Abstract
Objective
We propose a theoretical framework for EEG and evoked potential studies based on the single postulate that these data are composed of a combination of waves (as this term is used in the physical sciences) and thalamocortical network activity.
Methods
Using known properties of traveling and standing waves, independent of any neocortical dynamic theory, our simple postulate leads to experimental predictions, several of which have now been verified. A mathematical-physiological theory of “brain waves” based on known (but highly idealized) properties of cortical synaptic action and corticocortical fibers is used to support the framework.
Results
Brain waves are predicted with links between temporal frequencies and the spatial distributions of synaptic activity. Such dispersion relations, which essentially define more general phenomena as waves, are shown to restrict the spatial-temporal dynamics of synaptic action with many experimental EEG consequences.
Conclusions
The proposed framework accounts for several salient features of spontaneous EEG and evoked potentials.
Significance
We conjecture that wave-like behavior of synaptic action may facilitate interactions between remote cell assemblies, providing an important mechanism for the functional integration underlying conscious experience.
Keywords: traveling waves, standing waves, dispersion relation, propagation velocity, corticocortical fibers, physiological basis for EEG, synaptic action fields, consciousness, binding problem
Introduction
Scalp recorded electric potentials or electroencephalograms (EEGs) provide estimates of synaptic action at large scales closely related to behavior and cognition. Thus, EEG can provide a genuine window on the mind, especially if EEG properties are related to the underlying physiology. In this paper we focus on the wave-like dynamic behavior of EEG and show that the single postulate that EEG is actually composed partly of “waves” (as this term is used in the physical sciences) leads to a number of experimental predictions, several of which have now been verified. Furthermore, the wave-like behavior of EEG implies wave-like behavior of the underlying synaptic action fields that may facilitate interactions between cell assemblies believed responsible for conscious experience (Edelman 1978; Edelman and Tononi 2000).
A number of studies have presented experimental evidence that EEGs exhibit wave-like dynamic behavior. Waking EEG (Nunez 1974b, 1995), sleep EEG (Massimini et al 2004) and visually evoked potentials (Hughes et al 1992, 1995; Burkett et al 2000; Klimesch et al 2006; Nunez and Srinivasan 2006; Srinivasan et al 2006) appear to be partly composed of traveling waves. In addition, several experimental (Nunez et al 1976; Nunez 1995; Burkett et al 2000, Wingeier et al 2001; Wingeier 2004; Nunez and Srinivasan 2006) and theoretical (Nunez 1974a, 1989, 2000a, b; Nunez et al 2001) studies have suggested that EEG and evoked potentials are also partly composed of standing waves. This paper considers possible neurophysiological bases for these wave phenomena as follows:
Provide a theoretical framework for experimental studies of “brain waves” based only on general properties of known physical waves, independent of neocortical dynamic theory.
Demonstrate this general idea with a specific mathematical-physiological theory based on known (but highly idealized) properties of synaptic action in neocortex and its corticocortical (white matter) fiber systems.
Speculate on the possible roles of traveling and standing waves of synaptic action in facilitating interactions between brain networks.
Various features of the global theory of standing and traveling waves, first introduced by the senior author (Nunez 1972), have been published over the past 34 years. Issues addressed in past papers include the physiological idealizations and mathematical approximations required to obtain analytic solutions that lend themselves to interpretations of genuine EEG data. This paper contains a short review of the earlier material (refer especially to Appendix A), but mainly emphasizes that “brain waves” can be expected to have certain predictable dynamic properties, largely independent of the necessary physiological idealizations and mathematical approximations. Here we do not favor our specific global model over other models (whether complementary or competing) that may also predict large scale wave phenomena.
EEG is believed to be generated largely by synaptic current sources that we characterize as global fields of synaptic action (Nunez 1974a, 1981, 1995; 2000a,b; Nunez and Srinivasan 2006). These synaptic action fields are the (short-time) modulations in number densities of active excitatory and inhibitory synapses about background levels, analogous to sound waves, which are short-time modulations about background pressure. The word “field” used here and in the physical sciences denotes any continuous mathematical function of time and location, in this case the number densities of active excitatory and inhibitory synapses in each cortical tissue mass. Defined in this manner, the existence of these fields is non-controversial; the only open question is whether they are useful descriptors in neuroscience. We introduce the synaptic action fields for two reasons (1) The neocortical excitatory and inhibitory synaptic action fields are believed to underlie the electric (EEG) and magnetic fields (MEG) recorded at the scalp (2) We conjecture that these fields may act (top down) on networks, thereby facilitating important dynamic interactions between remote (and perhaps unconnected) networks. Here we use the label “top down” to indicate the hierarchical influence of large scale systems on smaller scale systems, consistent with its use in the physical sciences.
In apparent contrast to “wave-like” descriptions, many scientists have modeled neocortical dynamics in terms of cortical or thalamocortical networks (Wilson and Cowan 1973; Freeman 1975, 1992; Lopes da Silva 1991, 2000; Lumer et al 1997; Wright et al 2001). The wave-like global field and network pictures are not, however, incompatible as suggested by Fig. 1. Several theoretical studies based on genuine physiology and anatomy suggest that networks and global fields (including traveling and standing waves) can easily coexist in neocortex (Nunez 1981, 1989; 2000a,b; Jirsa and Haken 1997; 1999; Haken 1999; Liley et al 2002; Robinson et al 2003, 2004). We generally expect intracranial recordings to emphasize network activity at relatively small scales. Electrophysiological recordings are quite dependent on spatial scale, determined largely by electrode size in the case of intracranial recordings (Abeles 1982; Nunez 1995). By contrast, scalp recorded EEG dynamics emphasize global synaptic field dynamics due to the severe space averaging by volume conduction effects, mainly the physical separation between sources and sensors and the low conductivity skull (Nunez 1981; Nunez and Srinivasan 2006).
Figure 1.
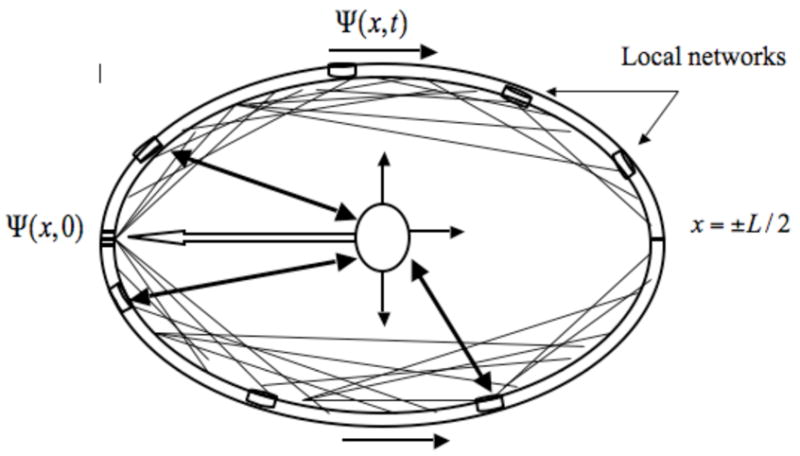
An idealized closed loop of neocortex is shown with location on the cortical circumference determined by the x coordinate that varies on the interval −L/2 < x < + L/2. An initial input Ψ(x,0) centered on x = 0, perhaps produced by a visual stimulus, reaches the cortex via the thalamus (inner ellipse). This stimulus causes two traveling wave pulses of excitatory synaptic action density Ψ(x, t) to propagate through superficial and mesial neocortical tissue with a characteristic speed determined largely by action potential propagation velocities along the corticocortical fibers, represented here by the light lines. The actual number in human neocortex is about 1010, a number sufficiently large to allow every macrocolumn (3 mm diameter) to be connected to every other macrocolumn in an idealized homogeneous system. Cell assemblies (neural networks) are assumed to be embedded in and interact with the synaptic action fields, which may facilitate interactions between remote networks. The thalamus participates in local networks (double arrows and cylinders) in addition to modulating cortical excitability by chemical or electrical input on long time scales (three light arrows). In the global theory proposed here this cortical excitability is determined by the control parameter β.
