Abstract
Latitudinal diversity gradients are first-order expressions of diversity patterns both on land and in the oceans, although the current hypotheses that seek to explain them are based chiefly on terrestrial data. We have assembled a database of the geographic ranges of 3,916 species of marine prosobranch gastropods living on the shelves of the western Atlantic and eastern Pacific Oceans, from the tropics to the Arctic Ocean. Western Atlantic and eastern Pacific diversities are similar, and the diversity gradients are strikingly similar despite many important physical and historical differences between the oceans. This shared diversity pattern cannot be explained by: (i) latitudinal differences in species range-length (Rapoport’s rule); (ii) species-area effects; or (iii) recent geologic histories. One parameter that does correlate significantly with diversity in both oceans is solar energy input, as represented by average sea surface temperature. If this correlation is causal, sea surface temperature is probably linked to diversity through some aspect of productivity. In this case, diversity is an evolutionary outcome of trophodynamic processes inherent in ecosystems, and not just a byproduct of physical geographies.
Latitudinal diversity gradients, peaking in the tropics and tailing off toward the poles, form the most striking large-scale biotic pattern shared by both marine and terrestrial systems. This pervasive pattern could thus provide a basis for addressing one of the central questions in ecological and macroevolutionary theory: the regulation of species diversity. However, although a multitude of explanatory hypotheses has been proposed to explain the latitudinal gradients (1–5), none has gained general acceptance. Comparative analyses are powerful methods for testing explanations of such large-scale biological patterns (see ref. 6), especially for those not amenable to direct experimentation, but marine latitudinal diversity patterns have rarely been evaluated in this fashion.
Marine prosobranch gastropods are the most diverse group of benthic marine invertebrates for which extensive comparative data are available. Our database consists of the latitudinal ranges of prosobranchs known to live in waters shallower than 200 m, thus embracing the continental shelf fauna, from the southern margins of the tropics to the Arctic Ocean along both coasts of the Americas (1,907 eastern Pacific species and 2,009 western Atlantic species). These data were compiled through an exhaustive search of the primary literature as well as from major museum collections (7, 8), and permit us to compare the distributions of large numbers of related species in two faunas that happen to be very similar in total species number, but that inhabit regions of very different coastal geographies, different hydrographies (e.g., washed by eastern vs. western boundary currents), and different biotic histories. These contrasts allow us to test several of the main hypotheses that seek to explain latitudinal diversity gradients, namely those that rely upon the effects of range size, of habitable area, and of the input of solar energy.
Strong latitudinal diversity gradients are present in both oceans (Fig. 1). The two diversity profiles are strikingly similar, with rich tropical provinces, steep gradients from tropical into temperate waters, and rather flat profiles farther to the north. A similar pattern is displayed by the Bivalvia, another diverse marine invertebrate group (5, 9, 10). The two latitudinal segments in which the profiles from the two oceans differ the most lie from about 0° to 10° N and from about 40° to 60° N; in both cases, Atlantic diversity is lower. The difference in the low-latitude profiles may reflect the environmental effects of discharge from South American rivers such as the Amazon and the Orinoco. Salinities are reduced over wide areas of the shelf off the river mouths, and significant terrigenous sediments blanket the shelves so that turbidity is high and hard substrates are scarce. These factors may contribute to an anomalously low species diversity in those regions (11). The difference in the other, cool-temperate latitudinal segments may be at least partly an artifact of north Pacific geography, for slightly different faunas occur in the Gulf of Alaska and the Bering Sea between 50° and 60° N (12). Although the similarity in total prosobranch diversities in the eastern Pacific and western Atlantic may be a coincidence, the similarity in diversity profiles suggests some general control on latitudinal diversity gradients.
Figure 1.
Latitudinal diversity gradient of eastern Pacific (□) and western Atlantic (•) marine prosobranch gastropods, binned per degree of latitude. The range of a species is assumed to be continuous between its range endpoints, so diversity for any given latitude is defined as the number of species whose latitudinal ranges cross that latitude.
