Abstract
Background
U19 is a potential tumor suppressor exhibiting frequent down-regulation and allelic loss in advanced human prostate cancer specimens. U19 has also been identified as ELL-associated factor 2 (EAF2) based on its binding to ELL, a fusion partner of MLL in acute myeloid leukemia. U19/EAF2 is a putative transcription factor with a transactivation domain and capability of sequence-specific DNA binding.
Methods
Yeast-two-hybrid-screening was used to identify U19/EAF2 binding partners. Co-immunoprecipitation and mammalian 1-hybrid assay were used to characterize a U19/EAF2 binding partner.
Results
FB1, an E2A fusion partner in childhood leukemia, was identified as a binding-partner of U19/EAF2. FB1 also binds to EAF1, the only homologue of U19/EAF2. FB1 also interacts and co-localizes with ELL in the nucleus. Interestingly, FB1 inhibited the transcription activity of U19/EAF2 but not EAF1.
Conclusions
FB1 is an important binding partner and a functional regulator of U19/EAF2, EAF1, and/or ELL.
Keywords: FB1, U19, EAF1, EAF2, ELL
Introduction
U19 is one of the novel androgen-responsive genes isolated using a PCR-based subtractive hybridization (1). Further characterization of U19 indicated that U19 is an apoptosis inducer with tumor-suppressive activity in prostate cancer (2). Overexpression of U19 in prostate cancer cell lines induced apoptosis. The expression of U19 is down-regulated in prostate cancer specimens. Allelic loss and promoter hypermethylation of U19 were detected in prostate cancer specimens, indicating that U19 could be inactivated genetically and/or epigenetically. Therefore, U19 has the potential to function as a tumor suppressor in the prostate (2).
Recently, U19 has been independently identified as EAF2, ELL associated factor 2 (3). ELL was first identified as a fusion partner gene of MLL (myeloid/lymphoid or mixed-lineage leukemia gene) in the (11;19)(q23;p13.1) translocation, a recurring chromosomal aberration in acute myeloid leukemia (4). ELL has subsequently been shown to function as an RNA polymerase II (Pol II) transcriptional elongation factor (5). EAF2, along with its homolog ELL-associated factor 1 (EAF1), was identified as an ELL-interacting protein from a yeast-two-hybrid screen (3). EAF2 is highly homologous to EAF1, with 58% identity and 74% amino acid conservation (3,6). EAF2 contains a transactivation domain in its C-terminal region. EAF2 and ELL co-localizes in a nuclear speckled pattern (3). A recently study of EAF2 expression during embryo development shows that EAF2 expression correlated with a differentiated status in the developing lens, suggesting that EAF2 might regulate lens maturation (7). Another study also cloned EAF2 as FESTA, a novel protein that interacts with the transcription elongation factor S-II (8). Thus, EAF2 has been shown to interact with two different elongation factors, ELL and S-II, suggesting that EAF2 might participate in the regulation of transcriptional elongation. In fact, a recent study showed that both EAF1 and EAF2 can enhance the transcriptional elongation activity of ELL (9). Identification of ELL and S-II as the binding partners of U19/Eaf2 suggests that one of the important functions of U19/EAF2 is to regulate transcription elongation. Identification and characterization of additional U19/EAF2-binding partners may provide further insights into the mechanisms of U19/EAF2 action.
The human FB1 was identified by virtue of its fusion with E2A in childhood leukemia (10). E2A is required for the development of committed B-lineage cells and regulates the expression of essential B-lineage genes at multiple stages of differentiation and activation (11). The exact functions of FB1 or FB1-E2A fusion in childhood leukemia development have not been determined yet (10). The rat FB1, named as Amida, was independently identified by virtue of its interaction with src, a neuro-specific immediate early gene (12). Amida contains novel nuclear localization signals and causes cell death and cell cycle arrest in cultured cells (12,13). Amida is predominantly expressed and developmentally regulated in rat testis (14). A recent study showed that recruitment of Amida by Par-4 (prostate apoptosis response-4) to the actin cytoskeleton leads to apoptosis, indicating the importance of Amida in Par-4 triggered apoptosis (15).
In this study, we report that FB1 interacts with U19/EAF2 and its homolog EAF1. FB1 also binds to and co-localizes with ELL in nuclear speckled patterns. Furthermore, we present evidence that FB1 inhibits the basal and ELL-stimulated transcriptional activity of U19/EAF2, but not EAF1. These observations suggest that FB1 is an important binding partner capable of modulating the functions of U19/EAF2 and ELL.
Materials and methods
Materials
The membranes for Western blot were from Schleicher & Schuell BioScience, Inc. (Keene, NH). X-ray films were from the Kodak Co. (Rochester, NY). COS-7 and HeLa cell line were purchased from ATCC (Rockville, MD) and were routinely maintained in DMEM (Dulbecco's Modified Eagle's Medium) with 10% FBS, 1% glutamine, 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) at 37 °C in a humidified atmosphere containing 5% CO2.