The conceptual framework adopted for this study is one in which the networks (or cell assemblies) underlying specific behavior and cognition are embedded in global fields of synaptic action (Nunez 2000a,b; Nunez and Srinivasan 2006). A sociological metaphor is perhaps appropriate here– social networks are embedded in a culture that influences the networks (top down). On the other hand, the culture is itself influenced (bottom up) by multiple interacting networks. The synaptic fields are believed driven by networks (bottom up), but may also act (top down) on the networks, thereby potentially facilitating interactions between remote networks to produce an (apparent) unified behavior and consciousness (“brain binding”). As in the sociological metaphor, no sharp distinction between fields and networks is implied; all synaptic action generates the fields and neurons with preferential functional connections form embedded networks. Thus, any active neuron contributes to the fields but also may be a member of multiple networks that functionally connect and disconnect on time scales in the range of perhaps 100 to 500 ms (Gevins and Cutillo 1986, 1995). Our distinction between field and network is partly one of spatial scale; at the very large spatial scale of scalp potentials, the continuous field variable is more closely related to experimental measures. Analogous dynamic phenomena have been widely studied in physical, biological, social and financial systems under the rubric “synergetics”, the so-called science of cooperation (Haken 1983; Ingber 1995). Top down/bottom up interactions in complex dynamic systems, including our postulated neocortical interactions, have been labeled “circular causality” by the founder of synergetics (Haken 1999).
Methods
What are “waves”?
In the neurosciences literature, the word “wave” is often used imprecisely. In order to make our case for genuine brain waves, we must be clear just what we mean by this term. Generally, the word “wave” indicates some disturbance that moves through a medium like air, water, plasma, tissue, or even empty space over long distances with minimal loss of energy or permanent distortion of the medium. The distinction between wave and nonwave can often be vague, however. For example, the passage of light through fog can change from wave propagation to a diffusion process if the fog becomes sufficiently thick. The simple and compound action potentials fit the above qualitative definition of “wave”, but they are not normally described as waves but rather in terms of the underlying membrane behavior, a nonlinear diffusion process. In this paper we are concerned with waves of synaptic action in a closed loop of idealized neocortex −L/2 < x < +L/2, where positive x indicates the upper cortical surface distance and negative x the mesial surface distance from primary visual cortex (Fig. 1).
Treatment of the human cortex/corticocortical fiber system as “closed” in the topological sense appears well justified based on the number (1010) of corticocortical fibers, a number sufficiently large to allow for complete connections at the macrocolumn scale, that is, every macrocolumn would be connected to every other macrocolumn in an idealized homogeneous corticocortical system (Braitenberg 1978; Braitenberg and Schuz 1991). The existence of preferential fiber paths prevents such complete connections at the macrocolumn scale; however, one may guess something close to complete connections at the very large scales (cms) appropriate for scalp EEG theory (Nunez 1995). Preferential (heterogeneous) connections may then be included in global theories as perturbations of the homogeneous model (Jirsa and Kelso 2000), but all large scale theories are expected to break down at smaller scales. Thus, one must be cautious with any attempts to extrapolate our predictions of large scale scalp waves to intracranial recorded waves that may be dominated by local morphology (depending also on electrode size). For example, waves with mm scale wavelengths are expected to propagate at much slower speeds, with delays perhaps due mainly to PSP rise and decay times (Wilson and Cowan 1973; Freeman 1975, 1992; Lopes da Silva and Storm van Leeuwen 1978; Lopes da Silva 1991, 1995).
For purposes of this introductory discussion, we consider waves in a one-dimensional infinite medium. A broad class of dynamic systems is governed (at least approximately) by linear differential or integral equations with an external input or forcing function F (x, t) of the form
| (1) |
Here ℜ indicates some arbitrary linear operator. For example, ℜ might describe the dynamic behavior of an ocean surface displacement Ψ(x, t)when acted on by wind forces F (x, t). ℜ might also describe the dynamic behavior of the excitatory synaptic action field Ψ(x, t) when forced by (subcortical) noise or a visual stimulus F (x, t). Although this linear approximation may appear severely restrictive in describing (generally nonlinear) brain dynamics, it is common practice in the physical sciences to apply linear approximations to systems known to be nonlinear by examining limiting cases where the system behaves in an approximately linear manner. Equation (1) is typically solved by taking the (double) Fourier transform Ψ(k, ω) of the function Ψ(x, t). This procedure is based on the Fourier integral theorem, which can be used to decompose any well behaved mathematical function into spatial (k) and temporal (ω) frequency components without reference to the underlying physical or biological process (if any). The solution to Eq (1) may be expressed generally in terms of the double integral
| (2) |
The dispersion function D(ω, k) is determined by the operator ℜ, that is, by the properties of the wave medium, and F(k, ω) is the double Fourier transform of the input function. The inverse of the dispersion function is a dynamic transfer function TD(ω, k) ≡ D(ω, k)−1, an extension of the idea commonly used in simple systems like electric circuits with only time as the independent variable. The inner integral in Eq (2) is determined by the singularities of the integrand. In most physical applications, the Fourier transform of the forcing function F(k, ω) and dispersion function D(ω, k) are entire functions of ω(meaning they are analytic everywhere in the complex ω plane) so that the integrand is singular only at the poles determined from
| (3) |
Solution of Eq (3) normally yields the resonant frequencies as a function of wavenumber, that is, the dispersion relation
| (4) |
Here the real part of the dispersion relation ωR(k) is the characteristic oscillation frequency of the field Ψ(x, t), and the imaginary part γ(k) is the damping of the field, both generally depend on the wavenumber k. Physical examples where dispersion relations may only exist in an approximate sense are discussed in Montgomery and Tidman (1964) and Nunez (1971). If a dispersion relation exists, Eq (2) may be expressed
| (5) |
Thus, rather than a general spatial temporal field, each spatial frequency k is associated with (usually) a single temporal frequency ω(k). More complicated dynamics may result in multiple ωb(k) or multiple branches of the dispersion relation. We here define a linear “wave” as any phenomenon for which (1) a dispersion relation exists or is at least well approximated and (2) the damping is relatively small, that is, γ(k) ≪ ωR(k). With this definition, “waves” propagate with minimal loss of energy to the medium. Note however, that according to condition (2), the field Ψ(x, t)may be a “wave” over some wavenumber range and a “non-wave” (e.g., diffusion) in other wavenumber ranges, depending on the relative magnitudes of γ(k) and ωR(k). Most of these results extend naturally to two or three spatial dimensions, although more complicated geometry complicates the mathematics, as in spherical or ellipsoidal shells representing neocortex (Nunez 1995).
Despite the severe restrictions on possible dynamic behavior dictated by the dispersion relation, Eq (5) still allows for very complicated dynamics; ocean waves provide one example (Nunez 2000a). Nevertheless, Eq (5) represents a substantial simplification over the general spatial-temporal behavior possible in complex systems, spatial-temporal chaos and spatial-temporal white noise being prominent examples. Equation (5) says that each wavenumber (spatial frequency) component k is associated with a corresponding temporal frequency component ω(k) or perhaps several temporal frequencies if the dispersion relation has multiple branches (multiple roots of Eq (3)). The wavenumber (k, cm−1) is related to the wave’s spatial wavelength (l, cm) by . The best known dispersion relation is ω(k) = vk, where v is the characteristic velocity of the wave medium. This relation for non-dispersive waves (ordinary sound waves and light in empty space, for example), describes waves that do not spread or distort as they propagate through the medium. However, most waves in nature are dispersive; they have more complicated dispersion relations that determine how they distort and how fast a localized pulse propagates. We emphasize that the dispersion relation is a property only of the wave medium; it is independent of the external input or forcing function F(x, t) and usually independent of boundary conditions.
Brain wave theory. Global fields of synaptic action
In order to provide an appropriate theoretical context for the experimental study of brain waves, we briefly outline a dynamic global model simplified to one spatial dimension (Nunez 1995), but emphasize that most of the general results in this paper are independent of the accuracy of this particular model. This model follows oscillations of synaptic action fields, which are defined as the continuous functions Ψe(x, t) and Ψi(x, t) representing the numbers of active excitatory and inhibitory synapses per unit area of cortex. The short-time modulations of these field variables are believed to be directly related to cortical or scalp recorded EEG (Nunez and Srinivasan 2006). In a series of publications over the past 34 years, this global model has predicted several general features of EEG, including standing wave oscillations of synaptic action in roughly the 6–15 Hz range and traveling waves with posterior- anterior propagation velocities in roughly the 5 to 10 m/sec range (Nunez 1974a, 1981, 1995).
In the purely global model, all network influences are neglected. For example, thalamocortical feedback loops and local cortical circuits are assumed nonexistent. Rather, we postulate simple input/output relations (linear or nonlinear-sigmoid) between synaptic action and action potentials fired in each mesoscopic tissue mass. In this greatly oversimplified model, delays are due only to finite propagation speeds in the longest corticocortical fibers (Nunez 1974a). We do not claim that this approximation is generally accurate, only that it provides a convenient entry point to the complexity of neocortical dynamics by focusing on the dynamic effects of corticocortical fiber systems. These systems are believed to mediate interactions in large-scale brain networks, a process sometimes termed “reentry”, which has been proposed as a basis for consciousness (Edelman 1978; Edelman and Tononi 2000).
