltrindole (GNTI) 2HCl (National Institute on Drug Abuse, Bethesda, MD) were dissolved in sterile water. Clocinnamox (CCAM) mesylate (provided by John W. Lewis, Ph.D., Professor, Department of Pharmacy and Pharmacology, University of Bath, Bath, United Kingdom), was dissolved in 3% lactic acid in sterile water. For systemic administration, all compounds were administered at a volume of 0.1 ml/kg. Doses are presented in the compound forms listed above.
Experimental Designs
Dose–Response of Butorphanol and Morphine
The first part of the study was to compare the dose–response curves of subcutaneously administered butorphanol (0.001–0.032 mg/kg) and morphine (0.1–3.2 mg/kg) for itch/scratching and antinociception. Each compound was administered subcutaneously in the monkey's back (i.e., around the scapular region) using a cumulative dosing procedure with a 35-min interinjection interval. The scratching activity was recorded from 20 to 35 min after each injection. The antinociceptive effects were measured starting 20 min after each injection. The same group of monkeys was involved in both behavioral assays, but each behavioral measurement was conducted on separate testing days with a 1-week interval. In addition, the antagonist studies were conducted to verify the role of MOR and KOR in butorphanol-induced itch/scratching and antinociception. A selective MOR antagonist, CCAM (0.1 mg/kg), or a selective KOR antagonist, GNTI (1 mg/kg), was administered intramuscularly 1 day before redetermination of the dose–response curve with subcutaneous butorphanol. The dose and pretreatment time for each opioid receptor antagonist were chosen based on previous studies showing that each antagonist produced selective functional antagonism for either MOR or KOR for 1–2 weeks.6,19,20 The interinjection interval between antagonists was 1 month to assure that antagonist effects had dissipated before starting the next experiment.
Effectiveness of Butorphanol in Attenuating Scratching
The second part of the study was to evaluate the effects of butorphanol on both subcutaneous and intrathecal morphine–induced itch/scratching and antinociception. Butorphanol (0.001–0.032 mg/kg) or vehicle (sterile water, 0.1 ml/kg) was administered subcutaneously 15 min before the measurement of the dose-related nature of subcutaneous morphine–induced itch. Pretreatment doses of butorphanol were given in a random order. The interval between test sessions or measurements was 1 week. In addition, butorphanol was administered subcutaneously 45 min after intrathecal administration of 32 μg morphine to determine its intervention effectiveness as an antipruritic. This dose for intrathecal morphine was chosen based on previous studies showing that it produced maximal scratching responses concurrently with full antinociception and it could be used to assess the ability of a drug to modulate intrathecal morphine–elicited scratching.5,6,10 The scratching activity was recorded during the 23rd to 38th min and 53rd to 8th min of each hour (i.e., 15 min per half hour) for 3 h after intrathecal administration. In a separate experiment, the tail-withdrawal latencies were determined at the same time points used in the measurement of scratching. Similarly, doses of butorphanol were given in a random order. The interinjection interval between intrathecal morphine was 2 weeks.
Antagonist Studies for Butorphanol's Anti-scratching Action
The third part of the study was to investigate the effects of KOR antagonists, GNTI and nor-BNI, on butorphanol modulation of behavioral effects produced by intrathecal morphine. The scratching activity was recorded during the first3h(i.e., 15 min per half hour) after intramuscular administration of either GNTI (1 mg/kg) or nor-BNI (3.2 mg/kg) to examine whether GNTI or nor-BNI alone increased scratching responses. After the GNTI administration, the effects of 0.032 mg/kg subcutaneous butorphanol on intrathecal morphine (32 μg)–induced scratching and antinociception were redetermined at 1 and 14 days. Likewise, effects of 0.032 mg/kg subcutaneous butorphanol on intrathecal morphine (32 μg)–induced scratching and antinociception were redetermined at 1, 14, and 28 days after the nor-BNI administration. The dose and pretreatment time for both antagonists were chosen based on previous studies showing that GNTI and nor-BNI produced selective KOR antagonism for 1 and 3 weeks, respectively.10,20,21 The interinjection interval between antagonists was 1 month, and nor-BNI was the last antagonist to be tested because of its long-acting KOR antagonism.21
Procedures
Scratching Responses
Scratching responses, inferred to be a response to an itch sensation,5,6 were recorded on videotape while the monkeys were in their home cages. Each recording session was conducted for 15 min/test session. A scratch was defined as one short-duration (< 1 s) episode of scraping contact of the forepaw or hind paw on the skin surface of other body parts. Scratches usually occurred repetitively at the same location. Scratching responses were scored by trained individuals who were blinded to experimental conditions. In addition, sedation was evaluated by cumulative time for eye closure or lying down at the bottom of the cage.
Antinociception
The warm water (50°C) tail-withdrawal assay was used to evaluate thermal antinociceptive effects of the test compound.6 Briefly, monkeys were seated in primate restraint chairs, and the lower part of their shaved tails (approximately 15 cm) were immersed in a thermal flask containing water maintained at 42°, 46°, or 50°C. Tail-withdrawal latencies were measured using a computerized timer by an experimenter who was blinded to experimental conditions. In each test session, monkeys were evaluated once with four temperatures given in a random order, and only the stimulus 50°C water was tested twice to confirm the full antinociceptive effect. If the monkeys did not remove their tails within 20 s (cutoff), the flask was removed and a maximum time of 20 s was recorded. Test sessions began with control determinations at each temperature. Subsequent tail-withdrawal latencies were determined at multiple time points after the drug administration.
Intrathecal Drug Delivery
Monkeys were positioned in primate restraint chairs and anesthetized by intravenous administration of propofol (2.5– 4.0 mg/kg for bolus infusion and 0.3–0.4 mg · kg−1 · min−1 for continuous infusion; AstraZeneca, Wilmington, DE).10 The lower back of the trunk was shaved and scrubbed with Betadine (Purdue Fredrick Co., Norwalk, CT). A spinal needle (22-gauge × 1½; Becton Dickinson, Franklin Lakes, NJ) was inserted into the subarachnoid space between L4 and L5 lumbar vertebras. Needle position was confirmed by a free flow of clear cerebrospinal fluid. A 1-ml solution of morphine was infused slowly through the spinal needle within 30 s. Monkeys normally recovered from anesthesia within 5–10 min after termination of the propofol infusion.