Vector construction
The full-length U19/EAF2 cDNA (2) was cloned into pEGFP N3 (Clontech, Palo Alto, CA) using primers 5′-GATATCAGATCTCGTCGACGCGCCACCATGAATAGCGCAGCGGGATTC-3′ and 5′-GATATCGGATCCTTGGTACCAAGCTTGTCATCACTGTCACTTCCTGA-3′ and restriction enzyme Bgl II and Kpn I to generate GFP tagged U19/EAF2. The full-length U19/EAF2 cDNA was also cloned into pLexA (Clontech) to generate pLexA-U19/EAF2. The cDNA sequence of the U19 exon III was cloned by PCR using primers 5′-GATATCAGATCTCGTCGACGCGCCACCATGGGTTCAACTCCACCAGTAACT-3′ and 5′-GATATCGGTACCAAGCTTTCTTGTTTTTTTTACAGT-3′, and then cloned into pEGFP-N3 using Bgl II and Kpn I to generate GFP tagged U19(III). The U19 exon III was also cloned into pLexA to generate pLexA-U19(III) (LexA DNA binding domain (BD)-U19(III) fusion protein) used as a bait for the yeast-two-hybrid screening. The N-terminal U19 (including exons I, II, and III) was cloned by PCR using primers 5′-GATATCAGATCTCGTCGACGCGCCACCATGAATAGCGCAGCGGGATTC-3′ and 5′-GATATCGGTACCAAGCTTTCTTGTTTTTTTTACAGT-3′, and then cloned into pEGFP-N3 using Bgl II and Kpn I. The C-terminal U19 (including exons IV, V, VI) was cloned by PCR using primers 5′-GATATCAGATCTCGTCGACGCGCCACCATGGTTGAAGGAAGCAGTAAAATT-3′ and 5′-GATATCGGATCCTTGGTACCAAGCTTGTCATCACTGTCACTTCCTGA-3′, and then cloned into pEGFP N3 using Bgl II and Kpn I. The full-length human EAF1 cDNA was cloned into pEGFP N3 by PCR using primers 5′-GAATTCAGATCTGCCACCATGAATGGGACCGCAAACCCGCTG-3′ and 5′-AAGCTTGGTACCGTCATCACTGTCACTGCCAGACT-3′ to generate GFP tagged EAF1. The full-length human FB1 cDNA was obtained from ATCC (I.M.A.G.E. clone: 3616596). Full-length human p53 cDNA was a gift from Dr. K.H. Vousden (16). Full-length human ELL cDNA was a gift from Dr. A. Shilatifard (17). Full-length cDNAs of U19/EAF2, and FB1 were cloned into pCMV-Tag2 vectors (Stratagene, La Jolla, CA) to generate Flag tagged constructs. Full-length cDNAs of FB1, P53, and ELL were cloned into pCMV-Myc (Clontech) to generate Myc tagged constructs. Full-length cDNA of ELL was cloned into pDsRed1-C1 vector to generate RFP tagged construct. Full-length cDNAs of U19/EAF2 and EAF1 were cloned into pM vector (Clontech) to generate Gal4 DNA binding domain fusion proteins of U19/EAF2 and EAF1.
Yeast-two-hybrid screening
The Matchmaker LexA two-hybrid system (Clontech, Palo Alto, CA) was used to identify proteins that interact with U19/EAF2. Exon III (the third exon of U19 from AA68 to AA114) of U19/EAF2 was cloned into the pLexA plasmid, creating a LexA DNA binding domain (BD)-U19(III) fusion protein. pLexA-U19(III) was transformed into the yeast strain (S. cerevisiae EGY48) using the lithium acetate method described in the manufacturer's protocol. The LexA DNABD-U19(III) fusion protein tested negative for autonomous transcriptional activity and therefore could be used in this yeast-two-hybrid system. U19(III) was used as the bait to screen a cDNA library generated from whole prostate glands pooled from 10 Caucasians, ages 14-60 (Clontech #HL4511AK) fused to the activation domain (AD) of the E. coli transcription factor B42. The pB42AD + prostate library was transformed into EGY48 yeast containing pLexA-U19(III) in a library scale transformation, using 0.5 mg of DNA following manufacturer's instructions. Two selective markers were used for screening, a LEU2 site integrated in the genome and a plasmid encoding the lacZ reporter, both of which contain a B42 minimal promoter flanked by LexA operators. Approximately 5 × 106 independent clones were screened for their ability to grow on leucine deficient media and to turn blue in the presence of X-gal. Positive clones were re-streaked on leucine deficient media containing X-gal twice to remove multiple plasmids containing library proteins. The plasmids were then isolated and sequenced. One of the clones was identified as the C-terminal region of FB1, E2A fusion partner in childhood leukemia.
Cell transfection and co-immunoprecipitation
COS-7 cells were plated on 10-cm Petri dishes and grown to approximately 95% confluency, and then transiently transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following manufacturer's instructions. At 30 hours after transfection, total cell extractions were prepared in 1 mL lysis buffer containing 50 mM Tris-HCl pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% Triton X-100, 1 mM Na3VO4, and a mix of protease inhibitor (Sigma, St. Louis, MO). Equal amounts of lysates were immunoprecipitated overnight at 4 °C with 40 μL Anti-c-Myc agarose affinity gel (Sigma) or 20 μL Anti-Flag M2-agarose affinity gel (Sigma). Immunocomplexes were collected by centrifugation, washed five times with 1x PBS (phosphate-buffered saline), separated by 12% SDS-PAGE gels and blotted to nitrocellulose membranes. Filters were blocked in TBS (Tris-buffered saline)-Tween plus 5% dried nonfat milk and immunoblotted with anti-Myc monoclonal antibody (1/2000) (Sigma), anti-Flag M2 monoclonal antibody (1/10,000) (Sigma), or Anti-GFP polyclonal antibody (1/5000) (Novus Biologicals, Littleton, CO). Western blotting was carried out as previously descried using ECL (enhanced chemiluminescence) (Amersham Biosciences, Piscataway, NJ) (18). All co-immunoprecipitation experiments were repeated at least three times independently.
Immunofluorescent microscopy
COS-7 or HeLa cells in 6-well plates were transfected with GFP (GFP-U19/EAF2, GFP-EAF1, and GFP-FB1) or RFP (RFP-ELL) tagged constructs, and, within 30 hours, directly observed with a Nikon Eclipse TE2000-U inverted fluorescent microscope (Melville, NY). Pictures were taken using PHOTOMETRICS® CoolSNAP fx digital camera (Roper Scientific, Inc. Trenton, NJ) and analyzed with MetaMorph software (Universal Imaging Corporation, Downingtown, PA). Representative images were shown in the figures.
