Abstract
Novel aluminum(III)- and zirconium(IV)-tetraphenylporhyrin (TPP) derivatives are examined as fluoride selective ionophores for preparing polymer membrane-based ion-selective electrodes (ISEs). The influence of t-butyl— or dichloro— phenyl ring substituents as well as the nature of the metal ion center (Al(III) vs. Zr(IV)) on the anion complexation constants of TPP derivative ionophores are reported. The anion binding stability constants of the ionophores are characterized by the so-called “sandwich membrane” method. All of the metalloporphyrins examined form their strongest anion complexes with fluoride. The influence of plasticizer as well as the type of lipophilic ionic site additive and their amounts in the sensing membrane are discussed. It is shown that membrane electrodes formulated with the metalloporphyrin derivatives and appropriate anionic or cationic additives exhibit enhanced potentiometric response toward fluoride over all other anions tested. Since selectivity toward fluoride is enhanced in the presence of both anionic and cationic additives, the metalloporphyrins can function as either charged or neutral carriers within the organic membrane phase. In contrast to previously reported fluoride-selective polymeric membrane electrodes based on metalloporphyrins, nernstian or near-nernstian (−51.2 to −60.1 mV decade−1) as well as rapid (t < 80s) and fully reversible potentiometric fluoride responses are observed. Moreover, use of aluminum(III)—t-butyltetraphenylporphyrin as the ionophore provides fluoride sensors with prolonged (7 months) functional life-time.
1. Introduction
During the past three decades, many ionophore-based polymeric membrane type ion-selective electrodes (ISEs) have been developed for simple potentiometric sensing of cations and anions in a wide range of samples. Generally it has been found that development of such sensors for detecting specific anions is far more challenging than for cations owing to the varied sizes and shapes of anionic species [1]. Ion-sensors for hydrophilic anions such as fluoride, phosphate and sulfate are especially difficult to devise owing to unfavorable partitioning of these ions from an aqueous sample phase into the organic polymeric membrane phase of the ISE. Only a very strong interaction between the host molecule (ionophore) and target anion in the membrane can induce a pronounced change in the potentiometric anion selectivity patterns observed with polymeric membranes.
The affinity of a given ion to ionophore is usually described as the ion-ionophore complex stability constant (β) and the magnitude of this value can be measured by a number of different methods [2–6]. One of the simplest and most relevant approaches for the determination of ion-ionophore stability constants is via the ‘sandwich membrane’ method which is based on EMF measurements across two sandwiched membranes of different compositions [7]. However, to date, this method has primarily been employed for determination of cation-ionophore stability constants, with only limited examples of the binding anions with ionophores being studied via this approach [6].
Similarly, thus far, relatively few useful fluoride-selective polymeric membrane electrodes have been reported [8–15]. Most suffer from practical problems such as high level of interference by lipophilic anions [11,12,15], super-nernstian response slopes, sluggish and not fully reversible response properties [8,9,10,14] and short use lifetimes of the electrodes [14]. Recent research has suggested that membranes formulated with Al(III) octaethylporhyrin (Al[OEP]) and Al(III) tetraphenylporphyrin (Al[TPP]) [14] exhibit very high potentiometric selectivity towards fluoride anions, and this has led also to the design of highly selective optical fluoride sensors with sub-micromolar fluoride ion detection limit [16,17]. However, when these same ionophores are employed in the potentiometric detection mode [14], the common problems cited above (sluggish response, non-nernstian response, etc.) are often observed.
It has been shown that incorporation of picket fence type metalloporphyrins (e.g. In[PFP] or Ga[PFP] [18] within ISE polymeric membrane leads to nernstian response for chloride and fluoride anions, respectively. The structure of these species prevent unwanted metalloporphyrin dimer-monomer equilibria within the membrane phase of the electrode, with such chemistry leading to non-ideal response properties. Similar studies with Al(III) picket fence porphyrin (Al[PFP]) to prevent dimer-monomer chemistry have also been reported [14]. However, it was found that Al[PFP] easily forms insoluble crystals within the membrane phase and also leaches from the membrane to the water sample; hence, in spite of excellent selectivity towards fluoride, electrodes based on Al(III)-[PFP] doped membranes are not analytically useful because of their continuous loss in slope, response range and selectivity over just a few days of operation.
To overcome the limitations related to the short lifetime of the Al[PFP]-based fluoride electrodes, and given the need to prevent dimer-monomer type ionophore equilibria in ISE membranes, alternate Zr(IV)- or Al(III)-porphyrin derivatives with enhanced lipophilicity as well as steric hindrance have now been prepared, Herein, we report results for membrane electrodes employing Zr(IV)- and Al(III) complexes of tetra(4-t-butyl)- and tetra-(2,6-dichloro)- derivatives of TPP as potential fluoride-selective ionophores. The influence of plasticizer and nature and amount of ionic site additives on the observed potentiometric responses, selectivity and detection limits for fluoride are discussed. Further, to better understand of anion-ionophore interactions that give rise to fluoride selectivity, the stability constants of the various Zr(IV) and Al(III) derivatives with fluoride and other anions are determined using the sandwich membrane method. It will be shown that these new fluoride selective ionophores provide electrodes with nernstian behavior, no evidence of dimermonomer chemistry within the organic membrane phase, and exhibit reasonably high potentiometric selectivity for fluoride ion.
2. Experimental
2.1. Reagents and chemicals
5,10,15,20-Tetrakis(4-t-butylphenyl)porphyrin [t-BTPP] and 5,10,15,20-tetrakis(2,6-dichlorophenyl)porphyrin (dClTPP) were purchased from Frontier Scientific (Logan, UT, USA). Zirconium(IV) complexes of these porphyrins were obtained by metallation of these compounds with ZrCl4 in benzonitrile (190 °C) for 24 h [19]. Aluminum(III) complexes of these porphyrins were prepared by metallation with diethylaluminum chloride in methylene chloride for 6 h at ambient temperature. Each of the final ionophore compounds were purified using silica gel column chromatography. The yields were 87%, 57%, 83% and 65% for Al[t-BTPP], Zr[t-BTPP], Al[dClTPP] and Zr[dClTPP], respectively. Structures of these metalloporphyrin ionophores are shown in the Figure 1.
Figure 1.
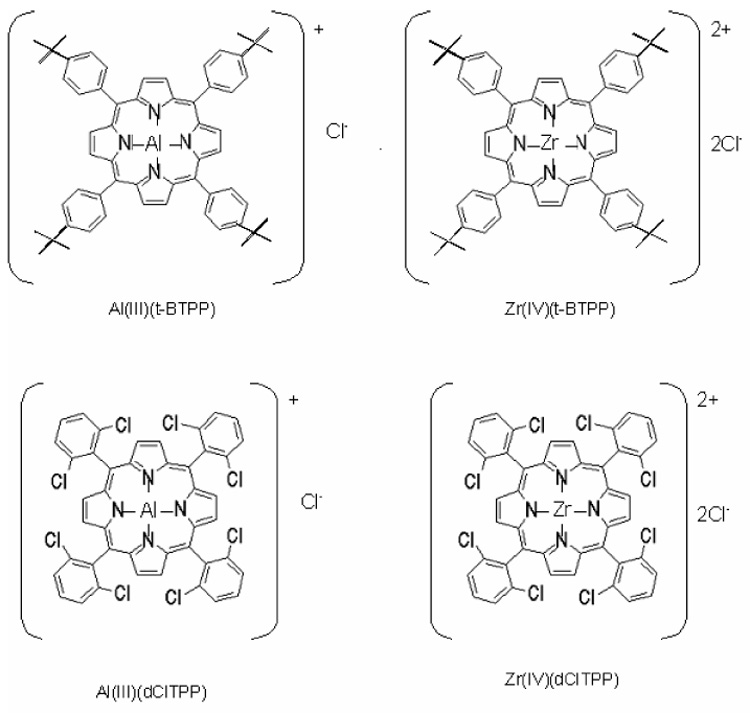
Structures of the metalloporphyrin ionophores examined for preparation of fluoride selective membrane electrodes.