Statistical Analysis
Mean values (mean ± SEM) were calculated from individual values for all behavioral endpoints. Comparisons were made for the same monkeys across all test sessions in the same experiment. For the dose–response curve for antinociception, individual tail-withdrawal latencies in 50°C water were converted to percentage of maximum possible effect. The formula of the percentage of maximum possible effect is defined as [(test latency – control latency)/(cutoff latency, 20 s – control latency)] × 100. ED50 values were calculated by least-squares regression with the portion of the dose– effect curves spanning the 50% maximum possible effect. The 95% confidence limits were also determined (P < 0.05). Mean ED50 values were considered to be significantly different when their 95% confidence limits did not overlap. Remaining data were analyzed by the analysis of variance followed by the Newman-Keuls test for multiple (post hoc) comparisons. The criterion for significance was set at P < 0.05.
Results
Dose-Response of Butorphanol and Morphine
Figure 1 compares the behavioral effects of butorphanol and morphine after subcutaneous administration. Butorphanol only slightly increased scratching responses. The peak effect was 142 ± 28 (mean ± SEM) scratches for 15 min evoked by 0.01 mg/kg subcutaneous butorphanol. In contrast, morphine dose-dependently increased scratching responses. The peak effect was 383 ± 81 scratches evoked by 1 mg/kg subcutaneous morphine (fig. 1A). Both butorphanol and morphine dose-dependently produced antinociception using 50°C water. Mean ED50 values (95% confidence limits) for butorphanol and morphine were 0.016 (0.014–0.018) and 0.93 (0.58–1.47) mg/kg, respectively (fig. 1B). These doses of butorphanol and morphine used in this study did not produce sedation.
Fig. 1.
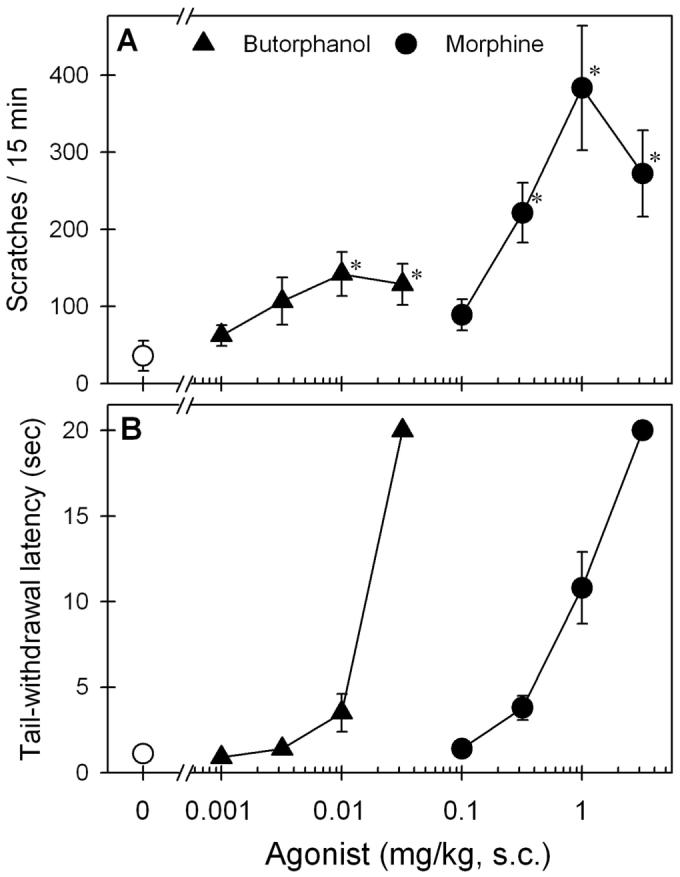
Comparison of behavioral effects produced by subcutaneous administration of butorphanol and morphine. (A) Dose–response curve for itch/scratching responses. (B) Dose–response curve for antinociception against 50°C water. Behavioral responses were determined using a cumulative dosing procedure. Each value represents mean ± SEM (n = 6). Symbols represent different dosing conditions for the same monkeys. * Significant difference from the vehicle condition (P < 0.05).
Figure 2 illustrates the effects of the KOR antagonist GNTI and the MOR antagonist CCAM on butorphanol-induced behavioral effects (F2,10 = 16.4, P < 0.05). Pretreatment with GNTI slightly elevated butorphanol-induced scratching (fig. 2A). However, GNTI did not change butorphanol-induced antinociception because the ED50 values for butorphanol with GNTI or vehicle pretreatment were similar (i.e., 0.016 [0.014–0.018] vs. 0.015 [0.014–0.018] mg/kg). In contrast, pretreatment with CCAM completely blocked butorphanol-induced scratching (fig. 2A). CCAM also antagonized butorphanol-induced antinociception because there was a significant rightward shift for the butorphanol dose–response curve (i.e., 0.083 [0.065–0.11] vs. 0.015 [0.014–0.018] mg/kg) after pretreatment with CCAM (fig. 2B).
Fig. 2.