For this paper we choose one-dimensional geometry (Nunez 1974a, Nunez and Srinivasan 2006) rather than a spherical (Katznelson 1981; Nunez 1995) or prolate spheroidal shell (roughly the shape of a Rugby football, Nunez 1995) to model neocortical dynamics based on the following. (1) Our experimental evidence of EEG waves has been obtained mainly for waves traveling in the anterior-posterior direction of one hemisphere. Estimates in other directions are more difficult due to the spatial limitations of scalp electrode placements. (2) Predictions of the proposed global theory depend strongly on the longest corticocortical fibers, which may run more in the anteriorposterior direction; the long corticocortical fiber systems are anisotropic. (3) Limitation to one spatial dimension simplifies the mathematics. Most of the general qualitative results can be extended to wave propagation in a closed shell (Nunez 1995, 2001a,b; Nunez and Srinivasan 2006).
The derived theoretical dispersion relation follows from a relatively straightforward analysis of synaptic action fields in a closed loop of idealized one dimensional cortex, essentially around the longest circumference as shown in Fig. 1. As outlined in Appendix A, the real and imaginary parts of the theoretical dispersion relation are (Nunez 1995, 2000a; Nunez and Srinivasan 2006)
| (6) |
The parameters estimated from physiology are as follows:
v the characteristic propagation velocity in corticocortical fibers ( ∼ 600 to 900 cm/sec)
k the wavenumber of each mode, , where L ∼ 50 cm is the circumference of the closed neocortical loop (Fig. 1)
λ the characteristic fall-off parameter with distance in fiber density in the long corticocortical systems, based on assumed exponential decay (∼ 0.1 cm−1)
β the background excitability of neocortex (dimensionless parameter, unknown)
In this theory the background excitability of neocortex β is determined by chemical or electrical input from the thalamus on long time scales as indicated in Fig. 1. This dimensionless parameter cannot now be accurately estimated from cortical physiology (Nunez 1995), but Eq (6b) indicates that all modes become unstable when β > 1. More accurate theory (including the spread in corticocortical velocities, for example) suggests γ = γ(k), that is, higher modes are more heavily damped for fixed β (Nunez 1995). We postulate that additional negative feedback from thalamus or cortex is recruited in healthy brains to prevent instability (possibly epilepsy), as discussed in Nunez and Srinivasan (2006). This type of local inhibition in response to excitatory input is characteristic of columnar organization in neocortex (Mountcastle 1997). With an assumed β 1and v ∼7.5 m/sec, the predicted fundamental mode frequency is
| (7) |
Higher (overtone) frequencies are also obtained from the dispersion relation (6); however, the spread in corticocortical velocities and other low pass filtering mechanisms may tend to suppress these higher modes (Nunez 1995). The phase velocity vP1 of predicted traveling waves of the fundamental mode k1is
| (8) |
Generally, we expect propagating wave packets composed of several (or perhaps many) modes kn having different phase velocities. In media that are nearly nondispersive, wave packets propagate at the group velocity vG = dω/dk; however, the group velocity has no simple physical meaning is media that are highly dispersive (Jackson 1975) as may be the case for brain waves. If a medium is sufficiently dispersive, wave pulses may become so distorted as they travel that it becomes difficult or impossible to define a propagation velocity.
Since volume conduction limits scalp recorded potentials to lower wavenumbers (longer wavelengths), we generally expect to observe waves with velocities in the general range of characteristic corticocortical propagation, apparently v 6 to 9 m/sec if the fibers are fully myelinated but perhaps somewhat lower based on evidence for incomplete myelination (see review in Nunez 1995). Because of cortical folding, propagation velocities along neocortex are expected to be about double propagation at the scalp (Nunez 1995). Scalp phase velocity estimates of 3 to 7 m/sec (Silberstein 1995a; Nunez 1995; Burkitt et al 2000) and 1 to 3 m/sec (Srinivasan et al 2006) have been observed in recordings of steady-state visual evoked potentials (SSVEP). Alpha rhythm phase velocities of 3 to 7 m/sec (Nunez 1995) and sleep waves of 1 to 7 m/sec have been reported (Massimini et al 2004). Scalp group velocity estimates for SSVEP of 3 to 7 m/sec and alpha rhythm of 3 to 5 m/sec (Wingeier 2001, 2004) have been obtained (see review in Nunez and Srinivasan 2006).
Theoretical predictions of EEG “propagation velocity” at the scalp are imprecise for several reasons, including (1) Phase and group velocities generally differ from v and from each other as in the example Eq (8). (2) Corticocortical propagation velocities are distributed rather than peaked at a single velocity v (Nunez 1995, Appendix A). (3) The head volume conductor acts as a spatial filter such that unprocessed scalp potentials represent only the long wavelength (small k) part of cortical source dynamics. Despite these limitations, the global theory outlined here is able to make the general prediction of traveling and standing waves with propagation velocities in the general range of v, as discussed in more detail in the following section.
Results and Discussion
Simulated pulses. Traveling and standing waves
Here we simulate traveling and standing waves produced by an external stimulus in a closed loop of wave medium with some dispersion relation on ω(k) as shown in Fig. 1. With the dispersion relation unspecified, our results depend only on the wave postulate, independent of any specific neocortical dynamic theory. We follow the modulation of excitatory synaptic action given in simplified notation by Ψ(x, t) ≡ δΨe(x, t). Consider an input pulse at t = 0with the initial Gaussian spatial shape of half width Δx ≪ L centered at x = 0, that is
| (9) |
As shown in Appendix B, the solution for times t > 0 is given by
| (10) |
Here the sum is over all allowed wavenumbers
| (11) |
Relation (11) is forced by periodic boundary conditions in the closed cortical loop, that is, only waves whose wavelengths ln are an integer fraction of the loop circumference L can persist, that is, . This condition is caused when traveling waves interfere to form standing waves. We emphasize that no wave reflection from boundaries occurs here, as in the common example of a stretched violin string where half integer wavelengths are also allowed. Equation (10) consists of two wave pulses, traveling in the positive and negative x directions as in the example of Fig. 2 based on the brain wave dispersion relation Eq (6). After about 30 ms, the two wave pulses begin to interfere to form standing waves. The solution given by Eq (10) is valid for any linear wave medium with dispersion relation ω(k).
Figure 2.

The spatial shapes of the initial Gaussian pulse (t = 0) given by Eq (9) and resulting traveling wave pulses at t = 20, 40 and 60 ms are shown. Positive and negative x correspond to upper and mesial cortex, respectively, as shown in Fig. 1. Wave interference begins to occur near t = 30 ms when the two dispersive pulses come together near frontal cortex resulting in standing waves. At t = 60 ms (lower plot) is two pulses have partly coalesced to form a single pulse with negative sign. The traveling wave solution for the synaptic action field Ψ(x, t) is given by Eq (10) and the proposed dispersion relation for brain waves, Eq (11). Parameters chosen for these plots are Δx = 4 cm, β =1, v = 7.5 m/sec, 1 0.1 cm−1 λ = and L = 50 cm
We apply Eq (10) to an imagined experiment with a visual stimulus represented by Eq (9) in a closed loop of cortex. Figure 3 illustrates a solution based on the brain wave dispersion relation Eq (6) for the predicted scalp potential Φ(x, t). Scalp potential is a spatially filtered version of the synaptic action field Ψ(x, t) ; this spatial filtering is based on a four shell model of volume conduction in the head (Nunez and Srinivasan 2006). At early times, roughly t < 30 ms, brain waves are predicted to travel away from the primary visual area across upper (x > 0) and mesial cortex (x < 0). After about 30 ms, the two pulses pass through each other near prefrontal cortex. If the medium were nondispersive (as well as undamped), these pulses would continue traveling around the closed loop indefinitely. However, wave dispersion causes the initial pulse to broaden and distort over time so that it soon fills the entire loop, forming standing waves composed of multiple modes (frequencies and corresponding wavenumbers). Figure 3 demonstrates that scalp potential Φ(x, t) is composed of multiple modes ωR(kn), but spatial filtering by the volume conducting head emphasizes the fundamental mode (near 9 Hz) except near the nodal lines of the fundamental standing wave. In this example, such nodal lines tend to occurs near x = ±L/4 or about ±12.5 cm. At these locations, higher mode oscillations are more evident.
Figure 3.