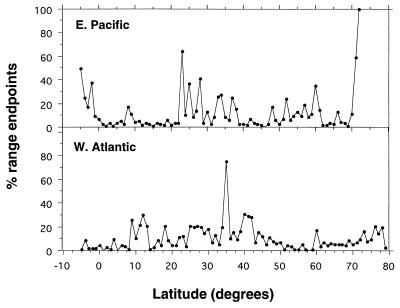
The profile similarities do not arise from similarities in the latitudinal distribution of species range endpoints. The latitudes of significant species turnovers differ quite significantly between the two coasts (Fig. 2). Points of maximal species turnover, often used to delimit biogeographic units such as bioprovinces, are located at boundaries between major water masses or types, commonly localized at topographic discontinuities such as those at Cabo San Lucas (22.52° N in the eastern Pacific) and Cape Hatteras (35.35° N in the western Atlantic). In the Pacific, the tropical fauna exhibits little geographic differentiation although the cool-temperate fauna is subdivided into several distinct biogeographic units, whereas in the Atlantic there are at least three distinct biogeographic units in the tropics but little geographic differentiation in cool-temperate latitudes (13, 14).
Figure 2.
Latitudinal distribution of the range endpoints of marine gastropods on the eastern Pacific and western Atlantic shelves, binned per degree of altitude. Each point represents the ratio, expressed as a percentage, of the number of species ranges that end at that latitude to the total number of species present at that latitude. Latitudes with the high concentrations of range endpoints represent provincial boundaries (14). Unlike the diversity trends, species range endpoints are distributed very differently along the two marine shelves.
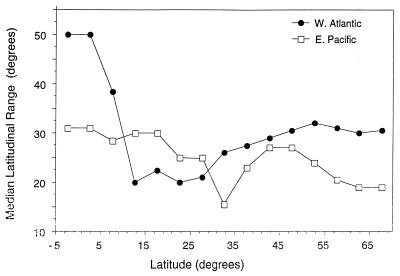
These data on the spatial distribution of species on the two shelves allow us to test proposed explanations for the latitudinal species diversity gradient. One hypothesis, termed Rapoport’s rule, attributes the gradient to a decrease in species’ range lengths toward low latitudes, resulting in progressively larger regional species pools along this trend. Support for this pattern has been uneven among taxa and regions (5, 15–17). For marine prosobranchs along both coasts, median latitudinal ranges of species are greatest, not in high but in low latitudes, a trend opposite to that predicted by Rapoport’s rule (Fig. 3). The latitudinal diversity trend in each ocean is largely independent of the number of provinces or the size of species’ geographic ranges. The Rapoport’s rule hypothesis cannot explain these marine diversity patterns.
Figure 3.
Median latitudinal ranges of western Atlantic (•) and eastern Pacific (□) marine gastropods. The data are binned in 5° of latitude; the value for each latitudinal segment is plotted at the middle of that segment. The median range for a particular latitudinal bin is calculated based on all species ranges that intersect that latitudinal segment.
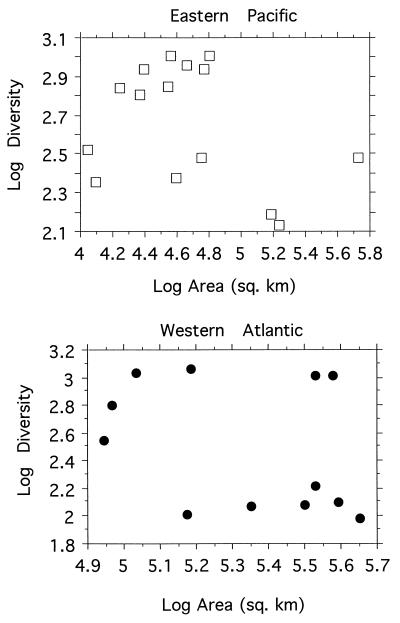
Geographical area is another major factor suggested as regulating global diversity trends, an argument that has been made most strongly for terrestrial organisms (2). We examined this hypothesis in two ways. First, we determined shelf areas along the two coasts in 5° latitudinal segments from 10° N, the southern margin of the Caribbean, to 72° N, approximately the border of the Arctic Ocean. We digitized a 1:107 scale Lambert Azimuthal equal area projection map (AAPG, 1977) for the eastern Pacific coast, and a 1:107 scale Lambert Zenithal equal area projection map (National Atlas of Canada, Base Map Series, North America, MCR 31, 1995) for the western Atlantic coast, down to the 200 m contour. Given the coarse scale of these maps and the associated measurement errors, the area values used here should be taken as relative and not absolute measures. Spearman rank correlations of shelf area vs. diversity show no significant relationship on either coast (Fig. 4).