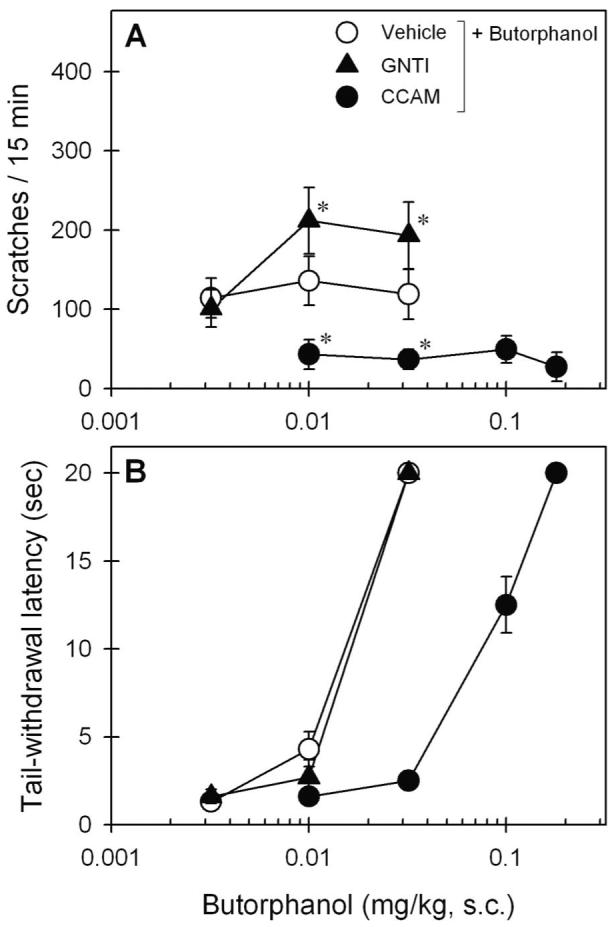
Effects of κ- and μ-opioid receptor antagonists on systemic butorphanol-induced behavioral effects in monkeys. A κ-opioid receptor antagonist (5′-guanidinylnaltrindole [GNTI], 1 mg/kg) or a μ-opioid receptor antagonist (clocinnamox [CCAM], 0.1 mg/kg) was administered intramuscularly 1 day before redetermination of the dose–response curve of subcutaneous butorphanol. (A) Dose–response curve for itch/scratching responses. (B) Dose–response curve for antinociception against 50°C water. Each value represents mean ± SEM (n = 6). Symbols represent pretreatment with different antagonists for the same monkeys. * Significant difference from the vehicle pretreatment condition (P < 0.05).
Effectiveness of Butorphanol in Attenuating Scratching
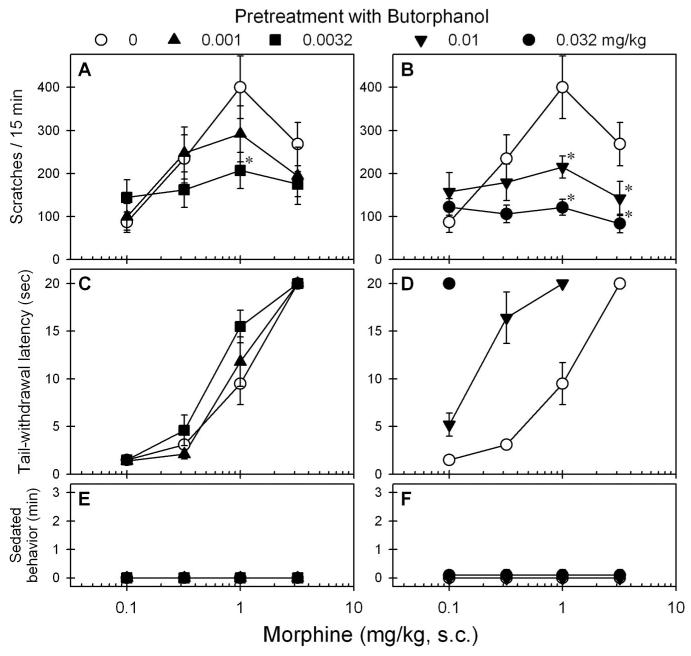
Figure 3 shows the effects of subcutaneous butorphanol on the morphine dose–response curves for scratching and antinociception. Pretreatment with butorphanol dose-dependently produced downward shifts of the morphine dose–response curve for scratching (F4,20 = 10.1, P < 0.05). Post hoc comparisons indicated that butorphanol from 0.0032 to 0.032 mg/kg significantly attenuated the peak scratching effect of 1 mg/kg subcutaneous morphine (figs. 3A and B). Pretreatment with butorphanol dose-dependently potentiated morphine-induced antinociception (figs. 3C and D). In particular, 0.01 mg/kg butorphanol significantly produced a leftward shift of the morphine dose–response curve for antinociception (i.e., ED50 values: 0.18 [0.10–0.32] vs. 1.0 [0.68–1.65] mg/kg). Furthermore, pretreatment with butorphanol did not increase the sedation score under these conditions (F4,20 = 1.0, P = 0.4) (figs. 3E and F).
Fig. 3.
Effects of butorphanol pretreatment on systemic morphine dose–response curves for scratching and antinociception. Butorphanol (mg/kg) was administered subcutaneously 15 min before the injection of the first dose of subcutaneous morphine. A and B: Effects of butorphanol on morphine-induced scratching responses. C and D: Effects of butorphanol on morphine-induced antinociception against 50°C water. E and F: Effects of butorphanol combined with morphine on the sedation. Each value represents mean ± SEM (n = 6). Symbols represent different experimental conditions for the same monkeys. * Significant difference from the vehicle pretreatment condition, subcutaneous morphine alone (P < 0.05). See figures 1 and 2 for other details.
Figure 4 shows the effects of subcutaneous butorphanol on intrathecal morphine–induced scratching and antinociception. Butorphanol intervention dose-dependently attenuated intrathecal morphine (32 μg)–induced scratching (F3,15 = 23.0, P < 0.05). Post hoc comparisons indicated that butorphanol from 0.01 to 0.032 mg/kg significantly attenuated scratching between time points 1.5 and 2.5 h (fig. 4A). Butorphanol did not alter intrathecal morphine–induced antinociception (F3,15 = 0.6, P = 0.6). Butorphanol intervention at the dose range from 0.01 to 0.032 mg/kg did not attenuate antinociception between time points 1 and 2.5 h (fig. 4B). In addition, butorphanol did not increase the sedation score (F3,15 = 1.0, P = 0.4) under these conditions (fig. 4C).
Fig. 4.