For preparation of polymeric ion-selective membranes, poly(vinyl chloride) (PVC), o-nitrophenyloctyl ether (o-NPOE), dioctylsebacate (DOS), potassium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (KTFPB), tridodecylmethylammonium chloride (TDMACl) and tetrahydrofuran (THF) were used as received from Fluka (Ronkonkoma, NY). Buffer species, including 4-morpholinoethanesulfonic acid (MES), glycine (Gly) and inorganic salts, as well as diethylaluminum chloride (1 M in hexane) were also obtained from Fluka. Solvents for syntheses carried out under an inert atmosphere were purified using standard procedures.
2.2. ISE Membrane Formulations and EMF measurements
The ion-selective membranes contained ionophore and lipophilic anionic or cationic additives (various quantities; see Table 1) in a PVC/plasticizer (1:2) polymeric matrix. All components were dissolved in 2 mL of THF and the mixture was then cast in a 24 mm i.d. glass ring on a glass slide. The solvent was allowed to evaporate overnight. Discs (5 mm o.d.) were then cut out from the parent membrane and mounted into Philips electrode bodies (ISE — 561) (Glasblaserei, W. Moller AG, Zurich, Switzerland). Electrochemical cell potentials were measured with the following galvanic cell:
Ag|AgCl(s)|KCl (3M)∥sample solution|ion-selective membrane|inner filling solution|AgCl(s)|Ag.
Table 1.
Membrane compositions and potentiometric response characteristics of fluoride-selective electrodes using the new Al(III) and Zr(IV) porphyrin complexes as ionophores. Membrane composition: ionophore (1 wt%), PVC/plasticizer (1:2) and lipophilic ionic additives (B = KTFPB, T = TDMACl)
| ID | Ionophore | Plasticizer | Additives amount / type [mol%] | Slope [mV decade−1] | Detection limit [M] |
|---|---|---|---|---|---|
| 1 | Al[t-BTPP] | o-NPOE | 10 / B | −59.2 ± 0.3 | 4·10−5 |
| 2 | 25 / B | −57.1 ± 0.2 | 1·10−4 | ||
| 3 | 50 / B | −55.2 ± 0.3 | 3·10−4 | ||
| 4 | 10 / T | −57.7 ± 0.2 | 2·10−4 | ||
| 5 | DOS | 10 / B | −59.0 ± 0.4 | 9·10−5 | |
| 6 | 25 / B | −57.7 ± 0.2 | 2·10−4 | ||
| 7 | 50 / B | −55,6 ± 0.3 | 4·10−4 | ||
| 8 | 10 / T | −59.1 ± 0.2 | 1·10−4 | ||
| 9 | Zr[t-BTPP] | o-NPOE | 10 / B | −58.1 ± 0.2 | 8·10−5 |
| 10 | 25 / B | −57.8 ± 0.3 | 1·10−4 | ||
| 11 | 50 / B | −57.5 ± 0.3 | 3·10−4 | ||
| 12 | 10 / T | −60.0 ± 0.3 | 6·10−5 | ||
| 13 | DOS | 10 / T | −60.2 ± 0.2 | 2·10−4 | |
| 14 | Al[dClTPP] | o-NPOE | 10 / B | −59.7 ± 0.3 | 1·10−4 |
| 15 | 25 / B | −56.2 ± 0.1 | 2·10−4 | ||
| 16 | 50 / B | −51.3 ± 0.3 | 3·10−4 | ||
| 17 | 10 / T | −58.2 ± 0.3 | 2·10−4 | ||
| 18 | DOS | 10 / B | −54,9 ± 0.4 | 2·10−4 | |
| 19 | 25 / B | −56.5 ± 0.3 | 3·10−4 | ||
| 20 | 50 / B | −51.2 ± 0.4 | 6·10−4 | ||
| 21 | 10 / T | −57.9 ± 0.3 | 2·10−4 | ||
| 22 | Zr[dClTPP] | o-NPOE | 10 / B | −60.1 ± 0.4 | 3·10−4 |
| 23 | 25 / B | −55.8 ± 0.2 | 1·10−3 | ||
| 24 | 50 / B | −52.2 ± 0.3 | 4·10−3 | ||
| 25 | 10 / B | −57.2 ± 0.4 | 5·10−4 | ||
| 26 | DOS | 10 / B | −56.8 ± 0.3 | 5·10−4 | |
| 27 | 25 / B | −55.4 ± 0.2 | 6·10−4 | ||
| 28 | 50 / B | −52.6 ± 0.3 | 2·10−3 | ||
| 29 | 10 / T | −58.6 ± 0.3 | 1·10−3 |
Two compositions of internal filling solutions were employed: Gly/H3PO4/NaCl buffer (pH = 3.0) or MES/NaOH buffer (pH = 5.5). The Gly/H3PO4/NaCl buffer with the addition of NaF (0.001 M) or the MES/NaOH buffer with added NaF (0.001 M) served as conditioning solutions for all electrodes examined with the respective internal solutions. When not in use, the electrodes were stored in the conditioning solution in the dark. All EMF values were measured at ambient temperature via PC computer coupled to a 16-channel potentiometric workstation (Lawson Labs. Inc.). The performance of the electrodes was examined by measuring the cell EMF for aqueous solutions of given anions over the concentration range of 10−7−10−1 mol·dm−3. For the preparation of calibration curves, the concentration of total fluoride ion species was used. Selectivity coefficients were calculated by the separate solution method (SSM) using the theoretical slope (−59.2 mV/decade) [20].
2.3. Determination of anion-metalloporphyrin formal stability constants
The measurement set-up for determining stability constants was the same as that described above for ISE measurements, except that two sandwiched membranes with different compositions were mounted on the Philips electrode body, as described in detail in [6]. For this purpose, two plasticized PVC membranes (the first one based on TDMACl (5 mmol·kg−1) only and the second one containing the given metalloporphyrin ionophore (10 mmol·kg−1) and KTFPB (5 mmol·kg−1) were prepared. A series of 5 mm i.d. membrane discs were then cut from the parent membranes and these discs were conditioned over 2 d in 10−2 mol·dm−3 solution of appropriate salt (NaF, NaCl, NaNO3, NaSCN, NaClO4, NaBr), prepared in Gly/H3PO4 buffer (pH = 3).