The simulated scalp potential Φ(x, t) due to traveling and standing waves of the synaptic action field Ψ(x, t) is shown at x = 0, 5, 10, 15, 20, 25 cm for the first second following application of a Gaussian pulse applied at t = 0 given by Eq (9). Scalp potential is estimated from synaptic action field by applying a head volume conductor transfer function TV (k) derived in Nunez and Srinivasan (2006), that is, Φ(k, ω) = TV (k) Ψ(k, ω), where TV (k) is a low pass spatial filter. The resulting first three mode frequencies obtained from Eq (6) are 9.1, 27.5 and 43.3 Hz. The fundamental mode tends to dominate in scalp potential as a result of the combined effects of the spatial filtering by the volume conductor and the linking of spatial to temporal frequencies in Ψ(x, t) by the dispersion relation. Exceptions occur near nodal lines of the fundamental mode that tend to occur near central regions x 10 – 15 cm. .
Experimental implications of EEG dispersion relations
We have demonstrated several expected relationships between cortical and scalp recorded potentials based on a single postulate: the cortical synaptic action field Ψ(x, t) is partly composed of genuine “waves”, that is, Ψ(x, t) satisfies a dispersion relation ω = ω(k) at large scales with effective damping that is weak (in the linear case) or zero (in the case of instability prevented by nonlinear negative feedback effects). These demonstrations make use of a simple, physiologically-based global wave theory of neocortex. We do not claim that this model comes close to capturing the full dynamic behavior of brain waves. Furthermore, we have neglected critical influences of cell assemblies (neural networks), which we picture as embedded in the global synaptic fields. Possible experimental support for this idea comes from studies suggesting that multiple alpha rhythms with similar or nearly identical dominant frequencies are generated by a combination of widely distributed global fields and local cortical “patches” that suggest the presence of local networks (Andrew and Pfurtscheller 1997; Florian et al 1998; Nunez 2000a; Nunez and Srinivasan 2006). A similar finding was obtained for SSVEPs (Srinivasan et al 2006a).
We include thalamocortical networks in the “local” category because (1) they are local from the viewpoint of a surface electrode (2) resonant frequencies are independent of global boundary conditions (3) resonant frequencies are believed to be independent of axonal delays. All three conditions distinguish local networks from global fields, but the distinction between (large) regional networks and global fields is not so sharply defined. The distinction between networks and fields is partly one of spatial scale. The categories may overlap at intermediate scales if the “field” label is applied to neural populations with preferential connections; however in contrast to the network label, the label “field” makes no assumption about neural connections.
We conjecture that network activity is dominant and brain waves cannot be observed at the scalp in brain states of moderate to demanding mental tasks in which the neocortical dynamic balance is shifted away from global coherence towards more functional segregation (Edelman 1978; Edelman and Tononi 2000). The best global wave candidates appear to be the states of resting alpha (only part of the alpha band), anesthesia, deeper sleep stages, and globally coherent epilepsy. Opening the eyes or complex mental calculations in the awake state may occur with enhanced cell assembly activity, thereby reducing global field behavior, at least as recorded on the scalp. However, responses of the brain to visual input exhibit both localized network and wavelike characteristics (Srinivasan et al 2006) suggesting that wave-like dynamics influences the processing of visual stimuli.
Whereas all genuine brain states are believed to reflect some mixture of global wave and cell assembly activity, the following summary of several experimental implications is based only on the single hypothesis that neocortical dynamics is partly composed of waves.
More relative power should be recorded on the cortex (or dura) at frequencies above the fundamental mode than on the scalp, a prediction with experimental support in the case of waking EEG, with much more relative power above the alpha band obtained in cortical recordings (Pfurtscheller and Cooper 1975; Nunez 1981). This is expected because the head volume conductor acts as a spatial filter, and the wave hypothesis links temporal frequencies to spatial frequencies through the dispersion relation; spatial filtering then implies temporal filtering as demonstrated in Fig. 3.
All other properties being equal, larger brains should produce lower fundamental mode frequencies. In the one-dimensional model, this prediction follows simply from Eqs (6) and (11), the larger the circumference of the cortical loop, the lower the mode frequencies provided the parameters v, λ, β are held constant; the general idea extends to ellipsoidal shells or other geometry used to model neocortex. This prediction has experimental support in the case of resting alpha rhythms (Nunez et al 1977; Nunez 1981, 1995).
We expect to observe cortical waves traveling away from primary visual areas as a result of visual stimuli. The propagation velocity is expected to be the general range of the bulk of corticocortical fiber speeds, something like 6 to 9 m/sec, within a factor of perhaps two or three due to both experimental and theoretical uncertainties. Substantial experimental support for this general prediction has been published (see citations in the Introduction and review in Nunez and Srinivasan 2006).
Short epochs of traveling waves (in variable directions) in roughly the same velocity range may be expected in spontaneous EEG. At least two groups have reported this phenomenon as cited in the Introduction.
We might search for direct evidence for the existence of dispersion relations from experimental spatial-temporal (frequency-wavenumber) spectra for scalp potentials at frequencies greater than the postulated fundamental mode. Such evidence has been advanced from three different laboratories for alpha rhythm above about 8 Hz (Nunez 1974b; Nunez 1981; Shaw 1991; Wingeier 2001, 2004) and SSVEP (Wingeier 2004; review by Nunez and Srinivasan 2006).
We expect EEG or MEG coherence between remote scalp locations to vary with cognition and behavior as a result of combined network and global field effects. By “remote” we mean EEG electrodes or MEG coils sufficiently far apart to minimize volume conduction effects (Gevins et al 1994; Nunez 1995; Nunez et al 1997, 1999; Srinivasan et al 1998; Srinivasan et al., 1999; Nunez and Srinivasan 2006). EEG (Nunez 1995; Nunez et al 2000) and SSVEP (Silberstein 1995a; Silberstein et al 2001, 2003, 2004) phase and coherence changes with mental tasks have been published. Conscious perception of a visual stimulus has been shown to occur with increased intrahemispheric MEG coherence (Srinivasan et al 1999) and phase-locking of frontal and occipital regions of the brain (Srinivasan and Petrovic 2006). Silberstein et al (2003, 2004) have emphasized the experimental finding that demanding mental tasks require decreased coherence between some regions together with increased coherence between other regions. These changes (coherence in the frequency domain or covariance in the time domain, Gevins and Cutillo 1986, 1995; Gevins et al 1997) are believed caused by some combination of cell assembly and global field interactions.
SSVEP’s are obtained by driving the visual system with a sinusoidally modulated flicker. Based only on the standing wave theory, we expect the spatial and temporal properties of SSVEP to be sensitive functions of both flicker frequency and spatial structure of the stimulus. Furthermore, the standing wave picture suggests that when the driving frequency matches the fundamental cortical resonant frequency, a large response may be expected at a distance of ½ wavelength from primary visual cortex, that is, near frontal cortex as demonstrated in Fig. 3. In the idealized case of no local networks and zero damping of the global field, this secondary region would have amplitudes equal to the responses near visual cortex, but we expect local visual network responses to add additional power. These predictions have some experimental support from cortical surface recordings of spontaneous EEG (Jasper and Penfield 1949; Nunez 1995) and scalp recordings of SSVEP (Silberstein 1995; Burkitt et al 2000; Nunez 2000a,b; Nunez and Srinivasan 2006; Srinivasan et al 2006). For example, some resonances are enhanced by attention, while others are suppressed by attention (Ding et al 2006); these networks exhibit different preferred frequencies, spatial distributions, and phase-locking.
One may question if this analysis simply represent a novel way of looking at process that are already understood rather than suggesting more profound implications for standard EEG methodology. We contend that the experimental predictions listed above imply that the general standing/traveling wave framework proposed here has substantial implications for future studies. Our central hypothesis is that local and regional networks can coexist naturally with widely distributed sources that we characterize in terms of global synaptic fields. Either of these overlapping and interacting processes may be emphasized with different experimental strategies. In both spontaneous and evoked potentials, MEG and EEG are generally sensitive to different source distributions (Nunez and Srinivasan 2006). Conventional (reference) EEG and evoked potentials are most sensitive to global fields. High-resolution EEG measures (such as the Laplacian) act as spatially band-pass filters of EEG, thereby reducing the contribution of global fields relative to network activity in gyral surfaces. MEG is mainly sensitive to isolated sources in sulcal walls; thus, MEG is relatively insensitive to global fields, except (potentially) the edges of large synchronous source regions.