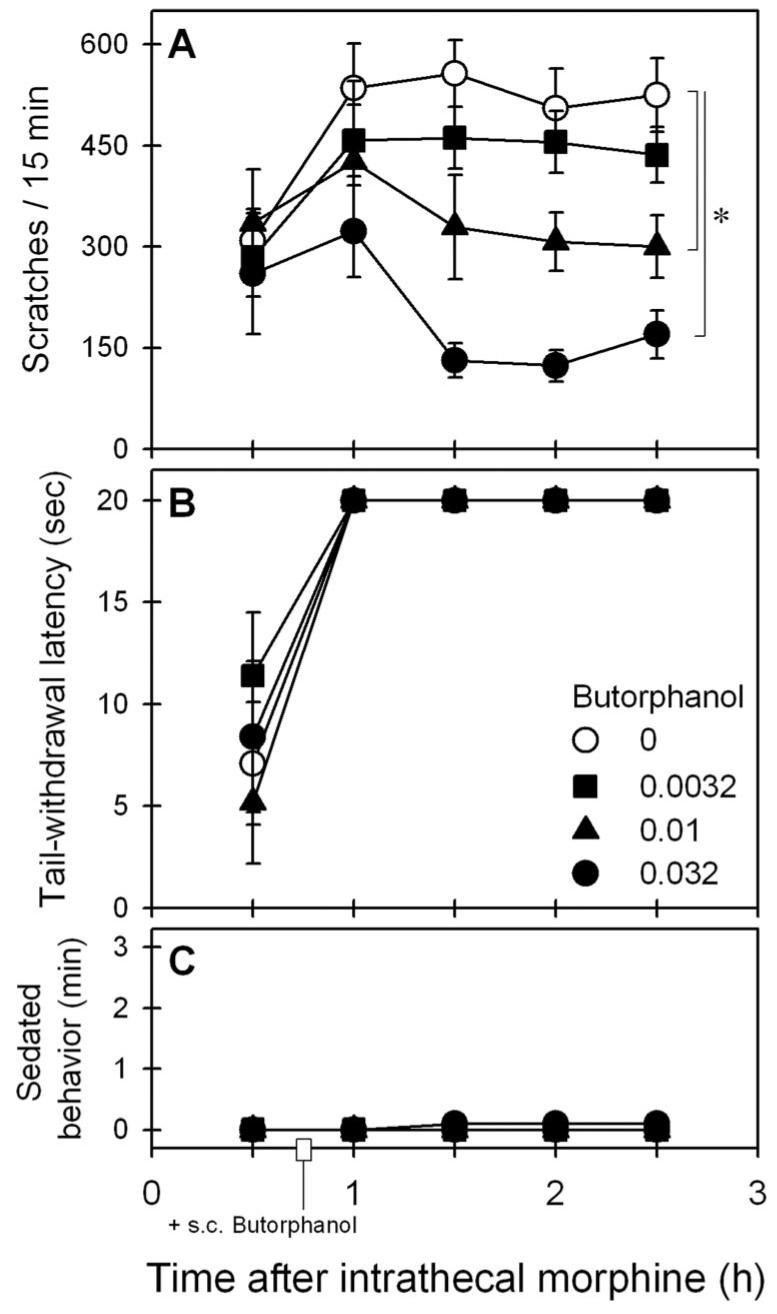
Effects of butorphanol intervention on intrathecal morphine–induced scratching and antinociception. Butorphanol (mg/kg) was administered subcutaneously 45 min after intrathecal administration of 32 μg morphine. A, B, and C represent effects of butorphanol on morphine-induced scratching, antinociception, and sedation, respectively. Each value represents mean ± SEM (n = 6). Symbols represent different experimental conditions for the same monkeys. * Significant difference from the vehicle condition between time points 1.5 and 2.5 h (P < 0.05). See Results section for other details.
Antagonist Studies for Butorphanol's Antiscratching Action
The scratching responses of monkeys were monitored for 3 h (i.e., 15 min per half hour) after monkeys received intramuscular administration of 1 mg/kg GNTI or 3.2 mg/kg nor-BNI. GNTI or nor-BNI alone did not increase scratching responses as compared with the vehicle-treated condition (GNTI: F1,5 = 0.2, P = 0.7; nor-BNI: F1,5 = 0.007, P = 0.9). The average number of scratches across different time points ranged from 21 ± 11 to 34 ± 14 scratches for 15 min per session. Figure 5 illustrates the antagonist effect of GNTI on the actions of butorphanol against intrathecal morphine. One-day pretreatment with 1 mg/kg GNTI significantly blocked the ability of 0.032 mg/kg subcutaneous butorphanol to attenuate intrathecal morphine (32 μg)–induced scratching (F3,15 = 28.1, P < 0.05). Post hoc comparisons indicated that 1-day pretreatment with GNTI only partially reversed the antiscratching effect of butorphanol because its effect was significantly different from the scratching responses produced by either intrathecal morphine alone or intrathecal morphine in combination with subcutaneous butorphanol (fig. 5A). The antagonist effect of GNTI subsided as 14-day pretreatment with GNTI was ineffective in blocking the antiscratching effect of butorphanol. Post hoc comparisons indicated there was no significant difference between effects of GNTI 14-day pretreatment (i.e., GNTI + [morphine + butorphanol]) and effects of intrathecal morphine with butorphanol (i.e., morphine + butorphanol) because the P values ranged from 0.6 to 0.9 across all time points during the test session. In addition, GNTI pretreatment did not change the effect of butorphanol on intrathecal morphine–induced antinociception (F3,15 = 1.0, P = 0.4) (fig. 5B).
Fig. 5.