Luciferase assay
GAL4 DNA binding domain fused EAF1 or U19/EAF2 were constructed using the pM vector (Clontech) and the full-length cDNA of EAF1 or U19/EAF2. The firefly luciferase reporter vector, pFR-Luc, was from Stratagene. The renilla luciferase vector, pRL-TK (Promega, Madison, WI), was used as a control vector for transfection efficiency. COS-7 cells in 12-well plates were transfected using Lipofectamine 2000 with 0.5 μg pM-EAF1 or pM-EAF2, 0.5 μg pFR-Luc, 0.1 μg pRL-TK together with or without 0.5 μg Myc-FB1 and/or 0.5 μg Myc-ELL. Empty pCMV-Myc vector was used to achieve equal amount of DNA transfected into each well. Total cell lysates were prepared from cells harvested 30 hours after transfection. Both the firefly and renilla luciferase activity were measured using a Dual-Luciferase Reporter Assay System from Promega following the manufacturer's instructions. Transfections and luciferase assays were carried out in triplicate and repeated more than three times independently. Relative luciferase activity was expressed as the ratio of the firefly luciferase activity to the renilla luciferase activity. All the data obtained from luciferase assays were analyzed using GraphPad Prism (GraphPad Software Inc., San Diego, CA). The statistical significance of differences in the data was calculated using Student's t-test.
Results
Identification of FB1 as a U19/EAF2 interacting protein partner in a yeast-two-hybrid screening
A yeast-two-hybrid screening was carried out to identify proteins that interact with U19/EAF2. U19 possesses transactivation activity in its C-terminal region (3). Therefore the full-length U19 is not suitable to be used as the bait in the screening. Previously we determined that the exon III (from AA68 to AA114) region of U19 is essential for the apoptotic activity of U19 (2,19). As a result, this region was chosen as the bait in a yeast-two-hybrid system. One of the clones isolated was FB1 (E2A fusion partner in childhood leukemia) (10,12). To verify the interaction of FB1 with U19 and to exclude the possibility that FB1 might activate reporters by itself, pAD-FB1 with pLexA-U19(III), pLexA-U19, and/or pLexA vectors were retransformed into yeast. These assays confirmed the specificity of the interaction of FB1 with U19(III) and U19 in the yeast-two-hybrid system.
To verify the interaction of FB1 with U19 in mammalian cells, COS-7 cells were transfected with GFP-U19 and Myc-FB1 constructs. As a control, empty GFP vector was also transfected with Myc-FB1. The cell lysates were immunoprecipitated with Myc-antibody conjugated agarose and then immunoblotted with GFP antibody. Figure 1A shows that Myc-FB1 co-precipitated GFP-U19, but not GFP. Reciprocally, Flag-U19 also co-precipitated Myc-FB1, further demonstrating the interaction between FB1 and U19 (Fig. 1B).
Fig. 1.
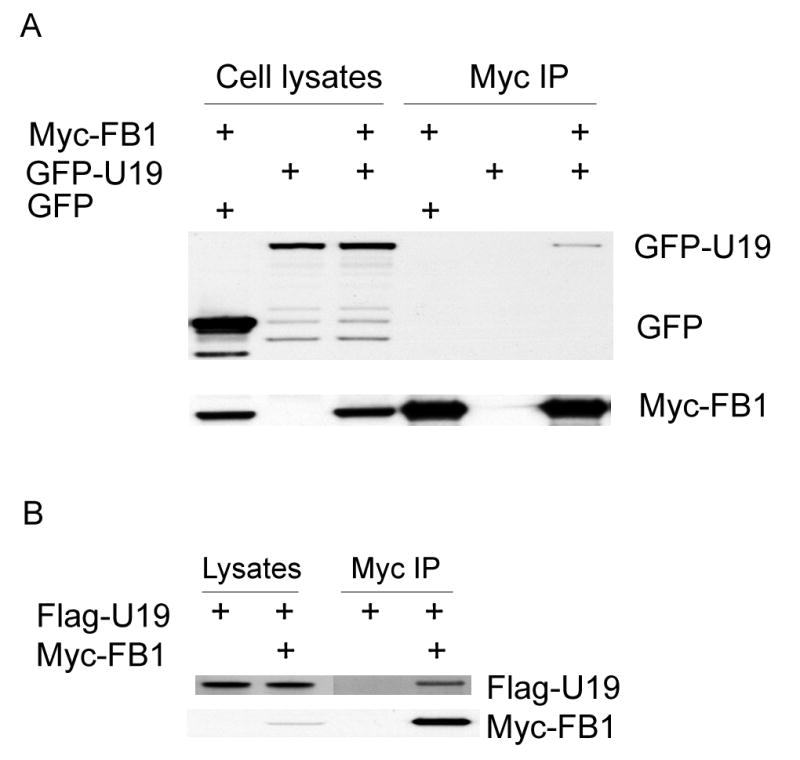
U19/EAF2 interaction with FB1. A. GFP-U19 was transiently expressed in COS-7 cells either alone or with Myc-FB1. COS-7 cells were also transfected with GFP and Myc-FB1. Myc-FB1 was then immunoprecipitated with Myc-antibody-conjugated agarose (Myc-IP). The cell lysates and immunocomplexes were analyzed by Western blot to detect GFP and GFP-U19 using GFP antibody and to detect Myc-FB1 using Myc antibody. Myc-FB1specifically co-precipitates GFP-U19, but not GFP. B. Flag-U19 was transiently expressed in COS-7 cells either alone or with Myc-FB1, which was then immnuoprecipitated with Myc-antibody-conjugated agarose (Myc-IP). The cell lysates and immnunocomplexes were analyzed by Western blot to detect Flag-U19 using Flag antibody and to detect Myc-FB1 using Myc antibody. Myc-FB1 specifically co-precipitates Flag-U19.
FB1 was identified by yeast two-hybrid screening using the conserved region of U19(III) as the bait. As expected, Fig. 2 showed that FB1 interacts with the N-terminal region of U19, which contains U19(III), but not the C-terminal region of U19.
Fig. 2.