To determine the stability constants for a given ionophore and a given anion, two cell EMF measurements, one for a membrane without ionophore and then for the two sandwiched membranes were carried out. The sandwiched membrane was made after drying of individual membranes, by attaching of the membrane with ionophore to the ionophore-free membrane. The segmented membrane was then mounted into a Philips electrode body (membrane with ionophore facing the sample solution) and immediately immersed into an appropriate salt solution (identical as that used for conditioning of the membrane). The potential was recorded as the mean EMF value over the last minute of a 10 min measurement period. The change in membrane potential values (EM) were calculated by subtracting the cell potential measured for a membrane without ionophore from the potential when the sandwiched membrane configuration was used. The formation constants were calculated according to Eq. 1 [6].
| (1) |
where: R+T is the concentration of lipophilic anion-exchanger in the ionophore-free segment, R−T is the concentration of lipophilic cation-exchanger in the segment containing ionophore (LT — concentration of ionophore).
3. Results and discussion
The chemical structures of the four newly synthesized compounds: Al[t-bTPP], Zr[t-bTPP], Al[dClTPP] and Al[dClTPP] examined as potential fluoride selective ionophores are shown in Figure 1. A number of different membrane formulations for each of the different ionophores were prepared (see Table 1) and examined for their response to fluoride and potential interfering anions (e.g., ClO4−, SCN−, NO3−, Br−, Cl−), pH influence, working response mechanism and lifetime.
As shown in Table 1, all four ionophores yield membrane electrodes that exhibit nernstian or near-nernstian response toward fluoride, regardless of plasticizer (o-NPOE or DOS) or whether anionic borate derivatives (B) or cationic quaternary ammonium sites (T) are added to the membrane cocktail. Figure 2 shows the EMF response to fluoride ion and potential interfering ions in Gly/H3PO4/NaCl buffer, pH 3.0, for membrane electrodes formulated with the three most promising formulations in terms of selectivity and detection limits (ID # 1, 2 and 10; see Table 1). Detailed discussions of membrane optimization, selectivity, response times, etc. for the various membranes examined are provided below.
Figure 2.
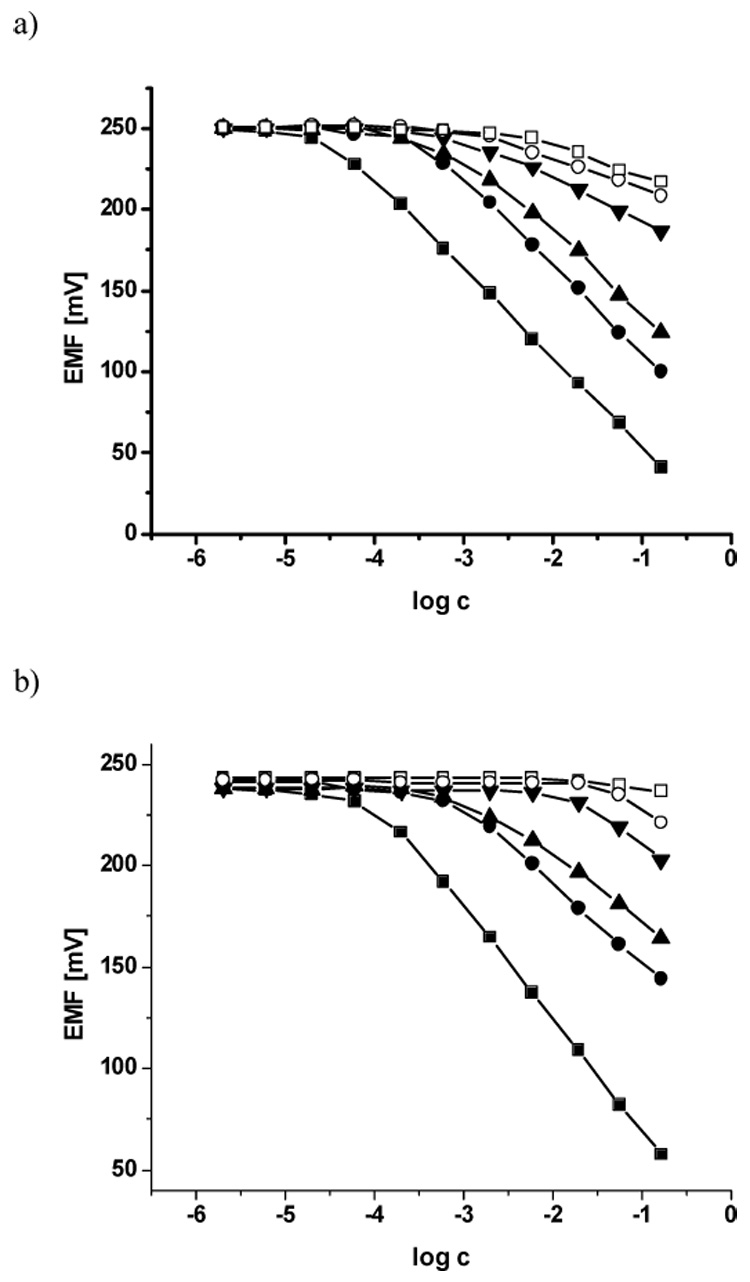
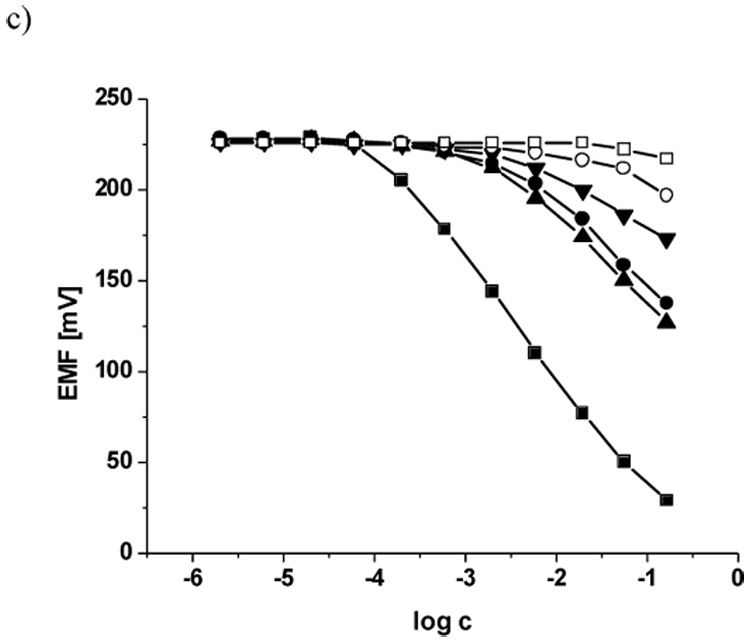
Typical calibration curves obtained for electrodes 1 (a), 2 (b) and 10 (c) in response to F− (■), SCN− (●), ClO4− (▲), NO3− (▼), Br−(○), Cl− (□) added to Gly/H3PO4 buffer pH 3.0.
3.1. Determination of working mechanism for ionophores tested
Knowledge of the ionophore working mechanism is a very important factor when optimizing membrane composition [21]. Anion-selective membranes doped with ionophores that function as charged carriers requires lipophilic anionic site additives (B, in Table 1), while cationic additives (T, in Table 1) are necessary in the case of ionophores that function as neutral carriers. It has been reported that Zr(IV)-porphyrins and other metalloporphyrins are able to function as both charged and neutral carriers [22–25]. To determine whether this so called “mixed-mode” mechanism also exists in the case of new ionophores reported hererin, a series of membranes containing 10 mol% of cationic or anionic additives (relative to ionophore) were prepared (see Table 1). Since the polarity of the plasticizer used for polymeric membrane preparation has also been shown to affect the working response mechanism, two plasticizers, o-NPOE (ε = 24 [26] and) DOS (ε = 4.6 [26]) were studied.
