Abstract
Cyclic nitroxides are a diverse range of stable free radicals that have unique antioxidant properties. Because of their ability to interact with free radicals, they have been used for many years as biophysical tools. During the past 15–20 years, however, many interesting biochemical interactions have been discovered and harnessed for therapeutic applications. Biologically relevant effects of nitroxides have been described including their ability to degrade superoxide and peroxide, inhibit Fenton reactions and undergo radical-radical recombination. Cellular studies defined the activity of nitroxides in vitro. By modifying oxidative stress and altering the redox status of tissues, nitroxides have been found to interact with and alter many metabolic processes. These interactions can be exploited for therapeutic and research use including protection against ionizing radiation, as probes in functional magnetic resonance imaging, cancer prevention and treatment, control of hypertension and weight, and protection from damage resulting from ischemia/reperfusion injury. While much remains to be done, many applications have been well studied and some are presently being tested in clinical trials. The therapeutic and research uses of nitroxide compounds are reviewed here with a focus on the progress from initial development to modern trials.
Keywords: Nitroxides, Tempol, Radiation, Oxidative Stress, Magnetic Resonance Imaging, Contrast Agents, Chemoprevention, Hypertension
Introduction
Cyclic nitroxides, also known as aminoxyls or nitroxyls, are stable free radicals stabilized by methyl groups at the α position in five-membered pyrrolidine, pyrroline or oxazolidine and six-membered piperidine ring structures (Figure 1A). The methyl groups confer stability to the nitroxide radicals by preventing radical-radical dismutation and also limit access to reactive substances, which can quench the radical species. The substituent groups on the ring (denoted by R-) produce a diverse range of compounds that can be directed to specific hydrophilic or hydrophobic regions in the cellular microenvironment. Figure 1B shows the various oxidation states of Tempol, one of the piperidine nitroxides. The redox transformations between the oxidation states of nitroxide, hydroxylamine and the oxoammonium cation are shown in Figure 1C. The nitroxide and oxoammonium pair acts as an efficient redox couple, which can support catalytic processes via reversible 1-electron redox reactions. The hydroxylamine and nitroxide forms do not constitute an effective redox couple and therefore are incapable of supporting catalytic processes. However, like other well known antioxidants such as ascorbate, the hydroxylamine can function as an efficient hydrogen atom donor and provide antioxidant defense.
Figure 1. General Structures of Nitroxide Compounds.
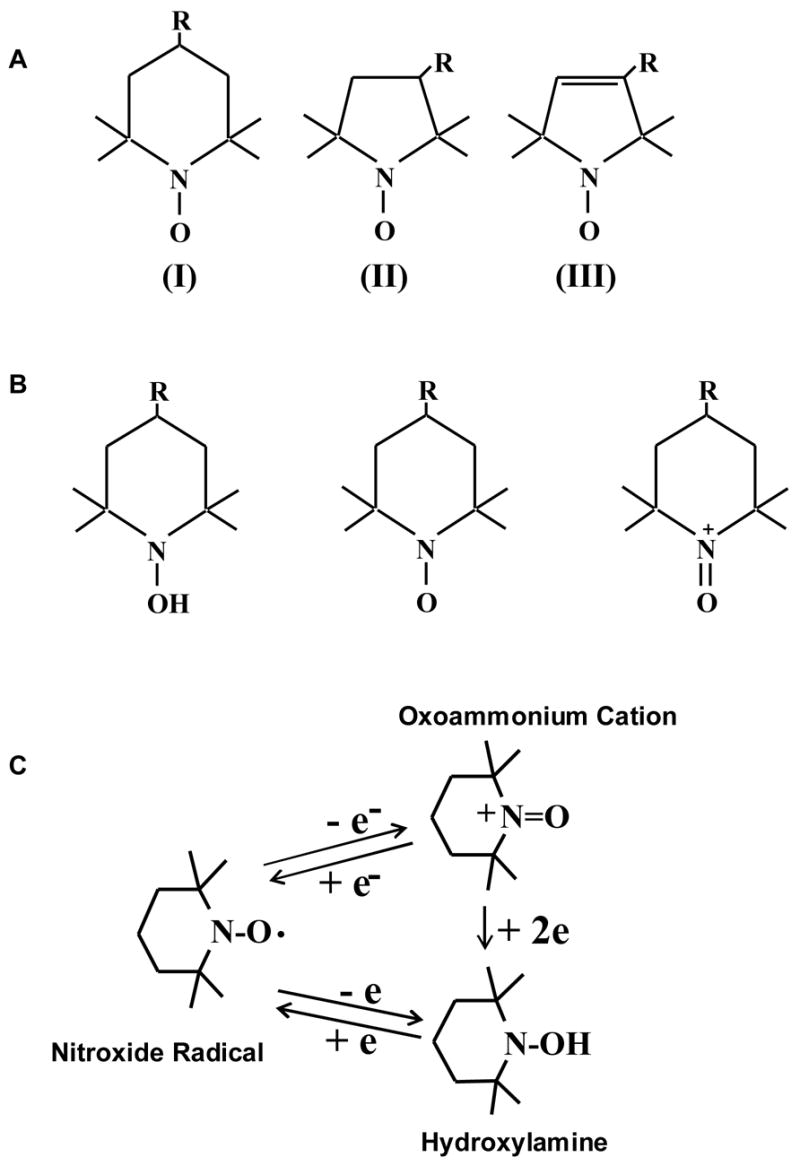
Nitroxide compounds are composed of five- or six-membered rings containing a nitrogen atom. The molecule bound to the nitrogen dictates the various properties of the compound. (A) Compound I belongs to the 6-membered piperidine ring class, compounds II and II belong to the saturated and unsaturated 5-membered ring classes respectively. (B) (C) Nitroxide compounds are found in vivo in an equilibrium between the nitroxide radical form which is detected by EPR, and the reduced form, known as the hydroxylamine, which is not detected. This equilibrium is dependent on the oxygen status and redox-status of the tissue milieu. Cellular redox processes convert the compound between the two states, thus the ratio of the two states is determined by the redox activity within the cell. (Adapted with permission from references # 21 and # 40)
Nitroxide radicals have long been utilized as biophysical tools for electron paramagnetic resonance (EPR) spectroscopic studies such as spin label/oxymetry and spin trapping because of their stability and their paramagnetic nature [1]. Studies have utilized the conversion of nitroxides to the hydroxylamine form to monitor cellular redox processes [2, 3]. This ability to participate in redox reactions enables nitroxides to protect against oxidative damage in several models ranging from cell systems to isolated organs to whole animal models including humans [4]. More recently, they have been recognized as redox-sensitive paramagnetic contrast agents in Magnetic Resonance Imaging (MRI) [5, 6]. In this review the chemical basis for the protective effects of nitroxides as well as the cellular and in vivo studies will be summarized.
Chemistry
SOD mimetic action
The initial observation that oxazolidine nitroxides are mimetics of the enzyme superoxide dismutase (SOD) [7] prompted further studies with the aim of extending this observation to other classes of nitroxides. The mechanism of the SOD mimetic activity is now understood to involve an oxoammonium/nitroxide redox couple [8].
| (1a) |
The 1-electron oxidation of the nitroxide radical (RR’NO•) by the protonated form of superoxide (HO2•) is pH dependent and converts the nitroxide to the corresponding oxoammonium cation (RR’NO+) [9]. The oxoammonium cation can then be reduced by O2•− back to the nitroxide radical.
| (1b) |
Combining equations 1a and 1b, yields the following:
| (1c) |
Similar to endogenous SOD, the nitroxide acts as a catalyst and is not consumed in the process of dismutation of O2•− to H2O2 and oxygen. The catalytic rate constants for the SOD mimetic action were determined for several nitroxides and, as expected, increased at lower pH values. Additionally, the rate constants were influenced by the redox mid-point potentials of the oxoammonium cation/nitroxide couple. The lower the mid-point potentials, the higher the rate constants within a range between 106 M−1s−1 to 108 M−1s−1 [9]. The catalytic rate constants for nitroxide SOD mimetic activity at physiological pH are 2–3 orders of magnitude lower than that of SOD itself. However, due to their low molecular weight (~200 kDa) nitroxides can accumulate intracellularly at concentrations that effectively decrease the steady state concentration of O2•− comparable to the activity of SOD. The rate determining step in this process is the oxidation of the nitroxide to the hydroxylamine form. The reduction of the oxoammonium cation back to the nitroxide by O2•− is reported to be faster than the corresponding reaction for SOD itself [10]. This may be the underlying reason that even upon prolonged exposure of nitroxides to a continuous flux of O2•− there is no apparent reduction in the EPR signal of the nitroxides even though the steady state levels of O2•− are decreased. Definitive EPR evidence for the oxidation of nitroxides by superoxide was obtained by including a 2-electron reducing agent NADH or NADPH in the reaction. In the presence of such agents, an SOD-inhibitable conversion of nitroxide to the corresponding hydroxylamine was observed with a concomitant oxidation of NADH or NADPH to NAD+ or NADP+ as shown in equation 1d [8, 9].
| (1d) |
Inhibition of lipid peroxidation
Cellular injury can be mediated by a free radical chain reaction called lipid peroxidation which causes membrane damage as well as oxidative modification of other critical targets. The process of lipid peroxidation occurs in three discrete steps. In the initiation step, cellular lipids (LH) are damaged by an oxidant free radical (X•).
| (2) |
This sets off a chain reaction whereby the initial lesion on the lipid molecule is propagated in the presence oxygen and redox-active transition metal complexes. These reactions are oxygen dependent and can inflict significant biologic damage until oxygen is depleted.
| (3a) |
| (3b) |
| (3c) |
| (3d) |
Finally, termination of the chain reaction occurs when the level of oxygen or other substrates is no longer sufficient to drive the cycle of reactions.
| (4a) |
| (4b) |
| (4c) |
Antioxidants afford protection by inhibiting the initiation reactions and by retarding the propagation of the chain reaction. Unlike other antioxidants that protect through stoichiometric reactions, nitroxides and hydroxylamines can inhibit lipid peroxidation by participating in redox reactions at every step [11]. Nitroxides (equation 5a) and hydroxylamines (equation 5b) inhibit the initiation step by interacting with the radical initiating species.
| (5a) |
| (5b) |
They also help to prevent the propagation of damage by interacting with oxidized lipid.
| (5c) |
| (5d) |
| (5e) |
Peroxidase activity
As previously mentioned, nitroxides were not only shown protect cells by acting as SOD mimics, but also to confer catalase-like behavior to heme proteins [12]. The ability of stable nitroxide radicals to detoxify hypervalent heme proteins such as ferrylmyoglobin (MbFeIV) produced in the reaction of metmyoglobin (MbFeIII) and H2O2 was evaluated by monitoring O2 evolution, H2O2 depletion, and redox changes of the heme prosthetic group. Depletion of H2O2 was enhanced by Tempol in a catalytic fashion, and a stiochiometric excess of Tempol enhanced catalase-like activity more than 4-fold. By shuttling between two oxidation states, the nitroxide radical and the oxoammonium cation, stable nitroxides enhance the catalase- like activity of MbFeIII, thus facilitating H2O2 dismutation and providing protection against oxidation by hypervalent heme proteins.
Inhibiting Fenton reactions
Oxidants such as hydroxyl (•OH) radicals can be generated from the transition metal-catalyzed Haber-Weiss reaction cycle for iron complexes.
| (6a) |
| (6b) |
Strong oxidants such as •OH radical are generated by the Fenton reaction (equation 6b). Nitroxides can prevent generation of •OH radicals from Fenton reactions by accepting the electron from the reduced metal complex as shown in equation 6c [13].
| (6c) |
Protection against radiation-induced radicals
The biological consequences of exposure to ionizing radiation are mediated by a series of physical, chemical, biochemical, and cellular responses initiated after the deposition of radiation energy in the medium. Some of these stages are represented in Figure 2. The energy associated with ionizing radiation is significantly greater than the bond energies of many molecules and can cause homolytic bond scission and the generation of secondary electrons. The time scale of the initial steps of energy deposition and molecular bond scission is on the order of 10−13 seconds. Since water is the main constituent of cellular matter, it is primarily the ionization of water that results in the production of secondary species with high reactivity and short life times (10−10 – 10−9 s) such as the •OH radical, aquated electrons, or hydrogen atoms. It is these secondary species that mediate the chemical reactions that damage biologically important molecules (BIMs). Equation 7 shows the primary radiolysis products that result from the ionization of water.
Figure 2. Sequence of Events Following Radiation Energy Absorption.
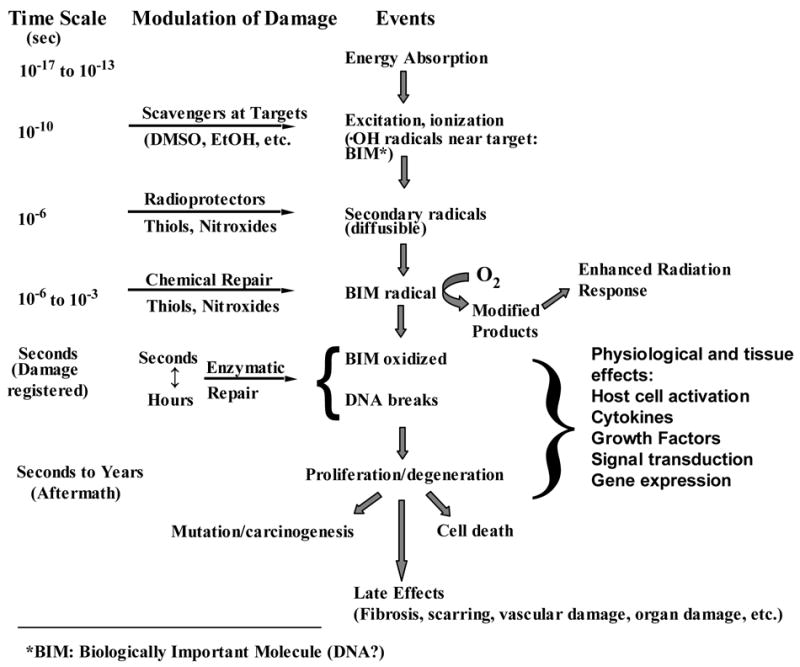
Following exposure to ionizing radiation, energy is absorbed by tissues very quickly. Secondary radicals are formed within 10−6 sec and it is these molecules that are intercepted by radioprotectors such as nitroxides and endogenous thiols. Between 10−6 to 10−3 sec the BIM radical is formed and is either repaired by thiols or nitroxides or fixed by oxygen. In this way, oxygen increases the biological effectiveness of radiation. BIM oxidation may either be repaired enzymatically or, in the case of DNA, strand breaks may occur. DNA or other BIM damage may prevent or alter cell division causing cell death or lack of proliferation. Alternately, mutated DNA may proliferate resulting in long term genetic effects including cancer. Other late effects such as fibrosis and vascular damage result from the permanent damage to other BIMs over the course of months to years.
| (7) |
Secondary radicals (X•) are then formed with life times in the microsecond range.
| (8) |
Antioxidants that scavenge the primary products of radiolysis can decrease the concentration of these highly reactive species and also the subsequent generation of secondary radicals at critical molecular targets. The cell membrane, enzymes and other proteins, and DNA are examples of BIMs that are possible critical targets in radiation-induced cytotoxicity, however DNA is thought be the crucial target which, when damaged, can result in both cell death and genetic alterations.
| (9) |
The use of radical scavengers (ScH) to eliminate primary radiolysis products close to chromatin/DNA (equation 10) or secondary radicals with intermediate reactivity (X•) that can diffuse to the DNA (equation 11) should provide protection against damage.
| (10) |
| (11) |
The scavenger radical, if sufficiently unreactive, will not cause any further biologic damage. In the absence of such scavengers, however, cellular oxygen can make the damage permanent, or “fix” the damage, by reacting with the radical on the target molecule to form the peroxyl radical. Some scavengers, in addition to scavenging reactive species and inhibiting damage, can also restore damaged target molecules by processes called chemical repair.
| (12) |
Nitroxides (RR’NO•) and hydroxylamines (RR’NOH) are radical scavengers that participate in electron transfer reactions (equations 13a and 13b) and are also involved in chemical repair through donation of hydrogen atoms (equations 14a and 14b). These interactions may underlie their protective effects.
| (13a) |
| (13b) |
| (14a) |
| (14b) |
For these reactions, the rate constant for nitroxides is expected to be higher than for their corresponding hydroxylamines. In addition to the above mentioned classes of reactions, nitroxides can also participate in radical-radical recombination reactions to provide additional detoxification pathways.
| (15a) |
| (15b) |
Although these reactions result in a modification of the target molecule, this can be repaired enzymatically.