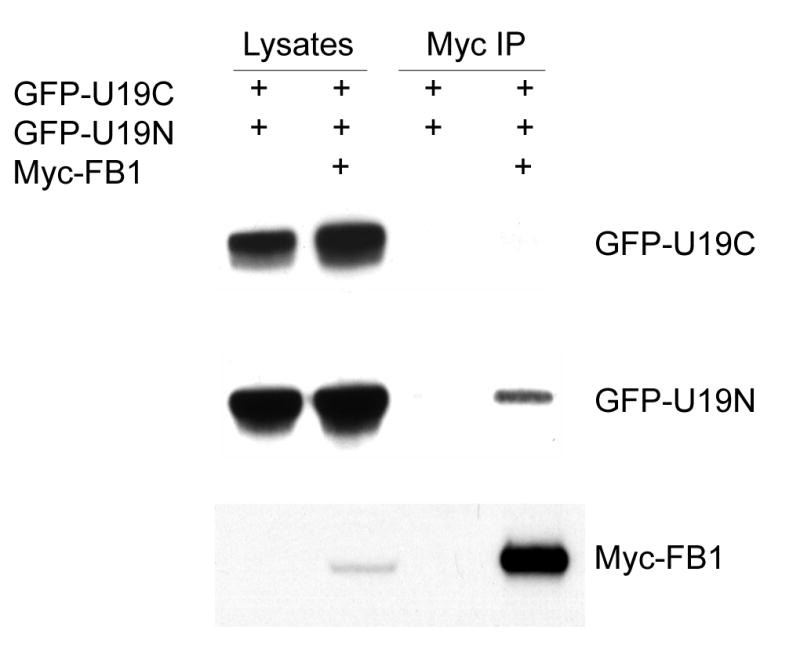
FB1 interaction with the N-terminal region of U19. A. Myc-FB1 was transiently expressed in COS-7 cells alone, with GFP-U19C (C-terminal region of U19), or with GFP-U19N (N-terminal region of U19). The cell lysates and immunocomplexes were analyzed by Western blot to detect Myc-FB1 using Myc antibody and to detect GFP-U19N and GFP-U19C using GFP antibody. Myc-FB1 specifically co-precipitates GFP-U19N, but not GFP-U19C.
FB1 interacts with EAF1
FB1 may also bind to EAF1 because EAF1 is highly homologous to U19/EAF2 (3). To test whether FB1 interacts with EAF1, COS-7 cells were co-transfected with GFP-EAF1 and Myc-FB1 and a co-IP experiment was performed. Figure 3 shows that Myc-FB1 co-precipitated GFP-EAF1, but not GFP, confirming the interaction of FB1 with EAF1. This result showed that FB1 interacts with EAF1, a homologue of U19/EAF2.
Fig. 3.
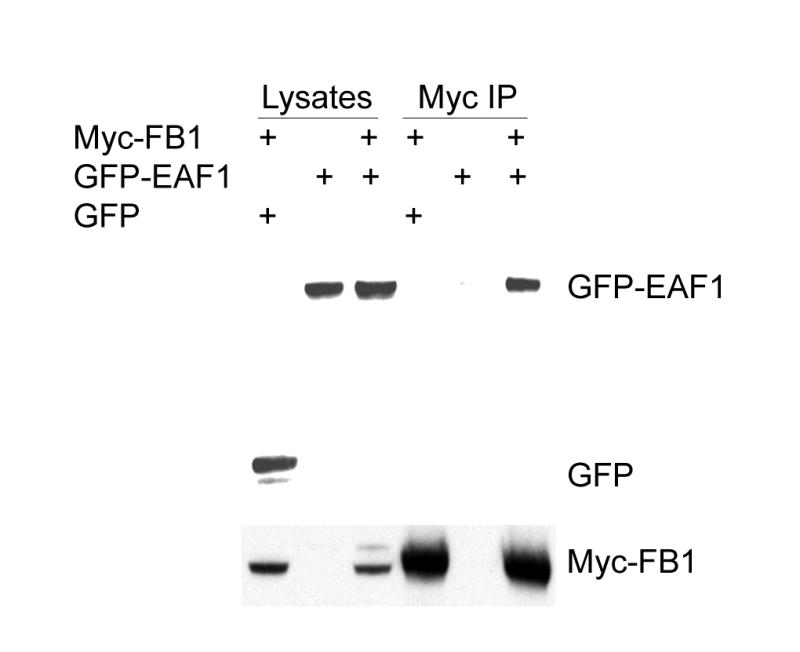
EAF1 interaction with FB1. GFP-EAF1 was transiently expressed in COS-7 cells either alone or with Myc-FB1. COS-7 cells were also transfected with GFP and Myc-FB1. Myc-FB1 was then immunoprecipitated with Myc-antibody-conjugated agarose (Myc-IP). The cell lysates and immunocomplexes were analyzed by Western blot to detect GFP and GFP-EAF1 using GFP antibody and to detect Myc-FB1 using Myc antibody. Myc-FB1specifically co-precipitates GFP-EAF1, but not GFP.
FB1 induces U19/EAF2, but not EAF1, into speckled nuclear patterns
Since EAF family proteins are localized into speckled nuclear patterns in the presence of their binding partner ELL (20), we tested if U19/EAF2 also forms nuclear speckles in the presence of FB1. Interestingly, GFP-U19 exhibited nuclear speckles in the presence of FB1 in both COS-7 and Hela cells (Fig. 4), suggesting that FB1 is able to recruit U19 to a nuclear structure.
Fig. 4.