As shown in the selectivity data illustrated in Figures 3a–c, it can be seen that each of the metalloporphyrin ionophores tested exhibit strong ionophore properties. Indeed, for all electrodes examined (IDs # 1–29), the selectivity varied considerably from the typical Hofmeister selectivity pattern observed when conventional dissociated ion-exchangers are employed within polymeric membranes (e.g., ClO4−>SCN−>NO3−>Br−>Cl−>F− [27]). However, for electrodes fabricated with the membranes containing anionic additives the differences in the selectivity order (F−>ClO4−~SCN−>NO3−>Cl−>Br−) are much more pronounced than for those with membranes possessing cationic sites (T) (i.e., ClO4−>SCN−>F−>NO3−>Cl−>Br−). Indeed, the presence of cationic additives yields membranes that exhibit strong interference (log KF,Y > 0) for very lipophilic anions such as perchlorate and thiocyanate.
Figure 3.
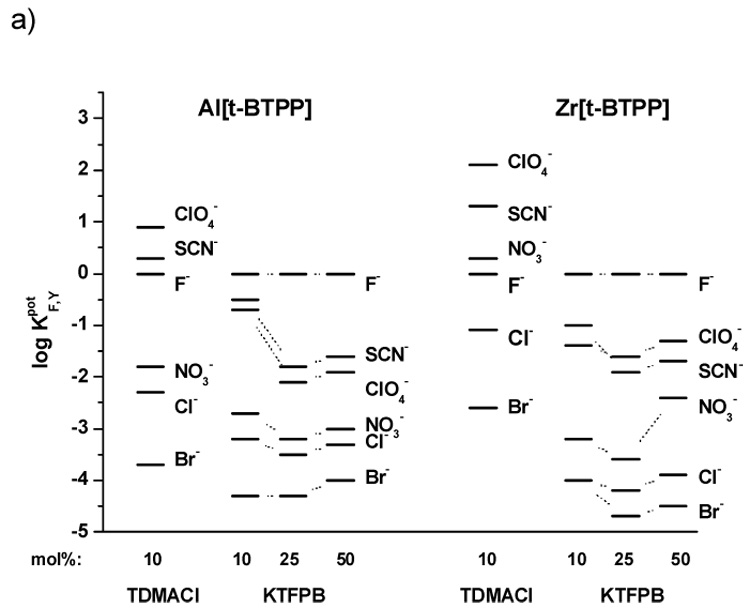
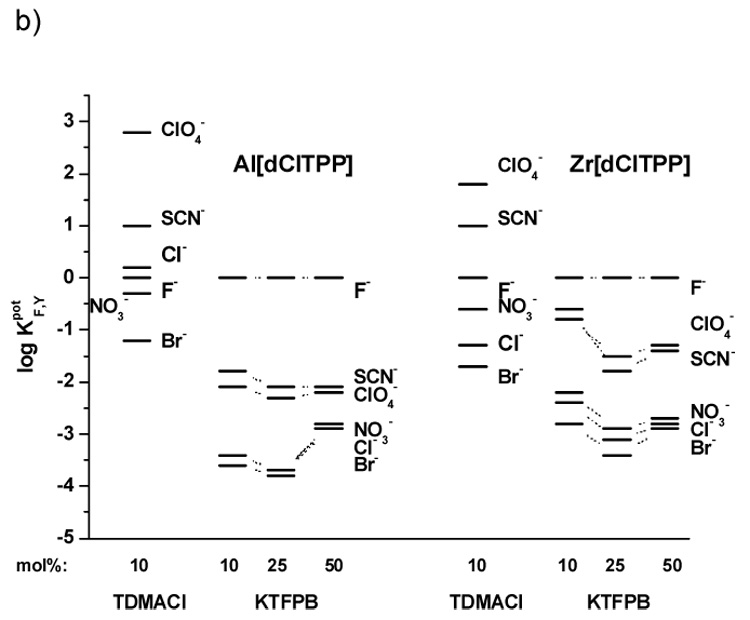
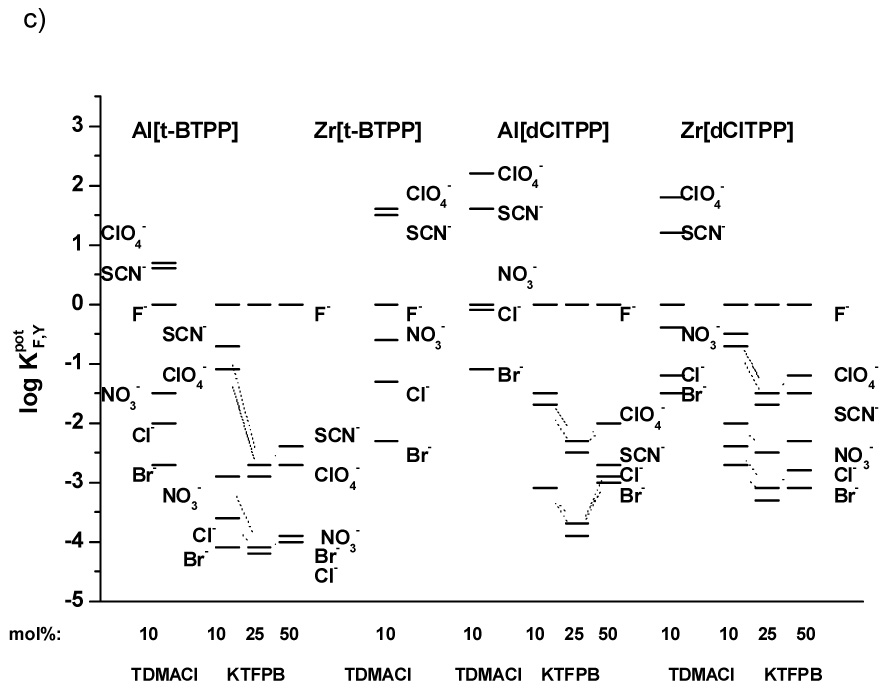
Potentiometric selectivity coefficients of electrodes based on: a) PVC/o-NPOE membranes with Al[t-BTPP] or Zr[t-BTPP]; b) PVC/o-NPOE membranes with Al[dClTPP] or Zr[dClTPP]; c) PVC/DOS membranes with Al[t-BTPP], Zr[t-BTPP], Al[dClTPP] or Zr[dClTPP]. Measurements carried out in pH 3.0 buffer.
These results suggest the strong affinity of fluoride to the Zr(IV)- and Al(III)-metal ion centers of the metalloporphyrin as well as the fact that a “mixed mode” mechanism applies to each of the ionophores tested. Moreover, these compounds can function as neutral or charged carriers regardless of the plasticizer employed to prepare the membrane. Given the higher selectivity of the membranes for fluoride when lipophilic anionic sites are added (e.g., electrodes 1, 9, 14, 22), it seems that operation of electrodes in the charged carrier response mode (with added borate derivative) is preferred to make analytically useful devices.