Compared to nitroxides, a fully reduced amine exhibits a modest sensitization to oxidative damage. This is presumably mediated by the generation of additional reactive species (RR’N• ) that possess sufficient reactivity to mediate further damage.
| (16) |
Additionally, the resulting reactive species may fix the damage by interacting with target molecules.
| (17) |
Evidence strongly suggests that radiation-induced cytotoxicity results from radiolytically generated reactive species. This evidence is based, in part, on the observed protective effects of aminothiols, which can scavenge reactive species. Studies suggest that a diradical intermediate with a life time on the order of 1 μs is involved in the formation of DNA double strand breaks which are felt to be the lethal lesion induced by ionizing radiation [14]. Reducing agents that can interfere with such species might provide radioprotection. As previously mentioned, primary species are highly reactive and short lived (10−9 s) while secondary species have an intermediate reactivity and life time (1 μs). Thiol compounds react with primary and secondary species with similar efficiencies and provide cytoprotection by scavenging oxidants that are generated close to the critical cellular target. Nitroxides do not react directly with thiols. However, they can undergo facile radical-radical recombination reactions with thiyl radicals. In order to elucidate the role of reactive species in the formation of DNA double strand breaks, it is necessary to use several reducing agents with different proclivities for reacting with radicals produced by radiation. Additionally, the agents employed should accumulate at all of the sites of radical production and should participate in reactions that produce oxidation products that are not significantly reactive.
Cellular Studies
Mutagenicity
Because of their radical nature, there was concern about the mutagenic and teratgogenic effects of nitroxides. To study this, the activity of a representative nitroxide, Tempol, was investigated using the xanthine-guanine phosphoribosyl transferase (XPRT) forward mutation assay in CHO AS52 cells [15]. These studies not only showed that Tempol was not mutagenic or toxic to AS52 cells, but that it was actually protective against the cytotoxic and mutagenic effects of H2O2 and hypoxanthine/xanthine oxidase. Tempol was also found to reverse chromosomal aberrations in human lymphocytes exposed to ionizing radiation and limited mutations caused by carcinogens such as neocarzinostatin [16]. These results demonstrated the benefit of using these compounds as protectors against free radical-mediated damage. In another study, Tempol was found to protect against radiation-induced chromosomal damage in peripheral blood lymphocytes [17].
Cellular protection against oxidative damage
To be considered an antioxidant, a compound must reduce levels of oxidants to minimize oxidative damage. While the SOD mimetic activity of nitroxides was recognized in the initial studies, the multiple modes by which this class of agents provides protection was first reported in 1990 [13]. In this study, several nitroxides and their corresponding hydroxylamines were tested for protective effects in Chinese hamster V79 lung fibroblasts exposed to hypoxanthine/xanthine oxidase under aerobic conditions. Under such conditions, this reaction generates copious amounts of O2•− and H2O2. As shown in Figure 3, Tempol and its corresponding hydroxylamine (Tempol-H) afforded significant protection to the cells from damage inflicted by these reactive oxygen species (ROS). The presence of extracellular SOD had no beneficial effects when added prior to or during exposure to HX/XO whereas the metal-chelator desferrioxamine and the enzyme catalase completely inhibited the damage. These results suggest additional modes of protection by nitroxides other than SOD-mimetic activity. To evaluate this, several nitroxides were tested as protectors in V79 cells exposed to bolus concentrations of H2O2 in the range 0–500 uM for 1 hour [13]. In the absence of protective agents, a H2O2 dose-dependent cytotoxicity was observed. Protective effects were observed with catalase, and metal-chelators also reversed the toxicity supporting the theory that generation of oxidants such as hydroxyl radicals derived from H2O2 can be catalyzed by metals. Tempol and Tempol-H were also protective suggesting that nitroxides and hydroxylamines offer protection against a variety of oxidants. Similar protective effects of nitroxides were observed in cells exposed to oxidants such as the organic hydroperoxide t-butyl hydroperoxide which generate alkoxyl, and alkyl peroxyl radicals indicating that nitroxides are effective in scavenging a wide variety of reactive intermediates [18].
Figure 3. Protection Against Oxidative Cytotoxicity.
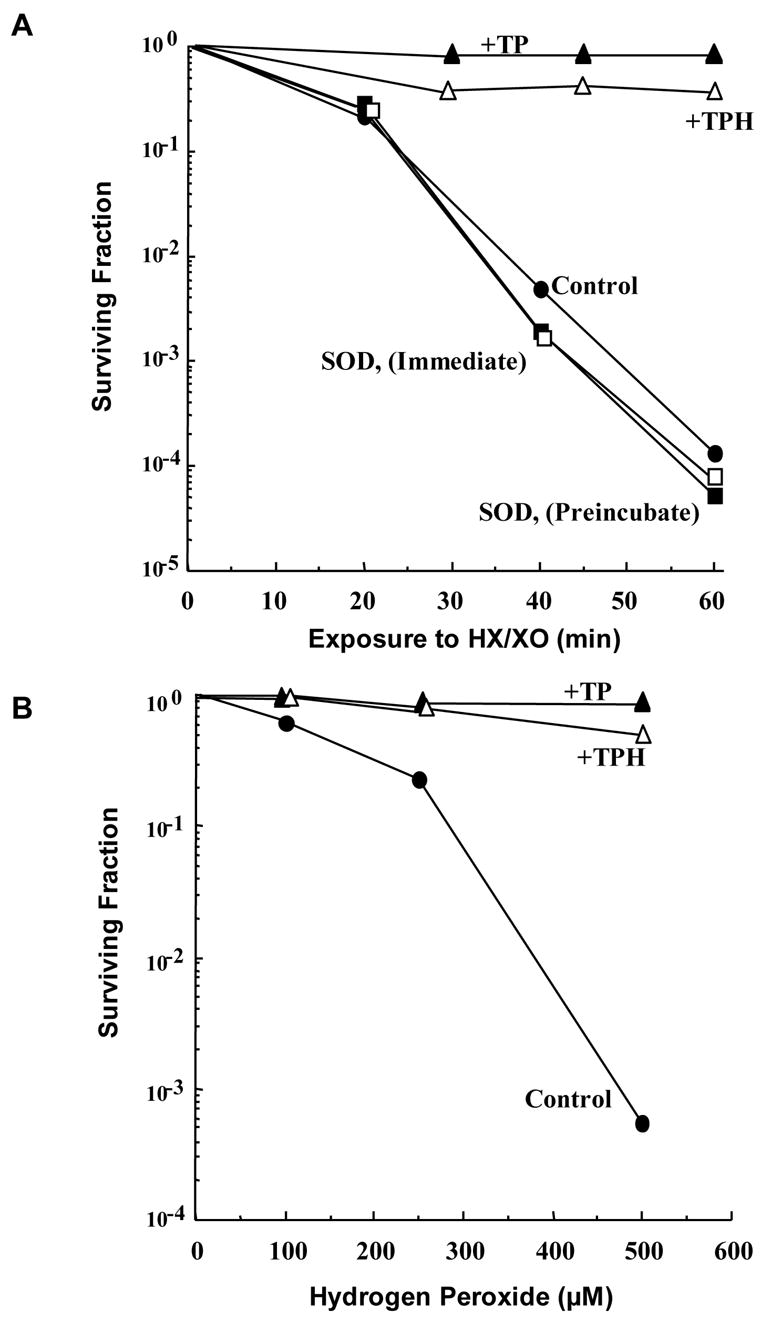
Chinese hamster V79 cells were exposed to 0.05 unit/mL xanthine oxidase (XO) + 0.5 mM hypoxanthine (HX) for up to 60 minutes or several concentrations of H2O2 in the presence of various additives: Control (–●–), Tempol (–▲–), TPH (–△–), SOD (immediate) (–□–), SOD (preincubated) (–■–). (A) SOD, which does not cross the cell membrane, does not protect against XO/HX-induced cytotoxicity. Tempol and TPH, which do enter the cell, protect against XO/HX and (B) H2O2-induced cytotoxicity. (Adapted with permission from reference # 13)
Nitroxides as modifiers of cellular radiation response
Because much of the damage caused by radiation in vitro results from the formation of free radicals it was hypothesized that nitroxides would ameliorate the damage caused by these radicals. An early radioprotection study showed that the administration of Tempol to Chinese Hamster cells exposed in culture to lethal doses of gamma-radiation resulted in a significant and dose-dependent protective effect with a protection factor of 2.5 compared to the untreated cells. Furthermore, it was found that although Tempol-H exhibited antioxidant effects against H2O2-induced radicals, it did not confer radiation protection even at a concentration of 100 mM. This suggests that nitroxides more readily react with the radical species produced by radiation than hydroxylamines [19]. To examine if this differential activity is a general phenomenon, three other nitroxide/hydroxylamine pairs were studied.[20] Plasmid DNA was exposed to the metal catalyzed Haber-Weiss reaction by incubating the plasmid DNA with hypoxanthine/xanthine oxidase under aerobic conditions. This generates O2−• in the presence of Cupric-phenathroline, which catalyzes the generation of H2O2 close to the target. In this study, it was found that the three nitroxide and hydroxylamine pairs were effective in inhibiting the H2O2-induced damage to DNA as monitored by the levels of relaxed form (Figure 4A). Once again, only the nitroxide protected against DNA resulting from ionizing radiation (Figure 4B). Analogous experiments were conducted in intact cells and again it was found that nitroxides and hydroxylamines effectively improved cell survival and prevented DNA double strand breaks following H2O2 exposure but that only nitroxides provided protection against ionizing radiation [20]. Taken together, these observations support the notion that while all radioprotectors are antioxidants, not all antioxidants provide radioprotection. The fact that nitroxides but not hydroxylamines are radioprotectors suggests that the radicals which lead to DNA lesions are more readily scavenged by nitroxides. This is presumably due, in part, to the efficiency with which nitroxides participate in radical-radical recombination reactions. Since conditions of elevated oxidative stress can exist in cells even after irradiation, nitroxides and hydroxylamines can exert protective effects by scavenging secondarily generated ROS resulting from radiation-induced damage.
Figure 4. Nitroxides Protect Against DNA Damage.
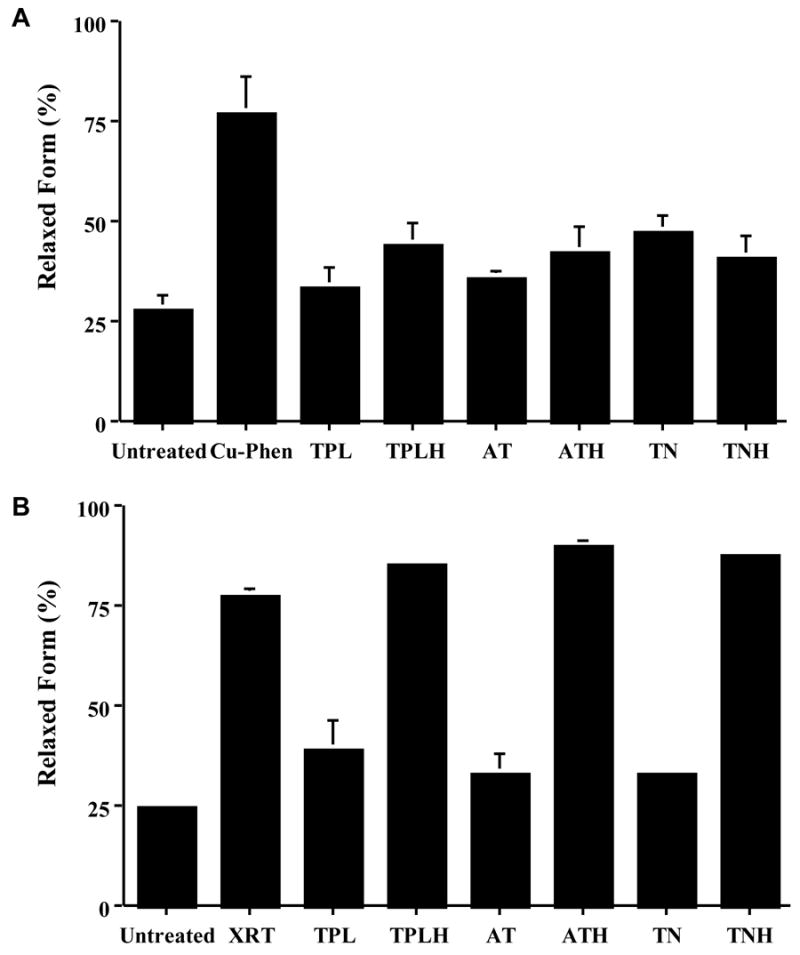
Copper-phenanthroline catalyzes DNA damage which can be represented as a decrease in the amount of the supercoiled form, or an increase in the relaxed form, of DNA. (A) Tempol (TPL), Tempol-H (TPLH), Tempamine (AT), Tempamine-H (ATH), Tempone (TN), and Tempone-H (TNH) all protect against this metal ion-catalyzed damage (Cu-Phen). (B) Only the nitroxide forms (TPL, AT and TN) protected against radiation-induced DNA damage. (Adapted with permission from reference # 20)
Structure activity relationship
The structural requirements for a nitroxide to function as an effective radioprotector were determined by large-scale, systematic screening of various nitroxides in an in vitro radiobiologic assay. The effect of ring size, substituents, and the ring oxidation state on radioprotection of mammalian cells under aerobic conditions were evaluated by evaluating the clonogenic viability of Chinese hamster lung fibroblast cells. Nitroxides of three different ring types were studied, namely the five-membered saturated pyrrolidine ring, the five-membered unsaturated pyrroline ring, and the six-membered saturated ring piperidine. The ring oxidation states tested were the nitroxide radical form (X = -O•) and its corresponding hydroxylamine (X = -OH) [21]. Several observations can be made from this study. First, as a class, nitroxides afford significant radioprotection while their corresponding hydroxylamines provide little or none. Second, the size and saturation of the ring have no influence on the observed radioprotective activity. Third, the substituents on the ring do significantly influence the radioprotective effects. Fourth, positively charged nitroxides have superior radioprotective activity compared to neutral or negatively charged nitroxides. It can be concluded, then, that because only nitroxides exhibit radioprotective effects, the chemical species causing radiation-induced cytotoxicity should have intermediate reactivity and life-time. Furthermore, like aminothiols, nitroxides with positively charged substituents provide enhanced radioprotection. This most likely occurs because the positively charged nitroxide can accumulate at sufficient concentrations at sites of potential damage, such as DNA, and prevent or repair the damage incurred.