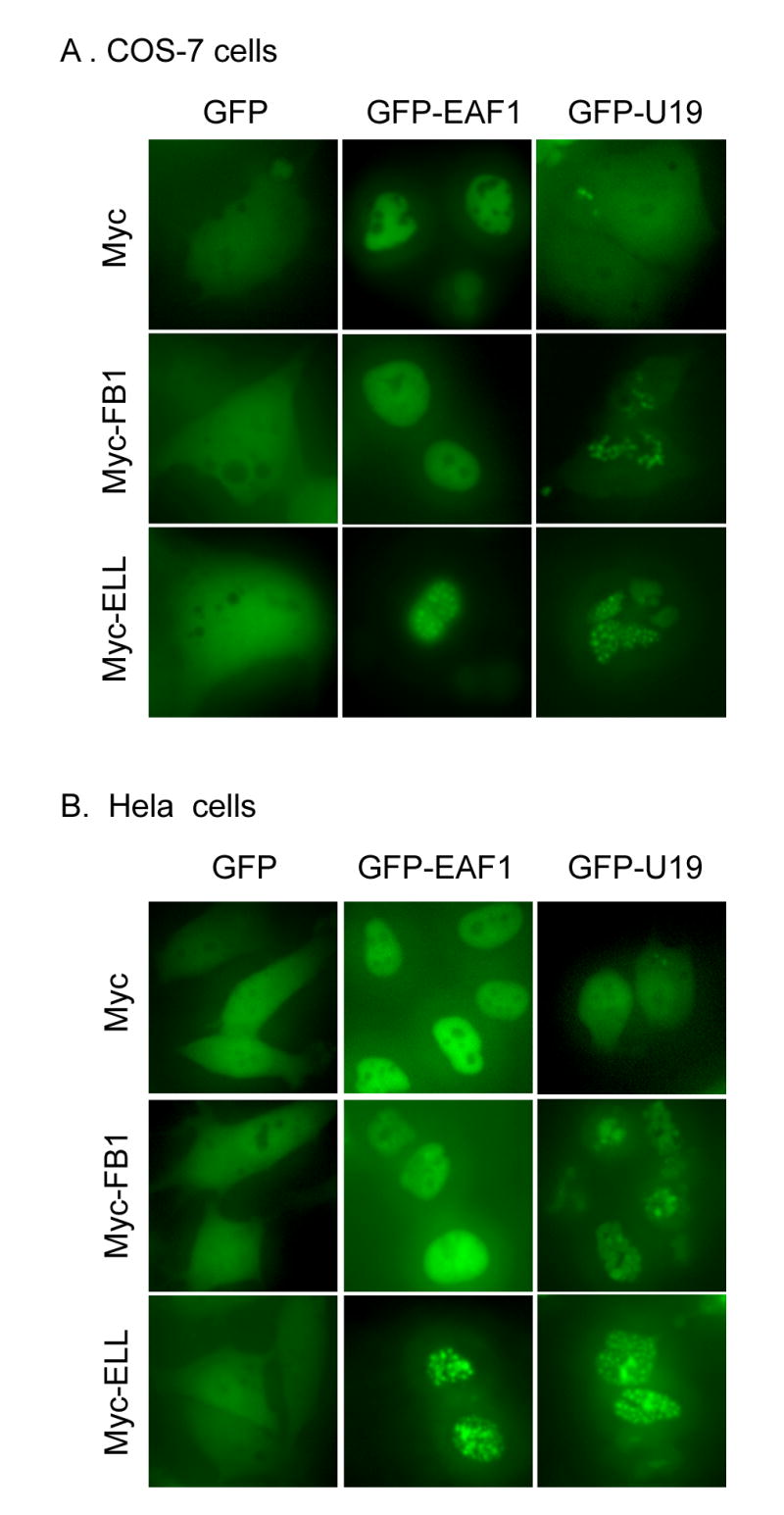
The effect of FB1 on intracellular localization of GFP-U19/EAF2 and GFP-EAF1. COS-7 (A) and Hela (B) cells were transiently transfected with indicated expression vectors. Transfected cells were then observed using fluorescent microscopy 24 hours after transfection. Representative images of transfected COS-7 (A) and Hela (B) cells are shown here.
We also tested if EAF1 can form speckled nuclear patterns in the presence of FB1. Unlikely GFP-U19, GFP-EAF1 subcellular localization was unaffected by FB1 co-transfection in both COS-7 and Hela cells (Fig. 4), suggesting that FB1 may not recruit EAF1 to a specific nuclear structure which was observed in cells co-transfected with both FB1 and U19. As a control, GFP-EAF1 formed speckled nuclear pattern in the presence of ELL under the same experimental conditions.
FB1 interacts with ELL
Both EAF1 and U19/EAF2 are ELL associated factors (3,6). The fact that FB1 interacts with both EAF1 and U19/EAF2 led us to investigate if FB1 also interacts with ELL. In order to test this hypothesis, a co-IP experiment was performed with Flag-FB1 and Myc-ELL. The cell lysates were immunoprecipitated with Myc-antibody conjugated agarose and then immunoblotted with Flag antibody. Figure 5A shows that FB1 interacts with ELL. As a control, no association was found between FB1 and p53 (data not shown). When RFP-ELL was co-transfected with GFP-FB1, co-localization was observed between these two tagged proteins, suggesting that ELL and FB1 coexist in the same nuclear structure, providing further evidence for their interaction (Fig. 5B).
Fig. 5.
