Abstract
192 IgG-saporin (SAP) was used to selectively destroy cholinergic neurons in the rostral basal forebrain (e.g., medial septum (MS) and vertical limb of the diagonal band of Broca (VDB)) and/or the caudal basal forebrain (e.g., nucleus basalis magnocellularis (NBM)) of ovariectomized Sprague-Dawley rats. The effects of these lesions on two different cognitive tasks, a delayed matching to position (DMP) T-maze task, and a configural association (CA) operant conditioning task, were evaluated and compared. Injecting SAP into either the MS or NBM significantly impaired acquisition of the DMP task. Analysis showed that the effects were due largely to an affect on response patterns adopted by the rats during training, as opposed to an effect on working memory performance. Notably, the impairment in DMP acquisition did not correlate with the degree of cholinergic denervation of the hippocampus. Despite the deficit, most animals eventually learned the task and reached criterion; however by the end of training, controls and animals that received SAP into either the MS or NBM appeared more likely to use an allocentric place strategy to solve the task, whereas animals that received SAP into both the MS and NBM were more likely to use an egocentric response strategy. Cholinergic lesions also produced a small but significant affect on acquisition of the CA task, but only with respect to response time, and only in the SAP-NBM-treated animals. SAP-NBM lesions also produced small but significant impairments in both the number of responses and response time during the acquisition of simple associations, possibly reflecting an effect on alertness or attention. Notably, the effects on CA acquisition were small, and like the effects on DMP acquisition did not correlate with the degree of cholinergic denervation of the hippocampus. We conclude that selective basal forebrain cholinergic lesions produce learning deficits that are task specific, and that cholinergic denervation of either the frontal cortex or hippocampus can affect response patterns and strategy in ways that affect learning, without necessarily reflecting deficits in working memory performance.
Keywords: 192IgG-saporin, T-maze, operant conditioning, learning, choline acetyltransferase
INTRODUCTION
Cholinergic projections from the basal forebrain to the hippocampus and frontal cortex play an important role in cognitive processes; however, the degree to which damage to specific cholinergic projections contributes to deficits within specific cognitive domains is less clear. In particular, it is often not clear the extent to which damage to one set of cholinergic projections produces deficits that are limited to a particular cognitive domain, or the extent to which deficits on a particular task reflect the selective loss of a particular set of cholinergic projections. This is due, in part, to the fact that different laboratories produce lesions in different ways, individual studies often lesion only one subset of basal forebrain cholinergic projections rather than comparing lesions of different subsets, and studies often test performance using only one cognitive task. This makes it difficult to compare the degree of impairment produced by different lesions across multiple cognitive domains.
In the present study, we compared the effects of selectively destroying cholinergic neurons in the rostral basal forebrain (e.g., medial septum (MS) and vertical limb of the diagonal band of Broca (VDB)) and in the caudal basal forebrain (e.g., nucleus basalis magnocellularis (NBM)) on acquisition of two different tasks, a delayed matching to position (DMP) T-maze task, and a configural association (CA) operant conditioning task, by the same set of animals. In addition, we chose to conduct these studies using ovariectomized female rats, rather than male rats which is more common. Ovariectomized female rats were used because (a) less is known about the effects of cholinergic lesions on cognitive performance in females than in males, (b) previous studies have demonstrated significant effects of cholinergic lesions on DMP acquisition in ovariectomized female rats (Gibbs, 2002), (c) studies have shown that estradiol replacement affects DMP acquisition in ovariectomized rats (Gibbs, 1999, 2000), and (d) some evidence suggests that loss of ovarian function may, over time, contribute to decreased basal forebrain cholinergic function as well as risk for age-related cognitive decline in postmenopausal women (Gibbs and Gabor, 2003). Since estradiol affects DMP acquisition in female rats, ovariectomized rats were used to avoid the confound of estradiol levels which vary across the estrous cycle.
Cholinergic neurons in the MS/VDB project primarily to allocortical fields and provide the primary cholinergic input to the hippocampus (Woolf, 1991), which is well known to play an important role in spatial learning and memory processes (Eichenbaum, 2006; Smith and Mizumori, 2006; Zola-Morgan and Squire, 1993). In addition, muscarinic receptor activation in the hippocampus and cortex have been shown to play an important role in memory consolidation (Power, Vazdarjanova, and McGaugh, 2003). Note that we have previously shown that cholinergic lesions in the MS/VDB impair acquisition of the DMP task in both males and females (Gibbs, 2002; Johnson, Zambon, and Gibbs, 2002), and have hypothesized that this is due specifically to the loss of hippocampal cholinergic inputs. In contrast, cholinergic neurons in the NBM project primarily to isocortical fields including frontal and prefrontal cortices (Woolf, 1991), which are known to be involved in directed attention, working memory, cognitive set switching, behavioral monitoring, and tasks requiring the ability to inhibit inappropriate responses (Collette, Hogge, Salmon, and Van der Linden, 2006; Dalley, Cardinal, and Robbins, 2004; Miller and Cummings, 1999).
Based on the anatomy of the cholinergic system, we predicted that acquisition of the DMP task would be impaired by MS/VDB (but not NBM) cholinergic lesions and that the severity of impairment would correlate specifically with the degree of cholinergic deafferentation of the hippocampus. While early studies suggested that the hippocampus also plays an essential role in configural learning (Rudy and Sutherland, 1989), subsequent studies showed that the critical neural system for configural associations is in the cortex with hippocampal outputs providing an important modulatory function (Rudy and Sutherland, 1995). Therefore, we predicted that acquisition of the CA task would be significantly affected by NBM cholinergic lesions, and that the severity of impairment would correlate with cholinergic deafferentation of the frontal cortex. Notably, the results generated a different picture, in which cortical projections from both the rostral and caudal cholinergic cell groups affected DMP acquisition, but had less effect on CA acquisition. In addition, the effects on DMP acquisition showed little correlation with cholinergic innervation of the hippocampus, and was more consistently correlated with cholinergic innervation of the frontal cortex. Analysis of the response patterns during acquisition of the DMP task suggest that the effects of the cholinergic lesions on DMP acquisition were due primarily to effects on response patterns and strategy selection rather than on interference with working memory processes. The results are consistent with several recent reports relating cholinergic activity to spatial learning, and provide novel insights about the effects of selective cholinergic lesions on these tasks.
METHODS
Animals
Eighty-nine adult (300-325g) ovariectomized Sprague-Dawley rats were purchased from Hilltop Laboratories and housed individually. All procedures were carried out in accordance with PHS policies and with the approval of the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Cholinergic Lesions
192 IgG-saporin (SAP; Advanced Targeting Systems, Inc., lot 24-87) was used to selectively destroy cholinergic neurons in the MS/VDB, and NBM. SAP was injected at a rate of 12 μL/hr into either (a) the MS (MS; +0.5 mm from Bregma, 0.0.lateral, -5.6 mm from dura; 0.2 or 0.24 μg in 1.0 μL; n=19), (b) bilaterally into the NBM (-2.4 mm from Bregma, 2.3 mm lateral, -7.0 mm from dura; 0.11 or 0.20 μg in 1.5 μL/side; n=23), or (c) into both the MS and NBM (n=12). Note that the doses of SAP were varied between animals in order to create variability in the degree of cholinergic cell loss, which is necessary for a correlational analysis. Controls received injections of normal saline into either the MS (1.0 μL, n=11), bilaterally into the NBM (1.5 μL/side, n=16) or into both the MS and NBM (n=8). These parameters were derived from both prior and pilot studies showing that low doses of SAP injected directly into the MS/VDB or NBM selectively destroys cholinergic neurons in these regions without destroying nearby noncholinergic cells (Baxter, Bucci, Gorman, Wiley, and Gallagher, 1995; Dornan, McCampbell, Tinkler, Hickman, Bannon, Decker, and Gunther, 1996; Johnson et al., 2002; Schliebs, Rossner, and Bigl, 1996).
DMP Training and Testing
After at least 2 weeks recovery, animals were handled daily, food restricted to 85% body weight, and then trained on the DMP task exactly as previously described (Gibbs, 1999). The t-maze consisted of an approach alley (4″ wide × 14″ long) and two goal arms (4″ wide × 12″ long). The walls of the maze were 5″ high and were constructed of black plexiglass. The top of the maze was constructed of clear plexiglass that allowed the animals to view the surrounding room, and was attached to the walls of the maze by metal hinges. Manually operated sliding doors were positioned 8″ down the approach alley and at the entrance to each goal arm.
Rats were adapted to the maze as previously described (Gibbs, 1999) and trained to run to the ends of the goal arms by using a series of 6 forced “choices” per day for 4 days, each rewarded with 4 food pellets (Formula 5TUM 45 mg pellets from Test Diets, Inc.; analogous to Formula A/1 pellets formerly from Research Diets, Inc.). Right and left arms were alternated in a random, balanced fashion to avoid the introduction of a side bias. Animals then began DMP training.
DMP training was performed in trial pairs. Initially, each rat received 8 trial pairs/day. The first trial of each pair consisted of a forced “choice” in which one goal arm was blocked, forcing the animal to enter the unblocked arm to receive food reward (2 pellets). The rat was then immediately returned to the approach alley. All arms of the maze were quickly (<5 sec) wiped down with 70% ethanol in order to minimize information from intramaze odor cues, and all arms were opened for the second trial. A choice was defined as an animal placing both front legs, and at least part of both rear legs, into a goal arm. Returning to the same arm visited on the forced trial resulted in food reward (4 pellets; the rat remained in the arm for 10-20 seconds while eating the food). Entering the incorrect arm resulted in no food reward and confinement to the arm for 60 seconds. Forced choices were selected in a random, balanced fashion to avoid the introduction of a side bias. Rats were run in squads of 4-6. After each trial pair, an animal was returned to its cage for 5-10 minutes while training proceeded with the other animals. Rats continued to receive 8 trial pairs/day until they reached a criterion of 15/16 correct choices over two consecutive days or until they had received 30 days of training.