To study the influence of the ring oxidation state in modifying the radiobiologic effects of nitroxides, clonogenic viability was tested using fixed concentrations of various nitroxides that varied only in their oxidation state. Three oxidation states were tested for a given ring; the nitroxide (X = O•), the one-electron reduced hydroxylamine (X = -OH), and the fully reduced secondary amine (X= -H). Once again the nitroxide form was an effective radioprotector whereas the corresponding hydroxylamine exhibited minimal protective effects despite the fact that both are chemically capable of scavenging free radicals. The data support the hypothesis that radiation-induced cytotoxicity is mediated, at least in part, by chemical species with lifetimes in the order of microseconds [14], which are more efficiently scavenged by nitroxides compared to the corresponding hydroxylamines.
The enhanced protection provided by nitroxides with positively charged ring substituents was demonstrated in experiments involving DNA binding studies [22]. Two of the tested nitroxides, Tempamine and 3-aminomethyl-PROXYL, bind strongly to DNA, which is an important target for both the direct and indirect cytotoxic effects of ionizing radiation. This study confirmed that nitroxides offer significant radioprotection that depends, in part, on the ability of the nitroxide to enter cells and their affinity for potential target molecules. For example, Tempol is a small molecule that enters cells readily while CAT-1, a larger and more hydrophilic nitroxide, does not [23]. In Figure 5A, the EPR spectra of Tempol and CAT-1 are shown in the presence and absence of the reducing agent chromium oxide (CrOx). In the presence of CrOx, which does not enter the cell, Tempol is not reduced and remains detectable whereas the CAT-1 signal is obliterated showing that Tempol is located intracellularly while CAT-1 remains outside the cell. As a result, Tempol protects cells against both radiation and H2O2 while CAT-1 does not (Figure 5B).
Figure 5. Intracellular and Extracellular Reduction of Nitroxides.
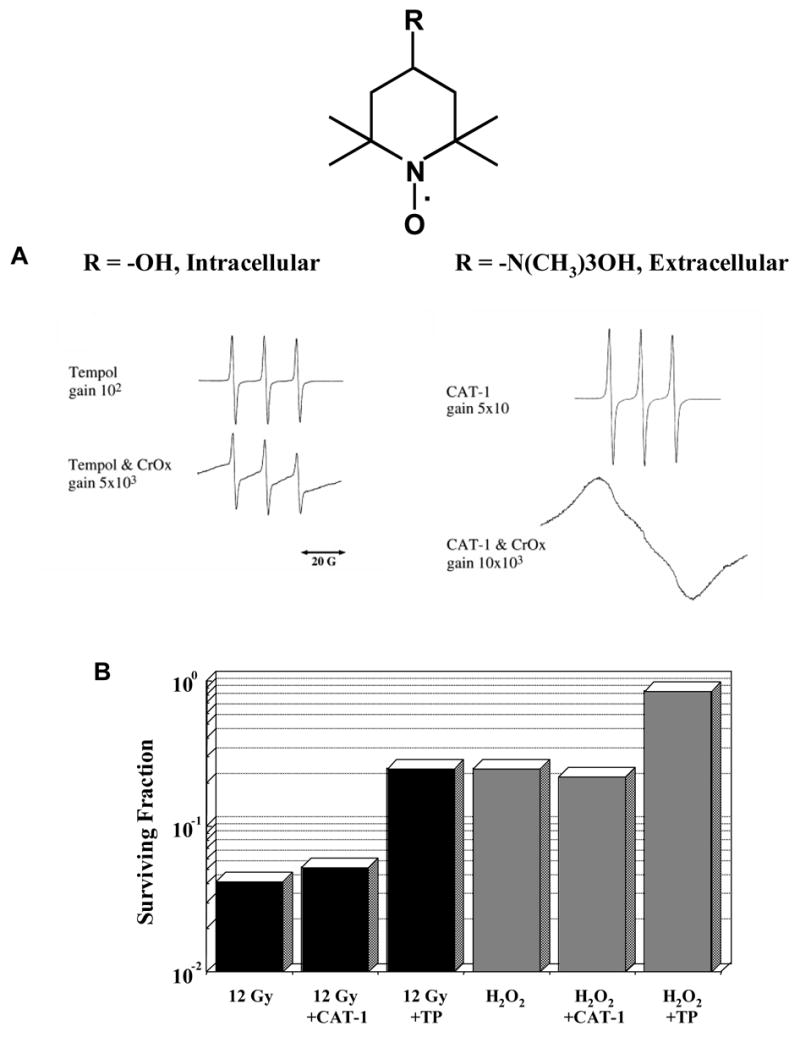
(A) The EPR spectra of Tempol and CAT-1 are shown in the absence of chromium oxide (CrOx). In the presence of the reducing agent CrOx, which does not enter the cell, Tempol remains detectable whereas the CAT-1 signal is obliterated showing that Tempol is located intracellularly while CAT-1 remains outside the cell. (B) The cytoprotective effect of nitroxide compounds is dependent on their ability to enter the cell. Tempol, which quickly crosses the cell membrane, protects cells against both radiation and H2O2 while CAT-1, which does not enter the cell, does not. (Adapted with permission from reference # 23)
Nitroxides and cell signaling
Promotion of apoptosis by radiation-induced free radical damage is one of the mechanisms implicated in radiation-induced cytotoxicity, particularly in hematopoietic cells. Tempol-treated TK6 human lymphoblastoid cells, which are known to undergo apoptosis in response to radiation exposure, demonstrated a decrease in cytotoxicity after exposure to 6 Gy dose of ionizing radiation [24]. Interestingly, Tempol had no effect on the accumulation of components of the apoptotic pathway such as cleaved Caspase-3 or cleaved PARP but did inhibit the radiation-induced increase in p53 observed in untreated control cells. In this same study, the antioxidant N-acetylcysteine was found to decrease cleaved Caspase-3 and cleaved PARP levels, but did not offer any significant protection from cytotoxicity. In a related experiment, nitroxides were found to protect against lipid peroxidation [25]. Radiation-induced free radicals can promote the peroxidation of lipids and the consequent degradation of liposomes, membranes and other lipid-based cellular machinery contributing to aging and other pathological conditions. Nitroxides were able to protect even the most susceptible, unsaturated lipids from damage while engendering no damage of their own.
Most nitroxides tested as antioxidants are administered in forms that lack substituent groups that allow localization to specific sub-cellular compartments. To attain nitroxide concentrations at sites of radical production sufficient to effectively scavenge the reactive species, the test nitroxide is usually administered at increasing concentrations. However, nitroxides with diverse ring substituents can attach to specific groups that help localize the nitroxide moiety to the desired sub-cellular location at effective concentrations at very low total doses. Proxyl nitroxides derivatized at the 3-position with alkylphosphonium groups were shown to retain their radical scavenging and SOD mimetic activity and localize effectively to mitochondria even when administered at concentrations ~3 orders of magnitude lower than those used with unsubstituted nitroxides. This suggests that a novel approach of using low total doses of certain nitroxides may achieve high local concentrations and provide protection against oxidative stress [26]. More recently, targeted nitrones, which are precursors of nitroxides, were synthesized [27]. Nitrones, which themselves are effective antioxidants, are transformed into nitroxides as a result of their radical scavenging activity which also can function to intercept reactive species.
Utsumi and colleagues have been developing nitroxides that can be targeted to specific regions based on the substituent groups at the 3-position of the proxyl ring [28, 29]. Blood-brain barrier permeable nitroxides were developed based on the proxyl moiety and have the ability to accumulate in the brain at sufficient levels to afford protection. Ester substituent groups at the 3-position made penetration of the blood-brain barrier possible after which endogenous esterases converted the nitroxide to a membrane impermeable species enabling the cells to retain the nitroxide at high levels sufficient for antioxidant activity.
Clinical Applications of Nitroxide Antioxidants
The protective effect of nitroxides has been elucidated in experiments beginning in the 1980’s [7] and has been shown to be the result of antioxidant activity both in vitro and in vivo [13]. Several clinical applications of these compounds have been discovered ranging from protection against ionizing radiation to functional imaging to cancer prevention.
Radioprotection
Radioprotection comprises several functions ranging from literally protecting individual cells from death after exposure to radiation to protection against the undesired clinical side-effects of radiation including fibrosis, alopecia, mucositis, skin damage and development of second malignancies. In fact, the most significant problem with the use of radiation as a therapeutic modality is the ability to administer a dose adequate to treat the targeted disease while avoiding development of these side effects. An ideal radioprotective agent would have several characteristics including minimal toxicity, ease of administration and selectivity for radioprotection of normal tissues compared to tumor. Several methods of achieving tumor specific activity have been employed by other treatment modalities, but the most consistent and reliable technique is to utilize a biochemical characteristic of tumors to bring about the desired effect.
While nitroxides had shown promise in in vitro studies, the therapeutic potential of any compound depends, of course, on its safety and efficacy in vivo. Pharmacologic evaluations were performed after administering doses of Tempol to C3H mice ranging from 100 to 500 mg/kg via intraperitoneal injection [30]. The maximum tolerated dose was found to be 275 mg/kg and the LD50 was 341 mg/kg with higher doses resulting in restless behavior, seizures and death within 60 minutes following injection. Animals surviving past 60 min did not subsequently develop any notable toxicity during the 30 day follow-up period. The whole blood concentration of Tempol peaked at 600 μl/ml and occurred at 5–10 min after injection. In these initial in vivo radioprotection studies, C3H mice were treated with Tempol then exposed to total body irradiation and followed for thirty days, a radiobiological time point that acts as a measure of bone marrow toxicity. The dose of radiation that caused 50% lethality at 30 days (LD50/30) for the non-treated mice was 7.84 Gy while the LD50/30 for the Tempol-treated mice was 9.97 Gy providing the first evidence that Tempol would function as an in vivo radioprotector (Figure 6A). A second study confirmed that Tempol protected against death from radiation compared to untreated mice and found that this effect was additive to the protective effect of stem cell factor which is given to aid in the reconstitution of the bone marrow following large doses of radiation [31].
Figure 6. In Vivo Pharmacology of Tempol and Tempol-H.
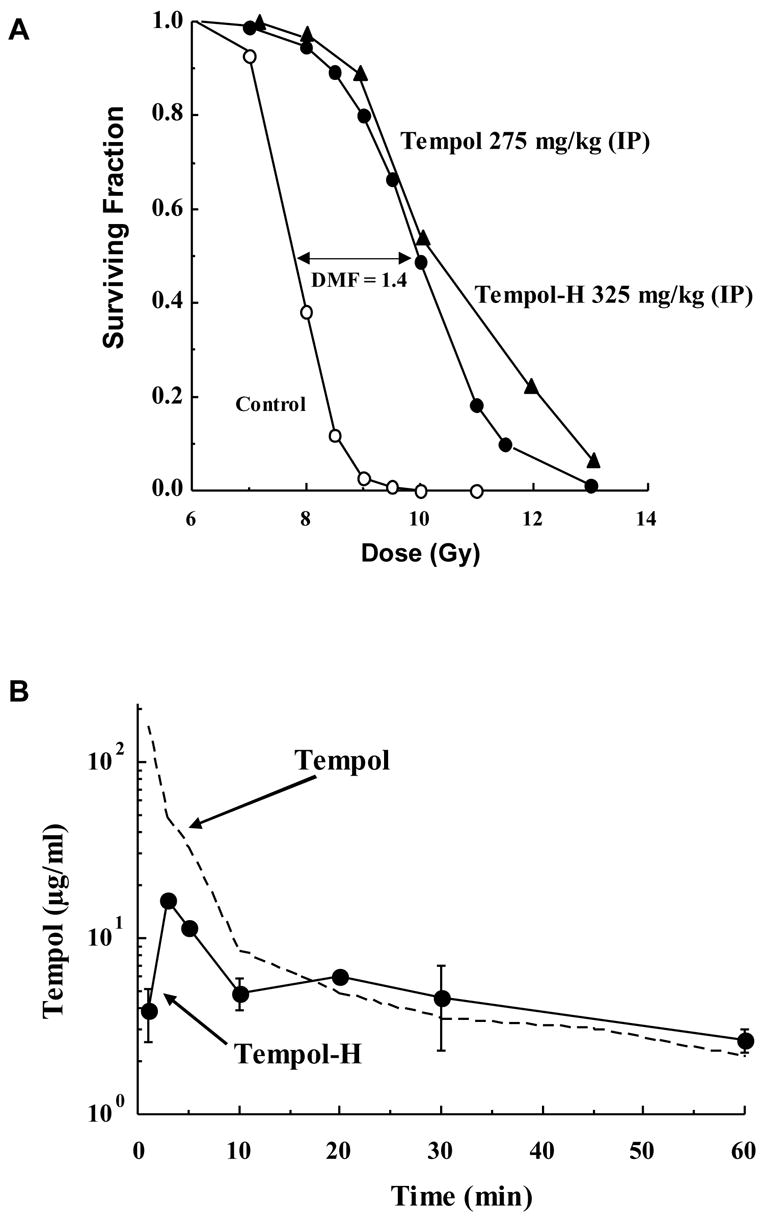
(A) Mice were exposed to whole-body radiation in the absence or presence of Tempol (275 mg/kg) or Tempol-H (325 mg/kg) administered via an intraperitoneal injection. Both Tempol and Tempol-H treatment provided significant radioprotection (dose modifying factor = 1.4) as compared to untreated controls. (B) Tempol-H is rapidly oxidized to Tempol in vivo, as measured by EPR, but has a lower initial peak drug level and less systemic toxicity. (Adapted with permission from references # 26 and # 28)
As would be expected, the hydroxylamine did not confer any radioprotectivity in vitro [19]. It was hypothesized, however, that following administration Tempol-H would be oxidized in vivo to the active, and radioprotective, form of the compound. This hypothesis, and the pharmacology of Tempol-H, was tested in a study in which Tempol-H was administered to C3H mice prior to whole body irradiation [32]. The maximally tolerated dose of Tempol-H was found to be 325 mg/kg and limiting toxicity was similar to Tempol including restlessness and seizure activity. As is the case in vitro, EPR spectroscopy can detect Tempol in the bloodstream in vivo while the reduced form, Tempol-H, cannot be detected. Using this technique, it was demonstrated that Tempol-H is rapidly oxidized in vivo to Tempol (Figure 6B). Although the peak level of the oxidized form is lower than when Tempol is administered directly, the pharmacokinetics were otherwise the same for Tempol-H as compared to Tempol itself with peak blood levels being observed 10 minutes after intraperitoneal injection. As in prior studies, only a small fraction of control mice survived total body irradiation to 9 Gy and none survived a 10 Gy dose while there was a dramatic increase in the surviving fraction of mice treated with Tempol-H (Figure 6A). Similar to the effects of Tempol itself, over 60% of the Tempol-H treated mice survived an 11 Gy dose of radiation and over 10% were alive at thirty days after exposure to a 13 Gy dose. This study demonstrated that reduced nitroxides could be safely administered and would be converted in vivo to the active, oxidized compound able to provide significant radioprotection.
