Abstract
Accumulating evidence suggests that estradiol might be responsible for the enhanced response to psychostimulants sometimes observed in females. In this study, 10 healthy pre-menopausal women who were using oral, hormone-based birth control learned to discriminate 15 mg/70 kg oral d-amphetamine from placebo. Once a discrimination criterion was met (i.e., ≥ 80% correct responding at the final time point for five consecutive sessions), a range of doses of oral d-amphetamine (0, 3.125, 7.5 and 15 mg/70 kg) was tested alone and in combination with sublingual estradiol (0 and 0.25 mg). Test sessions were conducted during the oral contraception placebo phase when levels of both estradiol and progesterone were at their lowest. d-Amphetamine functioned as a discriminative stimulus and produced prototypical stimulant effects (e.g., increased positive subject-rated drug effects, elevated cardiovascular measures). Estradiol enhanced the discriminative-stimulus effects of the low dose, but not higher doses of d-amphetamine. Estradiol also enhanced d-amphetamine effects on a subset of subjective-report ratings (i.e., VAS Like Drug and total score on the Stimulant subscale of the Adjective-Rating Scale). These findings provide limited support for the notion that estradiol increases sensitivity to the psychostimulant effects of drugs such as d-amphetamine.
Keywords: Estrogen, Amphetamine, Hormone, Menstrual Cycle, Drug Discrimination, Subjective
Introduction
Epidemiological data indicate that rates of the misuse of psychostimulants such as cocaine and amphetamines are higher in adult men than women (SAMHSA, 2005). This disparity in the prevalence of use between men and women might be due to socio-cultural factors (e.g., Van Etten et al., 1999). However, accumulating experimental evidence from laboratory studies in rats suggests that females might be more sensitive to some of the abuse-related effects of psychostimulants than males, and that gonadal sex hormones represent one component of the biological basis for these differences (reviewed in Lynch et al., 2002; Carroll et al., 2004).
Psychostimulants increase synaptic levels of dopamine (DA), serotonin and norepinephrine. Although each of these monoamine neurotransmitter systems is involved in the behavioral effects of psychostimulants, there is an extensive literature emphasizing the role of elevated mesocorticolimbic DA. Estradiol (i.e., 17β-estradiol), the most prominent estrogen in women, has been shown to enhance psychostimulant effects on dopaminergic function through both traditional genomic mechanisms (e.g., Zhou et al., 2002) and more rapid and direct means. For example, in vitro microdialysis studies have demonstrated that d-amphetamine-evoked DA release is decreased in striatal tissue from ovariectomized female rats, but administration of estradiol immediately reverses the effect of ovariectomy (Becker and Ramirez, 1981; Becker, 1990). Data from electrophysiological studies suggest that this immediate effect of estradiol depends on a G-protein coupled membrane receptor and is specific to certain estrogens such as estradiol (Balthazart and Ball, 2006).
Although many of the studies conducted in rats suggest that estradiol enhances the behavioral effects of psychostimulants (e.g., Lynch et al., 2002; Carroll et al., 2004, but see Caine et al., 2004), human studies have been less consistent with regards to the influence of estradiol levels across the menstrual cycle on the effects of psychostimulants. The self-reported effects of psychostimulants have been tested in women during the various phases of the menstrual cycle, including the early to mid follicular phase during which estrogen levels are initially low but increase and progesterone levels remain minimal, the late follicular phase during which estrogen peaks prior to ovulation but progesterone is still low, and the luteal phase during which levels of both hormones remain elevated. Two studies that compared the self-reported effects of d-amphetamine across the early-to-mid follicular and luteal phases found that some of the abuse-related subject-rated effects of d-amphetamine (e.g., High, Want More) were increased during the follicular phase and that these effects were positively correlated with estrogen levels (Justice and de Wit, 1999; White et al., 2002). In concordance with those results, women reported increased “positive” subject ratings following cocaine administration during the follicular, versus the luteal, phase (Sofuoglu et al., 1999; Evans et al., 2002; Evans and Foltin, 2006). In other studies, however, essentially no differences were observed when the subject-rated effects of d-amphetamine were compared during the early and late follicular phases (Justice and de Wit, 2000b) or when the subject-rated effects of cocaine were compared during the mid-follicular and luteal phases (Lukas et al., 1996; Mendelson et al., 1999). Similarly, when estradiol was administered via transdermal patch to women during the early follicular phase, most of the subject-rated effects of d-amphetamine were unaffected (Justice and de Wit, 2000a). Important to note is that there is evidence that progesterone masks the impact of estradiol on, or directly reduces, psychostimulant effects (e.g., Sofuoglu et al., 1999; White et al., 2002; Sofuoglu et al., 2004; Evans and Foltin, 2006), and the independent effects of estradiol are difficult to isolate across the menstrual cycle.