Figure 4.
Relationships between shelf area and diversity for eastern Pacific (Top) and western Atlantic marine prosobranchs. Spearman rank correlation for the eastern Pacific, P = 0.5; for the western Atlantic, P = 0.3. Spearman’s rho = −0.18 for the eastern Pacific, −0.31 for the western Atlantic. The relationships are not significant.
Second, one of the arguments in favor of an area effect in latitudinal diversity is simply that continental areas are greatest in low latitudes and decrease in high latitudes (refs. 18 and 19; but see ref. 20). This relation does not hold for the continental shelves along the Americas, however, where the greatest areas are in fact in high latitudes and areas decrease in low latitudes. For example, in the eastern Pacific, the shelf area from 5° S to 20° N, which includes most of the Panamic province, is 169,145 km2, while from 55° N to 70° N, which includes most of the subarctic and arctic provinces, it is 855,896 km2. Species diversity for the southern region can be tallied as 1,087 (1,097 − 10 species that are shared with the northern region), while that for the northern region is 313 (323 − 10 species). The latitudinal diversity gradient remains unaffected by the switch in the direction of increased area from the terrestrial to the marine shelf environments, falsifying the area-affect hypothesis, at least for latitudinal gradients in marine systems.
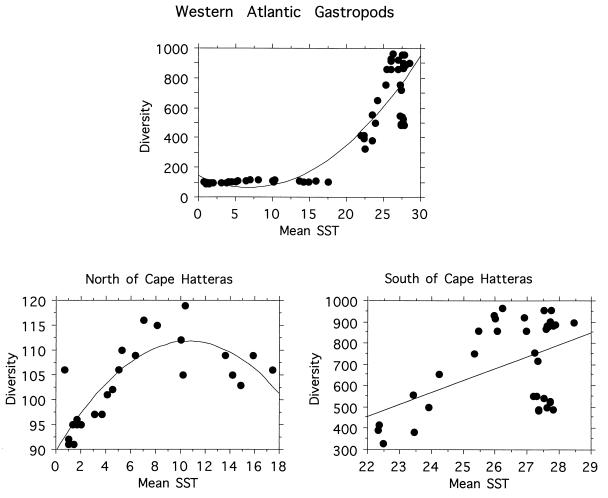
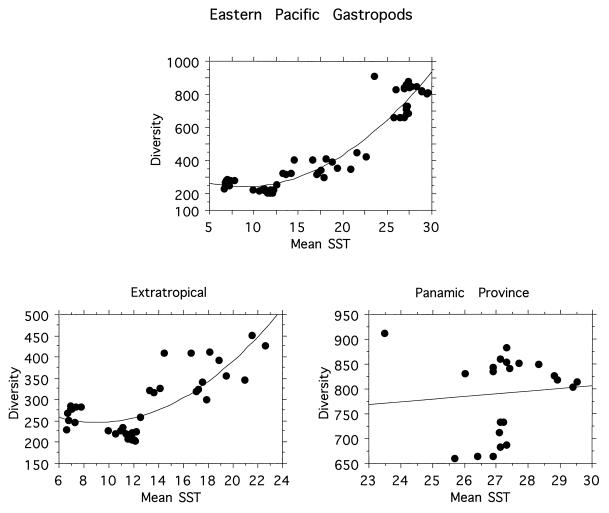
A third proposed explanation for the latitudinal diversity gradient holds that the pattern depends upon total or average amount of available energy (often taken as a function of solar radiation at the surface) (4, 21, 22). Support for this hypothesis again comes mainly from terrestrial ecosystems, where climatic variables such as mean annual temperature and associated parameters can account for much of the latitudinal variation in diversity (4, 21). A species/energy hypothesis has also been applied to tropical coral reefs (22). To test a species/energy hypothesis, we calculated an average sea surface temperature (SST) value for each degree of latitude from 5° S to 60° N along each coast, compiled from a monthly SST data set averaged over a 10-year period from October 1981 to December 1990 (P. N. Schweitzer, 1993, U.S. Geological Survey Digital Data Series DDS-10). The analyses reveal a strong relationship between mean SST and diversity patterns, especially in extratropical latitudes, along both coasts (Figs. 5 and 6). To further explore the relationship between mean SST and diversity, we also examined the relationships between residuals from regressions of latitude against mean SST, and regressions of latitude against diversity. These residuals, factoring out latitude, are also significantly related (for the eastern Pacific, R2 = 0.709, P < 0.0001; for the western Atlantic, R2 = 0.518, P < 0.0001), thereby further supporting the idea that some parameter that is correlated with mean SST is an important control on diversity in the systems we examined.