As previously mentioned, tissue specificity is extremely important when evaluating potential radiation dose modifying compounds. To determine if nitroxides protected normal tissues while leaving tumor cells vulnerable to the effects of radiation, radiation-induced fibrosarcoma (RIF-1) tumor cells were injected into the right hind leg of C3H mice [33]. Mice with tumors between 0.5–1 cm were injected with either PBS or Tempol solution 10 min prior to irradiation of the hind leg with doses from 10–60 Gy administered in a single fraction using a standard 9 MeV linear accelerator. Tumor size was then measured for up to two months to assess response to radiation. After thirty days, the radiation dose that resulted in 50% local control was 36.7 Gy in Tempol treated mice compared to 41.8 Gy in the control group and was not statistically different showing that Tempol administration had no effect on tumor regrowth and, therefore, does not compromise tumor control by radiation (Figure 7). This was felt to be the result of the fact that Tempol remains in the oxidized form in normal, well oxygenated tissues but is reduced in hypoxic tissue, such as tumor, to the biologically inactive hydroxylamine form.
Figure 7. Tempol Protects Normal Tissue but not Tumor.
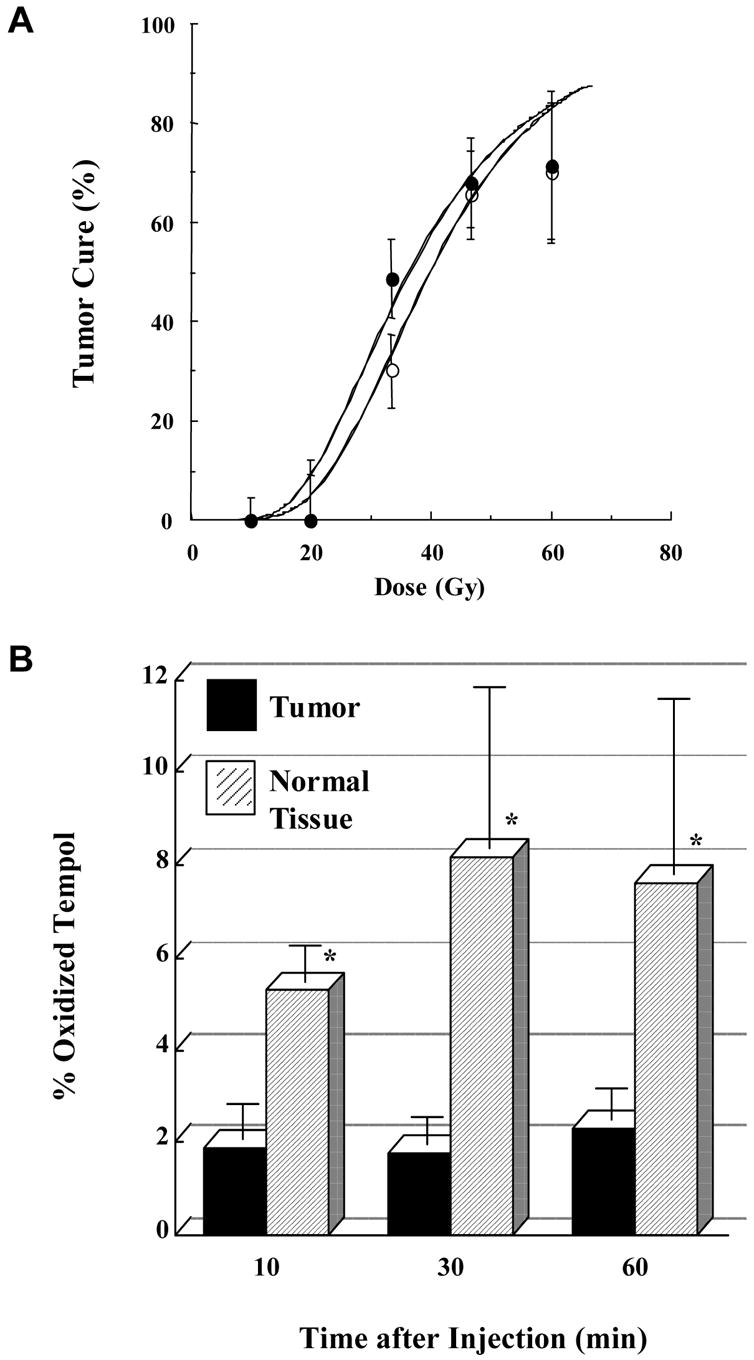
(A) Mice were injected with either PBS or Tempol solution prior to irradiation. After thirty days, tumor growth rates and the radiation dose that resulted in 50% local control were the same in mice treated with Tempol (
 ) compared to the control group that did not receive Tempol (
) compared to the control group that did not receive Tempol (
 ). (B) Tempol remains in the oxidized form in normal, well oxygenated tissues (bone marrow) (
). (B) Tempol remains in the oxidized form in normal, well oxygenated tissues (bone marrow) (
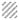 ) but is reduced in hypoxic tissue, such as tumor (■), to the inactive form of the compound. (Adapted with permission from reference # 26)
) but is reduced in hypoxic tissue, such as tumor (■), to the inactive form of the compound. (Adapted with permission from reference # 26)
Several nitroxides were selected for a trial in which C3H mice were irradiated to a single dose of 9 Gy then followed for 30 days to further evaluate the radioprotective effects [34]. After determining the maximally tolerated dose for each compound was determined they were administered via intraperitoneal injection and total body irradiation was administered within to a dose of 9 Gy. While only 15% of the untreated control group was alive at thirty days, the nitroxide groups showed significantly improved survival ranging from 35 to 100% depending on the nitroxide used. Surviving fraction at thirty days was also determined for several radiation doses using the nitroxide 3-carbamoyl-PROXYL. A small fraction of untreated mice survived irradiation to 8 and 9 Gy while none survived thirty days following doses of 10 or 11 Gy. Mice that received 3-carbamoyl-PROXYL showed essentially unchanged survival up to a dose of 9 Gy and a small fraction survived thirty days after receiving 11 Gy demonstrating a significant degree of radioprotection.
In addition to direct prevention of cytotoxicity, a radioprotective compound should also prevent the side-effects associated with radiation therapy. One such consequence of radiotherapy is the occurrence of xerostomia, decreased saliva production, in the treated area which can result in dental caries, infection, dysphagia and significant discomfort. Since the radioprotective effects of nitroxides appear to be selective for normal tissues, the ability of Tempol to prevent damage to the salivary glands from irradiation was studied [35]. Pilocarpine-induced salivary gland output eight weeks after a single dose of radiation was compared between C3H mice treated with Tempol and controls. The radiation dose dependent decrease in saliva production in the untreated cohort, was significantly ameliorated in the Tempol treated group. The effect of different administration routes on the observed pharmacokinetics of Tempol was tested by administering the agent via several routes and evaluating development of xerostomia [36]. The efficacy of intraperitoneal injection of Tempol was compared to administration by intramuscular, intravenous or subcutaneous injection and topical administration using a gel and mouthwash in C3H mice. All administration methods except the intramuscular route reduced radiation-induced salivary hypofunction by 50–60%.
Treatment with radiation also causes alopecia in patients, which can be very dramatic and can cause significant distress in patients, especially children. One of the first clinical applications of nitroxides to be evaluated was the ability to protect against radiation-induced alopecia. A Tempol solution applied topically to guinea pigs fifteen minutes prior to a 30 Gy single dose of ionizing radiation significantly increased the rate and extent of new hair growth compared to untreated skin and was not detectable in the blood or brain tissue [37]. The flanks of guinea pigs were then irradiated to 7 Gy daily for 10 days and Tempol was applied topically to the treatment group each day prior to irradiation. Although both Tempol-treated and untreated guinea pigs demonstrated hair loss, the hair density remained significantly greater in the Tempol treated group, and hair recovery was more rapid (Figure 8A). The clinical application of Tempol was evaluated in a pilot study at the University of Pennsylvania in which eleven patients were treated with topical Tempol prior to irradiation for brain metastases [38]. The Tempol-containing gel was well tolerated by all patients and not systemically absorbed to any significant degree. The application of Tempol resulted in significant reduction of hair loss as shown in Figure 8B.
Figure 8. Prevention of Radiation-induced Alopecia.
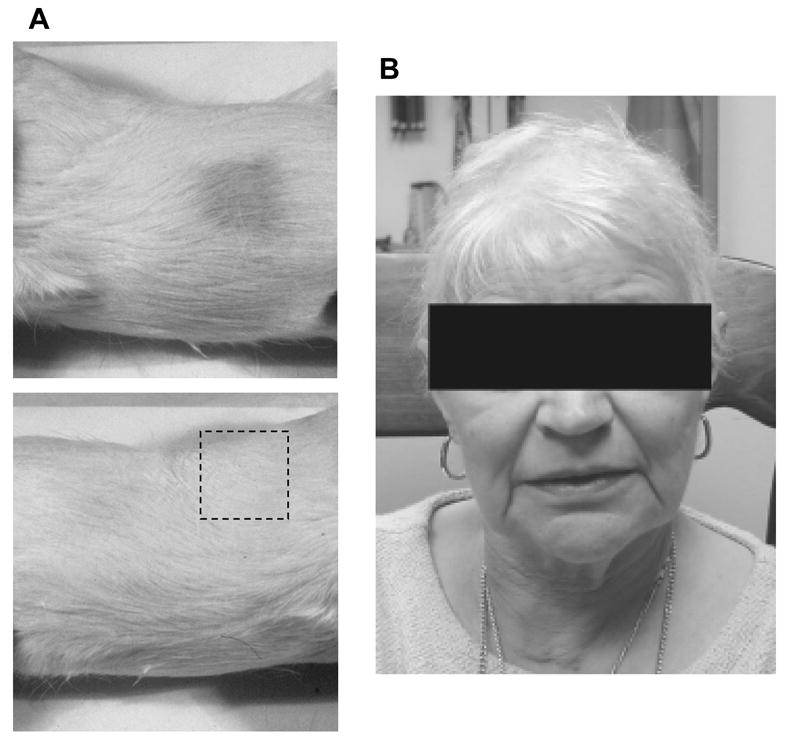
(A) The flanks of guinea pigs were irradiated to 7 Gy daily for 10 days without (top) or with (bottom) topical Tempol application each day prior to irradiation. Hair density remained significantly greater in the Tempol treated group (see dashed box), and hair recovery was more rapid. (B) Typically patients who undergo whole brain irradiation will experience complete alopecia 4–6 weeks post-treatment. Topical application of Tempol resulted in significant reduction of hair loss in this patient who underwent cranial irradiation. (Adapted with permission from reference # 33)
An overwhelming amount of evidence speaks to the radioprotective attributes of nitroxide compounds. They appear to be safe at doses below the maximally tolerated dose and exhibit tissue-specific protective effects. In light of this, nitroxides are being studied for use both in clinical radiation oncology as well as for use in radiological emergencies such as nuclear power plant disasters or terrorist attacks.
Functional imaging
By their nature, most cellular biological functions are exceedingly difficult to monitor in living organisms. In most cases, the observer is relegated to monitoring an effect or by-product of the interaction of interest. This is particularly true of complex functions that occur at the molecular level such as oxidation-reduction interactions. Recently developed techniques have enabled interrogation of tissue on the basis of tissue oxygenation, blood flow, levels of endogenous metabolites, and metabolism of exogenous tracers and provided opportunities to non-invasively observe tissues on a functional basis. The development of such “functional imaging” techniques has opened the door to a vastly improved understanding of certain diseases. For example, the pairing of MRI with positron emission tomography (PET) has allowed clinicians to correlate anatomic abnormalities with functional attributes to better identify metastatic lesions and areas of occult infection in patients. This leads to better and more patient specific treatment as therapy can be directed to treat the correct type of infection or the appropriate stage of cancer.
An extension of this is the use of techniques previously reserved for the laboratory such as EPR imaging which detects unpaired electrons in such species as transition metal complexes and free radicals [39–41]. Unpaired electrons are extremely rare in normal tissues, so little if any EPR signal is detected in biological tissues. While this was initially felt to be a limitation to the use of this technique in biological imaging, it was quickly discovered that agents containing unpaired electrons, or agents converted to such a compound in vivo, could be introduced into a living system and detected using frequencies similar to those used in MRI [42]. Following their administration in vivo, nitroxide compounds are found in an equilibrium between the nitroxide radical form which is detected by EPR, and the reduced hydroxylamine form which is not detected by EPR because of its diamagnetic nature [3, 43]. Because cellular redox processes convert the compound between the two states, the ratio of the two states is determined by the redox activity within the cell which is, in turn, dictated by the oxygen status and redox-status of the surrounding tissue [44]. Since only the oxidized form of the nitroxide can be detected using EPR, the signal intensity can be used as a surrogate marker for the relative amounts of the oxidized compound and, thus, the relative redox activity. In hypoxic cells, the hydroxylamine form is more prevalent resulting in a weak EPR signal whereas, the compound is oxidized to the radical form in well oxygenated tissues resulting in a stronger signal [2]. This property of nitroxides makes them ideal compounds for imaging intracellular redox metabolism.
The tissue specificity of nitroxide compounds has already been discussed with regard to protection against radiation-induced cytotoxicity. As mentioned, this is felt to be due to the relative abundance of the active nitroxide radical form in normal, well-oxygenated tissue as compared to tumor tissues. The first demonstration that EPR Imaging in combination with nitroxides could provide functional images regarding cellular metabolism in vivo was a study in which RIF-1 tumors were implanted into the thigh of mice then imaged with EPR utilizing intravenously injected nitroxides [45]. Two-dimensional images of the tumors revealed significant heterogeneity in both nitroxide distribution and rate of reduction. Furthermore, the nitroxides were reduced more quickly in tumor tissue than in normal muscle tissue and spin label oximetry confirmed a three-fold lower oxygen level within the tumor.