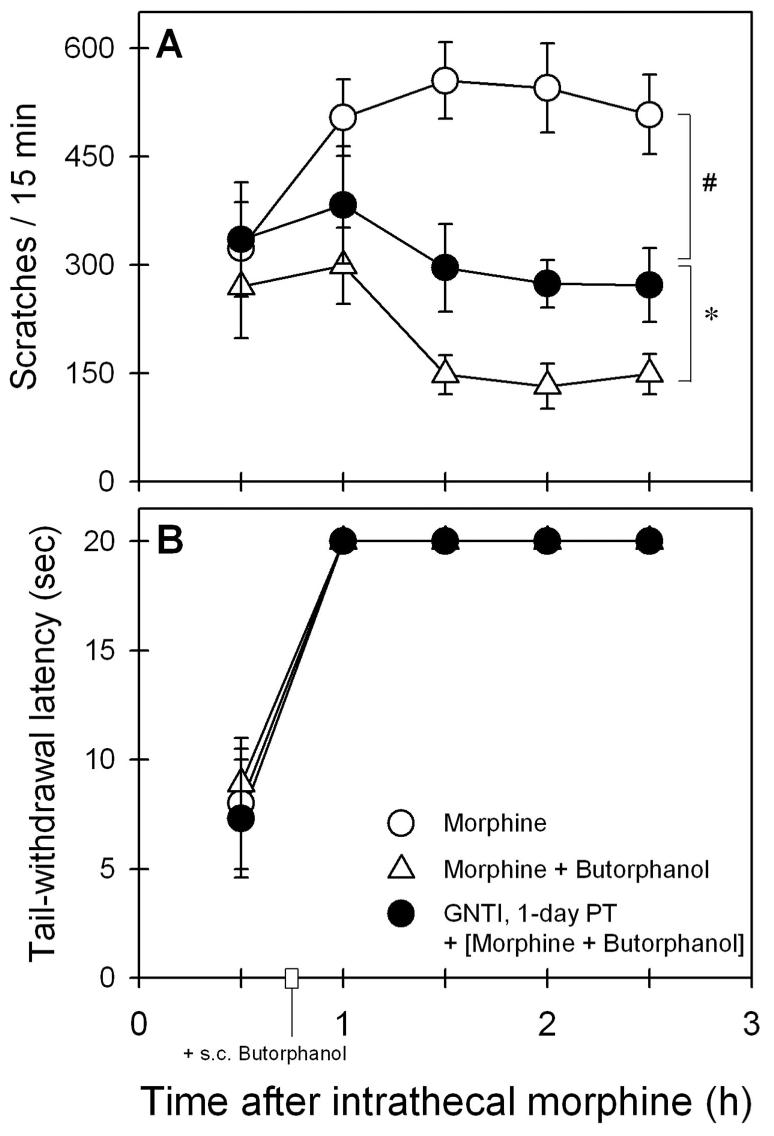
Antagonist effect of 5′-guanidinylnaltrindole (GNTI) on the actions of butorphanol against intrathecal morphine–induced scratching (A) and antinociception (B). Butorphanol (0.032 mg/kg) was administered subcutaneously 45 min after 32 μg intrathecal morphine. Filled symbols represent the effect of GNTI (1 mg/kg) 1-day pretreatment (PT) on the actions of subcutaneous butorphanol against intrathecal morphine. Each value represents mean ± SEM (n = 6). * Significant difference between experimental conditions, (GNTI + [morphine + butorphanol]) and (morphine + butorphanol). # Significant difference between experimental conditions, (GNTI + [morphine + butorphanol]) and (morphine alone). The effect of GNTI 14-day pretreatment was not shown for the sake of clarity because its effect was similar to that of (morphine + butorphanol). See Results section for other details.
Figure 6 illustrates the antagonist effect of nor-BNI on the actions of butorphanol against intrathecal morphine. Both 1- and 14-day pretreatment with 3.2 mg/kg nor-BNI significantly blocked the ability of 0.032 mg/kg subcutaneous butorphanol to attenuate intrathecal morphine (32 μg)–induced scratching (F4,20 = 30.6, P < 0.05). Post hoc comparisons indicated that either 1-day or 14-day pretreatment with nor-BNI only partially reversed the antiscratching effect of butorphanol because its effect was significantly different from the scratching responses produced by either intrathecal morphine alone or intrathecal morphine in combination with butorphanol (fig. 6A). The antagonist effect of nor-BNI subsided because 28-day pretreatment with nor-BNI was ineffective in blocking the antiscratching effect of butorphanol. Post hoc comparisons indicated that there was no significant difference between effects of nor-BNI 28-day pre-treatment (i.e., nor-BNI + [morphine + butorphanol]) and effects of intrathecal morphine with butorphanol (i.e., morphine + butorphanol) because the P values ranged from 0.2 to 0.9 across time points during the test session. In addition, nor-BNI pretreatment did not change the effect of butorphanol on intrathecal morphine–induced antinociception (F4,20 = 0.9, P = 0.5) (fig. 6B).
Fig. 6.
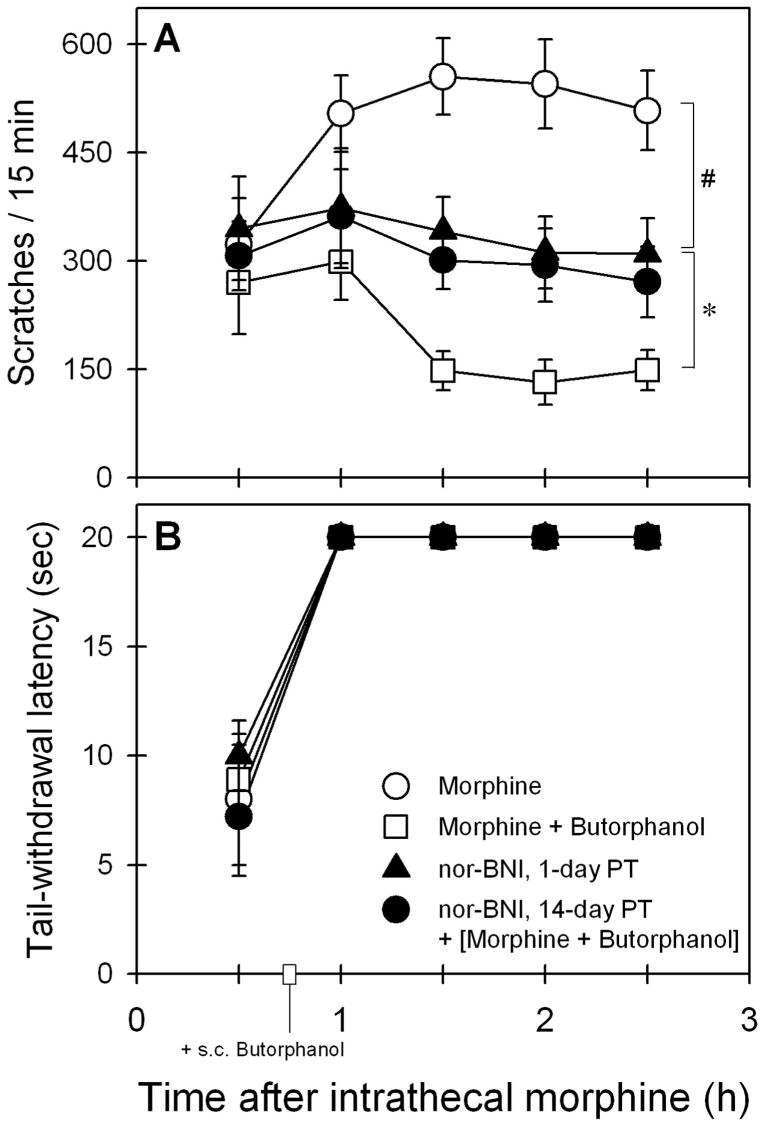
Antagonist effect of nor-binaltorphimine (nor-BNI) on the actions of butorphanol against intrathecal morphine–induced scratching (A) and antinociception (B). Butorphanol (0.032 mg/kg) was administered subcutaneously 45 min after 32 μg intrathecal morphine. Filled symbols represent the effects of nor-BNI (3.2 mg/kg) 1- or 14-day pretreatment (PT) on the actions of subcutaneous butorphanol against intrathecal morphine. Each value represents mean ± SEM (n = 6). * Significant difference between experimental conditions, (nor-BNI + [morphine + butorphanol]) and (morphine + butorphanol). # Significant difference between experimental conditions, (nor-BNI + [morphine + butorphanol]) and (morphine alone). The effect of nor-BNI 28-day pretreatment was not shown for the sake of clarity because its effect was similar to that of (morphine + butorphanol). See Results section for other details.