Post-Criterion Testing
One day after reaching criterion, animals received a probe trial during which the T-maze was rotated 180° (relative to extramaze cues) between the forced and open trial. This was done to assess whether rats were using a place strategy (relying on extramaze cues) or a response strategy (independent of extramaze cues) to perform the task. Animals relying on extramaze cues (i.e., place strategy) are expected to choose the arm located in the same position of the room that was previously visited. Animals relying on internal cues (i.e., response strategy) are expected to enter the same physical arm of the maze that was previously entered, even though it occupies a different position in the room (relative to extramaze cues) from that previously visited. For the purposes of analysis, selecting the arm located in the same position of the room was assigned a score of 0, whereas selecting the opposite arm was assigned a score of 1. Beginning one day after the probe trial, animals received four days of 8 trial pairs/day with successively increasing intertrial delays (day 1=minimal delay (same as training condition); day 2 = 30 seconds; day 3 = 60 seconds; day 4 = 90 seconds) to assess working memory performance.
CA Training
At the completion of DMP testing, rats were trained on an operant configural association (CA) negative patterning task as previously described (Butt, Allen, Arthur, Noble, Rea, and Rogers, 2000; Gibbs, 2005). Training was performed in operant chambers (Med. Associates, Inc., Georgia, VT) connected to a computer running Med-PC software. Each operant chamber contained a dim red house light, a ventilation fan, a 6W stimulus panel light, a speaker calibrated to present a 1500 Hz tone, a pellet dispenser, and a recessed food cup located immediately below the panel light. Entry into the food cup was monitored by a photosensor.
Rats were adapted to the chamber by receiving one 60 minute session during which they received a total of 16 food pellets delivered at intervals ranging from 2-6 minutes. CA training began the following day. Each rat received one training session per day for a total of 24 days. Twenty four days was selected based on pilot studies which showed that, in our hands, performance plateaus by 24 days and does not improve significantly with additional training (up to 40 days; Gibbs, unpublished observations). Each session lasted for a maximum of 110 minutes. During the session, rats received 30 presentations of a tone conditioning stimulus (CS), 30 presentations of a light CS, and 30 presentations during which the tone and the light were presented simultaneously. If an animal entered the food cup within 10 seconds of presentation of the tone or the light, the CS was discontinued and the animal received a food reward (one 45 mg pellet). If an animal entered the food cup when the light and the tone were presented simultaneously, the stimuli were discontinued, the house light was turned off for sixty seconds, and no food was delivered. This is referred to as a time out. The CS presentations were randomly distributed throughout the session and occurred at one of 30 randomly selected intertrial intervals ranging from 12 to 70 seconds. During each session, the measures that were recorded included (a) the number of responses (i.e., the number of times presentation of a stimulus resulted in the animal entering the food cup), and (b) response time (i.e., time between presentation of each CS and entry into the food cup).
ChAT Assays
The degree of cholinergic deafferentation was assessed by measuring ChAT activity within specific brain regions. After completing all training, animals were anesthetized with pentobarbital (100mg/kg; IP) and perfused with ice cold saline. Brains were removed, and tissues from the hippocampus, frontal cortex (FR1, FR2 and FR3 according to plate 5-9 of Paxinos & Watson (Paxinos and Watson, 1986)), and retrosplenial/entorhinal cortex (RS/E) were dissected, frozen on dry ice, and stored at -80°C until processed for ChAT activity as previously described (Johnson et al., 2002). The RS/E was included in part as a control, but also because it too receives cholinergic innervation from the basal forebrain, primarily from caudal regions of the MS and the diagonal band of Broca. Hence, some cholinergic cells projecting to the RS/E likely would be included in the most caudal portions of the MS/VDB lesions and in the most rostral portions of the NBM lesions. Briefly, frozen tissues were thawed and dissociated by sonication in a medium containing EDTA (10mM) and Triton X-100 (0.5%) and diluted to a concentration of 10 mg tissue/mL. An aliquot of each sample was used for the determination of total protein (Bradford, 1976). Three 5μl aliquots of each sample were incubated for 30 min. at 37 °C in a medium containing [3H] acetyl-CoA (50,000-60,000d.p.m./tube, final concentration 0.25 mM acetyl-CoA; Sigma Inc., St. Louis, MO), choline chloride (10.0 mM), physostigmine sulfate (0.2 mM), NaCl (300 mM), sodium phosphate buffer (pH 7.4, 50 mM), and EDTA (10 mM). The reaction was terminated with 4 mL sodium phosphate buffer (10 mM) followed by the addition of 1.6 ml of acetonitrile containing 5 mg/ml tetrephenylboron. The amount of [3H] acetylcholine produced was determined by adding 8 mL of EconoFluor scintillation cocktail (Packard Instruments, Meriden, CT) and counting total cpm in the organic phase using an LKB beta-counter. Background was determined using identical tubes to which no sample was added. For each sample, the three reaction tubes containing sample were averaged and the difference between total cpm and background cpm was used to estimate the total amount of ACh produced per sample. ChAT activity was then calculated for each sample as pmol ACh manufactured/hr/μg protein.
Data Analysis
ChAT activity
Because of the large number of samples, ChAT assays were conducted in multiple runs. Each run included samples from all treatment groups including controls. Values from each run were then normalized to percent change from the mean of the controls. This controlled for noise associated with interassay variability. The normalized data were then analyzed by ANOVA.
DMP Task
Days to criterion (DTC) on the DMP task was analyzed by Kruskal-Wallace nonparametric ANOVA and by the Dunn’s post-test. Performance during acquisition of the DMP task was blocked into ten 3-day blocks of training. Once an animal reached criterion, a value of 0.9375 (15/16) was recorded for performance on subsequent days. The blocked data were then analyzed by ANOVA with repeated measures on ‘Block’. The effects of rotating the maze 180° were analyzed by contingency table and Chi-square test. Performance during increased intertrial delays was analyzed by ANOVA with repeated measures on ‘Delay’.
Correlations with ChAT activity
One of the goals of this study was to test predictions about the relationship between cholinergic denervation of specific brain regions and behavioral performance. ChAT activity was used to quantify the degree of cholinergic denervation. In order to test whether ChAT activity correlated significantly with behavioral performance, we first performed a mixed model repeated measures ANCOVA including ChAT activity in each of the three regions of the brain as a covariate. In this case, a significant effect of ChAT activity would indicate that the degree of denervation correlates to a significant degree with behavioral performance; however, it would not reveal whether correlations exist within all treatment groups or during all phases of acquisition. If a significant effect of ChAT activity was observed, an ANCOVA including ChAT activity as a covariate was then conducted for each block of acquisition. This revealed on which blocks during acquisition ChAT activity correlated with behavioral performance. For those blocks in which the effect of ChAT activity was significant, the relationship between ChAT activity and performance was further analyzed by scatter plot and by Spearman’s Rho corrected for multiple comparisons. This was done separately for each treatment group, since combining data for all treatment groups can produce significant correlations that are non-meaningful with respect to the degree to which different levels of denervation influence performance within a specific treatment group. Spearman’s Rho was used, as opposed to Pearson correlation or simple regression, so that non-linear relationships would also be detected. Statistics were performed using JMPIN 5.1 for Macintosh and GraphPad Prism v3.0a. Significance was defined as p≤0.05.
Analysis of Response Patterns
During the course of DMP training, we observed that after 3-6 days of training, many of the rats began to adopt a turning strategy whereby they consistently entered either the right or left arm of the maze. To quantify this, we counted the total number of days during training that an animal chose the same arm of the maze 15 out of 16 times over a two day period. Any animal that met this criterion was defined as having developed a turning strategy during training. Chi-Square analysis was then used to compare the number of animals that developed this strategy, and ANOVA was used to compare the number of days that the strategy persisted, among the four treatment groups. To evaluate the contribution that number of days involved in a turning strategy made to effects of SAP lesions on DTC, the duration of the turning strategy was subtracted from DTC for each animal, and the results analyzed using Kruskal-Wallace non-parametric ANOVA. Finally, the relationship between cholinergic denervation and the duration of a turning strategy was further evaluated by ANCOVA using ChAT activity as a covariate. In this case, a significant effect of ChAT activity would indicate that the degree of denervation correlates to a significant degree with the duration of the turning strategy. If a significant effect of ChAT activity was detected, the relationship was further analyzed separately for each treatment group by scatter plot and by Spearman’s Rho as described above.
CA Task
Performance during acquisition of the CA task was blocked into eight 3-day blocks of training. Both the number of responses and response time to both simple and configural stimuli were analyzed by ANOVA with repeated measures on ‘Block’. Correlations between performance and ChAT activity were analyzed by ANCOVA, followed by scatter plot and Spearman’s Rho exactly as described above for the DMP task.