The influence of the plasticizer on the selectivity of the membrane electrodes can also be gleaned from data provided in Figure 3c (DOS) vs. that shown in Figure 3a, b (o-NPOE). For electrodes with membranes based on [dClTPP] derivatives and a more polar plasticizer environment (o-NPOE), better selectivity towards fluoride over interfering anions is obtained compared to membranes with less polar plasticizer (DOS). Interestingly, electrodes with membranes containing ionophores Al[t-BTPP] and Zr[t-BTPP] behave inversely, with better fluoride selectivity exhibited by membranes prepared with DOS as the plasticizer. However, in terms of lower detection limits (LDL), o-NPOE proved to be the optimal plasticizer for all the ionophore structures examined (see Table 1).
Since incorporation of lipophilic anion sites yields electrodes with better selectivity for fluoride, additional membranes were formulated with varying amounts of KTFPB (see Table 1). The optimum content of anionic additives in terms of LDL values is 10 mol% of KTFPB. Higher amounts of such sites shift the LDL to a higher concentration of flouride. For example, In the case of electrodes 1, 2, 3 prepared with the Al[t-BTPP] ionophore, the LDL is changed from 4·10−5 M for 10 mol% of KTFPB to 1·10−4 M for 25 mol% and 3·10−4 M for 50 mol% of additive. Some differences in the slopes of the electrodes when changing the amount of additive sites are also observed. With higher amoumts of KTFPB in the membrane phase, lower slopes are typical (for electrode 1 from −59.2 through −57.1 to −55.2 mV decade−1 for 10, 25 and 50 mol% of KTFPB, respectively). A similar trend is seen for the other electrodes tested (see Table 1).
The amount of lipophilic anionic sites in the membrane also influences the selectivity of the electrodes based on each of the metalloporphyrin ionophores examined. The optimal selectivity is observed using membranes possessing 25 mol% of additive (relative to ionophore) (see Fig. 3a, b and c). A lower concentration of KTFPB (e.g., 10 mol%) in the membranes results in decreasing selectivity for fluoride over all interfering anions. Incorporation of 50 mol% of sites increases the LDLs (shortening of the fluoride response linear range) as well as decreases membrane electrode selectivity.
Unfortunately, electrodes with optimized fluoride selectivity did not have the best detection limits. Some of the electrodes have better selectivity (e.g., electrodes 2, 4; see Fig. 3a and Table 1) while the others exhibit better LDLs (e.g., electrodes 1, 5; see Fig. 3a and Table 1). Among all electrode membrane formulations tested, the best selectivity for fluoride is observed for the electrode 6 (Al[t-BTPP] with DOS plasticizer and 25 mol% KFTPB).
3.2. The influence of pH on electrodes parameters
It is well known that most membrane electrodes based on metalloporhyrin ionophores have significant response to hydroxide ions [28,29]. Hydroxide has a great affinity to the metal ion center of examined metalloporphyrins (logKF-,OH-= 6.6, 6.9, 7.0 and 7.5 for electrodes 1, 9, 14 and 22 respectively) replacing the counterion of metalloporphyrin when in contact with water [30]. It can also block the interaction between the ionophore and the target anion. Thus, the effect of sample solution pH on the fluoride response characteristics of electrodes prepared with membranes containing each of the ionophores was investigated in detail. For this purpose, a glycine/H3PO4 buffer solution, pH 3.0, and a MES/NaOH buffer solution, pH 5.5, were examined as the background electrolyte solutions for potentiometric fluoride measurements.
For all membrane formulations examined, changing the sample pH from 3.0 to 5.5 results in a shortening of the electrode linear response range. For example, for the electrodes based on PVC/o-NPOE membranes formulated with Al[t-BTPP] as the ionophore along with 10 mol% of KTFPB, the LDL shifts significantly from 5·10−5 to 3·10−3 M. The interaction between the ionophore and OH− ion decreases the initial baseline of the calibration curve by approx. 75 mV. In the case of the electrode with the PVC/o-NPOE membrane doped with Zr[t-BTPP] and 10 mol% of KTFPB (electrode 9), the LDL shifts from 8·10−5 to 3·10−3 M. Similar behavior is observed for the electrodes based on Al- or Zr[dClTPP]. Generally, it was found that a change of pH from 3.0 to 5.5 increases the LDL by about two orders of magnitude. Therefore, the glycine/H3PO4 buffer solution, pH 3.0, is the preferred electrolyte for using these new fluoride selective electrodes. It should be noted that at this pH, a significant fraction of the fluoride ions are protonated ([A−]/[HA] = 0.72) and this influences the LDL observed, since free unprotonated fluoride is the species that is sensed by the membranes. Nonetheless, as with other metalloporphyrin-based electrochemical and optical sensors for fluoride reported previously [9,14,16,17], the tradeoff of using such a low pH for practical measurements of total fluoride concentration more than outweighs the greater loss in LDL owing to hydroxide interference that occurs when the pH of the test solution is raised to a higher value.
3.3. Response time and reversibility
Two other crucial parameters when evaluating the utility polymeric membrane ISEs are their response time and reversibility. Many of the previously reported fluoride-selective electrodes based on metalloporphyrins do not exhibit fast and reversible EMF response due to dimerization or aggregation equilibria of metalloporphyrins within the organic membrane phase [9,14]. To prevent dimer formation, sterically hindered metalloporphyrins have been suggested [14,18]. To date, only one such structure (Al(III) in picket fence porphyrin; Al[PFP]) has been examined for preparing fluoride selective electrodes, and it was shown that such a metalloporpyrin does not undergo dimer-monomer reactions in the membrane. In this work, two additional sterically hindered porphyrin structures ([dClTPP] and [t-BTPP]) are introduced, with the hope that dimer-monomer equilibria could also be eliminated. It should also be noted that these new Zr(IV)- and Al(III)- tetraphenylporphyrin derivatives are much easier and less time consuming to synthesize than the previous PFP-based complexes. The IUPAC preferred method to report response times is via a defined rate of potential change (ΔE/Δt). Accordingly, the response time was taken as the point when the differential quotient (ΔE/Δt) of the potential—time curve became smaller than a value (ΔE/Δt < 0.4). The measurements were carried out by adding the appropriate amount of fluoride to achieve changes in log aF ≤ 0.5 unit. The dynamic responses of representative electrodes with membranes containing Zr[t-BTPP] (membrane 9) and Al-[t-BTPP] (membrane 1) are presented in Figure 4. Indeed, the response times of the electrodes are relatively rapid (compared to other fluoride-selective polymer-based electrodes) and fully reversible. The response times are typically in the range range of 60–80 s (6.3·10−4 – 7·10−1 M) for the electrodes doped with anionic additives (KTFPB) and about 30 s for the electrodes doped with cationic additives (TDMACl), and these results are comparable to those reported earlier for Al(III)PFP as the fluoride ionophore [14].
Figure 4.