Thiol compounds such as glutathione (GSH) are important in maintaining the intracellular redox balance. GSH is a reducing agent present within cells and, in theory, is partly responsible for reactions that reduce nitroxide radicals to the hydroxylamine form. To further investigate the potential of EPR imaging, RIF-1 tumors were once again implanted into C3H mice and the EPR spectra of the nitroxide compound 3-CP were recorded from the tumor and compared to that observed in normal tissue and tumor treated with L-buthionine-S,R-sulfoximine (BSO), an inhibitor of GSH over time [46]. The nitroxide radical form decayed significantly more slowly in BSO treated tumor and normal tissue as compared to tumor implicating GSH in the reduction of nitroxide compounds within cells. Redox tumor maps were generated using nitroxides and EPR in mice whose thiol status was modulated using diethyl maleate [47]. As in the earlier study using BSO to deplete thiols, heterogeneity of redox status within tumor tissue was confirmed. These studies confirm both the feasibility of imaging nitroxide distribution in vivo and the ability to monitor local metabolism of nitroxides.
Initially, nitroxides were used exclusively in EPRI redox imaging; however, there are several limitations with the use of EPR imaging [9, 48, 49]. The advantages of MRI include the availability of MRI scanners, multi-slice imaging capability, enhanced spatial and temporal resolution, and co-registration of images of tissue redox status with anatomical information inherently available from MRI. Suitable contrast MRI agents provide important functional information such as blood flow and tissue perfusion [50]. Currently used agents used for T1-contrast enhancement contain paramagnetic entities such as the Gd3+ or Mn2+ complexes. Like these gadolinium-based agents, nitroxide radicals have a single unpaired electron and therefore have the potential to provide T1 contrast. The use of nitroxide radicals as T1 contrast agents in MRI was known [51–53] prior to their use as in vivo EPRI as probes [54], but were felt to be suboptimal as MRI contrast agents due to their rapid in vivo reduction to the undetectable diamagnetic products [55]. In addition to their biologic instability, nitroxide probes have only one unpaired electron, as compared to seven for Gd3+ resulting in a lower relaxivity. In light of these supposed shortcomings, nitroxide radicals were considered less effective contrast agents for MRI as it was making its entry into diagnostic radiology in the 1980s. However, with the time-efficient image data acquisition strategies standard in current MRI scanners, nitroxides are now being re-evaluated as functional redox-sensitive contrast agents.
Studies showed that nitroxides provide significant contrast enhancement in phantom objects and that the rate of decrease in intensity measured by T1-weighted MRI scans can be used as an indicator of metabolism of nitroxides as a function of time [5]. In vivo experiments using the nitroxide 3CP were carried out in tumor bearing mice to examine the differences in nitroxide metabolism in tumor and normal tissue [6]. The intensity of T1-weighted MR images as a function of time after intravenous administration of 3CP was found to decrease more quickly in tumor compared to normal tissue (Figure 9). When the intensity change is plotted as a function of time, the reduction rate in the normal leg ~60% of that observed in tumor. In this study, the advantageous pharmacokinetic distribution of nitroxides and their usefulness as MRI contrast agents was demonstrated for the first time.
Figure 9. Nitroxide Imaging Studies.
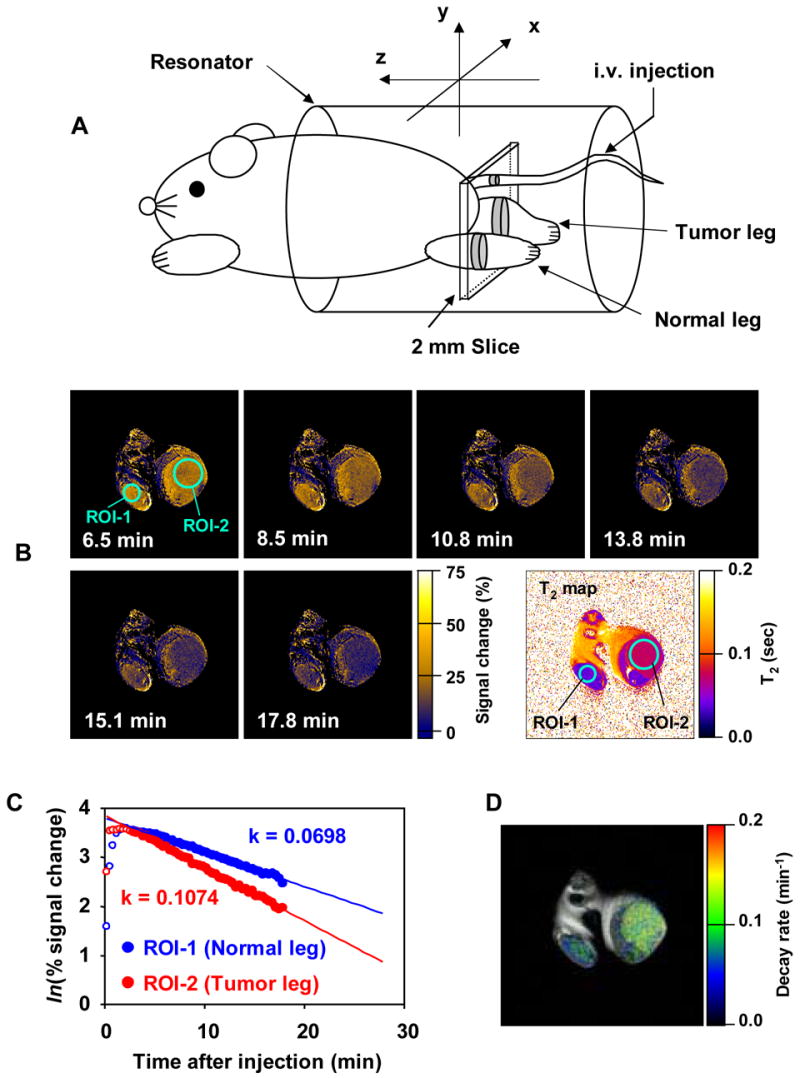
A) Experimental arrangement of the mouse in the MRI resonator and the slice selected to monitor the nitroxide levels used to examine the differences in nitroxide metabolism in tumor and normal tissue. (B) Sequence of T1-weighted MR images as a function of time after intravenous administration of 3CP. Signal intensity in normal (ROI-1) and tumor leg (ROI-2) increase after administration and reach a maximum at 8.5 min. Signal decreases faster in tumor region than in normal tissue. Intensity change plotted as a function of time in the normal leg was observed to be ~60% compared to that in tumor. (C) The rate of intensity change in each pixel was computed and (D) a parametric image redisplayed shows that tumor reduction globally is elevated compared to the normal tissue. (Adapted with permission from reference # 5)
In order to utilize nitroxides as MRI contrast agents, their reduction in a variety of normal tissues must be documented. The distribution and reduction of the nitroxides Tempol, 3CP, and carboxy-Proxyl were examined in MRI experiments [6]. While 3CP and Tempol are known to enter cells, carboxy-Proxyl is membrane impermeable and is restricted to extracellular regions. Using EPR imaging the spatial distribution of 3CP and carboxy-Proxyl was determined, but because of rapid bioreduction compared to the time taken for EPR imaging Tempol distribution could not be determined. Using MRI, it was possible to monitor the distribution and reduction of all three nitroxides. The reduction rates in normal tissue and tumor were calculated from these experiments. The two cell permeable nitroxides, 3CP and Tempol, displayed faster reduction in tumor compared to normal tissue while carboxy Proxyl exhibited no such differences and also had the longest half life of the three agents tested supporting the notion that nitroxide metabolism reflects intracellular redox status.
There are numerous potential applications of functional MRI in the study of cellular redox metabolism. It can be used to non-invasively probe the effect of ionizing radiation on various tissues, evaluating the impact of potentially radioprotective or radiosensitizing compounds, monitor patients undergoing radiotherapy to determine how the redox status in tumor and normal tissue changes as a function of time or whether free radical levels are likely to damage normal tissue, and to monitor the dose being administered to the area of interest. Furthermore, it may also play an important role in managing other conditions such as cerebral or cardiac ischemia or the deleterious effects of infection. While nitroxides as MRI contrast agents will need to be developed further before these clinical applications can be realized, the tremendous gains over the past several years demonstrate that rapid progress is possible.
Chemoprevention and anticancer activity
Despite the theoretical appeal, the clinical results of chemotherapeutic drugs targeted at specific biochemical pathways have been disappointing. Recently, nitroxide compounds have been found to act as chemopreventative agents by altering the entire redox milieu of the tissue at the cellular level rather than attacking a specific molecular target. A broad spectrum of oxidative stresses including environmental radiation exposure, chemical ingestion from foods, drugs and environmental pollutants, and some chronic diseases may overwhelm the cellular defense mechanisms precipitating sublethal cell damage, a process implicated in aging, certain neurological diseases and the development of cancer.
In a series of studies, C3H mice were given Tempol in their drinking water resulting in an incredible 75% reduction in tumor incidence in Tempol treated mice compared to control [56]. In the next study, Tempol was administered continuously in the food to Atm-deficient mice, a murine model for ataxia-telangiectasia [57]. Although Tempol did not change the characteristics of the thymic lymphomas these mice are predisposed to developing, it did significantly delay the onset resulting in an increased lifespan from 30.1 weeks to 62.4 weeks (Figure 10). Conversion of 2’7’-dichlorodihydrofluorescein (DCF) was used to measure the effect of Tempol on intracellular ROS. DCF fluorescence is significantly increased in thymocytes from Atm-deficient mice indicating increased ROS activity. Tempol decreased the DCF intensity indicating a reduction of ROS. In another study, the nitroxide 5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl (CTMIO) was administered to Atm-deficient mice and again a dramatic delay in the onset of thymic lymphomas was observed [58]. These studies added to the evidence that ROS play an important role in tumorigenesis and that nitroxides can potentially ameliorate this drive.
Figure 10. Tempol Increases Longevity of Atm-/- Mice.
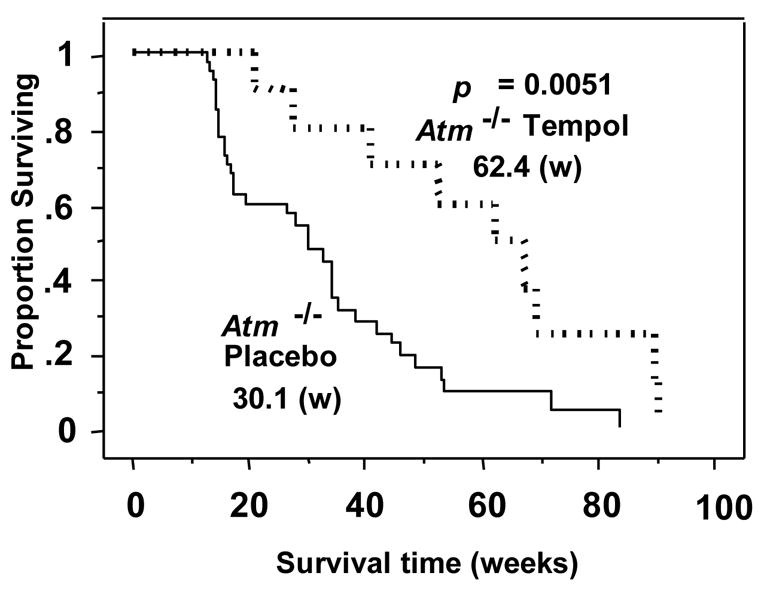
Survival curves of the Atm-/- mice fed placebo- or Tempol-containing mouse chow. Survival in weeks (w) is shown. Kaplan–Meier analysis shows that Atm-/- mice fed with a Tempol diet at weaning (
 ) lived significantly longer that those fed a placebo-containing (
) lived significantly longer that those fed a placebo-containing (
 ) diet. (Adapted with permission from reference # 53)
) diet. (Adapted with permission from reference # 53)
In a study using mice deficient in the p53 tumor suppressor gene Tempol once again, increased the latency of tumorigenesis, although this effect was less pronounced than in the Atm-deficient mice [59]. Unlike Atm-deficient mice, p53-deficient mice did not demonstrate increased thymic oxidative stress as measured by DCF, and Tempol did not further reduce these normal levels. Previous studies have shown that the p53 gene is activated by phosphorylation to suppress tumors in response to oxidative stress [60–62]. Tempol was found to phosphorylate p53 resulting in an increase in downstream anti-proliferative gene expression.
In addition to cancer prevention, nitroxides have been studied as a treatment for existing tumors. Although Tempol was previously shown to have no significant cytotoxicity, [16, 63] Tempol was found to have an antiproliferative effect on MCF-7 breast cancer cells [60]. Treated cells accumulated in G1 then paused in G2/M phase ultimately undergoing apoptosis as revealed by DNA fragmentation studies. A later study of HL60 leukemia cells that lack p53 tumor suppression revealed that Tempol induced a time and dose-dependent increase in expression of the downstream antiproliferative gene p21 resulting in G1 arrest [64, 65]. As a result of this finding, the anti-tumor effect of Tempol against glioma cells in anin vitro and in vivo murine xenograft model was studied [66]. Tumors from Tempol-treated mice treated showed increased evidence of apoptosis using a TUNEL assay and direct visualization of cells and showed decreased neovascularization on histological staining.
These results support the notion that the redox status of certain tumors and their microenviroment is significantly altered compared to that of normal tissue and that nitroxides can exploit this difference to treat various malignancies. While further study is required before nitroxides can be employed as anti-cancer agents, these results are promising and offer a glimpse into the potential therapeutic benefit of manipulating redox status of tumors with nitroxide compounds.
Ischemia-reperfusion injury and inflammation
Ischemic issue necessarily undergoes anaerobic metabolism resulting in the conversion of xanthine dehydrogenase to xanthine oxidase. When the blood supply is restored and blood rich in molecular oxygen is allowed to reperfuse the tissue, ROS are generated which can cause further tissue injury. This process is seen acutely during myocardial infarction and ischemic strokes however conditions such as ischemic colitis, vascular dementia, renal disease and other vascular diseases commonly seen in diabetics and hypertensive patients develop from tissue damage that accumulates over years and result partly from ischemia-reperfusion insults as well. Prevention of the ROS-induced damage could lessen the severity of these conditions and, in some cases, permit more aggressive therapies aimed at restoring blood flow to oxygen starved tissues.
Tempol, administered as an IV bolus in rat and rabbit models of myocardial infarction, reduced the size of infarcts by 30–40% [67]. Another nitroxide, TEMPO, decreased arrhythmia following experimentally induced cardiac ischemia in rats [68]. This beneficial effect was also observed in a study of gerbils in which cerebral ischemia was induced by occluding the common carotid arteries bilaterally [69]. Ischemic damage was detected post-mortem using immunohistochemical (IHC) staining for nitrotyrosine, an indicator of peroxynitrite production, and polyADP-ribose synthetase (PARS), an indicator of DNA breakage. Intraperitoneal administration of Tempol before and after reperfusion resulted in a significant decrease in IHC staining and a decrease in lipid peroxidation as demonstrated by a decrease in cerebral malondialdehyde levels. In another study, the middle cerebral artery of rats was occluded then Tempol was administered during the first 20 minutes of reperfusion [70]. Tempol treatment resulted in a significant, dose-dependent decrease in infarct size as measured by digital imaging of brain slices which were collected and fixed after four hours of reperfusion (Figure 11). Post-ischemic ROS formation and lipid peroxidation was also prevented using Tempol in a study of transient cerebral ischemia in rats as measured by assessing 2,3-dihydroxybenzoic acid levels and thiobarbituric acid reactive substances [71]. Acute subdural hematoma can also result in local cerebral ischemia and O2• − mediated damage. Tempol administration resulted in a 42% decrease in infarct size and 2,3-dihydrobenzoic acid levels in rats following experimentally induced subdural hematoma [72]. Similar trials studying renal protection were performed by clamping the renal pedicle in rats and Tempol again had a protective effect on kidney function after reperfusion [73].