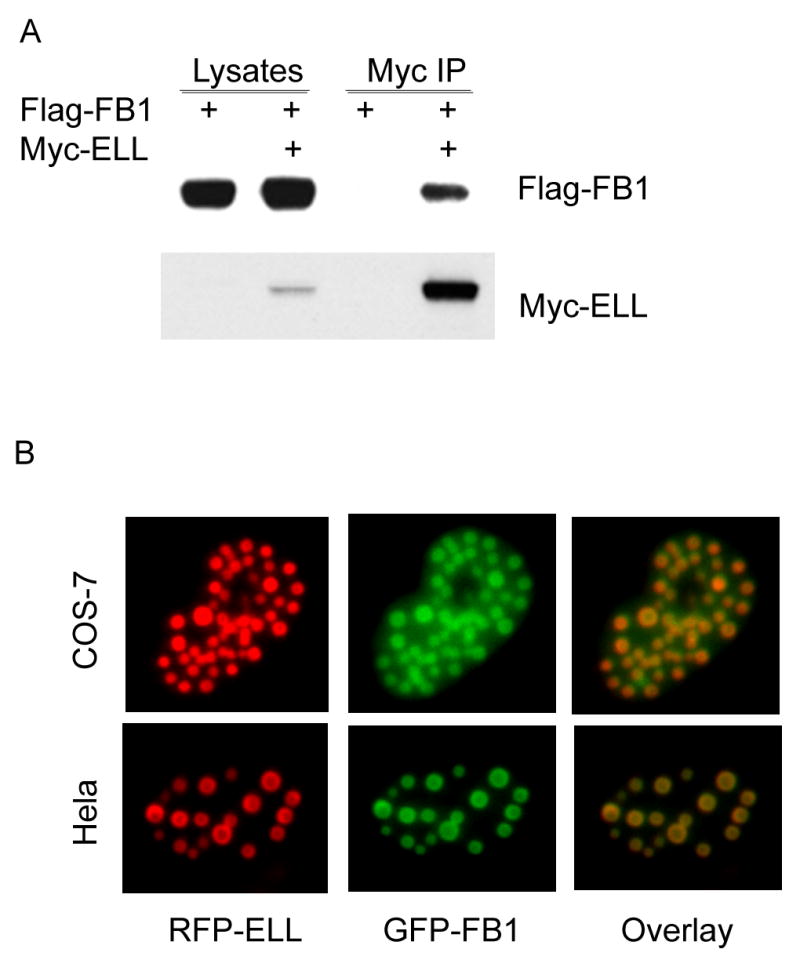
A. FB1 interaction with ELL. Flag-FB1 was transiently expressed in COS-7 cells either alone or with Myc-ELL, which was then immnuoprecipitated with Myc-antibody-conjugated agarose (Myc-IP). The cell lysates and immnunocomplexes were analyzed by Western blot to detect Flag-FB1 using Flag antibody and to detect Myc-ELL using Myc antibody. Myc-ELL specifically co-precipitated Flag-FB1. B. FB1 co-localization with ELL in nuclear speckles. GFP-FB1 was transiently transfected into COS-7 or HeLa cells with RFP-ELL and Myc-EAF1. Transfected cells were then observed under fluorescent microscope 24 hours after transfections. Overlay images were shown to demonstrate the co-localization of GFP-FB1 and RFP-ELL. Myc-EAF1 was used here to enhance the red fluorescent signal of RFP-ELL. Representative images of COS-7 or Hela nuclei are shown here.
FB1 inhibits the transcriptional activity of U19/EAF2 but not EAF1
Both EAF1 and U19/EAF2 have been shown to possess transactivation activity in a mammalian one-hybrid system (3,6). The same system was used to test the effect of FB1 on the transactivation activity of EAF1 and U19/EAF2. Fig. 6 shows that FB1 inhibited the transcriptional activity of U19, but not EAF1. When ELL was co-transfected with pM-EAF1 or pM-U19, it increased their transactivation activity, possibly due to the elongation activity of ELL (20). FB1 inhibited the transactivation of U19, but not EAF1, in the presence of ELL (Fig. 6). The inhibition of U19 transcriptional activity by FB1 was statistically significant either in the absence or presence of ELL co-transfection. Interestingly, the FB1 inhibition of U19/Eaf2 transactivation in the presence of ELL is ∼ 5-fold, more dramatic than the inhibition in the absence of ELL, ∼2-fold.
Fig. 6.
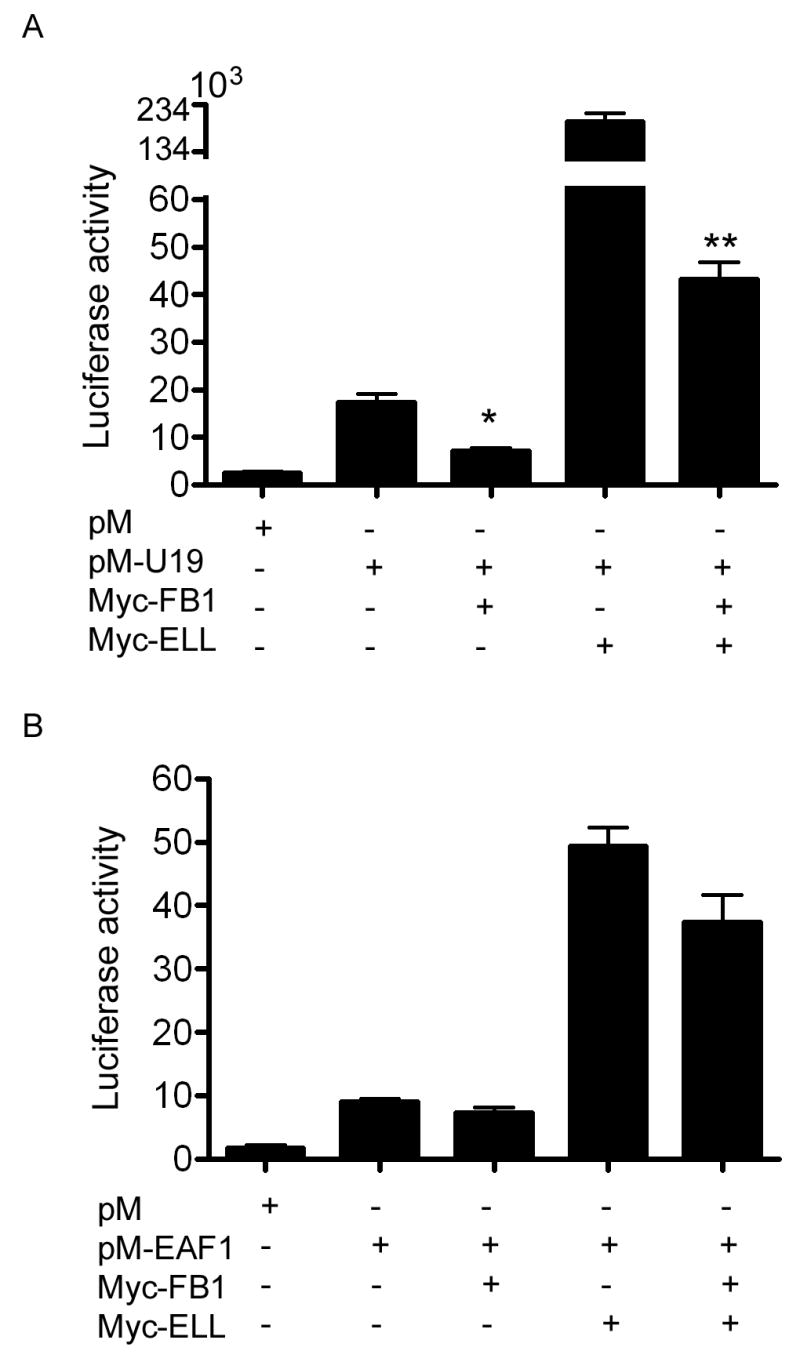
FB1 inhibition of the transcriptional activity of U19/EAF2, but not EAF1. COS-7 cell were transiently transfected with pM-U19 (A) or pM-EAF1 (B) either alone or with Myc-FB1, Myc-ELL, or Myc-FB1 and Myc-ELL. Relative luciferase activity is shown here. * p = 0.02; ** p < 0.0001.