RESULTS
ChAT Activity
No significant differences among the control groups were detected in any of the three brain regions analyzed (not shown). Therefore, the saline-treated controls were consolidated into one control group (n=35). Absolute levels of ChAT activity in the saline-treated controls is summarized in Table 1, and the effects of SAP lesions on relative levels of ChAT activity are summarized in Figure 1. As expected, injections of SAP into the MS and NBM produced significant decreases in ChAT activity in the hippocampus and frontal cortex, indicating a loss of cholinergic afferents to these structures. Notably, only animals that received SAP into the MS showed significant decreases in mean ChAT activity in the hippocampus, and only animals that received SAP into the NBM showed significant decreases in mean ChAT activity in the frontal cortex. Animals that received SAP into both the MS and NBM had the lowest mean ChAT activity in both the hippocampus and frontal cortex, and also showed a significant decrease in mean ChAT activity in the RS/E. Only the combined lesion group showed a significant decrease in ChAT activity in the RS/E relative to controls.
Table 1.
ChAT activity in saline-treated controls
| Range | Mean ± s.e.m. | |
|---|---|---|
| Hippocampus | 25.1 – 55.6 | 37.2 ± 1.2 |
| Frontal Cortex | 21.4 – 54.4 | 34.8 ± 1.5 |
| Retrosplenial/Entorhinal Cortex | 23.4 – 56.8 | 36.1 ± 1.3 |
Values are in pMol ACh produced / hr / μg protein
Figure 1.
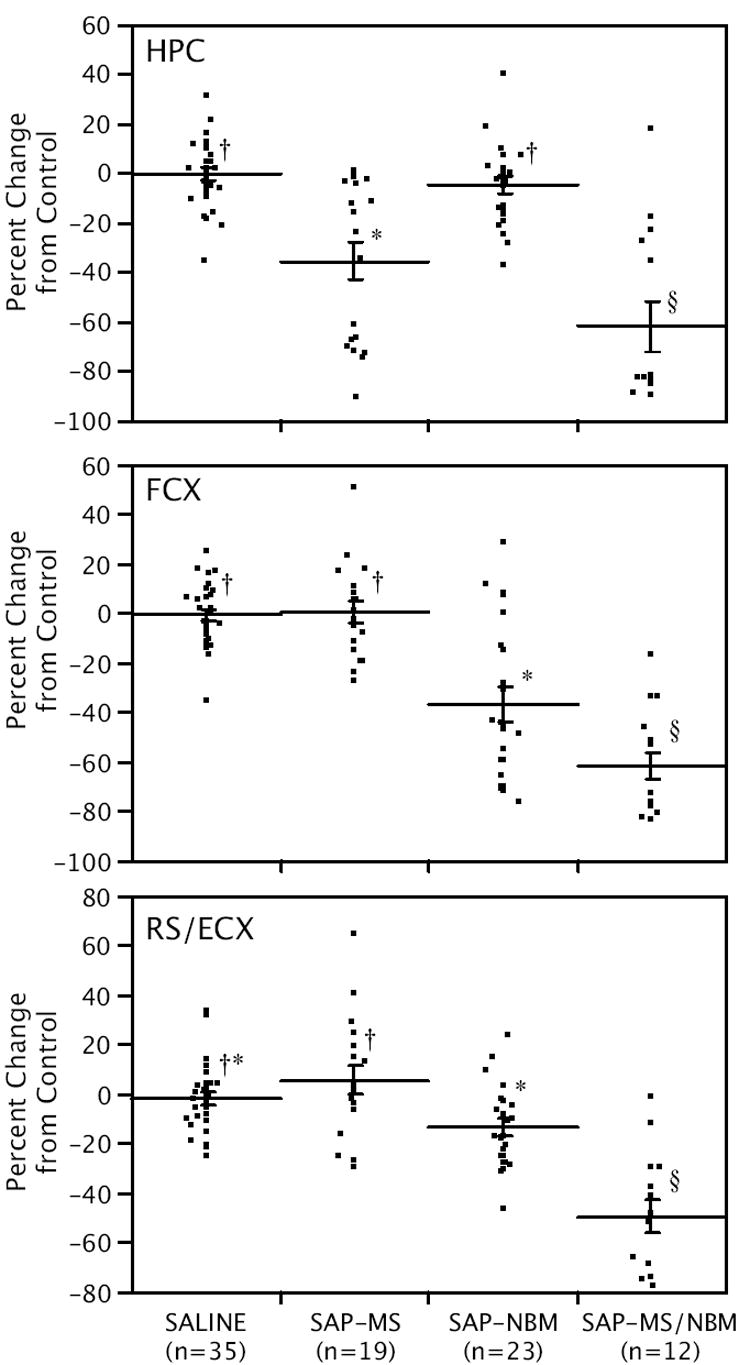
Scatter plots showing the effects of the SAP lesions on ChAT activity in the hippocampus (HPC), frontal cortex (FCX), and retrosplenial/entorhinal cortex (RS/E) relative to controls. Individual data points indicate percent change from controls. Lines show means ± s.e.m. Within each panel, groups that are not connected by the same symbol are significantly different from each other (p<0.05).
DMP Acquisition
All but three animals reached criterion on the DMP task. Two of the three received combined lesions in both the MS and NBM and one received SAP into the MS only. Again, no significant differences among the control groups were detected, and therefore the saline-treated controls were consolidated into one control group. SAP-treated animals required more days to reach criterion than saline-treated controls (Fig. 2). Non-parametric ANOVA revealed a significant effect of treatment (Chi-Square = 28.1, p<0.0001). Post-hoc comparisons indicate that each of the SAP-treated groups differed significantly from the controls, but did not differ significantly from each other.
Figure 2.
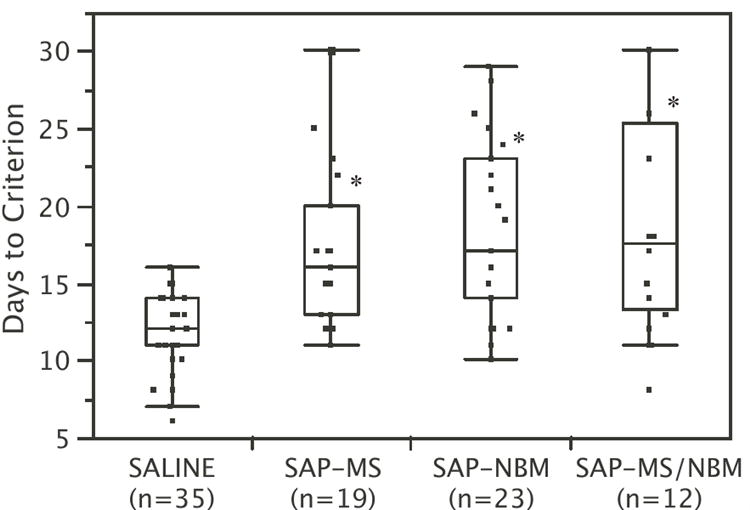
Box and Whisker plots summarizing the effects of SAP lesions on Days to Criterion (DTC) on the DMP task. Each box surrounds the upper and lower quartiles (i.e., middle 50% of the data). The line in the middle of the box shows the median. The lines extending from the box extend to the upper and lower adjacent values (within 1.5 times the interquartile range). *p<0.05 relative to saline-treated controls.
Examination of the learning curves (Fig. 3) shows that all four treatment groups performed at comparable levels below chance at the start of training, but that SAP-treated animals improved more slowly on average than saline-treated controls. ANOVA revealed a significant effect of ‘Treatment’ (F[3,85]=7.4, p=0.0002), a significant effect of ‘Block’ (F[9, 765]=309.0, p<0.0001), and a significant ‘Treatment’ × ‘Block’ interaction (F[27, 765]=3.7, p=0.0001). Post-hoc analyses revealed that saline-treated animals performed significantly better than SAP-treated animals on Blocks 3-7 of training. No significant differences between the three SAP-treated groups were detected.
Figure 3.
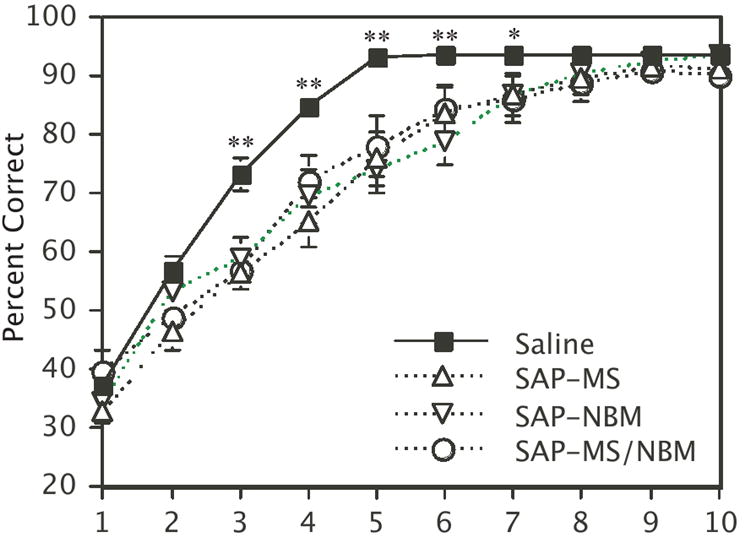
Plot showing the effects of the SAP lesions on the DMP learning curves Points represent the mean percent correct for each group ± s.e.m. **p<0.01, *p<0.05 for saline-treated animals relative to each of the SAP-treated groups.