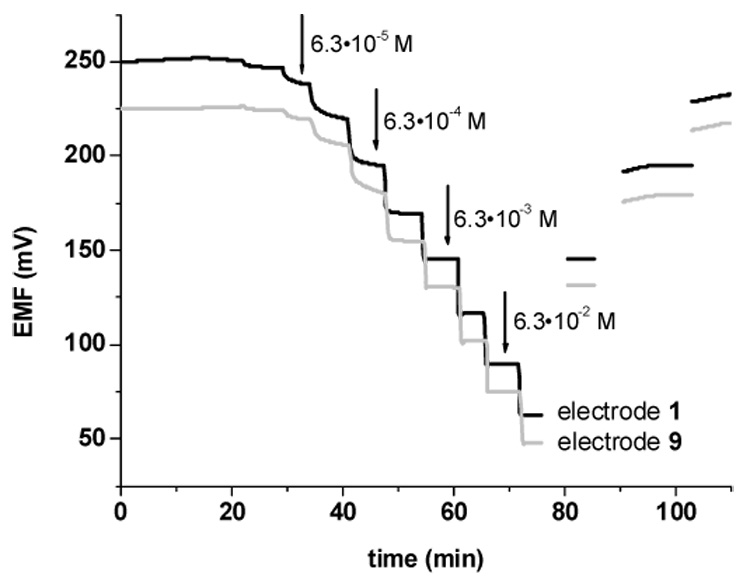
Dynamic fluoride EMF response and reversibility of electrodes with PVC/o-NPOE membranes containing ionophore Al[t-BTPP] (electrode 1) or Zr[t-BTPP] (electrode 9) and 10 mol% of KTFPB. Measurements carried out in pH 3.0 buffer.
The response properties observed for all electrodes (slopes of the calibration curves and dynamic response) suggest that there is no dimerization of the four different metalloporphyrin ionophores within the polymeric membrane phases of the electrodes. Further, UV-Vis absorption spectra of films of the same compositions as the sensing membranes were also recorded. Only one absorption peak corresponding to the expected soret band of metallopoprhyrins is observed, independent of the bathing fluoride concentration (data not shown). This supports the notion that dimerization or some other aggregation chemistry of the new metalloporphyrin ionophores does not take place in the films, and does not change as a function of fluoride concentration in the sample.
3.4. Lifetime of electrodes
The lifetime of polymeric membrane ion-selective electrodes is limited by leaching of the ionophore or additives from the organic membrane to the aqueous phase or by decomposition of membrane active components. As mentioned earlier, one of the goals of this work was to use metalloporphyrin structures that would not only prevent metalloporphyrin dimerization but would also increase lipophilicity via attachment of t-butyl or chlorine moieities to the phenyl rings of the tetraphenylporphyrin structures.
The various membrane electrodes were examined over a seven month period, in terms of selectivity coefficients, slopes and LDL values. The results for seven electrodes containing 10 mol% of KTFPB (membrane #s 1, 5, 9, 14, 18, 22, 26) are presented in the Table 2 and Figure 5. Incorporation of the t-butyl-substituents in the para position of TPP (see Fig. 1) significantly improves the lifetimes of electrodes 1 and 5. The slopes for the electrodes with the membranes doped with Zr[t-BTPP] and Al[t-BTPP] are more stable over time compared those of electrodes with membranes containing ionophores Al[dClTPP] and Zr[dClTPP], as well as previously reported fluoride selective ISEs using Zr(IV)- and Al(III)-[TPP] complexes [9,14]. For the electrodes with Zr[tBTPP], the slopes were acceptable for at least 30 d, with a decrease of only 2.7 mV/decade over this period. After this time, the slope changes at a higher rate. In contrast, electrodes 1 and 5 prepared with Al[t-BTPP] do not exhibit a measurable slope change over the entire seven month period. Further, the selectivity coefficients (Figure 5) and LDL for these electrodes are also maintained over this extended time period.
Table 2.
The changes in the examined electrodes fluoride response slopes in time (mV decade−1).
| ID | 1 | 5 | 9 | 14 | 18 | 22 | 26 |
|---|---|---|---|---|---|---|---|
| DAYS | |||||||
| 1 | −59.7 | −59.1 | −58.1 | −59.7 | −58.2 | −60.1 | −57.2 |
| 3 | −59.6 | −59.1 | −58 | −58.2 | −57.4 | −58.7 | −56.2 |
| 10 | −59.0 | −58.7 | −57.8 | −52.2 | −53.8 | −52.8 | −50.1 |
| 30 | −59.1 | −58.4 | −55.4 | −32.1 | −30.1 | −24.8 | −25.6 |
| 72 | −58.9 | −57.8 | −48.9 | n.m. | n.m. | n.m. | n.m. |
| 150 | −58.7 | −56.7 | −34.2 | n.m. | n.m. | n.m. | n.m. |
| 199 | −58.1 | −57.1 | −27.9 | n.m. | n.m. | n.m. | n.m. |
Figure 5.
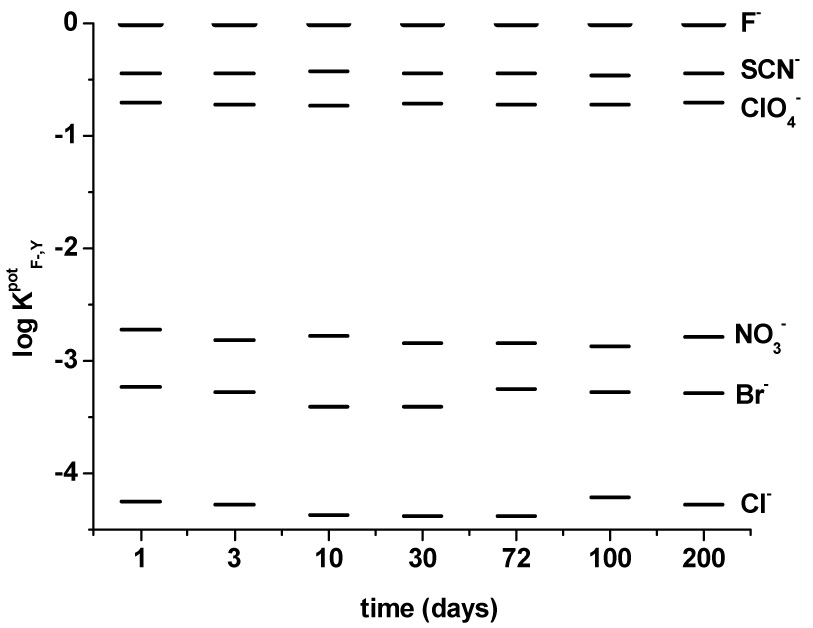
Changes in the selectivity coefficients for electrode with PVC/o-NPOE membrane doped with Al[t-BTPP] and 10 mol% of KTFPB (electrode 1) as a function of time.
The fluoride response slopes for electrodes 14, 18, 22, 26 with the membranes doped with the Al[dClTPP] and Zr[dClTPP] ionophore decrease by half within 30 d. Given the increased lipophilicity of these ionophores compared to the previously reported Al[TPP] and Zr[TPP] species [9,14], the lifetimes of these electrodes are surprisingly short. Further investigation of membranes with the M[dClTPP] derivatives revealed that there is no leaching of these ionophores; however, the fast decreasie of the observed slope values can be attributed to the rapid crystalization of the ionophore within the organic membrane phase. This phenomenon was observed no matter which plasticizer is used (see Figure 6). Indeed, even after only two days, growing crystals of these metalloporphyrins can be observed within the dry membranes as well as for those that remain in the contact with the aqueous phase. Hence, the uselife for of electrodes made with all membranes formulated with the [dClTPP] derivatives is unacceptably short owing to this insolubility of these ionophores in the organic polymer phase.