Figure 11. Tempol Reduces Cerebral Infarct Size.
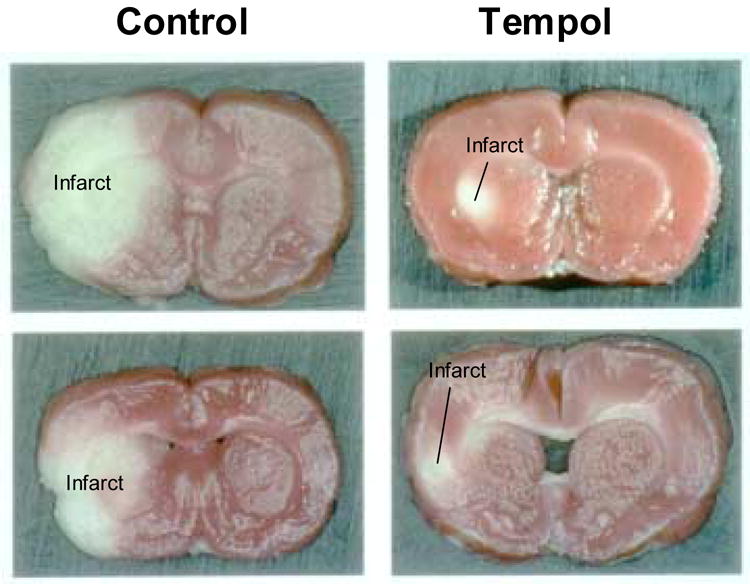
Cerebral infarcts were induced in rats by occluding the middle cerebral artery. After reperfusion, gross pathological specimens from Tempol treated rats revealed significantly smaller infarct size than in untreated animals. (Adapted with permission from reference # 66)
Regardless of the underlying pathology, all forms of shock are the result of poor tissue perfusion and the subsequent formation of toxic metabolites. There is mounting evidence that ROS play a role in shock-induced tissue injury. Toxic (septic) shock was induced in rats using lipopolysaccharide to determine whether Tempol could protect against systemic tissue injury [74]. Although an IV bolus of Tempol did not prevent circulatory collapse, it did reduce the development of kidney and liver dysfunction. In another study, severe hemorrhagic shock was induced in rats then reversed by transfusion and again, Tempol protected against the resulting multi-organ failure [75].
Much like perfusion-reperfusion injury, damage from inflammatory diseases is also mediated in part by ROS. Inflammation is the culmination of many processes all of which are designed to increase the ability of the body to respond to a perceived threat such as microbial invasion or repair of tissue destruction. Increased blood flow and vascular permeability cause the rapid accumulation of chemical and cellular mediators of inflammation, many of which generate ROS either as part of their protective mechanism or as a byproduct. While necessary to our survival, these processes sometimes cycle out of control leading to the undesired consequences of autoimmune and inflammatory disease. It has been hypothesized that nitroxides could decrease the inflammatory response and attenuate the damage caused by ROS released by immune effector cells. Tempol has been preliminarily shown to be effective in several models of inflammation including pancreatitis [49], pleurisy [76], arthritis [77], colitis [78], and uveoretinitis [79] and more studies are ongoing.
Neurodegenerative diseases
Parkinson’s disease is a disorder in which dopamine secreting neurons in primitive portions of the brain responsible for control of movement degenerate over time leaving the patient with progressively worsening motor dysfunction. ROS formation and the oxidation of dopamine are thought to contribute to this process [80–84]. One study found that Tempol protected dopamine secreting cells in vitro from 6-hydroxydopamine (6-OHDA)-induced apoptosis [85]. Tempol also protected mice from developing Parkinsonian symptoms induced by administration of 6-OHDA. A study of several toxic brain peptides associated with Alzheimer’s revealed that they were capable of oxidizing the nitroxide Tempone [86] although the significance of this finding is difficult to ascertain, however, as they do not spontaneously produce free radicals and the contribution of ROS to the development of Alzheimer’s is not well characterized [86].
Ocular damage
The nature of ROS-induced ocular damage is also being investigated. It is well known that clinicians who are chronically exposed to ionizing radiation, such as cardiologists and interventional radiologists, develop cataracts aver time. Several studies regarding the oxygen status of the retina have been published and studies suggest that cataract formation is largely due to oxidative stress [87] and elevated levels of H2O2 in the aqueous humor of cataract patients has been demonstrated [88]. In an early attempt to determine the mechanism by which ROS lead to cataract formation, it was found that Tempol prevented the H2O2-induced inhibition of cell growth, membrane blebbing, decreases in DNA-repair protein activation, and limited the amount of DNA damage [89]. It was later shown that Tempol also protected cultured glutathione-depleted lens epithelial cells from H2O2 toxicity [90]. As GSH-depletion is known to exist in most forms of cataract, it is argued that Tempol may prevent or slow the progression of this condition. A more recent study showed that topical application of Tempol-H decreased the formation of cataracts in an in vivo model in both rats and Rhesus monkeys [91].
Hypertension
During early studies of the radioprotective qualities of nitroxides, animal studies revealed that several nitroxides, when administered via the intraperitoneal route, resulted in a substantial decrease in mean arterial blood pressure [34]. This effect was recognized as being similar to that seen after nitric oxide (NO) administration which is well known to cause vasodilatation in vivo resulting in a decrease in arterial blood pressure. The assertion that nitroxides cause hypotension via a NO related mechanism was supported by studies performed on cultured endothelial cells [92]. NO release was measured using NO chemiluminescence following Tempol administration and it was found that nitroxides increase the amount of bioavailable NO.
Although the phenomenon had been described previously, the first well controlled investigation of the effect of nitroxides on blood pressure was a study of spontaneously hypertensive rats (SHR) in which Tempol was found to restore normal blood pressure [93]. Figure 12 shows the decrease in mean arterial blood pressure following administration of Tempol. The antihypertensive effect was demonstrated during both continuous infusion and when Tempol was administered via daily intraperitoneal injection. This effect was found to be dependent on the endogenous production and availability of NO because the NO synthesis blocker Nw-nitro-L-arginine methyl ester (L-NAME) abolished the antihypertensive effect. Since nitroxides are known to act as SOD mimics, the mechanism underlying the antihypertensive effect was felt to be inhibition of NO inactivation by O2•−.
Figure 12. Tempol Reduces Arterial Blood Pressure in a Dose-Dependent Manner.
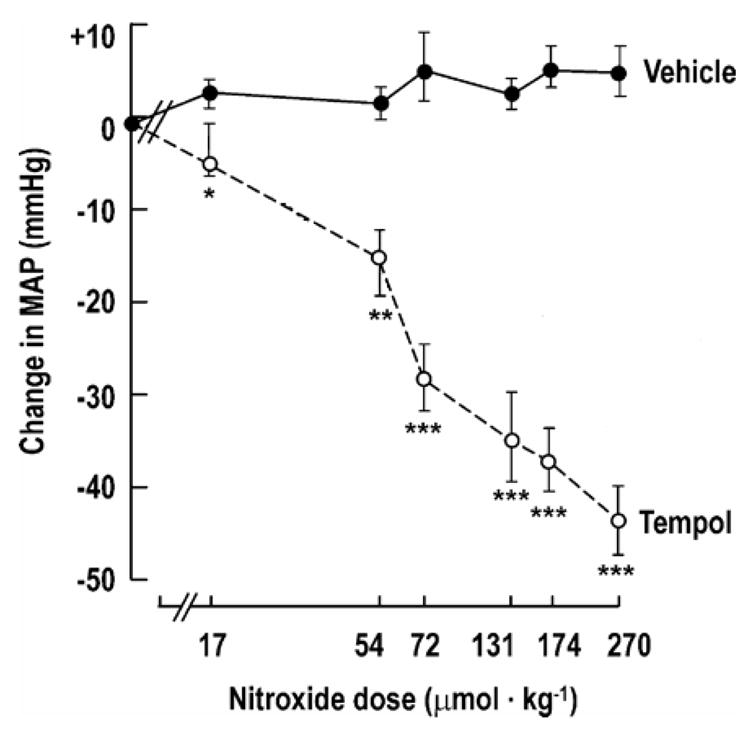
Means ± SE values for changes in mean arterial pressure (MAP) for SHR given Tempol (
 n = 6) or vehicle (
n = 6) or vehicle (
 n = 6). Compared with vehicle: *P < 0.05; **P < 0.01; ***P < 0.005. Tempol was infused intravenously over 10s. The MAP was recorded over the first 5 min and at 10 and 15 min. Tempol was administered in doses of 17, 54, 72, 174, or 270 μmol/kg under similar conditions. (Adapted with permission from reference # 92)
n = 6). Compared with vehicle: *P < 0.05; **P < 0.01; ***P < 0.005. Tempol was infused intravenously over 10s. The MAP was recorded over the first 5 min and at 10 and 15 min. Tempol was administered in doses of 17, 54, 72, 174, or 270 μmol/kg under similar conditions. (Adapted with permission from reference # 92)
A later study examined the antihypertensive effect of Tempol over a two week period and measured urinary excretion of 8-Iso prostaglandin F2-alpha, a measure of oxidative stress that is formed nonenzymatically from the attack of O2•− on arachidonic acid [94]. Again, Tempol corrected the blood pressure elevation in the SHR model of hypertension and the renal vasoconstriction associated with oxidative stress. A more comprehensive study of the effect of nitroxides on the cardiovascular system using Swan-Ganz catheterization in miniature pigs revealed similar results with regard to arterial blood pressure [95]. Systemic arterial resistance decreased causing a drop in blood pressure and a compensatory increase in heart rate resulting in an increase in the cardiac index. Several doses of the nitroxide compounds were administered and although the initial hemodynamic response was similar, the recovery time from hypotension back to normal blood pressure increased with increasing doses.
The direct interaction of nitroxides with NO was evaluated by incubating Tempol in a NO-saturated solution. No change in EPR signal was detected over time indicating that no interaction resulting in the degradation of Tempol was taking place confirming that nitroxides likely interfere with NO inactivation by scavenging oxygen free radicals rather than through direct interaction with NO itself.
A recent st udy clarified previous findings reporting that the six-membered ring piperidine nitroxides result in the observed hypotensive response while the five-membered ring pyrrolidine nitroxides do not [96]. This is felt to be due in part to the relative differences in lipophilicity and hydrophilicity since both are effective SOD mimics in vitro. Liposomal preparations of SOD itself restore the normal response of dysfunctional endothelium to acetylcholine after several days of treatment strongly suggesting that nitroxides could be formulated for use in the clinical setting of both acute hypertensive crisis as well as for long-term treatment of endothelial dysfunction, important factor in renal vascular diseases and coronary artery disease.
Nitric oxide has long been felt to play a role in both hypertension and endothelial dysfunction. Endothelial dysfunction is, in fact, defined as a paradoxical response of endothelial muscle to acetylcholine resulting in vasoconstriction rather than vasodilatation. Anomalous arterial constriction results in a decreased vascular compartment and increased vascular resistance which increases blood pressure placing increased strain on the heart and limiting blood supply to other vital organs. The subsequent stiffening of arterial walls and increased shear stress results in chronic damage and predisposes to plaque formation, the precursor of atherosclerotic heart disease. Prevention and even reversal of the initial step in the cascade, endothelial dysfunction, may be possible with the administration of nitroxides. Although these effects must be tested carefully in human patients, the swine models suggest that nitroxides can be safely administered to treat hypertension.
Weight control
An unanticipated result of long term nitroxide administration in several animal studies has been weight loss and prevention of obesity. In one study, C3H mice that were given Tempol in their drinking water at a concentration of 58mM maintained a normal healthy weight while sucrose-fed controls gained substantial weight very quickly (Figure 13) [56]. Caloric restriction is know to increase lifespan in mice [97–99]. In a long term study, Tempol treated mice had a significantly increased survival, 123 vs. 92.6 weeks, and remained more active as they aged [56]. Coat color and sheen was also superior in the Tempol treated group as they aged. In the initial phase of the study, it was noted that Tempol-treated mice ate less food, however in a modified study, Tempol was placed in bacon-flavored food instead of drinking water, and the weight gain and survival results were the same with no evidence of malnutrition or diarrhea. No metabolic differences were detected including thyroid stimulating hormone, follicle stimulating hormone, liver function tests, renal function tests, total protein and albumin levels. Glucose and insulin levels were also similar between all of the groups. In Tempol treated animals, leptin, which is produced by adipose tissue and relates to amount of adipose storage, was half that of the control animals. Several possible associations have been hypothesized, including mechanisms related to upregulation of heat shock protein-70 and uncoupling protein-2, but all require further study. The beneficial effect is clear, however, and may suggest a role for nitroxides in the treatment of obesity, diabetes, and for use in improving performance status in patients with cancer or other chronic diseases.
Figure 13. Tempol Controls Weight Gain.