The differences in spatial sensitivity of these measures imply that different temporal dynamics will be recorded in different experiments. Even the global fields alone can be expected to show different dynamics at different spatial scales as implied by the underlying dispersion relations. Local and regional networks in different parts of cortex are generally expected to produce distinct dynamic behavior; however, such network dynamics may partly reflect the common (top-down) influence of global fields. Furthermore, the local and regional networks are expected to be selectively recorded by high-resolution EEG and MEG depending on local cortical (dipole) orientation. This general picture is supported by our recent studies comparing conventional EEG, highresolution EEG and MEG in simultaneous recordings of spontaneous activity (Srinivasan et al 2006b) and SSVEP (Thorpe et al 2006). We suggest that promising measures of neocortical dynamic behavior, for example covariance of event related potentials (Gevins and Cutillo 1986, 1995; Gevins et al 1994) and EEG coherence (Nunez 1995; Srinivasan et al 1996, 1998, 1999; Nunez et al 1997, 1999, 2000; Srinivasan 1999; Silberstein et al 2001, 2003, 2004), call for physiological interpretation in terms of networks and fields (Nunez and Srinivasan 2006). Just to pick one issue, generalized increases in anteriorposterior coherence are best explained in terms of enhanced standing wave fields, whereas coherence changes between specific electrode pairs are better interpreted in terms of regional networks (Silberstein 1995a). Such interpretations are likely to impact the experimental designs of future studies. Our general framework also has implications for fMRI and PET, which appear to locate major hubs of widely distributed networks (Nunez and Silberstein 2001).
Cross scale relations. Scalp EEG versus intracortical recordings
We have emphasized the likely top-down influence of global fields on local and regional networks, while largely ignoring critical mechanisms at the local level like the columnar and laminar organization of neocortical neurons and thalamocortical interactions. For example, lateral inhibition is evidently critical to local network oscillations and provides an effective means of allowing local networks to remain semi-autonomous, at least in healthy brains. We readily acknowledge the critical importance of many local properties that we have largely ignored here in order to focus on global processes that are most evident in scalp recordings.
The potential Φ(r, t) at scalp location r due to brain sources can be expressed as the following volume integral over the entire brain (Nunez and Srinivasan 2006)
| (12) |
Here P(r′, t) is the tissue dipole moment per unit volume, that is, the meso-source strength at location r′ and time t. The integral in Eq (12) is weighted by the Green’s function G(r, r′), which accounts for all geometric and conductive properties of the volume conductor. G(r, r′) is large when the “electrical distance” (accounting for both actual distance and the effects of inhomogeneity and anisotropy) between recording location r and meso-source location r′ is small, implying that cortical sources generally make the largest contributions to scalp potentials. The current dipole moment per unit volume P(r, t) of a tissue mass is defined as a weighted space average of all the membrane micro-sources s(r, t) or s(r, w, t) within a volume element W (cortical column, for example)
| (13) |
The vector w locates each source within the volume element, whereas the vector r locates the center of the volume element. The sources s(r, w, t) are due to both synaptic action on membrane surfaces as well as the return currents required for current conservation. For purposes of this discussion, we may express the meso-source function in terms of the synaptic action fields
| (14) |
The vectors Ce(r) and Ci(r) depend on the local laminar structure of neocortex, that is, the distributions of excitatory and inhibitory synapses through the cortical depths as well as the capacitive/resistive properties of cortical cells. These three equations have several general implications (Nunez and Srinivasan 2006):
High spatial frequencies in the meso-source dynamics P(r, t) will not be recorded on the scalp because of positive and negative cancellations in Eq (12); the volume conductor acts as a low pass spatial filter. If the fields Ψe(r, t), Ψi(r, t), P(r, t) satisfy a dispersion relation, temporal filtering is expected as a byproduct of this spatial filtering.
Laplacian and dura image algorithms remove the very low spatial frequencies from scalp recorded potentials leaving only the intermediate spatial frequencies associated mostly with cortical patches with diameters in the several centimeters range. If brain waves consist only of very long wavelength activity (say 10 to 20 cm wavelengths), application of these algorithms will remove evidence of cortical waves, perhaps leaving only local network activity.
The very low spatial frequencies that dominate scalp recordings may not be observed with intracranial electrodes that are mainly sensitive to the local fields Ψe(r, t), and Ψi(r, t). There is no guarantee that networks contributing to these fields will be observed on the scalp.
Maximum meso-source strengths P(r, t) occur when inhibitory and excitatory synapses are widely separated as implied by Eq (13). By contrast, a near uniform mixture of micro-sources approximates a closed field. The so-called stationary or moving dipole solutions are based on the assumption that P(r, t) ≈ 0 at all but a few isolated locations either because the synaptic action fields are near zero of because closed field effects in Eqs (13) and (14).
The mathematical construct of fields is more appropriate for modeling the spatial distribution of synaptic action over the cortex. Network descriptions are most appropriate for the horizontal and vertical interactions underlying a cortical column (Mountcastle 1997). At regional scales, both network and field descriptions may be appropriate depending on context. The field description makes most sense in the context of EEG recordings.
Speculations on the significance of global synaptic fields for conscious experience
We view neocortical source dynamics in terms of rapidly changing cell assemblies (or networks) embedded within a global environment. We express this global environment in terms of synaptic and action potential fields; the traveling and standing waves are special cases of these global fields. Scalp potentials evidently provide signatures of some (generally unknown) combination the synaptic action fields and network activity, but are strongly biased towards dynamics with low spatial frequencies, suggesting substantial contribution from globally coherent synaptic fields. We further conjecture that both bottom up (networks to global) and top down (global to networks) interactions provide important contributions to the neocortical dynamics of behavior and cognition. Such twoway interactions appear to facilitate substantially brain binding, the coordination of separate functions into unified behavior and consciousness.
The global wave picture substantially overlaps views expressed by other neuroscientists. For example, a quantitative measure of complexity has been proposed as a measure of consciousness and brain binding (Edelman and Tononi 2000); these authors state
…high values of complexity correspond to an optimal synthesis of functional specialization and functional integration within a system. This is clearly the case for systems like the brain--different areas and different neurons do different things (they are differentiated) at the same time they interact to give rise to a unified conscious scene and to unify behaviors (they are integrated).
In this view complexity (and by implication, cognition) tends to maximize between the extremes of isolated networks and global coherence. We find this a compelling working hypothesis. It then follows that local network dynamics, interactions between networks, and interactions between networks and global fields are important for brain function. Different experimental designs and methods of data analysis can, however, bias EEG (or MEG) physiological interpretations in either local or global directions. Extracranial recordings of EEG and MEG inherently emphasize global dynamics because of volume conductor spatial filtering (Nunez and Srinivasan 2006). By contrast, analysis methods that “localize” sources of EEG signals that are typically widely distributed can lead to biased physiological interpretations.
Silberstein (1995b) has taken this general local/global dynamic picture a step further in the physiological and clinical directions by suggesting several ways in which brainstem neurotransmitter systems might act to change the coupling strength between global fields and local or regional networks. He outlines how different neurotransmitters might alter the coupling strengths by their selective actions at different cortical depths, thereby selectively altering resonance properties based on cortical morphology. For example, in the context of our global model, noradrenaline, dopamine, and acetylcholine appear to be well positioned in the appropriate cortical layers to reduce our corticocortical coupling parameter β and increase local network (cortical or thalamocortical) coupling, effecting a shift from more global to more locally dominated dynamics, a shift from more “hypercoupled” to more “hypocoupled” states. In contrast, 5-HT could reduce local loop gain and move the cortex to a more hypercoupled state. Silberstein further conjectures that several diseases may be manifestations of hypercoupled or hypocoupled dynamic states brought on by faulty neurotransmitter action. For example, the association of (positive) schizophrenia symptoms with hypocoupled states is consistent with observations of a therapeutic response to dopamine receptor blockers and prominent beta activity likely due to enhanced local networks. Again, healthy consciousness is associated with a proper balance between local, regional and global mechanisms.
Finally we cite a general mathematical theory that suggests how “soft-wired” brain networks might continually interact and disconnect on roughly 10 to 100 ms time scales. A general class of weakly connected oscillators was studied by Hoppensteadt and Izhikevich (1998) and Izhikevich (1999). The main attraction of this approach is that very minimal restrictions need be placed on the oscillators—the results are largely independent of specific network model. These scientists showed that the individual oscillators cannot substantially interact unless certain resonant relations exist between the characteristic frequencies of the autonomous oscillators. Since the “oscillators” are largely arbitrary, the analysis can be applied to interactions between local or regional networks or between networks and the global synaptic action fields. The studies outlined here raise the speculation that consciousness is a resonance phenomenon in reentrant networks that continuously form and disconnect at multiple spatial and temporal scales. Standing and traveling brain waves may be manifestations of this process at the very large scales accessible with EEG recordings, providing one mechanism to effect a largescale functional integration of neocortex.
Appendix A. Outline of neocortical dynamic global theory
A.1 Brief description
The label global theory is used here to indicate mathematical models in which delays in the cortico-cortical fibers forming most of the white matter in humans provide the underlying time scale for the large scale EEG dynamics. Periodic boundary conditions are generally essential to global theories because the cortical-white matter system of each hemisphere is topologically close to a spherical shell. This is in marked contrast to local theory, which refers to mathematical models of cortical or thalamocortical interactions for which corticocortical propagation delays are neglected. The underlying time scales in local theories are typically postsynaptic potential rise and decay times due to membrane capacitive-resistive properties. Thalamocortical networks are also “local” from the viewpoint of a surface electrode, which cannot distinguish purely cortical from thalamocortical networks. These theories are also “local” in the sense of being independent of global boundary conditions.