When ChAT activity in each of the three brain regions was included as a covariate, the analysis revealed that ChAT activity in the frontal cortex co-varied significantly with the effects of Treatment on performance. Analysis revealed a significant effect of Treatment (F[3,82]=7.6, p<0.0001), a significant effect of ChAT activity in the frontal cortex (F[1,82]=5.8, p<0.02), a significant effect of Block (F[9,74]=69.8, p<0.0001), and a significant Treatment x Block interaction (F[27,228]=2.1, p<0.002). ANCOVA performed on each Block revealed a significant effect of Treatment on Blocks 2-6, and a significant effect of ChAT activity in the frontal cortex on Blocks 4-6. Correlations with ChAT activity in the hippocampus and RS/E were not statistically significant (hippocampus: F[1,82]=3.0, p=0.09; RS/E: F[1,82]=0.53, p=0.47); however, there was a significant interaction between Block and ChAT activity in the RS/E irrespective of treatment (F[9,74]=2.2, p<0.05).
Scatter plots and Spearman’s analysis were used to evaluate further the relationship between ChAT activity and DMP performance within each treatment group for each of Blocks 1-7. As expected from the ANCOVA, there were no significant correlations between DMP performance and ChAT activity in the hippocampus in any of the treatment groups on any of the Blocks. However, the analysis revealed significant negative correlations between performance and ChAT activity in the frontal cortex during Blocks 2-6 in animals that received SAP into the NBM, and a similar correlation on Blocks 3 and 4 (and a trend on Block 5) in animals that received SAP into the MS. In all of these cases, correlations were in the negative direction. Note that a negative correlation indicates that performance declined as ChAT activity increased. The only other significant correlation was observed on Block 7, and was a positive correlation between performance and ChAT activity in the RS/E of animals that received SAP into both the MS and NBM.
Effects of rotating the maze 180°
Eighty-five of the original 89 animals underwent post-criterion testing. After reaching criterion, each rat received a probe trial during which the maze was rotated 180° (relative to extramaze cues) between the forced and open trials. As described above, animals relying on extramaze cues are expected to choose the arm located in the same position of the room that was previously visited (scored as ‘0’), whereas animals relying on internal cues are expected to enter the same physical arm of the maze that was previously visited (scored as ‘1’), even though it occupies a different position in the room (relative to extramaze cues) from the position previously visited. During the probe trial, average performance was 0.429 for saline-treated controls, 0.412 for SAP-MS, and 0.565 for SAP-NBM-treated animals. These values differed significantly from criterion performance (0.9375, 15/16 correct when the maze is not rotated; p<0.05 in all cases), but did not differ significantly from chance (0.50). This indicates that by the time rats reached criterion, rotating the maze disrupted arm choice in each of these groups, indicating that rats in these groups used extramaze cues to a significant degree in making arm choices (see discussion below). In contrast, average performance for the SAP-MS/NBM group during the probe trial was 0.80. Analysis shows that this does not differ significantly from criterion performance, indicating that by the time rats reached criterion, animals in this group were using an egocentric (i.e., response) strategy.
Effects on Working Memory
One day after rotating the maze, rats received four days of testing with increasing intertrial delays (delay between the forced and open choice) of <5 (day 1), 30 (day 2), 60 (day 3), and 90 (day 4) seconds. Performance on day 1 (minimal delay) did not differ significantly for any group from the criterion performance achieved prior to the probe trial. In contrast, the performance of all groups decreased significantly as a result of increasing the intertrial delay (Figure 4). ANOVA revealed no significant effect of ‘Treatment’ (F[3,81]=1.4, p=0.26), a significant effect of ‘Delay’ F[3,243]=54.4, p<0.0001), and no significant interaction between ‘Treatment’ and ‘Delay’ (F[9,243]=0.7, p=0.73).
Figure 4.
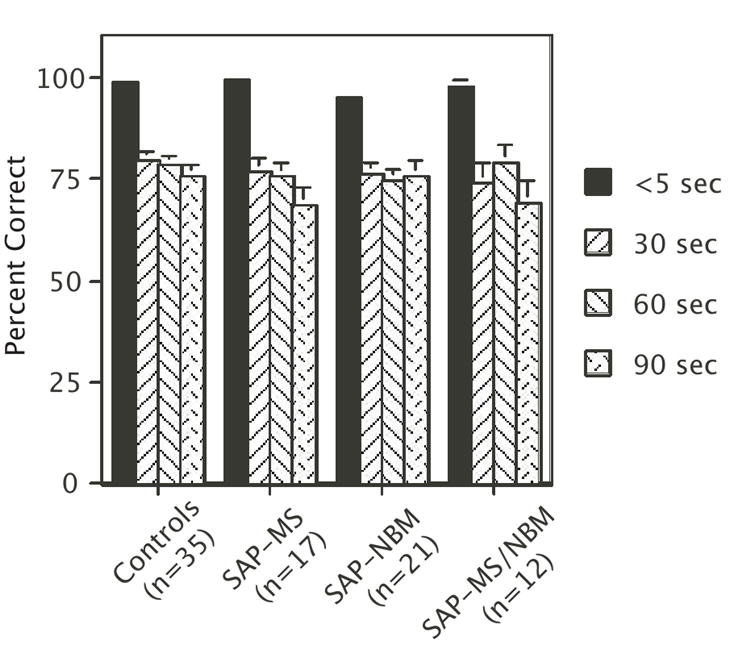
Bar graph summarizing the effects of increasing the intertrial delay on performance of the DMP task Bars represent mean percent correct ± s.e.m. Note that there was a main effect of Delay on performance, but no affect of Treatment and no interaction between Treatment and Delay (see text for statistics).
Analysis of Response Patterns
As mentioned above, we observed that after 3-6 days of training many of the rats appeared to adopt a turning strategy. In each case, once an animal adopted a turning strategy it persisted in that strategy until the criterion for defining a turning strategy was no longer met. Chi-Square analysis revealed that animals in each of the SAP-treated groups were significantly more likely to adopt a turning strategy (79-87%) than saline treated controls (51.4%) (Table 2). In addition, the number of days that animals displayed a turning strategy was significantly greater for SAP-treated animals than for saline-treated controls (Figure 5A). For number of days, ANOVA revealed a significant effect of Treatment (F[3, 85]=6.0, p<0.001), and Tukey analysis confirmed that saline-treated animals differed significantly from each of the SAP-treated groups.
Table 2.
Percentage of animals that adopted a persistent turn strategy during DMP training
| Treatment | n | % |
|---|---|---|
| Saline | 35 | 51.4 |
| SAP-MS | 19 | 79.0 |
| SAP-NBM | 23 | 87.0 |
| SAP-MS/NBM | 12 | 83.3 |
Pearson Chi-square = 10.8, p<0.02
Figure 5.
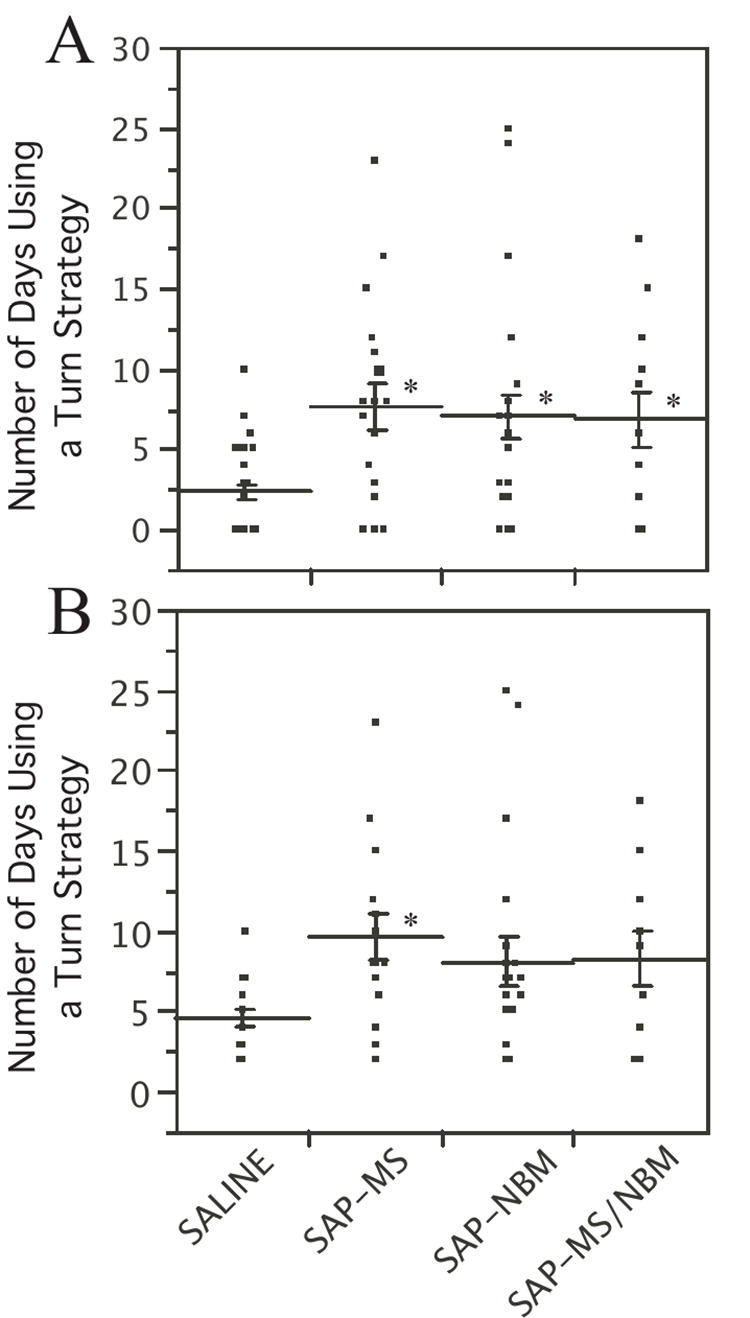
Scatter plot showing the number of days that rats engaged in a turning strategy during DMP training Lines indicate mean ± s.e.m. Panel A shows data for all rats. Panel B shows data only for rats that met the definition of having adopted a turning strategy. *p<0.05 relative to saline-treated controls.