Discussion
EAF family proteins and their binding partner ELL play important roles in development and carcinogenesis (2,4,7,17). Elucidating the mechanism by which EAF-ELL axis is regulated will facilitate further studies on the roles of EAF-ELL axis in animal development and cancer progression. In this paper, we report the identification and characterization of FB1 as a novel binding partner of EAF1, U19/EAF2, and ELL.
Previously we reported that U19/EAF2 is an apoptosis inducer in the prostate cancer cells in vitro (2). Our data suggested that U19/EAF2 induction of apoptosis requires downstream genes since the apoptosis induction by U19/EAF2 can be blocked by cycloheximide, a protein synthesis inhibitor. Interestingly, U19/EAF2 is still capable of inducing apoptosis even when its transactivation domain is deleted (19). Thus, U19 might activate other transcription factors through protein-protein interactions, which provides basis for a yeast-two-hybrid screening for candidate U19 interacting proteins.
FB1 was identified as a binding partner of U19/EAF2 in a yeast two-hybrid screen. We verified the FB1 binding to U19/EAF2 using co-immunoprecipitation of co-transfected Myc-, Flag-, or GFP-tagged proteins. We further showed that FB1 also binds to the other member of the EAF family, EAF1, which shares 58 % identity and 74% similarity with U19/EAF2. Thus, FB1 could modulate both members of the EAF family.
FB1 is a novel protein with no strong homology with known proteins (10) and is present in the nucleus predominantly (15). Rat FB1, called Amida, causes both apoptosis and cell cycle arrest in COS-7 cells (12,13). Since Aimda is involved in the apoptosis induced by Par-4, which was reported to play a role in prostate cancer apoptosis (15), FB1 has potential to modulate the sensitivity of prostate cancer cells to apoptotic agents. However, FB1 had little effect on the apoptotic activity of U19/EAF2 in different cell lines, including HeLa and prostate cancer cell line PC3 (data not shown). Therefore, other U19/EAF2 interacting proteins such as ELL may be required for the apoptotic activity of U19/EAF2. Although FB1 may not modulate U19/EAF2-mediated apoptosis, it may affect other functions of U19/EAF2, such as activation of transcription.
EAF1 and U19/EAF2 are potential transcription factors with a transactivation domain in its C-terminus and the transactivation of both proteins is enhanced by their binding partner ELL, which could function as a transcription elongation factor (9). Using a mammalian-one-hybrid system, we showed that FB1 inhibited the transcriptional activity of U19/EAF2 either in the absence or presence of ELL. However, FB1 had no significant effect on the transactivation activity by EAF1 either in the absence or presence of ELL. This observation indicates that FB1 modulates the transcriptional activity of U19/EAF2 and EAF1 differently. In addition, U19/EAF2, but not EAF1, formed speckled nuclear pattern when co-transfected with FB1. This finding provided further evidence for differential regulation of U19/EAF2 and EAF1 by FB1.
Interestingly, co-immunoprecipitation showed that FB1 also interacts with ELL, the binding partner of EAF proteins. This raised a possibility that FB1, EAFs, and ELL can be present in the same complex. FB1 co-localizes with ELL in nuclear speckles (Fig. 5). ELL and EAF1 are components of the Cajal bodies (CB) (21), raising a possibility that FB1 may be also present in the Cajal Bodies. ELL is essential in early animal development because ELL gene knockout in the mouse resulted in embryonic lethality (22). ELL is also involved in tumor development because MLL-ELL translocation occurs in acute myeloid leukemia. Identification of FB1 as a binding partner for EAFs and ELL suggests that FB1 may play a role in early animal development and carcinogenesis by modulating the EAF-ELL axis. These findings provide basis for in vivo studies to determine the physiological functions of FB1.
In conclusion, we identified FB1 as a U19/EAF2 interacting protein partner. FB1 is also found to interact with EAF1 and ELL. FB1 inhibits the transcriptional activity of U19/EAF2, but not EAF1. Our findings suggest that FB1 is an important modulator of ELL-EAF axis, with ability to differentially modulate EAF1 and U19/EAF2 activities.
Acknowledgments
We thank members of Wang lab for critical reading. This work was supported in part by NIH R01 DK51193 and NIH P50 CA90386 Prostate Cancer SPORE.