A brief outline of the simplest version of the global theory follows. More detail may be found in Nunez (1995) and Nunez and Srinivasan (2006). These references also discuss methods to combine local and global theories. The global theory proposes equations for three field variables:
- δΨe(r, t), ≡ Ψ(r, t)
the modulation of excitatory synaptic action density
- δΨi(r, t)
the modulation of inhibitory synaptic action density
- δΘ(r, t)
the modulation of action potential density
The excitatory synaptic action density Ψe(r, t) is defined as the number of active excitatory synapses per unit area of cortical surface in the two-dimensional version of theory or number per unit length of a cortical strip in the one-dimensional version. The modulation of cortical synaptic output δΨe(r, t) due to modulation of action potential input δΘ(r, t) about background is considered first. In the linear version of the theory, a dynamic transfer function (or its inverse, the dispersion function) is obtained from a basic integral equation relating these two variables as indicated below. The dispersion relation is determined by the poles of the transfer function in the usual manner of linear systems analysis. An equivalent linear differential equation in the variable δΨe(r, t), ≡ Ψ(r, t) follows directly from the dispersion relation. Nonlinear versions of this equation are suggested based on apparently plausible physiological arguments, especially the postulate that instability is prevented in healthy brains by recruitment of additional inhibitory mechanisms (negative feedback) from thalamus or contiguous cortex (lateral inhibition).
A.2 Derivation of a dispersion relation for an idealized one-dimensional medium
T he excitatory synaptic action at cortical location r may be expressed in terms of an inner integral over the cortical surface and outer integral over distributed axon propagation speeds as
| (A.1) |
The corticocortical and thalamocortical fibers are believed to be exclusively excitatory (or nearly so). Thus, (A.1) is based on the simple, non-controversial idea that excitatory synaptic action at cortical location r is due to excitatory sub-cortical input δΨ0 (r, t) plus excitatory action potential density δΘ(r, t) integrated over the entire neocortex. The inner integrals in (A.1) are over cortical surface coordinates, but action potentials at location r1 produce synaptic activity at location r only after a delay that is proportional to cortical separation distance and inversely proportional to axon speed v1. Distances are defined on an equivalent smooth cortex. All the complications of white matter (corticocortical) fiber tracts (after smoothing) are included in the distribution function Γ(r, r1, v1). The outer integral is over generally distributed corticocortical propagation velocities v1.
In the simplest one-dimensional version of the global theory (Nunez 1972, 1974a), axons are assumed to run only in the anterior-posterior direction of each hemisphere (perhaps not such a bad assumption based on known anatomy). The corticocortical axons are parceled into M fiber systems with connection densities that fall off exponentially with separation |x − x1|, that is
| (A.2) |
where ρm is the number of excitatory synapses per m-system axon. By substituting (A.2) into (A.1) and taking the spatial-temporal Fourier transform based on an infinite medium, one obtains
| (A.3) |
By expanding an assumed sigmoid relationship between action potential density and synaptic action, the additional relation may also be obtained (Jirsa and Haken 1997)
| (A.4) |
Dispersion relation obtained with linear theory
Here we consider the linear limiting case α → 0. We can shed a little light on the origin of the parameter β in (A.4) as follows. The relation between inhibitory synaptic action field Ψi(x, t ) and action potential density provides an additional equation relating inhibitory action to action potential density; however, this relation is much simpler than (A.1) since inhibitory synapses occur only on (local) intracortical axons so zero inhibitory delays are assumed. That is, for the scalp waves of moderate to long wavelength k ≪ λi, the equivalent of (A.1) is simply (Nunez 1995)
| (A.5) |
The third equation relating the three field variables is the basic linear approximation (Nunez 1974a, 1995)
| (A.6) |
Here the Q’s are defined as the partial derivatives indicating the incremental changes in action potential output from a neural mass due to excitatory or inhibitory input. The incremental increase in action potentials produced in a cortical tissue mass is assumed proportional to the weighted difference between incremental increases in excitatory synaptic input and inhibitory synaptic input. Essentially, we expand about a fixed background state of the brain (or linear portion of a sigmoid function) where the above partial derivatives are assumed to be constant over the short time scales of interest. An infinite cortex is assumed to allow for easy Fourier transform and the above three equations combined so that the basic input-output relation for cortex may be expressed in terms of Fourier transform variables as
| (A.7) |
Consider the case where all but one (m = M) cortico-cortical axon systems have infinite propagation speeds (zero global delays). The dispersion function then takes on the form
| (A.8) |
where the cortical excitatory function is defined by
| (A.9) |
Here the sum in the denominator of (A.9) is over all excitatory systems with negligible global delays. If we restrict the analysis to long wavelengths such that
| (A.10) |
the excitability function becomes an excitability parameter B(k)→ 2β, which simplifies the following expressions. To first approximation, we assume only a single propagation speed for the Mth fiber system, that is
| (A.11) |
The complex frequency is given in terms of real and imaginary parts by
| (A.12) |
This yields the dispersion relation in terms of real and imaginary frequencies
| (A.13) |
These are identical to Eqs (6) with the subscript M indicating the longest fiber system omitted and noting that positive and negative real frequencies are physically identical.
Several caveats concerning this oversimplified theory
The dispersion function for an infinite medium approximates the dispersion function for closed loop of length L provided e−λM L/2 ≪ 1 (Srinivasan 1995; Nunez 1995). Assuming a cortical loop circumference L 50 cm and characteristic length for fiber density fall-off in the longest corticocortical system of 10 cm, e−λM L/2 0.08, thus, this approximation my be well justified.
-
If corticocortical velocities in the Mth system are distributed rather than peaked at a single characteristic velocity vM, Eq (A.8) has no simple analytic solution. To address this issue, numerical solutions to this dispersion relation were obtained based on the following fit to experimental data which peaks at the velocity v0 (Nunez 1995)
(A.14) Here the numerical factor is required to force(A.15) The predicted frequencies are similar to those expected from Eq (A.13) with v → v0, that is, ωR(k) kv0 for waves with zero damping; however, the damping γ(k) increases with wavenumber when velocities are distributed. Oscillations with zero damping only occur for a more excitable neocortex, for example, roughly 1 < βγ=0 < 2 for the modes 1 < k/λ < 4 as compared to 1 βγ=0 =1 when the fibers have only the single propagation velocity v0.
The frequency of each mode decreases as the background excitability of the cortex β increases, suggesting a possible influence on EEG changes associated with sleep and anesthesia. These predicted linear waves become unstable when β > βγ=0, but nonlinear theory suggests several physiological mechanisms in healthy brains to limit the growth in wave amplitudes (Nunez 1995; Jirsa and Haken 1997).
A dispersion relation ω = ω(n) has been derived from (A.1) for standing waves in a homogeneous and isotropic spherical shell of radius R, where n is the degree of the spherical harmonic function Ynm (θ, φ) (Katznelson 1981; Nunez 1995). Weakly damped standing waves with frequencies in the same general range as those of this onedimensional theory are also obtained, that is, with a fundamental mode ω(1) v/R. By contrast to the one-dimensional waves, the spherical shell waves exhibit multiple branches of the dispersion relation so that higher modes also have frequencies ω(n) v/R in addition to their more conventional overtone frequencies. Our understanding of the anisotropic nature of corticocortical fibers is not sufficient to judge whether the one dimensional or spherical shell models are more likely to be accurate (Nunez and Srinivasan 2006).