To determine whether the effect on the number of days was due predominantly to the difference in the percentage of animals that adopted the turning strategy as opposed to a difference in duration of the turning strategy, we repeated the analysis including only those animals that adopted a turning strategy. In this case, there was a significant effect of Treatment (F[3, 59]=2.9, p<0.05), and a significant difference between SAP-MS and saline-treated animals (Figure 5B), suggesting that at least some of this difference is, in fact, due to an effect on the duration of the turning strategy.
To determine whether the differences in duration of the turning strategy were sufficient to account for the effects of treatment on DMP acquisition, we subtracted the number of days that an animal displayed a turning strategy from the number of days required to reach criterion (Figure 6). Kruskal-Wallace non-parametric ANOVA revealed that after subtracting the number of days spent using a turning strategy from DTC, no significant effect of Treatment was observed (Chi-square = 3.3, p=0.34). This indicates that the effect of the SAP lesions on DTC was due predominantly to an effect on the amount of time that animals spent engaged in a persistent turning strategy.
Figure 6.
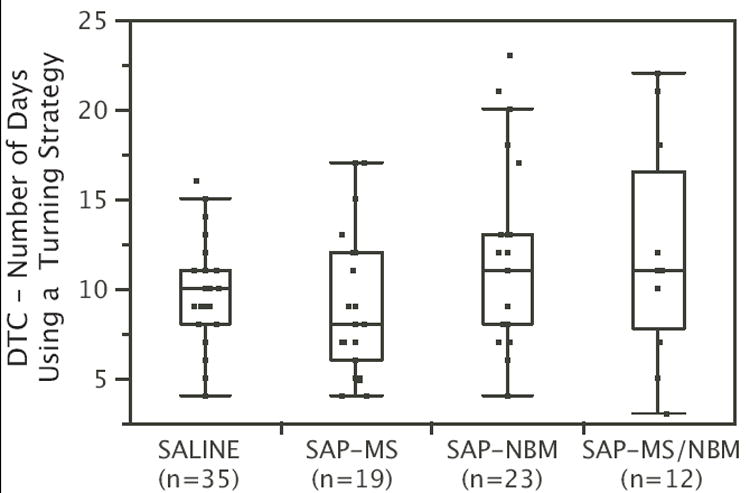
Box and whisker plots showing DTC minus the number of days that rats engaged in a turning strategy during DMP training. Each box surrounds the upper and lower quartiles (i.e., middle 50% of the data). The line in the middle of the box shows the median. The lines extending from the box extend to the upper and lower adjacent values (within 1.5 times the interquartile range). Note that after subtracting the number of days engaged in a turning strategy, SAP lesions no longer had a significant effect on DTC.
Correlations between strategy and ChAT activity
Next we looked for correlations between duration of the turning strategy and ChAT activity in each of the three brain regions. ANCOVA revealed that ChAT activity in the frontal cortex (but not the HPC or RS/E) co-varied significantly with the effects of Treatment on the number of days that animals displayed a turning strategy (effect of Treatment: F[3,56]=6.66, p<0.001; effect of ChAT-FCX: F[1, 56]=13.8, p<0.001).
The relationship between ChAT activity in the frontal cortex and the number of days that animals displayed a turning strategy was further analyzed for each treatment group by scatter plot and by Spearman’s Rho. This analysis revealed a significant positive correlation, but only for animals in the SAP-NBM group (p<0.001 by Spearman Rho). Note that a positive correlation indicates that higher ChAT activity in the frontal cortex was associated with a greater number of days during which animals displayed the turning strategy. Collectively, these findings indicate that SAP-lesions significantly affected the duration of the turning strategy and that this effect can correlate with cholinergic innervation of the frontal cortex.
CA Acquisition
Of the original 89 rats, seven rats (4 SAP-NBM-treated animals and 3 SAP-MS/NBM-treated animals) had to be removed from the study prior to completion of the CA training either due to health problems, or to technical problems with the equipment. Among the remaining 82 rats, all treatment groups demonstrated acquisition of both simple and configural associations in the CA task (Figures 7 & 8). Acquisition of the simple associations (e.g., response to light, response to tone) occurred very rapidly and achieved maximal response (30/30 correct responses) from most animals within 1-2 blocks of training (Figures 7A-B). Acquisition of the negative patterning component (e.g., lack of response to simultaneous presentation of light + tone) took longer, and tended to stabilize at a group average of 22-25 incorrect responses out of thirty presentations after 24 training sessions (Figure 7C). The variance in each group was high, as indicated by the fact that during Block 8 some animals in each group were performing the negative patterning component very well, and some were performing poorly (Figure 7D). Similar patterns were observed for response time (Figure 8). For example, the time to respond to either a tone or a light stimulus decreased significantly during the first two to three blocks of training and then stabilized (Figure 8A-B). In contrast, response time to the configural stimulus decreased during the first 2-3 blocks of training and then increased significantly (Figure 8C). The variability in performance observed after 24 days of training was high as illustrated in Figure 8D.
Figure 7.
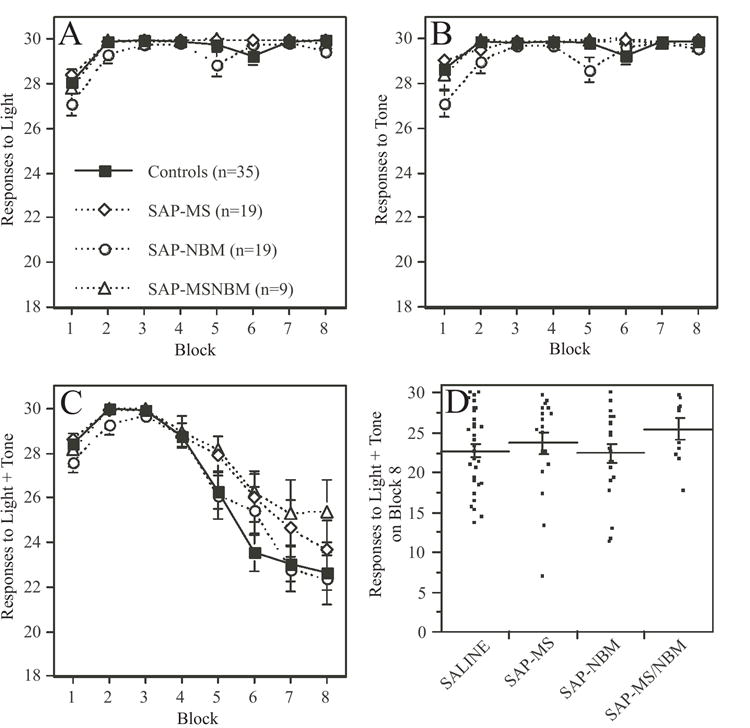
Plots summarizing the effects of the SAP lesions on the number of responses to the simple and configural stimuli during acquisition of the CA task The data are grouped into eight 3-day blocks of training. In panels A-C, each data point represents a group mean ± s.e.m. Panels A and B show the mean number of responses (maximum of 30) to presentation of either the light or the tone. Note that animals quickly learn to associate light or tone with food reward. ANOVA revealed that animals in the SAP-NBM group performed slightly worse than animals in the other groups (see text for statistics). Panel C shows the mean number of responses (maximum of 30) to presentation of the combined stimulus of light + tone. Note that animals initially increased their responses to the configural stimulus, but over time reduce their responses as they learned to distinguish between the lack of reward associated with responding to the configural stimulus versus the positive reward associated with responding to the simple stimuli. There was no significant effect of Treatment on responses to the configural stimulus. Panel D is a scatter plot showing each rat’s mean number of responses to the combined stimulus on Block 8 of training. Lines indicate mean ± s.e.m. Note the considerable variance in responses after 24 days of training.
Figure 8.