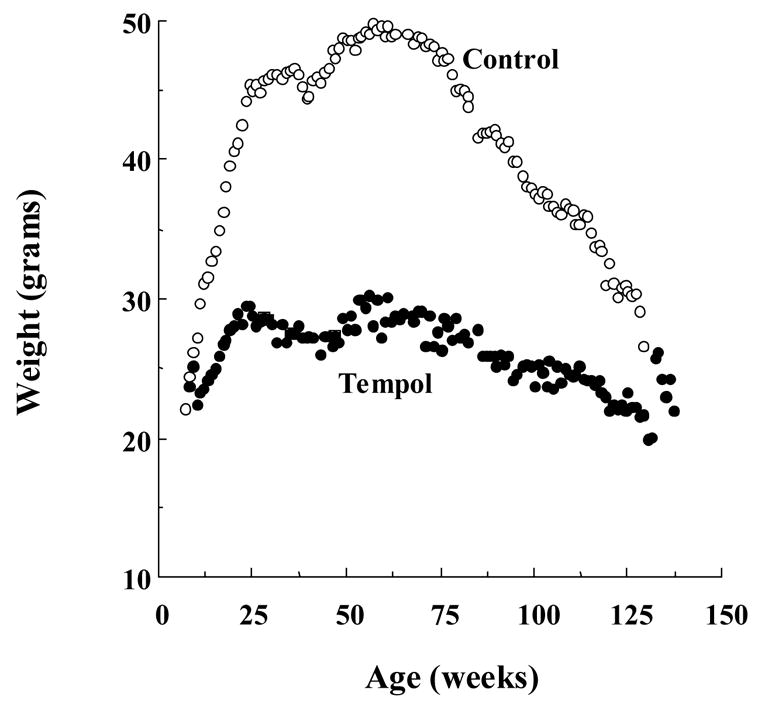
Tempol/sucrose treated mice (●, 58mM in drinking water) gained weight more slowly and maintained a normal healthy weight while the sucrose-fed controls (○) gained substantial weight very quickly and maintained higher weight throughout their lifespan. (Adapted with permission from reference # 52)
Conclusion
Although nitroxide radicals have long been utilized as biophysical tools for ESR spectroscopic studies and spin labeling oximetry, over the past two decades, research involving nitroxide compounds has flourished. As more studies are performed, more is revealed about these fascinating compounds, and more potential applications are uncovered. Experiments involving these compounds have ranged from radioprotection studies that revealed much about the antioxidant nature of these compounds and opened the door to their use as therapeutic agents to imaging studies that exploit the innate polarity of these compounds for use as MRI contrast agents. Nitroxides appear to have the ability to delay cancer formation and progression and someday may even be used to treat certain tumors. Damage from acute and chronic ischemic conditions could potentially be lessened with nitroxide compounds and their use in aging and weight control have yet to be fully explored. This wide variety of applications of nitroxide compounds all ties back to the ability of these compounds to alter the cellular redox state by scavenging oxygen free radicals and destroying O2•− because, incredibly, all of these seemingly disparate disease processes are related in some way to free radicals. Nitroxides provide an unprecedented ability to study the mechanisms underlying many disease states by interfering with a single important cellular metabolic phenomenon. With continued study, there is no doubt that even more experimental and clinical applications for nitroxides will be found.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McConnell HM. In: Spin Labeling: Theory and Applications. Berliner L, editor. New York: Academic Press; 1976. [Google Scholar]
- 2.Swartz HM, Chen K, Pals M, Sentjurc M, Morse PD. 2nd Hypoxia-sensitive NMR contrast agents. Magn Reson Med. 1986;3:169–174. doi: 10.1002/mrm.1910030126. [DOI] [PubMed] [Google Scholar]
- 3.Swartz HM. Principles of the metabolism of nitroxides and their implications for spin trapping. Free Radic Res Commun. 1990;9:399–405. doi: 10.3109/10715769009145700. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein S, Samuni A, Russo A. Reaction of cyclic nitroxides with nitrogen dioxide: the intermediacy of the oxoammonium cations. J Am Chem Soc. 2003;125:8364–8370. doi: 10.1021/ja035286x. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto K, Hyodo F, Matsumoto A, Koretsky AP, Sowers AL, Mitchell JB, Krishna MC. High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin Cancer Res. 2006;12:2455–2462. doi: 10.1158/1078-0432.CCR-05-2747. [DOI] [PubMed] [Google Scholar]
- 6.Hyodo F, Matsumoto K, Matsumoto A, Mitchell JB, Krishna MC. Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res. 2006;66:9921–9928. doi: 10.1158/0008-5472.CAN-06-0879. [DOI] [PubMed] [Google Scholar]
- 7.Samuni A, Krishna CM, Riesz P, Finkelstein E, Russo A. A novel metal-free low molecular weight superoxide dismutase mimic. J Biol Chem. 1988;263:17921–17924. [PubMed] [Google Scholar]
- 8.Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci U S A. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishna MC, Russo A, Mitchell JB, Goldstein S, Dafni H, Samuni A. Do nitroxide antioxidants act as scavengers of O2-. or as SOD mimics? J Biol Chem. 1996;271:26026–26031. doi: 10.1074/jbc.271.42.26026. [DOI] [PubMed] [Google Scholar]
- 10.Kocherginsky N, Swartz H. Nitroxide Spin Labels - Reactions in Biology and Chemistry. Boca Raton: CRC Press; 1995. [Google Scholar]
- 11.Miura Y, Utsumi H, Hamada A. Antioxidant activity of nitroxide radicals in lipid peroxidation of rat liver microsomes. Arch Biochem Biophys. 1993;300:148–156. doi: 10.1006/abbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- 12.Krishna MC, Samuni A, Taira J, Goldstein S, Mitchell JB, Russo A. Stimulation by nitroxides of catalase-like activity of hemeproteins. Kinetics and mechanism. J Biol Chem. 1996;271:26018–26025. doi: 10.1074/jbc.271.42.26018. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell JB, Samuni A, Krishna MC, DeGraff WG, Ahn MS, Samuni U, Russo A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry. 1990;29:2802–2807. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- 14.Milligan JR, Ng JY, Wu CC, Aguilera JA, Fahey RC, Ward JF. DNA repair by thiols in air shows two radicals make a double-strand break. Radiat Res. 1995;143:273–280. [PubMed] [Google Scholar]
- 15.DeGraff WG, Krishna MC, Russo A, Mitchell JB. Antimutagenicity of a low molecular weight superoxide dismutase mimic against oxidative mutagens. Environ Mol Mutagen. 1992;19:21–26. doi: 10.1002/em.2850190105. [DOI] [PubMed] [Google Scholar]
- 16.DeGraff WG, Krishna MC, Kaufman D, Mitchell JB. Nitroxide-mediated protection against X-ray- and neocarzinostatin-induced DNA damage. Free Radic Biol Med. 1992;13:479–487. doi: 10.1016/0891-5849(92)90142-4. [DOI] [PubMed] [Google Scholar]
- 17.Johnstone PA, DeGraff WG, Mitchell JB. Protection from radiation-induced chromosomal aberrations by the nitroxide Tempol. Cancer. 1995;75:2323–2327. doi: 10.1002/1097-0142(19950501)75:9<2323::aid-cncr2820750922>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Samuni A, Mitchell JB, DeGraff W, Krishna CM, Samuni U, Russo A. Nitroxide SOD-mimics: modes of action. Free Radic Res Commun. 1991;12–13(Pt 1):187–194. doi: 10.3109/10715769109145785. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JB, DeGraff W, Kaufman D, Krishna MC, Samuni A, Finkelstein E, Ahn MS, Hahn SM, Gamson J, Russo A. Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, tempol. Arch Biochem Biophys. 1991;289:62–70. doi: 10.1016/0003-9861(91)90442-l. [DOI] [PubMed] [Google Scholar]
- 20.Xavier S, Yamada K, Samuni AM, Samuni A, DeGraff W, Krishna MC, Mitchell JB. Differential protection by nitroxides and hydroxylamines to radiation-induced and metal ion-catalyzed oxidative damage. Biochim Biophys Acta. 2002;1573:109–120. doi: 10.1016/s0304-4165(02)00339-2. [DOI] [PubMed] [Google Scholar]
- 21.Krishna MC, DeGraff W, Hankovszky OH, Sar CP, Kalai T, Jeko J, Russo A, Mitchell JB, Hideg K. Studies of structure-activity relationship of nitroxide free radicals and their precursors as modifiers against oxidative damage. J Med Chem. 1998;41:3477–3492. doi: 10.1021/jm9802160. [DOI] [PubMed] [Google Scholar]
- 22.Hahn SM, Wilson L, Krishna CM, Liebmann J, DeGraff W, Gamson J, Samuni A, Venzon D, Mitchell JB. Identification of nitroxide radioprotectors. Radiat Res. 1992;132:87–93. [PubMed] [Google Scholar]
- 23.Samuni AM, DeGraff W, Krishna MC, Mitchell JB. Cellular sites of H2O2-induced damage and their protection by nitroxides. Biochim Biophys Acta. 2001;1525:70–76. doi: 10.1016/s0304-4165(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 24.Samuni AM, DeGraff W, Cook JA, Krishna MC, Russo A, Mitchell JB. The effects of antioxidants on radiation-induced apoptosis pathways in TK6 cells. Free Radic Biol Med. 2004;37:1648–1655. doi: 10.1016/j.freeradbiomed.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Samuni AM, Barenholz Y. Stable nitroxide radicals protect lipid acyl chains from radiation damage. Free Radic Biol Med. 1997;22:1165–1174. doi: 10.1016/s0891-5849(96)00509-6. [DOI] [PubMed] [Google Scholar]
- 26.Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, Kalyanaraman B. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med. 2005;39:567–583. doi: 10.1016/j.freeradbiomed.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Kalyanaraman B. Synthesis and ESR studies of a novel cyclic nitrone spin trap attached to a phosphonium group-a suitable trap for mitochondria-generated ROS? Free Radic Res. 2007;41:1–7. doi: 10.1080/10715760600911147. [DOI] [PubMed] [Google Scholar]
- 28.Yamada K, Inoue D, Matsumoto S, Utsumi H. In vivo measurement of redox status in streptozotocin-induced diabetic rat using targeted nitroxyl probes. Antioxid Redox Signal. 2004;6:605–611. doi: 10.1089/152308604773934369. [DOI] [PubMed] [Google Scholar]
- 29.Sano H, Naruse M, Matsumoto K, Oi T, Utsumi H. A new nitroxyl-probe with high retention in the brain and its application for brain imaging. Free Radic Biol Med. 2000;28:959–969. doi: 10.1016/s0891-5849(00)00184-2. [DOI] [PubMed] [Google Scholar]
- 30.Hahn SM, Tochner Z, Krishna CM, Glass J, Wilson L, Samuni A, Sprague M, Venzon D, Glatstein E, Mitchell JB, et al. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992;52:1750–1753. [PubMed] [Google Scholar]
- 31.Liebmann J, DeLuca AM, Epstein A, Steinberg SM, Morstyn G, Mitchell JB. Protection from lethal irradiation by the combination of stem cell factor and tempol. Radiat Res. 1994;137:400–404. [PubMed] [Google Scholar]
- 32.Hahn SM, Krishna MC, DeLuca AM, Coffin D, Mitchell JB. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic Biol Med. 2000;28:953–958. doi: 10.1016/s0891-5849(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 33.Hahn SM, Sullivan FJ, DeLuca AM, Krishna CM, Wersto N, Venzon D, Russo A, Mitchell JB. Evaluation of tempol radioprotection in a murine tumor model. Free Radic Biol Med. 1997;22:1211–1216. doi: 10.1016/s0891-5849(96)00556-4. [DOI] [PubMed] [Google Scholar]
- 34.Hahn SM, DeLuca AM, Coffin D, Krishna CM, Mitchell JB. In vivo radioprotection and effects on blood pressure of the stable free radical nitroxides. Int J Radiat Oncol Biol Phys. 1998;42:839–842. doi: 10.1016/s0360-3016(98)00317-4. [DOI] [PubMed] [Google Scholar]
- 35.Vitolo JM, Cotrim AP, Sowers AL, Russo A, Wellner RB, Pillemer SR, Mitchell JB, Baum BJ. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res. 2004;10:1807–1812. doi: 10.1158/1078-0432.ccr-03-0194. [DOI] [PubMed] [Google Scholar]
- 36.Cotrim AP, Sowers AL, Lodde BM, Vitolo JM, Kingman A, Russo A, Mitchell JB, Baum BJ. Kinetics of tempol for prevention of xerostomia following head and neck irradiation in a mouse model. Clin Cancer Res. 2005;11:7564–7568. doi: 10.1158/1078-0432.CCR-05-0958. [DOI] [PubMed] [Google Scholar]
- 37.Goffman T, Cuscela D, Glass J, Hahn S, Krishna CM, Lupton G, Mitchell JB. Topical application of nitroxide protects radiation-induced alopecia in guinea pigs. Int J Radiat Oncol Biol Phys. 1992;22:803–806. doi: 10.1016/0360-3016(92)90528-p. [DOI] [PubMed] [Google Scholar]
- 38.Metz JM, Smith D, Mick R, Lustig R, Mitchell J, Cherakuri M, Glatstein E, Hahn SM. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin Cancer Res. 2004;10:6411–6417. doi: 10.1158/1078-0432.CCR-04-0658. [DOI] [PubMed] [Google Scholar]
- 39.Halpern HJ, Spencer DP, van Polen J. Imaging Radio Frequency Electron-Spin-Resonance Spectrometer with High Resolution and Sensitivity for in vivo Measurements . Rev Sci Instrum. 1989;60:1040–1050. [Google Scholar]
- 40.Kuppusamy P, Chzhan M, Vij K, Shteynbuk M, Lefer DJ, Giannella E, Zweier JL. Three-dimensional spectral-spatial EPR imaging of free radicals in the heart: a technique for imaging tissue metabolism and oxygenation. Proc Natl Acad Sci U S A. 1994;91:3388–3392. doi: 10.1073/pnas.91.8.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, Walczak T, Wu M, Swartz HM. Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci U S A. 1993;90:5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leunbach I. On a novel MRI technique (OMRI) for the determination of tissue parameters. Acta Anaesthesiol Scand Suppl. 1997;110:121–122. doi: 10.1111/j.1399-6576.1997.tb05526.x. [DOI] [PubMed] [Google Scholar]
- 43.Samuni A, Krishna CM, Mitchell JB, Collins CR, Russo A. Superoxide reaction with nitroxides. Free Radic Res Commun. 1990;9:241–249. doi: 10.3109/10715769009145682. [DOI] [PubMed] [Google Scholar]
- 44.Krishna MC, Samuni A. The effect of oxygen at physiological levels on the detection of free radical intermediates by electron paramagnetic resonance. Free Radic Res Commun. 1993;18:239–247. doi: 10.3109/10715769309145873. [DOI] [PubMed] [Google Scholar]
- 45.Kuppusamy P, Afeworki M, Shankar RA, Coffin D, Krishna MC, Hahn SM, Mitchell JB, Zweier JL. In vivo electron paramagnetic resonance imaging of tumor heterogeneity and oxygenation in a murine model. Cancer Res. 1998;58:1562–1568. [PubMed] [Google Scholar]
- 46.Ilangovan G, Manivannan A, Li H, Yanagi H, Zweier JL, Kuppusamy P. A naphthalocyanine-based EPR probe for localized measurements of tissue oxygenation. Free Radic Biol Med. 2002;32:139–147. doi: 10.1016/s0891-5849(01)00784-5. [DOI] [PubMed] [Google Scholar]
- 47.Yamada KI, Kuppusamy P, English S, Yoo J, Irie A, Subramanian S, Mitchell JB, Krishna MC. Feasibility and assessment of non-invasive in vivo redox status using electron paramagnetic resonance imaging. Acta Radiol. 2002;43:433–440. doi: 10.1080/j.1600-0455.2002.430418.x. [DOI] [PubMed] [Google Scholar]