Appendix B. Traveling wave pulse in a dispersive medium
Here we consider a pulsed input to a closed loop of some wave medium occupying the closed loop −L/2 < x < +L/2. The dynamics of this medium are assumed to be governed by the following forced linear differential or integral equation where ℜ indicates some arbitrary linear operator
| (B.1) |
Expand both sides of Eq (B.1) in the Fourier series
| (B.2) |
Take the Fourier transform of Eq (B.2a)
| (B.3) |
Here the coefficients Ψn(ω) are obtained from Eq (B.1)
| (B.4) |
The dispersion function Dn(ω, kn) or dynamic transfer function is determined by the linear operator ℜ. The inverse Fourier transform of Ψn(ω) is given by
| (B.5) |
If all singularities of the integrand are the poles p = 1,2,…, P, a dispersion relation(s) exists and Eq (5) reduces to a sum over the poles
| (B.6) |
So that the solution Eq (B.1) becomes
| (B.7) |
The forcing function is
| (B.8) |
If an impulse is applied at t = 0, F(x, t) = G(x)δ(t)
| (B.9) |
We may force the medium with a Gaussian shaped pulse (our guess as to the spatial distribution of the visual input to neocortex)
| (B.10) |
We obtain the Fourier transform of this input
| (B.11) |
Here the approximation to the integral is valid for . Substitute Eq (B.11) into Eq (B.7) to obtain
| (B.12) |
We have used the following to obtain Eq (B.12)
| (B.13) |
Consider the case where the zero mode is non-oscillatory and the dispersion relation involves two poles ±ωR(kn) + γ(kn), then Eq (B.12) becomes
| (B.14) |
Note that the variable Ψ(x, t) ≡ δΨe(x, t) is defined as the short time modulation of the excitatory synaptic action density about its background level. The expression after the arrow is obtained because the first exponential term adds or subtracts to the constant background of excitatory synaptic action, depending on the sign of γ(0). If linear instability γ(0) > 0 occurs, this background grows until limited by nonlinear effects. This may cause the cortical excitability parameter β also to increase past the point of linear instability for higher modes γ(kn) >0. We conjecture that as healthy brains approach instability, additional negative feedback is recruited from thalamus or cortex to prevent such linear instability. The end result may be quasi-linear limit cycle-like modes that propagate with effectively zero damping γ(kn) ≅ 0 in some brain states as outlined in earlier publications (Nunez 1995, 2000a,Nunez b; Nunez and Srinivasan 2006).
Footnotes
Citation
Nunez, P. L., & Srinivasan, R. (2006). A theoretical basis for standing and traveling brain waves measured with human EEG with implications for an integrated consciousness. Clin Neurophysiol, 117(11), 2424-2435.
References
- Abeles M. Local Cortical Circuits. New York: Springer-Verlag; 1982. [Google Scholar]
- Andrew C, Pfurtscheller G. On the existence of different alpha band rhythms in the hand area of man. Neuroscience Letters. 1997;222:103–106. doi: 10.1016/s0304-3940(97)13358-4. [DOI] [PubMed] [Google Scholar]
- Braitenberg V. Cortical architechtonics. General and areal. In: Brazier MAB, Petsche H, editors. Architectonics of the Cerebral Cortex. New York: Raven Press; 1978. pp. 443–465. [Google Scholar]
- Braitenberg V, Schuz A. Statistics and Geometry. New York: Springer-Verlag; 1991. Anatomy of the Cortex. [Google Scholar]
- Burkitt GR, Silberstein RB, Cadusch PJ, Wood AW. The steady-state visually evoked potential and travelling waves. Clinical Neurophysiology. 2000;111:246–258. doi: 10.1016/s1388-2457(99)00194-7. [DOI] [PubMed] [Google Scholar]
- Cooper R, Winter AL, Crow HJ, Walter WG. Comparison of subcortical, cortical, and scalp activity using chronically indwelling electrodes in man. Electroencephalography and Clinical Neurophysiology. 1965;18:217–228. doi: 10.1016/0013-4694(65)90088-x. [DOI] [PubMed] [Google Scholar]
- Ding J, Sperling G, Srinivasan R. Attentional modulation of SSVEP power depends on the network tagged by the flicker frequency. Cerebral Cortex. 2006;16:1016–1029. doi: 10.1093/cercor/bhj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM. The Mindful Brain: Cortical Organization and the Group-Selective Theory of Higher Brain Functions. Cambridge: MIT; 1978. [Google Scholar]
- Edelman GM, Tononi G. A Universe of Consciousness. New York: Basic Books; 2000. [Google Scholar]
- Florian G, Andrew C, Pfurtscheller G. Do changes in coherence always reflect changes in functional coupling? Electroencephalography and Clinical Neurophysiology. 1998;106:87–91. doi: 10.1016/s0013-4694(97)00105-3. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Mass Action in the Nervous System. New York: Academic Press; 1975. [Google Scholar]
- Freeman WJ. Predictions on neocortical dynamics derived from studies in paleocortex. In: Basar E, Bullock TH, editors. Induced Rhythms in the Brain. Birhauser: 1992. [Google Scholar]
- Gevins AS, Cutillo BA. Signals of cognition. In: Lopes da Silva FH, editor. Handbook of Electroencephalography and Clinical Neurophysiology. Vol. 2. Amsterdam: Elsevier; 1986. pp. 335–381. [Google Scholar]
- Gevins AS, Le J, Martin N, Brickett P, Desmond J, Reutter B. High resolution EEG: 124-channel recording, spatial enhancement, and MRI integration methods. Electroencephalography and Clinical Neurophysiology. 1994;90:337–358. doi: 10.1016/0013-4694(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Gevins AS, Cutillo BA. Neuroelectric measures of mind. In: Nunez PL, editor. Neocortical Dynamics and Human EEG Rhythms. New York: Oxford University Press; 1995. pp. 304–338. [Google Scholar]
- Gevins AS, Smith ME, McEvoy L, Yu D. High-resolution mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cerebral Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Haken H. Synergetics: An Introduction. 3. Springer-Verlag; 1983. [Google Scholar]
- Haken H. What can synergetics contribute to the understanding of brain functioning? In: Uhl C, editor. Analysis of Neurophysiological Brain Functioning. Berlin: Springer-Verlag; 1999. pp. 7–40. [Google Scholar]
- Hoppensteadt FC, Izhikevich EM. Thalamo-cortical interactions modeled by weakly connected oscillators: Could brain use FM radio principles? Biosystems. 1998;48:85–92. doi: 10.1016/s0303-2647(98)00053-7. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Ikram A, Fino JJ. Characteristics of traveling waves under various conditions. Electroencephalography and Clinical Neurophysiology. 1995;26:7–22. doi: 10.1177/155005949502600104. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Kuruvilla A, Kino JJ. Topographic analysis of visual evoked potentials from flash and pattern reversal stimuli: evidence for “traveling waves”. Brain Topography. 1992;4:215–228. doi: 10.1007/BF01131153. [DOI] [PubMed] [Google Scholar]
- Ingber L. Statistical mechanics of multiple scales of neocortical interactions. In: Nunez PL, editor. Neocortical Dynamics and Human EEG Rhythms. New York: Oxford University Press; 1995. pp. 628–681. [Google Scholar]
- Izhikevich EM. Weakly connected quasi-periodic oscillators, FM interactions, and multiplexing in the brain. SIAM Journal of Applied Mathematics. 1999;59:2193–2223. [Google Scholar]
- Jackson JD. Classical Electrodynamics. New York: Wiley; 1975. [Google Scholar]
- Jasper HD, Penfield W. Electrocorticograms in man: Effects of voluntary movement upon the electrical activity of the precentral gyrus. Archiv Fur Psychiatrie and Zeitschrift Neurologie. 1949;183:163–174. [Google Scholar]
- Jirsa VK, Haken H. A derivation of a macroscopic field theory of the brain from the quasi-microscopic neural dynamics. Physica D. 1997;99:503–526. [Google Scholar]
- Jirsa VK, Kelso JAS, Fuchs A. Traversing scales of brain and behavioral organization III Theoretical modeling. In: Uhl C, editor. Analysis of Neurophysiological Brain Functioning. Berlin: Springer-Verlag; 1999. pp. 107–125. [Google Scholar]
- Jirsa VK, Kelso JAS. Spatiotemporal pattern formation in continuous systems with heterogeneous connection topologies. Physical Review E. 2000;62:8462–8465. doi: 10.1103/physreve.62.8462. [DOI] [PubMed] [Google Scholar]
- Katznelson RD. Normal modes of the brain: Neuroanatomical basis and a physiological theoretical model. In: Nunez PL, editor. Electric Fields of the Brain: The Neurophysics of EEG. 1. New York: Oxford University Press; 1981. pp. 401–442. [Google Scholar]
- Klimesch W, Hanslmayr S, Sauseng P, Gruber W, Doppelmayr M. The P1 and travelling alpha waves: Evidence for evoked oscillations, private communication from W. Klimesch to PL Nunez. 2006 doi: 10.1152/jn.00876.2006. [DOI] [PubMed] [Google Scholar]
- Law SK, Nunez PL, Wijesinghe RS. High resolution EEG using spline generated surface Laplacians on spherical and ellipsoidal surfaces. IEEE Transactions on Biomedical Engineering. 1993;40:145–153. doi: 10.1109/10.212068. [DOI] [PubMed] [Google Scholar]