Figure 6.
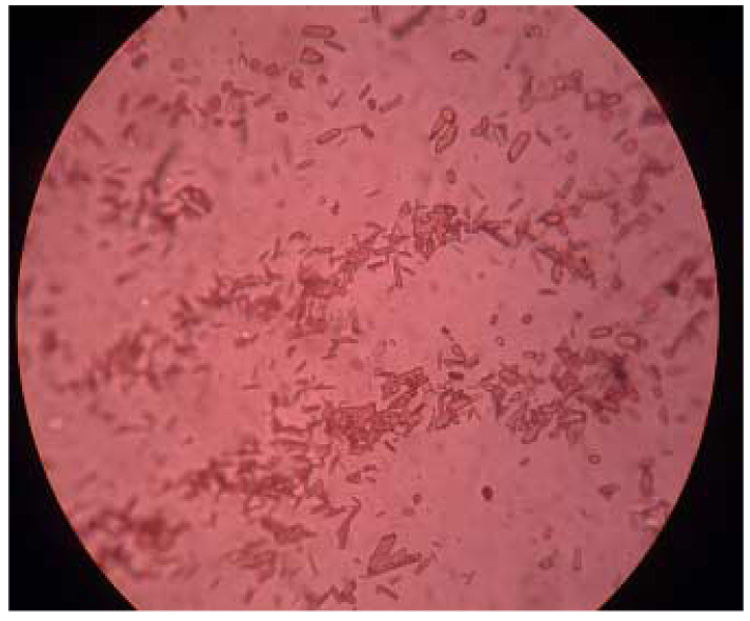
A picture showing crystallized Al[dClTPP] within a PVC/o-NPOE membrane.
3.5. Stability constants
It is well established that the formation constants of ion-ionophore complexes in the organic membrane phase are the critical parameter that influence the observed selectivity of polymeric membrane ISEs [23]. Most classical methods useful for determining ion-ionophore complex stability constants (e.g., NMR, etc.) suffer from the drawback that they are typically carried out in phases that are not identical to the membrane phase if the ISEs. In contrast, the segmented sandwich method proposed by Bakker and coworkers [6] enables the determination of ion-ionophore stability constants within the same PVC/plasticizer matrix employed to fabricate the ISE.
The values of the formation constants (β) for various anions with the four different ionophores in both DOS and o-NPOE plasticized membranes are presented in the Figure 7. Since earlier Al(III)- and Zr(IV)-porphyrin complexes used as ionophores in membrane electrodes exhibited enhanced fluoride selectivity [9,14], and fluoride is also preferred in all electrodes tested in this study, it is not surprising that each of the ionophores tested form their strongest complexes with fluoride. However, the values measured are somewhat greater than expected. Indeed, given such strong complexation with fluoride, it is surprising that the EMF responses of the new fluoride electrodes are fully reversible (as shown in Fig. 4).
Figure 7.
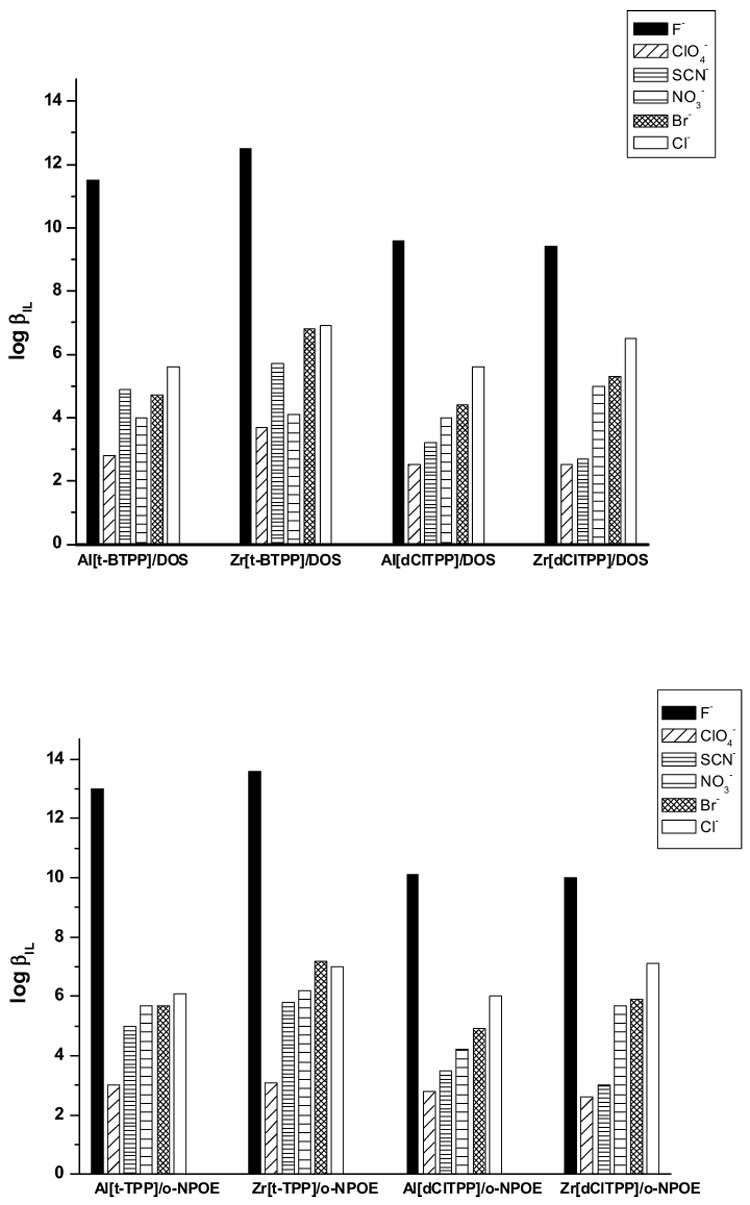
Formal complex formation constants, logβIL, obtained with the examined ionophores using the segmented sandwich method. Standard deviation < 0.3 (based on at least three replicate measurements).
It is known that the positive charge density of the metal ion center of metalloporphyrins can be increased by introducing electrophilic groups within the structure of the surrounding porphyrin ligand [21,31]. Thus, Al[dClTPP] and Zr[dClTPP] with Cl—substituents should interact stronger with all anions than Al[t-BTPP] and Zr[t-BTPP], respectively. However, the values obtained are opposite this expected trend. Indeed, for all anions, the logβ values are higher for the t-butyl— substituted metalloporphyrins than for dichloro— substituted ones. As mentioned above, the Al[dClTPP] and Zr[dClTPP] ionophores were not stable in the membrane phase and tend to crystallize, so the actual soluble concentration of these ligands were likely lower than the mass of compound dissolved in the original cocktail used to cast the membranes. This fact could result in the lower values of the stability constants measured for these ionophores.
When comparing the anion complexation constants of Al[t-BTPP] to Zr[t-BTPP]) or Al[dClTPP] to Zr[dClTPP], it is clear that the stronger complexes are formed by the Zr(IV)-tetraphenylporphyrin derivatives. For the two Zr(IV)-based ionophores the strongest complexes with all anions are formed with Zr[t-BTPP], although differences in stability constants between all the ligands examined not very significant.