- 48.Kuppusamy P, Li H, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, Krishna MC, Mitchell JB. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002;62:307–312. [PubMed] [Google Scholar]
- 49.Sledzinski Z, Wozniak M, Antosiewicz J, Lezoche E, Familiari M, Bertoli E, Greci L, Brunelli A, Mazera N, Wajda Z. Protective effect of 4-hydroxy-TEMPO, a low molecular weight superoxide dismutase mimic, on free radical toxicity in experimental pancreatitis. Int J Pancreatol. 1995;18:153–160. doi: 10.1007/BF02785889. [DOI] [PubMed] [Google Scholar]
- 50.Dunn JF, O'Hara JA, Zaim-Wadghiri Y, Lei H, Meyerand ME, Grinberg OY, Hou H, Hoopes PJ, Demidenko E, Swartz HM. Changes in oxygenation of intracranial tumors with carbogen: a BOLD MRI and EPR oximetry study. J Magn Reson Imaging. 2002;16:511–521. doi: 10.1002/jmri.10192. [DOI] [PubMed] [Google Scholar]
- 51.Brasch RC. Work in progress: methods of contrast enhancement for NMR imaging and potential applications. A subject review. Radiology. 1983;147:781–788. doi: 10.1148/radiology.147.3.6342034. [DOI] [PubMed] [Google Scholar]
- 52.Brasch RC, London DA, Wesbey GE, Tozer TN, Nitecki DE, Williams RD, Doemeny J, Tuck LD, Lallemand DP. Work in progress: nuclear magnetic resonance study of a paramagnetic nitroxide contrast agent for enhancement of renal structures in experimental animals. Radiology. 1983;147:773–779. doi: 10.1148/radiology.147.3.6844613. [DOI] [PubMed] [Google Scholar]
- 53.Brasch RC, Nitecki DE, Brant-Zawadzki M, Enzmann DR, Wesbey GE, Tozer TN, Tuck LD, Cann CE, Fike JR, Sheldon P. Brain nuclear magnetic resonance imaging enhanced by a paramagnetic nitroxide contrast agent: preliminary report. AJR Am J Roentgenol. 1983;141:1019–1023. doi: 10.2214/ajr.141.5.1019. [DOI] [PubMed] [Google Scholar]
- 54.Berliner LJ, Fujii H, Wan XM, Lukiewicz SJ. Feasibility study of imaging a living murine tumor by electron paramagnetic resonance. Magn Reson Med. 1987;4:380–384. doi: 10.1002/mrm.1910040410. [DOI] [PubMed] [Google Scholar]
- 55.Keana JF, Pou S. Nitroxide-doped liposomes containing entrapped oxidant: an approach to the "reduction problem" of nitroxides as MRI contrast agents. Physiol Chem Phys Med NMR. 1985;17:235–240. [PubMed] [Google Scholar]
- 56.Mitchell JB, Xavier S, DeLuca AM, Sowers AL, Cook JA, Krishna MC, Hahn SM, Russo A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 57.Schubert R, Erker L, Barlow C, Yakushiji H, Larson D, Russo A, Mitchell JB, Wynshaw-Boris A. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum Mol Genet. 2004;13:1793–1802. doi: 10.1093/hmg/ddh189. [DOI] [PubMed] [Google Scholar]
- 58.Gueven N, Luff J, Peng C, Hosokawa K, Bottle SE, Lavin MF. Dramatic extension of tumor latency and correction of neurobehavioral phenotype in Atm-mutant mice with a nitroxide antioxidant. Free Radic Biol Med. 2006;41:992–1000. doi: 10.1016/j.freeradbiomed.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 59.Erker L, Schubert R, Yakushiji H, Barlow C, Larson D, Mitchell JB, Wynshaw-Boris A. Cancer chemoprevention by the antioxidant tempol acts partially via the p53 tumor suppressor. Hum Mol Genet. 2005;14:1699–1708. doi: 10.1093/hmg/ddi181. [DOI] [PubMed] [Google Scholar]
- 60.Gariboldi MB, Lucchi S, Caserini C, Supino R, Oliva C, Monti E. Antiproliferative effect of the piperidine nitroxide TEMPOL on neoplastic and nonneoplastic mammalian cell lines. Free Radic Biol Med. 1998;24:913–923. doi: 10.1016/s0891-5849(97)00372-9. [DOI] [PubMed] [Google Scholar]
- 61.Cheng WH, Zheng X, Quimby FR, Roneker CA, Lei XG. Low levels of glutathione peroxidase 1 activity in selenium-deficient mouse liver affect c-Jun N-terminal kinase activation and p53 phosphorylation on Ser-15 in pro-oxidant-induced aponecrosis. Biochem J. 2003;370:927–934. doi: 10.1042/BJ20021870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito K, Nakazato T, Yamato K, Miyakawa Y, Yamada T, Hozumi N, Segawa K, Ikeda Y, Kizaki M. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004;64:1071–1078. doi: 10.1158/0008-5472.can-03-1670. [DOI] [PubMed] [Google Scholar]
- 63.Ankel EG, Lai CS, Hopwood LE, Zivkovic Z. Cytotoxicity of commonly used nitroxide radical spin probes. Life Sci. 1987;40:495–498. doi: 10.1016/0024-3205(87)90116-0. [DOI] [PubMed] [Google Scholar]
- 64.Gariboldi MB, Rimoldi V, Supino R, Favini E, Monti E. The nitroxide tempol induces oxidative stress, p21(WAF1/CIP1), and cell death in HL60 cells. Free Radic Biol Med. 2000;29:633–641. doi: 10.1016/s0891-5849(00)00347-6. [DOI] [PubMed] [Google Scholar]
- 65.Monti E, Supino R, Colleoni M, Costa B, Ravizza R, Gariboldi MB. Nitroxide TEMPOL impairs mitochondrial function and induces apoptosis in HL60 cells. J Cell Biochem. 2001;82:271–276. doi: 10.1002/jcb.1160. [DOI] [PubMed] [Google Scholar]
- 66.Gariboldi MB, Ravizza R, Petterino C, Castagnaro M, Finocchiaro G, Monti E. Study of in vitro and in vivo effects of the piperidine nitroxide Tempol--a potential new therapeutic agent for gliomas. Eur J Cancer. 2003;39:829–837. doi: 10.1016/s0959-8049(02)00742-6. [DOI] [PubMed] [Google Scholar]
- 67.McDonald MC, Zacharowski K, Bowes J, Cuzzocrea S, Thiemermann C. Tempol reduces infarct size in rodent models of regional myocardial ischemia and reperfusion. Free Radic Biol Med. 1999;27:493–503. doi: 10.1016/s0891-5849(99)00100-8. [DOI] [PubMed] [Google Scholar]
- 68.Gelvan D, Saltman P, Powell SR. Cardiac reperfusion damage prevented by a nitroxide free radical. Proc Natl Acad Sci U S A. 1991;88:4680–4684. doi: 10.1073/pnas.88.11.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuzzocrea S, McDonald MC, Mazzon E, Siriwardena D, Costantino G, Fulia F, Cucinotta G, Gitto E, Cordaro S, Barberi I, De Sarro A, Caputi AP, Thiemermann C. Effects of tempol, a membrane-permeable radical scavenger, in a gerbil model of brain injury. Brain Res. 2000;875:96–106. doi: 10.1016/s0006-8993(00)02582-8. [DOI] [PubMed] [Google Scholar]
- 70.Rak R, Chao DL, Pluta RM, Mitchell JB, Oldfield EH, Watson JC. Neuroprotection by the stable nitroxide Tempol during reperfusion in a rat model of transient focal ischemia. J Neurosurg. 2000;92:646–651. doi: 10.3171/jns.2000.92.4.0646. [DOI] [PubMed] [Google Scholar]
- 71.Kato N, Yanaka K, Hyodo K, Homma K, Nagase S, Nose T. Stable nitroxide Tempol ameliorates brain injury by inhibiting lipid peroxidation in a rat model of transient focal cerebral ischemia. Brain Res. 2003;979:188–193. doi: 10.1016/s0006-8993(03)02918-4. [DOI] [PubMed] [Google Scholar]
- 72.Kwon TH, Chao DL, Malloy K, Sun D, Alessandri B, Bullock MR. Tempol, a novel stable nitroxide, reduces brain damage and free radical production, after acute subdural hematoma in the rat. J Neurotrauma. 2003;20:337–345. doi: 10.1089/089771503765172291. [DOI] [PubMed] [Google Scholar]
- 73.Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58:658–673. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 74.Leach M, Frank S, Olbrich A, Pfeilschifter J, Thiemermann C. Decline in the expression of copper/zinc superoxide dismutase in the kidney of rats with endotoxic shock: effects of the superoxide anion radical scavenger, tempol, on organ injury. Br J Pharmacol. 1998;125:817–825. doi: 10.1038/sj.bjp.0702123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mota-Filipe H, McDonald MC, Cuzzocrea S, Thiemermann C. A membrane-permeable radical scavenger reduces the organ injury in hemorrhagic shock. Shock. 1999;12:255–261. doi: 10.1097/00024382-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 76.Cuzzocrea S, McDonald MC, Filipe HM, Costantino G, Mazzon E, Santagati S, Caputi AP, Thiemermann C. Effects of tempol, a membrane-permeable radical scavenger, in a rodent model of carrageenan-induced pleurisy. Eur J Pharmacol. 2000;390:209–222. doi: 10.1016/s0014-2999(99)00910-3. [DOI] [PubMed] [Google Scholar]
- 77.Cuzzocrea S, McDonald MC, Mota-Filipe H, Mazzon E, Costantino G, Britti D, Mazzullo G, Caputi AP, Thiemermann C. Beneficial effects of tempol, a membrane-permeable radical scavenger, in a rodent model of collagen-induced arthritis. Arthritis Rheum. 2000;43:320–328. doi: 10.1002/1529-0131(200002)43:2<320::AID-ANR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 78.Cuzzocrea S, McDonald MC, Mazzon E, Dugo L, Lepore V, Fonti MT, Ciccolo A, Terranova ML, Caputi AP, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces dinitrobenzene sulfonic acid-induced colitis. Eur J Pharmacol. 2000;406:127–137. doi: 10.1016/s0014-2999(00)00623-3. [DOI] [PubMed] [Google Scholar]
- 79.Zamir E, Zhang R, Samuni A, Kogan M, Pe'er J. Nitroxide stable radical suppresses autoimmune uveitis in rats. Free Radic Biol Med. 1999;27:7–15. doi: 10.1016/s0891-5849(99)00026-x. [DOI] [PubMed] [Google Scholar]
- 80.Bonnet AM, Houeto JL. Pathophysiology of Parkinson's Disease . Biomed Pharmacother. 1999;53:117–121. doi: 10.1016/S0753-3322(99)80076-6. [DOI] [PubMed] [Google Scholar]
- 81.Martinez M, Martinez N, Hernandez AI, Ferrandiz ML. Hypothesis: can N-acetylcysteine be beneficial in Parkinson's disease? Life Sci. 1999;64:1253–1257. doi: 10.1016/s0024-3205(98)00472-x. [DOI] [PubMed] [Google Scholar]
- 82.Merad-Boudia M, Nicole A, Santiard-Baron D, Saille C, Ceballos-Picot I. Mitochondrial impairment as an early event in the process of apoptosis induced by glutathione depletion in neuronal cells: relevance to Parkinson's disease. Biochem Pharmacol. 1998;56:645–655. doi: 10.1016/s0006-2952(97)00647-3. [DOI] [PubMed] [Google Scholar]
- 83.Spencer JP, Jenner P, Daniel SE, Lees AJ, Marsden DC, Halliwell B. Conjugates of catecholamines with cysteine and GSH in Parkinson's disease: possible mechanisms of formation involving reactive oxygen species. J Neurochem. 1998;71:2112–2122. doi: 10.1046/j.1471-4159.1998.71052112.x. [DOI] [PubMed] [Google Scholar]
- 84.Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang Q, Smith AD, Pan S, Tyurin VA, Kagan VE, Hastings TG, Schor NF. Neuroprotective effects of TEMPOL in central and peripheral nervous system models of Parkinson's disease. Biochem Pharmacol. 2005;70:1371–1381. doi: 10.1016/j.bcp.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 86.Dikalov SI, Vitek MP, Maples KR, Mason RP. Amyloid beta peptides do not form peptide-derived free radicals spontaneously, but can enhance metal-catalyzed oxidation of hydroxylamines to nitroxides. J Biol Chem. 1999;274:9392–9399. doi: 10.1074/jbc.274.14.9392. [DOI] [PubMed] [Google Scholar]
- 87.Sies H. Oxidative stress: oxidants and antioxidants. London, U.K: Academic Press; 1991. [DOI] [PubMed] [Google Scholar]
- 88.Spector A, Garner WH. Hydrogen peroxide and human cataract . Exp Eye Res. 1981;33:673–681. doi: 10.1016/s0014-4835(81)80107-8. [DOI] [PubMed] [Google Scholar]
- 89.Reddan JR, Sevilla MD, Giblin FJ, Padgaonkar V, Dziedzic DC, Leverenz V, Misra IC, Peters JL. The superoxide dismutase mimic TEMPOL protects cultured rabbit lens epithelial cells from hydrogen peroxide insult. Exp Eye Res. 1993;56:543–554. doi: 10.1006/exer.1993.1068. [DOI] [PubMed] [Google Scholar]
- 90.Reddan JR, Giblin FJ, Kadry R, Leverenz VR, Pena JT, Dziedzic DC. Protection from oxidative insult in glutathione depleted lens epithelial cells. Exp Eye Res. 1999;68:117–127. doi: 10.1006/exer.1998.0606. [DOI] [PubMed] [Google Scholar]
- 91.Zigler JS, Jr, Qin C, Kamiya T, Krishna MC, Cheng Q, Tumminia S, Russell P. Tempol-H inhibits opacification of lenses in organ culture . Free Radic Biol Med. 2003;35:1194–1202. doi: 10.1016/s0891-5849(03)00505-7. [DOI] [PubMed] [Google Scholar]
- 92.Zollner S, Haseloff RF, Kirilyuk IA, Blasig IE, Rubanyi GM. Nitroxides increase the detectable amount of nitric oxide released from endothelial cells. J Biol Chem. 1997;272:23076–23080. doi: 10.1074/jbc.272.37.23076. [DOI] [PubMed] [Google Scholar]
- 93.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- 94.Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension. 1999;33:424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]
- 95.Hahn SM, Sullivan FJ, DeLuca AM, Bacher JD, Liebmann J, Krishna MC, Coffin D, Mitchell JB. Hemodynamic effect of the nitroxide superoxide dismutase mimics. Free Radic Biol Med. 1999;27:529–535. doi: 10.1016/s0891-5849(99)00099-4. [DOI] [PubMed] [Google Scholar]
- 96.Patel K, Chen Y, Dennehy K, Blau J, Connors S, Mendonca M, Tarpey M, Krishna M, Mitchell JB, Welch WJ, Wilcox CS. Acute antihypertensive action of nitroxides in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R37–43. doi: 10.1152/ajpregu.00469.2005. [DOI] [PubMed] [Google Scholar]
- 97.McCay CM, Crowell CB, Maynard LA. The Effect of Retarded Growth upon the Length of Life Span and upon the Ultimate Body Size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 98.Osborne TB, Mendel LB, Ferry EL. THE EFFECT OF RETARDATION OF GROWTH UPON THE BREEDING PERIOD AND DURATION OF LIFE OF RATS. Science. 1917;45:294–295. doi: 10.1126/science.45.1160.294. [DOI] [PubMed] [Google Scholar]
- 99.Weindruch R. Caloric restriction and aging. Sci Am. 1996;274:46–52. doi: 10.1038/scientificamerican0196-46. [DOI] [PubMed] [Google Scholar]