- Liley DTJ, Cadusch PJ, Dafilis MP. A spatially continuous mean field theory of electrocortical activity network. Computation in Neural Systems. 2002;13:67–113. [PubMed] [Google Scholar]
- Lopes da Silva FH. Neural mechanisms underlying brain waves: from membranes to networks. Electroencephalography and Clinical Neurophysiology. 1991;79:81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva FH. Dynamics of electrical activity of the brain, local networks, and modulating systems. In: Nunez PL, editor. Neocortical Dynamics and Human EEG Rhythms. Oxford University Press; 1995. pp. 249–271. [Google Scholar]
- Lopes da Silva FH. Dynamics of EEGs as signals of neuronal populations: models and theoretical considerations. In: Niedermeyer E, Lopes da Silva FH, editors. Electroencephalography. Basic Principals, Clinical Applications, and Related Fields. 5. London: Williams and Wilkins; 2000. pp. 76–92. [Google Scholar]
- Lopes da Silva FH, Storm van Leeuwen W. The cortical source of the alpha rhythm. Neuroscience Letters. 1977;6:237–241. doi: 10.1016/0304-3940(77)90024-6. [DOI] [PubMed] [Google Scholar]
- Lumer E, Edelman GM, Tononi G. Neural dynamics in a model of the thalamocortical system. I. Layers, loops and the emergence of fast synchronous rhythms. Cerebral Cortex. 1997;7:207–227. doi: 10.1093/cercor/7.3.207. [DOI] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. The Journal of Neuroscience. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery DC, Tidman DA. Plasma Kinetic Theory. New York: McGraw Hill; 1964. pp. 51–63. [Google Scholar]
- Mountcastle VB. Perceptual Neuroscience: The Cerebral Cortex. Academic Press; 1997. [Google Scholar]
- Nunez PL. Longitudinal wave propagation in a plasma containing an external time dependent electric field. Physics of Fluids. 1971;14:633–639. [Google Scholar]
- Nunez PL. The brain wave equation: A model for the EEG. American EEG Society Meeting; Houston. 1972. [Google Scholar]
- Nunez PL. The brain wave equation: A model for the EEG. Mathematical Biosciences. 1974a;21:279–297. [Google Scholar]
- Nunez PL. Wave-like properties of the alpha rhythm. IEEE Transactions on Biomedical Engineering. 1974b;21:473–482. [Google Scholar]
- Nunez PL. Electric Fields of the Brain: The Neurophysics of EEG. 1. New York: Oxford University Press; 1981. [Google Scholar]
- Nunez PL. Generation of human EEG by a combination of long and short range neocortical interactions. Brain Topography. 1989;1:199–215. doi: 10.1007/BF01129583. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Neocortical Dynamics and Human EEG Rhythms. New York: Oxford University Press; 1995. [Google Scholar]
- Nunez PL. Toward a large-scale quantitative description of neocortical dynamic function and EEG (Target article) Behavioral and Brain Sciences. 2000a;23:371–398. doi: 10.1017/s0140525x00003253. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Neocortical dynamic theory should be as simple as possible, but not simpler (Response to 18 commentaries on target article) Behavioral and Brain Sciences. 2000b;23:415–437. [Google Scholar]
- Nunez PL, Silberstein RB. On the relationship of synaptic activity to macroscopic measurements: Does co-registration of EEG with fMRI make sense? Brain Topography. 2001;13:79–96. doi: 10.1023/a:1026683200895. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG, Second Edition. New York: Oxford University Press; 2006. [Google Scholar]
- Nunez PL, Reid L, Bickford RG. The relationship of head size to alpha frequency with implications to a brain wave model. Electroencephalography and Clinical Neurophysiology. 1977;44:344–352. doi: 10.1016/0013-4694(78)90309-7. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, micro-current sources, multiscale measurements, and global binding of local networks. Human Brain Mapping. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Cooper R. Frequency dependence of the transmission of the EEG from cortex to scalp. Electroencephalography and Clinical Neurophysiology. 1975;38:93–96. doi: 10.1016/0013-4694(75)90215-1. [DOI] [PubMed] [Google Scholar]
- Robinson PA, Whitehouse RW, Rennie CJ. Nonuniform corticothalamic continuum model of electroencephalographic spectra with application to split alpha peaks. Physical Review E. 2003;68:021922-1–10. doi: 10.1103/PhysRevE.68.021922. [DOI] [PubMed] [Google Scholar]
- Robinson PA, Rennie CJ, Rowe DL, O’Conner SC. Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Human Brain Mapping. 2004;23:53–72. doi: 10.1002/hbm.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GR. Ph.D. Dissertation. Alberta, Canada: The University of Alberta; 1991. Spherical Harmonic Analysis of the Electroencephalogram. [Google Scholar]
- Silberstein RB. Steady-state visually evoked potentials, brain resonances, and cognitive processes. In: Nunez PL, editor. Neocortical Dynamics and Human EEG Rhythms. Oxford University Press; 1995a. pp. 272–303. [Google Scholar]
- Silberstein RB. Neuromodulation of neocortical dynamics. In: Nunez PL, editor. Neocortical Dynamics and Human EEG Rhythms. Oxford University Press; 1995b. pp. 591–627. [Google Scholar]
- Silberstein RB, Nunez PL, Pipingas A, Harris P, Danieli F. Steady state visually evoked potential (SSVEP) topography in a graded working memory task. International Journal of Psychophysiology. 2001;42:219–232. doi: 10.1016/s0167-8760(01)00167-2. [DOI] [PubMed] [Google Scholar]
- Silberstein RB, Danieli F, Nunez PL. Fronto-parietal evoked potential synchronization is increased during mental rotation. NeuroReport. 2003;14:67–71. doi: 10.1097/00001756-200301200-00013. [DOI] [PubMed] [Google Scholar]
- Silberstein RB, Song J, Nunez PL, Park W. Dynamic sculpting of brain functional connectivity is correlated with performance. Brain Topography. 2004;16:240–254. doi: 10.1023/b:brat.0000032860.04812.b1. [DOI] [PubMed] [Google Scholar]
- Srinivasan R. Spatial structure of the human alpha rhythm: global correlation in adults and local correlation in children. Clinical Neurology. 1999;110:1351–1362. doi: 10.1016/s1388-2457(99)00080-2. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL, Tucker DM, Silberstein RB, Cadusch PJ. Spatial sampling and filtering of EEG with spline-Laplacians to estimate cortical potentials. Brain Topography. 1996;8:355–366. doi: 10.1007/BF01186911. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL, Silberstein RB. Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE Transactions on Biomedical Engineering. 1998;45:814–825. doi: 10.1109/10.686789. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Russell DP, Edelman GM, Tononi G. Frequency tagging competing stimuli in binocular rivalry reveals increased synchronization of neuromagnetic responses during conscious perception. Journal of Neuroscience. 1999;19:5435–5448. doi: 10.1523/JNEUROSCI.19-13-05435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Bibi FA, Nunez PL. Steady-state visual evoked potentials: distributed local sources and wave-like dynamics are sensitive to flicker frequency. Brain Topography. 2006;18:167–187. doi: 10.1007/s10548-006-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Winter W, Nunez PL. Source analysis of EEG oscillations using high-resolution EEG and MEG. Chapter 3. In: Neuper C, Klimesch W, editors. Progress in Brain Research. Vol. 159. 2006. pp. 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Petrovic S. MEG phase follows conscious perception during binocular rivalry induced by visual stream segregation. Cerebral Cortex. 2006;16:597–608. doi: 10.1093/cercor/bhj016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe S, Nunez PL, Srinivasan R. Identification of wave-like spatial structure in the SSVEP: comparisons of EEG and MEG. Statistics in Medicine. 2006 doi: 10.1002/sim.2969. in press. [DOI] [PubMed] [Google Scholar]
- Wilson HR, Cowan JD. A mathematical theory of the functional dynamics of cortical and thalamic nervous tissue. Kybernetik. 1973;13:55–80. doi: 10.1007/BF00288786. [DOI] [PubMed] [Google Scholar]
- Wingeier BM, Nunez PL, Silberstein RB. Spherical harmonic decomposition applied to spatial-temporal analysis of human high-density electroencephalogram. Physical Review E. 2001;64:051916-1–9. doi: 10.1103/PhysRevE.64.051916. [DOI] [PubMed] [Google Scholar]
- Wingeier BM. Ph.D. Dissertation. Tulane University; 2004. A High resolution Study of Coherence and Spatial Spectral in Human EEG. [Google Scholar]
- Wright JJ, Robinson PA, Rennie CJ, Gordon E, Bourke PD, Chapman CL, Hawthorn N, Lees GJ, Alexander D. Toward an integrated continuum model of cerebral dynamics: the cerebral rhythms, synchronous oscillations and cortical stability. Biosystems. 2001;63:71–88. doi: 10.1016/s0303-2647(01)00148-4. [DOI] [PubMed] [Google Scholar]