The effect of the plasticizer polarity on the stability constants of ion-ionophore complexes was also examined. In the more polar membrane matrix (PVC/o-NPOE), the logβ values for all four ionophores and each anion tested were found to be higher then those obtained for the less polar matrix (PVC/DOS) (see Figure 7). A similar plasticizer effect was reported previously for cation binding by cation-selective ionophores [32]. The highest differences are observed for the complexes of Al[t-BTPP], and Zr[t-BTPP], with fluoride (Δlogβ >1), while the differences in logβ for the other complexes are less pronounced (< 0.5).
4. Conlusions
Herein, it has been shown that Al(III)- and Zr(IV)- [t-BTPP] and [dClTPP] compounds can be used as ionophores to prepare polymeric membrane ion-selective electrodes that show enhanced potentiometric selectivity towards fluoride and improved life-times. These new ionophores can function via a charged carrier or neutral carrier mechanism, depending on the nature of lipophilic sites added to the membrane. Studies on the influence of sample solution pH on the performance of the electrodes indicate that the best pH for fluoride response is pH 3.0. All of the electrodes studied exhibit relatively short response times and their response to fluoride is fully reversible. Most notable is that the response characteristics for membrane electrode prepared with the Al[t-BTPP] ionophore are maintained for at least seven months. The functional lifetime of electrodes doped with compounds Al[dClTPP] and Zr[dClTPP] is limited by significant crystallization within the polymer membrane phases. The ionophore-ion stability constants determined using potentiometric sandwich method suggest that each of the metalloporphyrin ionophores examined form their strongest complexes with fluoride. Based on this work it appears that electrodes prepared with membranes doped with the Al[t-BTPP] ionophore can be the most analytically useful among the four ionophores examined owing to optimal fluoride selectivity and very stable EMF response over an extended time period.
Acknowledgement
We thank the National Institutes of Health (US) for partial support of this work (GM 028882-26).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antonisse MMG, Reinhoudt DN. Electroanalysis. 1999;11:1035. [Google Scholar]
- 2.Bakker E, Willer M, Lerchi M, Seiler K, Pretsch E. Anal. Chem. 1994;66:516. [Google Scholar]
- 3.Bakker E, Pretsch E. J. Electrochem. Soc. 1997;144:L125. [Google Scholar]
- 4.Bakker E, Pretsch E. Anal. Chem. 1998;70:295. doi: 10.1021/ac970903j. [DOI] [PubMed] [Google Scholar]
- 5.Shultz MM, Stefanova OK, Mokrov SB, Mikhelson KN. Anal. Chem. 2002;74:510. doi: 10.1021/ac015564f. [DOI] [PubMed] [Google Scholar]
- 6.Qin Y, Bakker E. Talanta. 2002;58:909. doi: 10.1016/s0039-9140(02)00405-8. [DOI] [PubMed] [Google Scholar]
- 7.Bakker E, Mi Y. Anal. Chem. 2003;71:5279. doi: 10.1021/ac9905930. [DOI] [PubMed] [Google Scholar]
- 8.Górski Ł, Malinowska E. Anal. Chim. Acta. 2005;540:159. [Google Scholar]
- 9.Malinowska E, Górski Ł, Meyerhoff ME. Anal. Chim. Acta. 2002;468:133. [Google Scholar]
- 10.Steinle ED, Schaller U, Meyerhoff ME. Anal. Sci. 1998;14:79. [Google Scholar]
- 11.Antonisse MMG, Snellink-Ruel BHM, Ion AC, Reinhoudt DN. J. Chem. Soc. Perkin Trans. 1999;2:1211. [Google Scholar]
- 12.Perdikaki P, Tsagkatakis I, Chaniotakis NA, Altmann R, Jurkschat K, Reeske G. Anal. Chim. Acta. 2002;467:197. [Google Scholar]
- 13.Mitchell-Koch JT, Malinowska E, Meyerhoff ME. Electroanalysis. 2005;17:1347. [Google Scholar]
- 14.Mitchell-Koch JT, Pietrzak M, Malinowska E, Meyerhoff ME. Electroanalysis. 2006;18:551. [Google Scholar]
- 15.Chandra S, Ruzicka A, Svec P, Lang H. Anal. Chim. Acta. 2006;577:91. doi: 10.1016/j.aca.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Badr IHA, Meyerhoff ME. J. Am. Chem. Soc. 2005;127:5318. doi: 10.1021/ja0500153. [DOI] [PubMed] [Google Scholar]
- 17.Badr IHA, Meyerhoff ME. Anal. Chem. 2005;77:6719. doi: 10.1021/ac050987t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinle ED, Amemiya S, Bühlmann P, Meyerhoff ME. Anal. Chem. 2000;72:5766. doi: 10.1021/ac000643x. [DOI] [PubMed] [Google Scholar]
- 19.Berezin BD, Łomowa TN. Zurnal Nieorganicieskoj Chimii. 1981;26:379. [Google Scholar]
- 20.Bakker E, Pretsch E, Bühlmann P. Anal. Chem. 2000;72:1127. doi: 10.1021/ac991146n. [DOI] [PubMed] [Google Scholar]
- 21.Shahrokhian S, Seifi H, Bagherzadeh M, Mousavi SR. Chem. Phys. Chem. 2004;5:652. doi: 10.1002/cphc.200301025. [DOI] [PubMed] [Google Scholar]
- 22.Shahrokhian S, Hamzehloei A, Bagherzadeh M. Anal. Chem. 2002;74:3312. doi: 10.1021/ac020099n. [DOI] [PubMed] [Google Scholar]
- 23.Bakker E, Pretsch E, Bühlmann P. Chem. Rev. 1997;97:3083. doi: 10.1021/cr940394a. [DOI] [PubMed] [Google Scholar]
- 24.Bakker E, Malinowska E, Schiller RD, Meyerhoff ME. Talanta. 1994;41:881. doi: 10.1016/0039-9140(94)e0041-o. [DOI] [PubMed] [Google Scholar]
- 25.Górski Ł, Malinowska E, Meyerhoff ME. Talanta. 2004;63:101. doi: 10.1016/j.talanta.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Eugster R, Rosatzin T, Rusterholz B, Aebersold B, Pedrazza U, Ruegg D, Schmid A, Spichiger UE, Simon W. Anal. Chim. Acta. 1994;289:1. [Google Scholar]
- 27.Hofmeister F. Arch. Exp. Pathol. Pharmakol. 1888;24:247. [Google Scholar]
- 28.Zhang W, Malinowska E, Parzuchowski P, Meyerhoff ME. Anal. Chem. 2002;74:4548. doi: 10.1021/ac0202536. [DOI] [PubMed] [Google Scholar]
- 29.Qin W, Parzuchowski P, Zhang W, Meyerhoff ME. Anal. Chem. 2003;75:332. doi: 10.1021/ac0205356. [DOI] [PubMed] [Google Scholar]
- 30.Hattori H, Hoshino M, Wakii T, Yuchi A. Anal. Chem. 2004;76:5056. doi: 10.1021/ac049592k. [DOI] [PubMed] [Google Scholar]
- 31.Fuji H. J. Am. Chem. Soc. 1993;115:4641. [Google Scholar]
- 32.Qin Y, Mi Y, Bakker E. Anal. Chim. Acta. 2000;421:207. [Google Scholar]