Discussion
The first part of the study showed that systemic butorphanol alone produced an antinociceptive effect with slight itch/scratching responses in monkeys. This finding is similar to clinical studies reporting that butorphanol produces analgesia with a low incidence of itch.22,23 A MOR-selective antagonist, CCAM, blocked both butorphanol-induced itch and analgesia, indicating that MOR mainly mediated both itch and analgesia produced by butorphanol (fig. 2). In addition, a KOR-selective antagonist, GNTI, slightly potentiated butorphanol-induced itch without affecting its analgesia, indicating that butorphanol's KOR agonist actions were responsible for its antipruritic response. Given that butorphanol has high affinity and partial efficacy for both MOR and KOR,14,15 these results indicate that a partial KOR-agonist action may inhibit butorphanol-induced itch mediated by MOR activation. Previous studies have demonstrated that MOR, not other opioid receptor subtypes, mediates itch evoked by a variety of opioid analgesics and that activation of KOR inhibits MOR agonist–induced itch without attenuating analgesia in primates.5,6,10 It seems likely that butorphanol's concurrent activation of both MOR and KOR with partial efficacy contributes to its limited pruritic effect.
The second part of the study showed that systemic butorphanol was effective in attenuating systemic or intrathecal morphine–induced itch (figs. 3 and 4). Importantly, systemic butorphanol either potentiated or maintained morphine-induced analgesia without producing sedation. In contrast, butorphanol dose-dependently produced downward shifts in the dose–response curves of systemic morphine–induced itch. This changing pattern of the dose–response curves is similar to that produced by selective KOR agonists that showed a functional antagonism of itch.10 In contrast, MOR antagonists produced only parallel rightward shifts in the dose–response curves of morphine-induced itch, indicating that antipruritic effects of MOR antagonists are produced at MOR in a competitive and reversible manner.5,24 Nevertheless, these findings support clinical studies showing that butorphanol is effective in alleviating opioid-induced itch without reducing analgesia.16-18 Taken from the first two parts of the study, it is possible that the MOR population or/and efficacy required for producing pruritus is greater that that for antinociception. Most partial MOR agonists produce full antinociception measured by the 50°C warm water tail-withdrawal assay in monkeys.25 Therefore, the ability of butorphanol to inhibit morphine-induced pruritus but not morphine-induced analgesia may be expected.
The third part of the study demonstrated that both partial KOR and MOR agonist actions contributed to the antipruritic effect of butorphanol against intrathecal morphine–induced itch (figs. 5 and 6). KOR-selective antagonists, GNTI and nor-BNI, only partially reversed the antipruritic effect of butorphanol, and nor-BNI produced a longer duration of KOR antagonism. Nor-BNI at the same dose (i.e., 3.2 mg/kg) was able to reverse completely the antipruritic effect of U-50488H, a full KOR agonist, on intrathecal morphine–induced itch.10 In addition, nor-BNI pretreatment did not modulate intrathecal morphine–induced pruritus, indicating that morphine does not have the KOR agonist action.6 These findings indicate that the partial MOR agonist action may also contribute the antipruritic effect of butorphanol (i.e., low-efficacy ligands antagonize high-efficacy ligands' action in producing itch sensation). This notion is supported by clinical trials showing that a partial MOR agonist, nalbuphine, is effective in attenuating spinal opioid–induced itch while maintaining spinal opioid analgesia.8,9 Given that MOR is the dominant receptor population for producing analgesia, these results concur with animal and human studies indicating that partial MOR agonists have an advantage over MOR antagonists as supplemental agents to treat itch in patients receiving spinal opioid analgesics.5,7-9
Itch is a significant clinical problem that receives different pharmacologic treatments with limited success, and there are many potential mediators involved in itch sensation.26,27 MOR antagonists are effective in alleviating itch in patients with chronic cholestasis, implying the involvement of endogenous opioids in this type of itch.28 Furthermore, the potential antipruritic effect of opioid partial agonists such as butorphanol was raised in treating this population of patients.29 The pharmacologic profile of butorphanol is different from those of selective full MOR or KOR agonists. For example, the subjective and psychophysical effects of systemic butorphanol are not similar to that produced by either the MOR agonist morphine or the KOR agonist enadoline in humans.30,31 In addition, butorphanol produced a variety of behavioral and physiologic effects in primates, suggesting that it has a mixed partial agonist action at both MOR and KOR.25,32 The current study demonstrates that butorphanol's partial agonist action at both MOR and KOR contributes to its antipruritic effect. It is interesting to note that itch/scratching can be elicited by other MOR agonists with different chemical structures and that the MOR is responsible for opioid analgesic–elicited pruritus at the cellular level.7 It will be valuable to further study the effects of butorphanol against pruritus elicited by other MOR agonists.