Abbreviations
- EAF1
ELL associated factor 1
- EAF2
ELL associated factor 2
- ELL
11-19 lysine-rich leukemia gene
- FB1
E2A fusion partner in childhood pre-B cell acute lymphoblastic leukemia
- COS-7
African green monkey kidney cells, transformed by SV40 origin defective mutant
- HeLa
Human cervix epithelioid carcinoma
- GFP
green fluorescent protein
- RFP
red fluorescent protein
- co-IP
co-immunoprecipitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang Z, Tufts R, Haleem R, Cai X. Genes regulated by androgen in the rat ventral prostate. Proc Natl Acad Sci U S A. 1997;94(24):12999–13004. doi: 10.1073/pnas.94.24.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao W, Zhang Q, Jiang F, Pins M, Kozlowski JM, Wang Z. Suppression of prostate tumor growth by U19, a novel testosterone-regulated apoptosis inducer. Cancer Res. 2003;63(15):4698–4704. [PubMed] [Google Scholar]
- 3.Simone F, Luo RT, Polak PE, Kaberlein JJ, Thirman MJ. ELL-associated factor 2 (EAF2), a functional homolog of EAF1 with alternative ELL binding properties. Blood. 2003;101(6):2355–2362. doi: 10.1182/blood-2002-06-1664. [DOI] [PubMed] [Google Scholar]
- 4.Thirman MJ, Levitan DA, Kobayashi H, Simon MC, Rowley JD. Cloning of ELL, a gene that fuses to MLL in a t(11;19)(q23;p13.1) in acute myeloid leukemia. Proc Natl Acad Sci U S A. 1994;91(25):12110–12114. doi: 10.1073/pnas.91.25.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shilatifard A, Lane WS, Jackson KW, Conaway RC, Conaway JW. An RNA polymerase II elongation factor encoded by the human ELL gene. Science. 1996;271(5257):1873–1876. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- 6.Simone F, Polak PE, Kaberlein JJ, Luo RT, Levitan DA, Thirman MJ. EAF1, a novel ELL-associated factor that is delocalized by expression of the MLL-ELL fusion protein. Blood. 2001;98(1):201–209. doi: 10.1182/blood.v98.1.201. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Wu X, Zhuang F, Jiang S, Jiang M, Liu YH. Expression of murine ELL-associated factor 2 (Eaf2) is developmentally regulated. Dev Dyn. 2003;228(2):273–280. doi: 10.1002/dvdy.10367. [DOI] [PubMed] [Google Scholar]
- 8.Saso K, Ito T, Natori S, Sekimizu K. Identification of a novel tissue-specific transcriptional activator FESTA as a protein that interacts with the transcription elongation factor S-II. J Biochem (Tokyo) 2003;133(4):493–500. doi: 10.1093/jb/mvg065. [DOI] [PubMed] [Google Scholar]
- 9.Kong SE, Banks CA, Shilatifard A, Conaway JW, Conaway RC. ELL-associated factors 1 and 2 are positive regulators of RNA polymerase II elongation factor ELL. Proc Natl Acad Sci U S A. 2005;102(29):10094–10098. doi: 10.1073/pnas.0503017102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brambillasca F, Mosna G, Colombo M, Rivolta A, Caslini C, Minuzzo M, Giudici G, Mizzi L, Biondi A, Privitera E. Identification of a novel molecular partner of the E2A gene in childhood leukemia. Leukemia. 1999;13(3):369–375. doi: 10.1038/sj.leu.2401338. [DOI] [PubMed] [Google Scholar]
- 11.Kee BL, Quong MW, Murre C. E2A proteins: essential regulators at multiple stages of B-cell development. Immunol Rev. 2000;175:138–149. [PubMed] [Google Scholar]
- 12.Irie Y, Yamagata K, Gan Y, Miyamoto K, Do E, Kuo CH, Taira E, Miki N. Molecular cloning and characterization of Amida, a novel protein which interacts with a neuron-specific immediate early gene product arc, contains novel nuclear localization signals, and causes cell death in cultured cells. J Biol Chem. 2000;275(4):2647–2653. doi: 10.1074/jbc.275.4.2647. [DOI] [PubMed] [Google Scholar]
- 13.Gan Y, Taira E, Irie Y, Fujimoto T, Miki N. Arrest of cell cycle by amida which is phosphorylated by Cdc2 kinase. Mol Cell Biochem. 2003;246(12):179–185. [PubMed] [Google Scholar]
- 14.Gan Y, Taira E, Irie Y, Tanaka H, Ichikawa H, Kumamaru E, Miki N. Amida predominantly expressed and developmentally regulated in rat testis. Biochem Biophys Res Commun. 2001;288(2):407–412. doi: 10.1006/bbrc.2001.5779. [DOI] [PubMed] [Google Scholar]
- 15.Boosen M, Vetterkind S, Koplin A, Illenberger S, Preuss U. Par-4-mediated recruitment of Amida to the actin cytoskeleton leads to the induction of apoptosis. Exp Cell Res. 2005;311(2):177–191. doi: 10.1016/j.yexcr.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7(3):683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 17.Johnstone RW, Gerber M, Landewe T, Tollefson A, Wold WS, Shilatifard A. Functional analysis of the leukemia protein ELL: evidence for a role in the regulation of cell growth and survival. Mol Cell Biol. 2001;21(5):1672–1681. doi: 10.1128/MCB.21.5.1672-1681.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang F, Yang L, Cai X, Cyriac J, Shechter I, Wang Z. Farnesyl diphosphate synthase is abundantly expressed and regulated by androgen in rat prostatic epithelial cells. J Steroid Biochem Mol Biol. 2001;78(2):123–130. doi: 10.1016/s0960-0760(01)00086-3. [DOI] [PubMed] [Google Scholar]
- 19.Hahn J, Xiao W, Jiang F, Simone F, Thirman MJ, Wang Z. Apoptosis induction and growth suppression by U19 is mediated through its ELL-binding domain. Prostate. doi: 10.1002/pros.20481. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao W, Jiang F, Wang Z. ELL binding regulates U19/Eaf2 intracellular localization, stability, and transactivation. Prostate. 2006;66(1):1–12. doi: 10.1002/pros.20309. [DOI] [PubMed] [Google Scholar]
- 21.Polak PE, Simone F, Kaberlein JJ, Luo RT, Thirman MJ. ELL and EAF1 are Cajal body components that are disrupted in MLL-ELL leukemia. Mol Biol Cell. 2003;14(4):1517–1528. doi: 10.1091/mbc.E02-07-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitani K, Yamagata T, Iida C, Oda H, Maki K, Ichikawa M, Asai T, Honda H, Kurokawa M, Hirai H. Nonredundant roles of the elongation factor MEN in postimplantation development. Biochem Biophys Res Commun. 2000;279(2):563–567. doi: 10.1006/bbrc.2000.3970. [DOI] [PubMed] [Google Scholar]