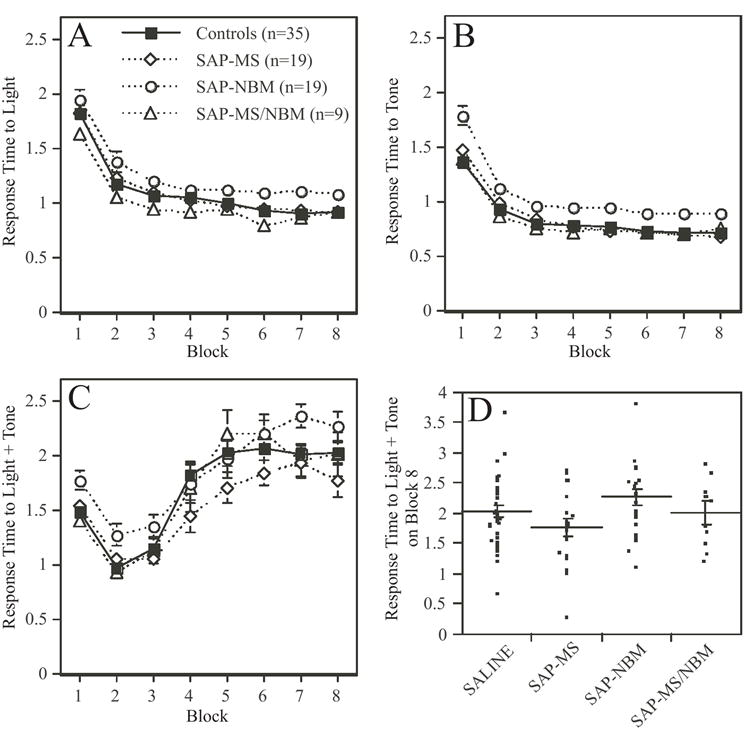
Plots summarizing the effects of the SAP lesions on response time to simple and configural stimuli during acquisition of the CA task The data are grouped into eight 3-day blocks of training. In panels A-C, each data point represents a group mean ± s.e.m. Panels A and B show the mean response time to presentation of either the light or the tone. Note that animals quickly learn to associate light or tone with food reward, as indicated by the decrease in response time. ANOVA revealed that animals in the SAP-NBM group performed slightly worse than animals in the other groups (see text for statistics). Panel C shows the mean response time to presentation of the combined stimulus of light + tone. Note that animals initially decreased their response time to the configural stimulus, but over time they increased their response time as they learned to distinguish between the lack of reward associated with responding to the configural stimulus versus the positive reward associated with responding to the simple stimuli. ANOVA revealed a significant effect of Treatment on response time to the configural stimulus, which was due to a significant difference between the SAP-MS and SAP-NBM groups. Panel D is a scatter plot showing each rat’s mean response time to the combined stimulus on Block 8 of training. Lines indicate mean ± s.e.m. Note the considerable variance in response time after 24 days of training.
ANOVA revealed a highly significant effect of Block on all measures examined (p<0.0001 in all cases). In addition, ANOVA revealed significant effects of Treatment on the acquisition of both simple and configural associations. With respect to the simple associations, ANOVA revealed significant effects of Treatment on both the number of responses to light (F[3,77]=5.1, p<0.005) and to tone (F[3,77]=9.1, p<0.0001), as well as response time to tone (F[3,77]=5.6, p<0.05). In each case, SAP-NBM-treated animals performed slightly but significantly less well than all other groups. In addition, a significant Treatment x Block interaction was observed with respect to the number of responses to tone (F[21,539]=2.3, p<0.05). With respect to the configural association, ANOVA revealed a significant effect of Treatment on response time to light+tone (F[3,77]=3.2, p<0.05). Post-hoc comparisons revealed that only the SAP-MS and SAP-NBM-treated groups differed significantly from each other. No other significant effects were detected.
When ChAT activity in each of the three brain regions was included as a covariate, analysis revealed that ChAT activity in the frontal cortex (but not the HPC or RS/E) co-varied significantly with the effects of Treatment on response time to light + tone (F[1,75]=4.9, p<0.05), and interacted significantly with Block (F[7,518]=3.3, p<0.005). When the ANCOVA was broken down by Block, a significant effect of Treatment was observed on Blocks 1, 2, and 4, with a significant effect of ChAT activity in the frontal cortex on Blocks 1, 3, 4, and 5. No other correlations between ChAT activity and measures of CA performance were detected by ANCOVA.
To further examine the relationship between ChAT activity and response time to light + tone within specific Blocks, scatter plots and Spearman’s Rho analysis were conducted separately for each treatment group. This analysis revealed significant correlations in the saline-treated controls on Blocks 1-4, and in the SAP-NBM-treated animals on Blocks 4-5. In each case correlations were negative, indicating that response time decreased as ChAT activity in the frontal cortex increased. Since a decrease in response time to light + tone represents poorer performance, this indicates that performance on the configural component of the task decreased as ChAT activity in the frontal cortex increased. No other significant correlations between ChAT activity on measures of CA acquisition were detected.
DISCUSSION
The data show that lesioning cholinergic neurons in the MS and NBM using the immunotoxin 192IgG-SAP produced deficits in DMP acquisition. In addition, SAP lesions in the NBM (but not the MS or MS/NBM) produced small but significant reductions in responses to simple associations in an operant conditioning task, and had a small but significant effect on response time to a learned configural association. In contrast, the cholinergic lesions did not significantly affect performance decrements on the DMP task produced by increasing the intertrial delay. Since the configural association task relies on the same motivational element (i.e., food reward) as the T-maze task, we can conclude that the deficits in DMP acquisition, particularly in response to the SAP-MS and SAP-MS/NBM lesions, were not due to an effect on the animals’ willingness to work for food. In addition, since the SAP lesions only minimally affected acquisition of the configural association, we conclude that the lesions did not have a severe effect on the animals’ ability to learn rules or to distinguish between appropriate and inappropriate choices. Therefore, we conclude that the deficits in DMP acquisition reflect task-selective effects of the cholinergic lesions, consistent with the hypothesis that cholinergic projections contribute to performance within specific cognitive domains.
Effects on spatial working memory
As stated above, while the SAP lesions affected acquisition of the DMP task, these same lesions did not alter the effect of increasing the intertrial delay. Traditionally, one way of testing for an effect on working memory performance is to show that performance decreases with an increase in intertrial delay. The fact that performance declined with an increase in intertrial delay demonstrates, as expected, that performance on this task requires intact spatial working memory. The fact that the SAP-lesions had no impact on the effect of the delays suggests that any lesion-induced effect on spatial working memory was not sufficient to affect performance on this task, and does not account for the lesion-induced deficits in acquisition. This is consistent with our previous reports, as well as with other studies that have reported little effect of selective cholinergic deafferentation of the hippocampus on working memory performance in rodents (Baxter et al., 1995; Baxter and Gallagher, 1996; Chappell, McMahan, Chiba, and Gallagher, 1998; McMahan, Sobel, and Baxter, 1997). We conclude from this that the effects of the SAP lesions on DMP acquisition are not due to a significant impairment in spatial working memory.
Effects on Response Patterns and Turning Strategy
The analysis of response patterns suggests that the deficit in DMP acquisition most likely is related to effects on strategies adopted during training. Note in Figure 3 that at the start of training, all groups performed at a level below chance, reflecting the rodents’ natural tendency to alternate (i.e., to not revisit a location recently visited), but that most of the animals reached chance levels of performance within 3-6 days of training. In many cases, this coincided with the adoption of a persistent turning strategy whereby animals consistently entered either the right or left arm of the maze. This is similar to the observations by Pych et al. who reported that some rats tested on a reinforced spontaneous alternation task adopted a persistent turning strategy (Pych, Chang, Colon-Rivera, and Gold, 2005a). A similar result was also recently reported by Fitz et al. (Fitz, Gibbs, and Johnson, 2006) using gonadally intact male rats. Note that in the DMP task, adoption of the persistent turning strategy results in increased success early on during training; however the success rate plateaus at 50%. In order to improve further, animals must alter their response pattern and adopt yet a different strategy. Therefore, the time it takes an animal to reach criterion is, in part, a function of the likelihood that an animal will adopt a persistent turning strategy, as well as the ability of the animal to break away from that response pattern and adopt a more successful strategy.
Our analysis shows that SAP lesions significantly increase the likelihood that rats will adopt a turning strategy during DMP training. In addition, SAP lesions increased the time that rats persisted with the turning strategy before switching to a more successful strategy. When the duration of the turning strategy was subtracted from the number of days to reach criterion, no significant effects of treatment on DTC were detected. These findings suggest that the effects of the SAP lesions on DMP acquisition can be explained entirely by effects on response pattern, strategy selection, and the ability to alter strategy (i.e., cognitive flexibility) during training, as opposed to effects on working memory.
Effects on Ultimate Strategy Selection
While it is clear that animals with SAP lesions were more likely to adopt and retain a turning strategy during training, it is also clear that nearly all of the rats did eventually reach criterion, indicating an ability over time to adopt a more successful strategy. This raises the question of whether rats in each of the treatment groups ultimately adopted the same response strategy. Analysis of the probe trial indicates that rotating the maze after rats had reached criterion significantly disrupted arm choice to approximately chance levels in controls, SAP-MS and SAP-NBM-treated groups. This could indicate any of three possibilities: a) each animal in each of these groups were equally likely to use either a response strategy or a place strategy in making a choice; b) half of the animals in each group were strongly disposed to using a response strategy, and half were strongly disposed to using a place strategy; or c) rotating the maze caused the animals to become confused and to choose an arm at random. There is no way to distinguish between these possibilities from the current data; however, we can conclude that by the time rats reached criterion, a significant number of animals in each of these groups were using extramaze cues to a significant degree in making arm choices, consistent with the use of an allocentric strategy. In contrast, arm choice in the SAP-MS/NBM-treated group was not significantly disrupted by rotating the maze. Analysis of the arm choices indicates that rats in this group ultimately adopted an egocentric (i.e., response) strategy. These data show that while each of the SAP lesions appeared to produce similar deficits in DMP acquisition, rats with denervation of the hippocampus or frontal cortex were more likely to ultimately adopt an allocentric strategy, whereas rats with combined denervation of the hippocampus and frontal cortex were more likely to ultimately adopt an egocentric strategy. One possibility is that the ability to adopt an allocentric strategy requires intact cholinergic innervation of either the hippocampus or frontal cortex. The analysis of correlations between behavioral performance and ChAT activity (see below) support the hypothesis that cholinergic innervation of the frontal cortex plays a role in predisposing rats to adopt an allocentric strategy.
Correlations between ChAT activity and DMP acquisition
Initially, we predicted that cholinergic lesions of the MS, but not the NBM, would significantly impair DMP acquisition in association with a deficit in working memory, and that the severity of the effect would correlate significantly with cholinergic denervation of the hippocampus. This, in fact, was not the case. Cholinergic lesions in either the MS or the NBM produced a significant impairment in DMP acquisition, which did not reflect a deficit in working memory. In addition, these effects were not additive, suggesting that the two lesions might produce deficits via a common underlying mechanism.