It is worth noting that KOR activation produces several functions opposite to MOR activation in primates. For example, MOR agonists produce euphoria and KOR agonists produce dysphoria.31,33 MOR agonists produce antidiuretic effects and KOR agonists produce diuresis.34,35 MOR agonists produce pruritus and KOR agonists produce antipruritic effects.10,36,37 Interestingly, butorphanol produces neither euphoria nor dysphoria in humans.38 Butorphanol does not cause diuresis25 and it produces low incidence of itch in primates (the current study).22,23 These findings strengthen the notion that a mixed MOR/KOR agonist may have a therapeutic advantage over a selective MOR agonist. Given that MOR and KOR are abundant in the primate central nervous system,39,40 an opioid agonist with mixed actions of high efficacy at MOR and low efficacy at KOR may be a strong analgesic with less side effects and abuse liability.
In summary, this study reveals butorphanol's dual actions at both MOR and KOR by using selective MOR and KOR antagonists in monkeys. It further demonstrates that butorphanol is effective in blocking systemic or spinal morphine–evoked itch/scratching responses while maintaining morphine-induced analgesia. Both partial MOR and KOR agonist actions of butorphanol may contribute to its antipruritic effect. These findings support the therapeutic potential of partial MOR or/and KOR agonists as antipruritics in the context of spinal opioid analgesia. More importantly, the study encourages the research and development of novel opioid agonists with dual actions at both MOR and KOR with different degrees of intrinsic efficacy.
Acknowledgments
The authors thank Justin Knorr, B.S., Timothy Zdrodowski, B.S., and Tina Frese, B.S. (Research Assistants, Department of Pharmacology, University of Michigan, Ann Arbor, Michigan), for technical assistance.
Supported by grant Nos. DA-000254 and DA-013685 from the United States Public Health Service, Bethesda, Maryland.
References
- 1.Dougherty PM, Staats PS. Intrathecal drug therapy for chronic pain: From basic science to clinical practice. Anesthesiology. 1999;91:1891–918. doi: 10.1097/00000542-199912000-00044. [DOI] [PubMed] [Google Scholar]
- 2.Bennett G, Serafini M, Burchiel K, Buchser E, Classen A, Deer T, Du Pen S, Ferrante FM, Hassenbusch SJ, Lou L, Maeyaert J, Penn R, Portenoy RK, Rauck R, Willis KD, Yaksh T. Evidence-based review of the literature on intrathecal delivery of pain medication. J Pain Symptom Manage. 2000;20:S12–36. doi: 10.1016/s0885-3924(00)00204-9. [DOI] [PubMed] [Google Scholar]
- 3.Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276–310. [PubMed] [Google Scholar]
- 4.Waxler B, Dadabhoy ZP, Stojiljkovic L, Rabito SF. Primer of postoperative pruritus for anesthesiologists. Anesthesiology. 2005;103:168–78. doi: 10.1097/00000542-200507000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Ko MCH, Naughton NN. An experimental itch model in monkeys: Characterization of intrathecal morphine–induced scratching and antinociception. Anesthesiology. 2000;92:795–805. doi: 10.1097/00000542-200003000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko MCH, Song MS, Edwards T, Lee H, Naughton NN. The role of central mu opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther. 2004;310:169–76. doi: 10.1124/jpet.103.061101. [DOI] [PubMed] [Google Scholar]
- 7.Rawal N, Schott U, Dahlstrom B, Inturrisi CE, Tandon B, Sjostrand U, Wennhager M. Influence of naloxone infusion on analgesia and respiratory depression following epidural morphine. Anesthesiology. 1986;64:194–201. doi: 10.1097/00000542-198602000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Cohen SE, Ratner EF, Kreitzman TR, Archer JH, Mignano LR. Nalbuphine is better than naloxone for treatment of side effects after epidural morphine. Anesth Analg. 1992;75:747–52. [PubMed] [Google Scholar]
- 9.Wang JJ, Ho ST, Tzeng JI. Comparison of intravenous nalbuphine infusion versus naloxone in the prevention of epidural morphine-related side effects. Reg Anesth Pain Med. 1998;23:479–84. doi: 10.1016/s1098-7339(98)90031-1. [DOI] [PubMed] [Google Scholar]
- 10.Ko MCH, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, Woods JH, Naughton NN. Activation of k-opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J Pharmacol Exp Ther. 2003;305:173–9. doi: 10.1124/jpet.102.044909. [DOI] [PubMed] [Google Scholar]
- 11.Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H. Antipruritic activity of the κ-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259–64. doi: 10.1016/s0014-2999(01)01588-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li PG, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of three distinct kappa ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–30. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 13.Wikstrom B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M, Kawashima Y, Ueno K, Mori A, Ueno Y. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16:3742–47. doi: 10.1681/ASN.2005020152. [DOI] [PubMed] [Google Scholar]
- 14.Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the μ opioid receptor. J Pharmacol Exp Ther. 1996;278:1121–7. [PubMed] [Google Scholar]
- 15.Remmers AE, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. J Pharmacol Exp Ther. 1999;288:827–33. [PubMed] [Google Scholar]
- 16.Lawhor CD, McNitt JD, Fibuch EE, Joyce JT, Leadley RJ., Jr Epidural morphine with butorphanol for postoperative analgesia after cesarean delivery. Anesth Analg. 1991;72:53–7. doi: 10.1213/00000539-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Dunteman E, Karanikolas M, Filos KS. Transnasal butorphanol for the treatment of opioid-induced pruritus unresponsive to antihistamines. J Pain Symptom Manage. 1996;12:255–60. doi: 10.1016/0885-3924(96)00154-6. [DOI] [PubMed] [Google Scholar]