Figure 5.
Relationships between mean annual SST and diversity for western Atlantic marine gastropods. (Upper) All data points combined. The second order polynomial regression gives a significant relationship, R2 = 0.82, P < 0.0001. (Lower, Right) The relationship south of Cape Hatteras (i.e., tropical and subtropical species). R2 = 0.24, P = 0.007. (Lower, Left) The relationship north of Cape Hatteras, R2 = 0.668, P = 0.0002. Data are spatially autocorrelated so the regression statistics should be used only for comparative purposes.
Figure 6.
Relationships between mean annual SST and diversity for eastern Pacific marine gastropods. (Upper) All data points combined. The second order polynomial relationship gives a significant relationship, R2 = 0.92, P < 0.0001. (Lower, Right) The relationship for the Panamic province (tropical species). Relationship is not significant, R2 = 0.009, P = 0.66. (Lower, Left) The relationship for the extratropical species, R2 = 0.63, P = 0.001. Data are spatially autocorrelated so the regression statistics should be used only for comparative purposes.
Historical events have also been invoked to explain the latitudinal diversity gradient (1, 9, 23), but macroevolutionary aspects of this gradient remain underexplored. The comparison of western Atlantic and eastern Pacific diversity patterns allows us to evaluate the effects of extinctions on the latitudinal diversity gradient. Molluscan faunas in the eastern Pacific show about 15% species extinctions over the last 2 million years (latest Pliocene-Recent) (24, 25). Pleistocene glacial-interglacial climate swings in the Pacific did not cause increased extinctions, but rather induced extensive species range shifts (26). In the tropical western Atlantic, however, the cooling of the marine climate with the onset of glacial cycles appears to have brought intense biotic turnover, perhaps because species were trapped within the Caribbean region (27). Early Pleistocene extinction rates in the western Atlantic were about twice those in the eastern Pacific (27–29); however, originations must also have accelerated, as western Atlantic diversity now appears to be similar to the preglaciation levels (28), and even exceeds eastern Pacific values. Thus the marine data show (i) that the latitudinal diversity gradient is not a simple consequence of recent geologic history, because the two oceans had different histories yet exhibit similar diversity trends today; and (ii) that the western Atlantic diversity gradient was resilient to an intense turnover episode, thereby suggesting an evolutionary dynamic underlying the latitudinal patterns, such that speciation compensated for heightened extinction rates.
The latitudinal diversity gradients in one of the most diverse benthic macroinvertebrate groups on the eastern Pacific and western Atlantic shelves therefore cannot be explained by the sizes of habitat area or by latitudinal differences in species range lengths. Environmental variables that exhibit globally pervasive latitudinal trends and that have been proposed to influence evolutionary dynamics are chiefly related to solar radiation, such as temperature, productivity, and seasonality (4, 21, 22, 30). Here we have shown that diversity trends are significantly correlated with mean SST along both coasts, although the relationship is weak or nonsignificant in tropical latitudes. Clearly, the relationships between diversity and other variables such as primary productivity and the trophodynamics within communities need to be quantified, although this will be difficult because of correlations among variables (22). Finally, the mechanisms that link those variables to evolutionary rates, including speciation and extinction, are poorly understood; whether the latitudinal differences are best viewed as tropical enhancement or high-latitude damping of evolutionary rates is still unclear (9, 31). A few studies suggest higher turnover rates in the tropics (32), but this needs to be confirmed. Comparative inter-ocean and across-time analyses for living and fossil biotas are becoming increasingly feasible, and should yield a better understanding of this fundamental biodiversity pattern.
Acknowledgments
We thank Mike Foote, University of Chicago, and Jere Lipps, University of California, Berkeley, for manuscript review, and J. H. McLean and L. T. Groves, Natural History Museum of Los Angeles County, for access to their collections. This work was supported by National Science Foundation Grants EAR90-05744, EAR91-96068, and EAR93-17114.