Analysis of ChAT activity in the hippocampus and frontal cortex demonstrated that the lesions were fairly selective with respect to denervating one structure or the other. This suggests that the deficit produced by the NBM lesions was not due to partial destruction of cells in the MS. As expected, animals that received lesions in both the MS and NBM showed significant cholinergic denervation of both the hippocampus and frontal cortex, as well as a significant reduction in ChAT activity in the RS/E. The RS/E receives cholinergic inputs from cells located from the caudal MS through the diagonal band of Broca. Hence, it is not surprising that a significant reduction in ChAT activity in the RS/E was observed following combined lesions of the MS and NBM. Notably, no correlation between DMP acquisition and ChAT activity in the hippocampus was detected in any treatment group with the exception of performance on Block 4 of training in SAP-MS-treated animals. This strongly suggests that the deficit in DMP acquisition observed in SAP-NBM-treated animals was not due to accidental cholinergic deafferentation of the hippocampus. Furthermore these data indicate that the degree of cholinergic denervation of the hippocampus is not a strong predictor of DMP impairment, contrary to the original hypothesis.
Notably, our analysis did reveal a significant negative correlation between DMP acquisition and the degree of cholinergic denervation in the frontal cortex of the SAP-NBM-treated animals on Blocks 1-6 of training, and in SAP-MS-treated animals on Blocks 3-4 of training. Note that a negative correlation indicates a decrease in performance accuracy associated with increased ChAT activity. Similar correlations were not observed in controls, or in SAP-MS/NBM animals. This suggests that when cholinergic afferents to either the hippocampus or frontal cortex become impaired, there is an interesting relationship between the degree of cholinergic denervation of the frontal cortex and performance. The biological significance of this unclear. One possibility is that the ratio of cholinergic activity in these two structures, as opposed to the absolute level of cholinergic activity in either structure alone, influences performance. Hence, when cholinergic inputs to one of the two structures is compromised, having fewer cholinergic inputs in the remaining structure may help to achieve a more balanced ratio. In addition, levels of cholinergic activity, or the ratio of acetylcholine release in the frontal cortex relative to other structures, may bias the system towards one response strategy versus another (see below). Notably, correlations were detected primarily with ChAT activity in the frontal cortex, not the hippocampus. One possibility is that cholinergic innervation of the cortex may function as a rheostat, modulating cortical activity as needed to adopt specific response strategies. Note that this is consistent with studies in humans which indicate that cholinergic neurotransmission modulates activity in sensory and frontal cortical regions in a task-dependent manner (Thiel, 2003); however, direct evidence that cholinergic input to the frontal cortex influences strategy selection will require further studies.
It is interesting that correlations between ChAT activity in the frontal cortex and DMP performance were not observed in animals that received combined lesions of both the MS and NBM. One possibility is that when both structures are significantly compromised, animals adopt alternate strategies which rely on different neural circuits, and hence are not reflected by ChAT activity in the frontal cortex. This is consistent with the fact that by the time rats reached criterion, controls as well as SAP-MS and SAP-NBM animals were more likely to adopt an allocentric strategy, whereas animals with combined lesions were more likely to adopt an egocentric strategy. Therefore, even though most of the animals did eventually reach criterion, animals with combined lesions adopted a different response strategy, which did not correlate with ChAT activity in the frontal cortex. Collectively, these data are consistent with the idea that cholinergic innervation of either the frontal cortex or hippocampus influences the likelihood that rats will adopt an allocentric strategy. It must be emphasized that ChAT activity as measured here is not a measure of cholinergic activity, but rather is an index of the degree of cholinergic denervation. Additional studies, like the microdialysis studies discussed below, are needed to test the hypothesis that cholinergic activity in specific cortical areas can influence an animal’s predisposition to adopt an allocentric strategy.
Comparisons with previous findings
The idea that different neural circuits underlie different types of learning strategies, and that the activity of specific cholinergic projections can reflect the use of different strategies, is not new. Evidence in rodents, as well as humans, suggests that cortical and diencephalic structures can underlie the acquisition of specific types of place learning or specific forms of declarative memory (e.g., simple recognition of familiarity), whereas extrapyramidal structures can underlie specific types of response learning or habit learning (e.g., probabilistic classification learning) (Squire, 1998). It is well known that rodents can use a variety of strategies to solve T maze tasks (Dudchenko, 2001), and that the strategy employed can change throughout the course of acquisition. For example, in a study by Packard & McGaugh (1996) rats trained to approach a baited arm in a cross-maze initially used a place strategy, and then shifted to a response strategy, indicating that with extended training there was a shift in learning mechanisms controlling behavior. Notably, the data also showed that inactivation of the hippocampus selectively interfered with place learning, whereas inactivation of the caudate nucleus selectively interfered with response learning, indicating that the hippocampus and caudate are involved in different types of learning strategies.
In addition, studies by Gold and co-workers have shown that the release of acetylcholine in the hippocampus and caudate nucleus changes during training, and that the ratio of ACh release in the hippocampus and caudate can accurately reflect the use of place and response strategies (see Gold, 2003 for review). For example, McIntyre et al. (McIntyre, Marriott, and Gold, 2003) reported that when rats were trained on a maze that could be learned using either a place or a response strategy, rats that used a response strategy displayed a lower ratio of ACh release in the hippocampus/striatum than rats that used a place strategy. In a subsequent study, Pych et al. (Pych et al., 2005a) reported that rats tested on a food-rewarded spontaneous alternation task initially used a spatial working memory strategy, and then shifted to a persistent turning strategy. Measurements of ACh release in the hippocampus and striatum during learning showed that as rats adopted the persistent turning strategy, the ratio of ACh release in the hippocampus/striatum steadily decreased. More recently, Pych et al. (Pych, Chang, Colon-Rivera, Haag, and Gold, 2005b) showed that when rats were trained on place or response versions of food rewarded maze tasks, ACh release in the striatum was greater in rats trained on the response tasks than in rats trained on the place tasks. While ACh release in the hippocampus also increased, this effect was lessened when rats were trained on a response task under cue-poor (low salience extramaze cues) conditions. In addition, high baseline levels of ACh release in the hippocampus predicted rapid learning in a cue-rich condition and slow learning in a cue-poor condition. Among other things, these the data demonstrate a relationship between the ratio of cholinergic activity in different structures and performance strategy. Whether or not the ratio of cholinergic activity in the frontal cortex vs. hippocampus or striatum similarly influences strategy is not yet know.
As mentioned above, with respect to the DMP task, the time it takes an animal to reach criterion is, in part, a function of the likelihood that an animal will adopt a persistent turning strategy, as well as the ability of the animal to break away from that response pattern and adopt a more successful strategy. Some evidence suggests that this type of behavioral flexibility is a function of cholinergic activity in the dorsal striatum as opposed to cholinergic innervation of cortical structures. For example, Ragozzino (2003) reported that ACh release in the dorsal striatum correlated with reversal learning of a spatial discrimination task, and that blockade of muscarinic receptors in the dorsal striatum did not block acquisition of an egocentric spatial discrimination task, but did block reversal learning of the task. This suggests that cholinergic activity in the striatum is associated with the ability to inhibit one response pattern in order to adopt another. Further studies will need to investigate whether cholinergic input to the frontal cortex also plays a role in strategy switching, particularly as it pertains to the DMP task.
Summary of effects on the DMP task
Collectively, the data show that cholinergic lesions impair acquisition of the DMP task, and that this effect is due primarily to an affect on strategy during training. In particular, cholinergic lesions appear to impair DMP acquisition specifically by affecting the likelihood that animals will adopt a turning strategy during training, as well as the ability of animals to eventually abandon that strategy and adopt a strategy that produces a greater rate of success. The data also suggest that when cholinergic neurons in either the MS or NBM are impaired, there is an interesting relationship between performance and the degree of cholinergic denervation of the frontal cortex. A similar relationship was not detected in the controls, or in animals with combined lesions of the MS and NBM. Further studies are needed to elucidate the significance of these relationships with respect to the predisposition to adopt allocentric vs. response strategies.
Effects on CA acquisition
In contrast to the effects of SAP lesions on DMP acquisition, these same lesions had much less effect on CA acquisition. Specifically, cholinergic lesions of cells in the NBM, but not the MS or MS/NBM, produced small but significant reductions in responses and response time during simple associations. This may reflect an affect of the lesions on either perception or alertness and attention. This is not surprising given that several studies have demonstrated significant effects of cholinergic NBM lesions on measures of attention (Everitt and Robbins, 1997; Sarter, Bruno, and Givens, 2003). In addition, there was a small but significant effect of Treatment on response time to the configural stimulus, but not on the number of responses to the stimulus. Post-hoc analysis showed that the effect on response time was due to a difference between SAP-MS and SAP-NBM-treated animals, but that none of the SAP-treated groups differed significantly from the saline-treated controls. ANCOVA revealed a significant correlation between ChAT activity in the frontal cortex and response time to the configural stimulus, but only in SAP-NBM-treated animals. These data indicate that, in contrast to the effects on DMP acquisition, the SAP lesions had only a minor effect on acquisition of the configural task, and that this effect was on response time.