- 18.Gunter JB, McAuliffe J, Gregg T, Weidner N, Varughese AM, Sweeney DM. Continuous epidural butorphanol relieves pruritus associated with epidural morphine infusions in children. Paediatric Anaesth. 2000;10:167–72. doi: 10.1046/j.1460-9592.2000.00447.x. [DOI] [PubMed] [Google Scholar]
- 19.Zernig G, Butelman ER, Lewis JW, Walker EA, Woods JH. In vivo determination of mu opioid receptor turnover in rhesus monkeys after irreversible blockade with clocinnamox. J Pharmacol Exp Ther. 1994;269:57–65. [PubMed] [Google Scholar]
- 20.Negus SS, Mello NK, Linsenmayer DC, Jones RM, Portoghese PS. Kappa opioid antagonist effects of the novel kappa antagonist 5′-guanidinonaltrindole (GNTI) in an assay of schedule-controlled behavior in rhesus monkeys. Psychopharmacology. 2002;163:412–9. doi: 10.1007/s00213-002-1038-x. [DOI] [PubMed] [Google Scholar]
- 21.Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–76. [PubMed] [Google Scholar]
- 22.Ackerman WE, Juneja MM, Kaczorowski DM, Colclough GW. A comparison of the incidence of pruritus following epidural opioid administration in the parturient. Can J Anaesth. 1989;36:388–91. doi: 10.1007/BF03005335. [DOI] [PubMed] [Google Scholar]
- 23.Palacios QT, Jones MM, Hawkins JL, Adenwala JN, Longmire S, Hess KR, Skjonsky BS, Morrow DH, Joyce TH. Post-caesarean section analgesia: A comparison of epidural butorphanol and morphine. Can J Anaesth. 1991;38:24–30. doi: 10.1007/BF03009159. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Naughton NN, Woods JH, Ko MCH. Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol. 2003;14:501–8. doi: 10.1097/01.fbp.0000095082.80017.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butelman ER, Winger G, Zernig G, Woods JH. Butorphanol: Characterization of agonist and antagonist effects in rhesus monkeys. J Pharmacol Exp Ther. 1995;272:845–53. [PubMed] [Google Scholar]
- 26.Biro T, Ko MC, Bromm B, Wei ET, Bigliardi P, Siebenhaar F, Hashizume H, Misery L, Bergasa NV, Kamei C, Schouenborg J, Roostermann D, Szabo T, Maurer M, Bigliardi-Qi M, Meingassner JG, Hossen MA, Schmelz M, Steinhoff M. How best to fight that nasty itch: From new insights into the neuroimmunological, neuroendocrine, and neurophysiological bases of pruritus to novel therapeutic approaches. Exp Dermatol. 2005;14:225–40. doi: 10.1111/j.0906-6705.2005.0321a.x. [DOI] [PubMed] [Google Scholar]
- 27.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–47. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 28.Bergasa NV, Talbot TL, Alling DW, Schmitt JM, Walker EC, Baker BL, Korenman JC, Park Y, Hoofnagle JH, Jones EA. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology. 1992;102:544–9. doi: 10.1016/0016-5085(92)90102-5. [DOI] [PubMed] [Google Scholar]
- 29.Bergasa NV. Treatment of the pruritus of cholestasis. Curr Treat Options Gastroenterol. 2004;7:501–8. doi: 10.1007/s11938-004-0009-1. [DOI] [PubMed] [Google Scholar]
- 30.Zacny JP, Lichtor JL, Thapar P, Coalson DW, Flemming D, Thompson WK. Comparing the subjective, psychomotor, and physiological effects of intravenous butorphanol and morphine in healthy volunteers. J Pharmacol Exp Ther. 1994;270:579–88. [PubMed] [Google Scholar]
- 31.Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology. 2001;157:151–62. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- 32.Vivian JA, DeYoung MB, Sumpter TL, Traynor JR, Lewis JW, Woods JH. k-Opioid receptor effects of butorphanol in rhesus monkeys. J Pharmacol Exp Ther. 1999;290:259–65. [PubMed] [Google Scholar]
- 33.Kumor KM, Haertzen CA, Johnson RE, Kocher T, Jasinski D. Human psychopharmacology of ketocyclazocine as compared with cyclazocine, morphine, and placebo. J Pharmacol Exp Ther. 1986;238:960–8. [PubMed] [Google Scholar]
- 34.Peters GR, Ward NJ, Antal EG, Lai PY, deMaar EW. Diuretic actions in man of a selective kappa opioid agonist: U-62,066E. J Pharmacol Exp Ther. 1987;240:128–31. [PubMed] [Google Scholar]
- 35.Weiskopf RB, Reid IA, Fisher DM, Holmes MA, Rosen JI, Keil LC. Effects of fentanyl on vasopressin secretion in human subjects. J Pharmacol Exp Ther. 1987;242:970–3. [PubMed] [Google Scholar]
- 36.Bailey PL, Rhondeau S, Schafer PG, Lu JK, Timmins BS, Foster W, Pace NL, Stanley TH. Dose–response pharmacology of intrathecal morphine in human volunteers. Anesthesiology. 1993;79:49–59. doi: 10.1097/00000542-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose–response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–44. doi: 10.1097/00000542-199902000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Dershwitz M, Rosow CE, DiBiase PM, Zaslavsky A. Comparison of the sedative effects of butorphanol and midazolam. Anesthesiology. 1991;74:717–24. doi: 10.1097/00000542-199104000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Sim-Selley LJ, Daunais JB, Porrino LJ, Childers SR. Mu and kappa1 opioid-stimulated [35S]guanylyl-5′-O-(gamma-thio)-triphosphate binding in cynomolgus monkey brain. Neuroscience. 1999;94:651–62. doi: 10.1016/s0306-4522(99)00344-9. [DOI] [PubMed] [Google Scholar]
- 40.Ko MCH, Lee H, Harrison C, Clark MJ, Song HF, Naughton NN, Woods JH, Traynor JR. Studies of mu-, kappa-, and delta-opioid receptor density and G protein activation in the cortex and thalamus of monkeys. J Pharmacol Exp Ther. 2003;306:179–86. doi: 10.1124/jpet.103.050625. [DOI] [PubMed] [Google Scholar]