ABBREVIATION
- SST
sea surface temperature
References
- 1.Rhode K. Oikos. 1992;65:514–527. [Google Scholar]
- 2.Rosenzweig M L. J Mamm. 1992;73:715–730. [Google Scholar]
- 3.Stevens G C. Am Nat. 1989;133:240–256. [Google Scholar]
- 4.Turner J R G, Lennon J J, Greenwood J J D. In: Aspects of the Genesis and Maintenance of Biological Diversity. Hochberg M E, Clobert R, Barbault R, editors. Oxford: Oxford Univ. Press; 1996. pp. 199–220. [Google Scholar]
- 5.Roy K, Jablonski D, Valentine J W. Proc Natl Acad Sci USA. 1994;91:8871–8874. doi: 10.1073/pnas.91.19.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricklefs R E, Schluter D, editors. Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Chicago: Univ. of Chicago Press; 1993. [Google Scholar]
- 7.Jablonski D, Valentine J W. Paleobiology. 1990;16:126–142. [Google Scholar]
- 8.Rosenberg G. Am Malacol Bull. 1993;10:257–266. [Google Scholar]
- 9.Stehli, F. G., Douglas, R. G. & Newell, N. D. Science 164, 947–949. [DOI] [PubMed]
- 10.Bernard F R, McKinnell S M, Jamieson G S. Geol Soc Canada Spec Publ Fish Aquat Sci. 1991;112:1–60. [Google Scholar]
- 11.Altena C C van R. Zool Verhandl. 1969;101:1–49. [Google Scholar]
- 12.Vermeij G J. Veliger. 1990;33:346–354. [Google Scholar]
- 13.Briggs J C. Marine Zoogeography. New York: McGraw–Hill; 1974. [Google Scholar]
- 14.Valentine J W. Limnol Oceanogr. 1966;11:198–211. [Google Scholar]
- 15.Rohde K, Heap M, Heap D. Am Nat. 1993;142:1–16. [Google Scholar]
- 16.Letcher A U, Harvey P H. Am Nat. 1994;144:30–42. [Google Scholar]
- 17.Brown J H, Stevens G C, Kaufman D M. Annu Rev Ecol Syst. 1996;27:597–623. [Google Scholar]
- 18.Flessa K W. Paleobiology. 1975;1:189–194. [Google Scholar]
- 19.Rosenzweig M L, Sandlin E A. Oikos. 1977;80:172–176. [Google Scholar]
- 20.Rhode K. Oikos. 1997;79:169–172. [Google Scholar]
- 21.Wright C H, Currie D J, Maurer B A. In: Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Ricklefs R E, Schluter D, editors. Chicago: Univ. of Chicago Press; 1993. pp. 64–74. [Google Scholar]
- 22.Fraser R H, Currie D J. Am Nat. 1996;148:138–154. [Google Scholar]
- 23.Pianka E R. Am Nat. 1966;100:33–46. [Google Scholar]
- 24.Stanley S M, Addicott W O, Chinzei K. Geology. 1980;8:422–426. [Google Scholar]
- 25.Valentine J W. Paleobiology. 1989;15:83–94. [Google Scholar]
- 26.Roy K, Jablonski D, Valentine J W. Geology. 1995;23:1071–1074. [Google Scholar]
- 27.Stanley S M. Palaios. 1986;1:17–36. [Google Scholar]
- 28.Allmon W D, Rosenberg G, Portell R W, Schindler K S. Science. 1993;260:1626–1629. doi: 10.1126/science.260.5114.1626. [DOI] [PubMed] [Google Scholar]
- 29.Jackson J B C. In: Extinction Rates. Lawton J H, May R M, editors. Oxford: Oxford Univ. Press; 1995. pp. 45–54. [Google Scholar]
- 30.Valentine J W. Evolutionary Paleoecology of the Marine Biosphere. Englewood Cliffs, NJ: Prentice–Hall; 1973. [Google Scholar]
- 31.Jablonski D. Nature (London) 1993;364:142–144. [Google Scholar]
- 32.Flessa K W, Jablonski D. In: Evolutionary Paleobiology. Jablonski D, Erwin D H, Lipps J H, editors. Chicago: Univ. of Chicago Press; 1996. pp. 376–397. [Google Scholar]