Why the cholinergic lesions had a relatively mild effect on the CA task is unknown. Given that the task requires animals to distinguish between simple and complex stimuli and to select an appropriate response, and given that cortical circuits are essential for configural associations (Rudy and Sutherland, 1995), we anticipated that cholinergic deafferentation of the frontal cortex would significantly impair acquisition of this task. Consistent with out results, Moran (1992) reported that systemic injection of the muscarinic cholinergic antagonist methylscopolamine had no effect on acquisition of a similar negative patterning task, but did impair retention of the task. These findings suggest that central cholinergic systems are not a necessary substrate for the formation of configural associations, particularly in an operant chamber where negative patterning places few demands on spatial information or spatial strategy.
In contrast, Butt and co-workers have reported that either excitotoxic lesions of the NBM, or selective cholinergic lesions of the NBM using 192IgG-SAP, can significantly impair acquisition of a negative patterning task, without producing impairment on a similar transverse patterning task (Butt and Bowman, 2002; Butt and Hodge, 1997; Butt, Noble, Rogers, and Rea, 2002). There are several possible explanations for the disagreement between these results. First, all of the studies by Butt were conducted using Long-Evans rats, whereas the current study as well as the study by Moran used Sprague Dawley rats. While a strain-dependent difference may be unlikely, it cannot be excluded. Second, all of the studies by Butt were conducted using gonadally intact males, whereas the current study was conducted using ovariectomized females. There are many examples of sex differences, as well as gonadal hormone effects, on cognitive performance in both rodents and higher species, including humans (Daniel, 2006; Dohanich, 2002; Janowsky, 2006; Sherwin, 2003, 2006). Such differences may account for the discrepancies here. So far, our studies have not detected any significant effects of gonadectomy or gonadal hormone treatment on acquisition of the CA task (Gibbs, 2005; Gibbs and Gabor, 2003); however, the possibility of a sex difference on this task with respect to the effects of cholinergic lesions cannot be excluded. Third, in the present study, all rats underwent training on the DMP task before being trained on the CA task. This introduces the possibility that DMP training in some way negated the effect of the cholinergic lesions on CA acquisition, perhaps by producing some sort of a priming effect. Any of these possibilities may explain the relatively mild effect of the cholinergic lesions on CA acquisition observed in the present study.
Summary
The results show that lesions of cholinergic neurons in either the MS or NBM significantly impair acquisition of a DMP T-maze task, and that this effect is due largely to an affect on response patterns adopted by the rats during training. In contrast, the same lesions produced little impairment on a configural association operant conditioning task. Notably, the deficit in DMP acquisition did not correlate significantly with cholinergic denervation of the hippocampus. In the SAP-MS and SAP-NBM lesion groups, evidence for a negative correlation between performance and the degree of cholinergic denervation in the frontal cortex was detected. We hypothesize that cholinergic activity in the frontal cortex may play an important role in finetuning cortical activity in a way which influences the likelihood to adopt an allocentric strategy. Further studies are needed to identify the role that cholinergic projections to specific regions of the isocortex play in mediating strategy selection.
Acknowledgments
We wish to acknowledge the excellent technical assistance provided by Rhianon Mauk and Douglas Nelson. Advice with respect to the statistical analyses was provided by Levent Kirisci, Assistant Professor of Pharmaceutical Sciences. This work was supported by NIH grant R01 AG021471.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav Neurosci. 1995;109(4):714–22. doi: 10.1037//0735-7044.109.4.714. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M. Intact spatial learning in both young and aged rats following selective removal of hippocampal cholinergic input. Behav Neurosci. 1996;110(3):460–7. doi: 10.1037//0735-7044.110.3.460. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principal of protein dye binding. Analyt Biochem. 1976;72:248–253. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Butt AE, Allen K, Arthur K, Noble C, Rea T, Rogers J. A test of negative patterning reveals selective impairment in configural association learning in rats with 192 IgG-saporin lesions of the nucleus basalis magnocellularis. Soc for Neurosci. 2000;26 program #563.5. [Google Scholar]
- Butt AE, Bowman TD. Transverse patterning reveals a dissociation of simple and configural association learning abilities in rats with 192 IgG-saporin lesions of the nucleus basalis magnocellularis. Neurobiol Learn Mem. 2002;77(2):211–33. doi: 10.1006/nlme.2001.4013. [DOI] [PubMed] [Google Scholar]
- Butt AE, Hodge GK. Simple and configural association learning in rats with bilateral quisqualic acid lesions of the nucleus basalis magnocellularis. Behav Brain Res. 1997;89(12):71–85. doi: 10.1016/s0166-4328(97)00062-4. [DOI] [PubMed] [Google Scholar]
- Butt AE, Noble MM, Rogers JL, Rea TE. Impairments in negative patterning, but not simple discrimination learning, in rats with 192 IgG-saporin lesions of the nucleus basalis magnocellularis. Behav Neurosci. 2002;116(2):241–55. doi: 10.1037//0735-7044.116.2.241. [DOI] [PubMed] [Google Scholar]
- Chappell J, McMahan R, Chiba A, Gallagher M. A re-examination of the role of basal forebrain cholinergic neurons in spatial working memory. Neuropharmacol. 1998;37:481–487. doi: 10.1016/s0028-3908(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139(1):209–21. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28(7):771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18(10):787–95. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Dohanich GP. Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Farbach SE, Rubin RT, editors. Hormones, brain and behavior. Vol. 1. San Diego: Academic Press; 2002. pp. 265–327. [Google Scholar]
- Dornan WA, McCampbell AR, Tinkler GP, Hickman LJ, Bannon AW, Decker MW, Gunther KL. Behav Brain Res. Vol. 82. 1996. Comparison of site-specific injections into the basal forebrain on water maze and radial arm maze performance in the male rat after immunolesioning with 192 IgG saporin; pp. 93–101. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA. How do animals actually solve the T maze? Behav Neurosci. 2001;115(4):850–60. [PubMed] [Google Scholar]
- Eichenbaum H. Remembering: functional organization of the declarative memory system. Curr Biol. 2006;16(16):R643–5. doi: 10.1016/j.cub.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fitz NF, Gibbs RB, Johnson DA. The role of septo-hippocampal cholinergic lesion, place versus response strategy, and acquisition of a delayed matching to position T-maze task. Annual Meeting of the Society for Neuroscience; 2006. Program Number 751.20. http://www.sfn.org/index.cfm?pagename=abstracts_ampublications§ion=publications. [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Hormones & Behavior. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol of Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal Forebrain Cholinergic Neurons are Necessary for Estrogen to Enhance Acquisition of a Delayed Matching-To-Position T-maze Task. Horm & Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48(3):268–77. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and Cognition: Applying Preclinical Findings to Clinical Perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003;80(3):194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn Sci. 2006;10(2):77–82. doi: 10.1016/j.tics.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Zambon NJ, Gibbs RB. Selective lesion of cholinergic neurons in the medial septum by 192 IgG-saporin impairs learning in a delayed matching to position T-maze paradigm. Brain Research. 2002;943:132–141. doi: 10.1016/s0006-8993(02)02623-9. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol Learn Mem. 2003;79(2):177–83. doi: 10.1016/s1074-7427(02)00014-x. [DOI] [PubMed] [Google Scholar]
- McMahan RW, Sobel TJ, Baxter MG. Selective immunolesions of hippocampal cholinergic input fail to impair spatial working memory. Hippocampus. 1997;7(2):130–6. doi: 10.1002/(SICI)1098-1063(1997)7:2<130::AID-HIPO2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Miller BL, Cummings JL. The Frontal Lobes: Functions and Disorders. New york: Guilford Press; 1999. [Google Scholar]
- Moran PM. Scopolamine deficits in negative patterning discrimination: evidence for a role of the central cholinergic system in retention but not acquisition of non-spatial configural association learning. Behav Brain Res. 1992;48(2):187–97. doi: 10.1016/s0166-4328(05)80156-1. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 2. London: Academic Press; 1986. The Rat Brain in Stereotaxic Atlas. [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem. 2003;80(3):178–93. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, Gold PE. Acetylcholine release in hippocampus and striatum during testing on a rewarded spontaneous alternation task. Neurobiol Learn Mem. 2005a;84(2):93–101. doi: 10.1016/j.nlm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, Haag R, Gold PE. Acetylcholine release in the hippocampus and striatum during place and response training. Learn Mem. 2005b;12(6):564–72. doi: 10.1101/lm.33105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. Acetylcholine actions in the dorsomedial striatum support the flexible shifting of response patterns. Neurobiol Learn Mem. 2003;80(3):257–67. doi: 10.1016/s1074-7427(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behav Brain Res. 1989;34(12):97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: an appraisal and reconfiguration. Hippocampus. 1995;5(5):375–89. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem. 2003;80(3):245–56. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Rossner S, Bigl V. Immunolesion by 192IgG-saporin of rat basal forebrain cholinergic system: a useful tool to produce cortical cholinergic dysfunction. Progress in Brain Research. 1996;109:253–64. doi: 10.1016/s0079-6123(08)62109-3. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Steroid hormones and cognitive functioning in aging men: a mini-review. J Mol Neurosci. 2003;20(3):385–93. doi: 10.1385/JMN:20:3:385. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138(3):1021–6. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006;16(9):716–29. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems. C R Acad Sci III. 1998;321(23):153–6. doi: 10.1016/s0764-4469(97)89814-9. [DOI] [PubMed] [Google Scholar]
- Thiel CM. Cholinergic modulation of learning and memory in the human brain as detected with functional neuroimaging. Neurobiol Learn Mem. 2003;80(3):234–44. doi: 10.1016/s1074-7427(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci. 1993;16:547–63. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]