The present study was undertaken to further evaluate the ability of estradiol to modify the behavioral effects of psychostimulants and possibly clarify some of the inconsistent results from previous human laboratory studies. Instead of comparing the effects of d-amphetamine across menstrual cycle, exogenous sublingual estradiol (0 and 0.25 mg) was administered to healthy pre-menopausal women during the placebo phase of their oral contraceptive cycle, when hormone levels are at their lowest. Sublingual administration was chosen because it provides a rapid increase in estradiol and should provide consistent estradiol levels across test conditions. A drug-discrimination task was used to assess the discriminative-stimulus effects of oral d-amphetamine (0, 3.125, 7.5, 15 mg/70 kg) alone and in combination with estradiol. In drug discrimination procedures, responses are differentially reinforced depending on the presence or absence of specific interoceptive drug stimuli. These reinforcement contingencies result in the development of stimulus control over responding by the presence or absence of interoceptive drug effects. This procedure has been widely adopted, largely due to concordance with receptor binding studies and high degree of pharmacological specificity (Holtzman and Locke, 1988). Furthermore, it is thought that the combination of a drug-discrimination task and self-report questionnaires might be more sensitive to drug effects than self-report questionnaires alone (reviewed in Kelly et al., 2003). Therefore, a battery of self-report questionnaires, as well as a performance task and cardiovascular measures of physiological activity, were incorporated to more fully characterize the effects of the combination of d-amphetamine and estradiol.
Methods
Subjects
Healthy, adult, pre-menopausal women were recruited through advertisements placed on the University of Kentucky campus and in the local community. All potential subjects completed a brief telephone interview or an internet-based questionnaire addressing general medical and legal status. Respondents who reported good health, occasional stimulant use (e.g., caffeine), and use of a oral, hormone-based contraceptive that included 5–7 consecutive days of placebo administration were contacted by telephone and invited to participate.
During an orientation and medical screening day, subjects completed a battery of medical and psychological questionnaires (e.g., Kelly et al., 2006), and underwent blood chemistry, complete blood count, liver function and urinalysis tests. Urine samples were also screened for drugs of abuse and pregnancy (cocaine, benzodiazepine, barbiturate, marijuana, amphetamine and opiate, OnTrack TesTstik Bar, Varian, Inc., Lake Forest, CA; Clearview HCG II, Unipath, Ltd., Inverness Medical North America, Princeton, NJ). Breath samples were screened for recent alcohol use (Alco-Sensor III, Intoximeters, Inc., St. Louis, MO). Subjects were excluded if they had a history of serious medical illness (e.g., cardiovascular disease, neurological or psychiatric disorder, including drug dependence) or if there was any indication of elevated medical risk associated with administration of the study drugs.
Eighteen women were enrolled. Seven of these subjects were unable to reliably discriminate 15 mg/70 kg of d-amphetamine from placebo and were excluded from further participation. One subject withdrew for reasons unrelated to the study protocol. The data from these subjects were not included in the analysis. Ten subjects (nine Caucasian, one Asian) completed this protocol. Subjects ranged in age from 18 to 30 years (median = 21) and in body mass index from 19.4 to 29.8 (median = 22.4). The oral contraceptives used by these subjects included: Triphasil® (one subject), Ortho Cyclen® (one subject), Ortho Tri-Cyclen® (two subjects), Ortho Tri-Cyclen Lo® (two subjects), Yasmin® (three subjects) and Demulen® (one subject). Seven subjects were non-smokers; one subject reported smoking two cigarettes per day and two subjects reported infrequent cigarette use. Alcohol use ranged from 0 to 14 drinks per week (median = 5.3). All subjects reported caffeine use in the past year; daily use ranged from 0 to 120 mg per week (median = 80.0). Other lifetime psychostimulant use included cocaine (two subjects), amphetamine (two subjects) and diet pills (three subjects). Other lifetime drug use included marijuana (six subjects), benzodiazepines (two subjects) and hallucinogens (two subjects). Illicit drug use for all subjects was infrequent and in almost all cases did not occur in the month prior to study participation.
Subjects received $20 for participation in each session and were able to earn an additional $20 based on their performance (see below). At the end of their participation, subjects also received $40 per session for successfully completing the entire study and for adhering to the drug and alcohol use restrictions.
This study was conducted in accordance with the Helsinki Declaration of 1975. The Institutional Review Board of the University of Kentucky Medical Center approved the study and the informed consent document. All subjects provided sober, written informed consent and the confidentiality of their personal information was maintained throughout.
Design
A double-blind, placebo-controlled, randomized design was used to examine the effects of three within-subject variables [oral d-amphetamine dose (0, 3.125, 7.5 and 15.0 mg/70 kg), sublingual estradiol dose (0 and 0.25 mg) and time (0, 30, 60, 90, 120, 150 and 180 minutes post dose)]. Doses of oral d-amphetamine and sublingual estradiol were chosen based on previous research. A comparable dose (i.e., 15 mg) to the training dose of d-amphetamine used in the present study (i.e., 15 mg/70 kg) has been shown to function as a discriminative stimulus and produce positive subject rated effects (Rush et al., 1998, 2003, 2004; Lile et al., 2005a,b). A study that assessed the time course for d-amphetamine plasma concentrations found that peak levels occurred 2–3 h following oral administration and remained elevated for the duration of the 4-h experiment (Angrist et al., 1987). With respect to estradiol, a pharmacokinetic study demonstrated that sublingual administration of 0.25 mg resulted in peak serum estradiol levels of approximately 300 pg/mL, which are comparable to what is observed during the late follicular phase (Price et al., 1997). That study also showed that the time to maximum concentration for 0.25 mg estradiol was one hour or less (one hour was the earliest time point assessed) and the terminal half-life was approximately 8 hours (Price et al., 1997). Estradiol tablets were always administered immediately prior to d-amphetamine capsule administration, for two reasons. First, this dosing strategy allowed for less complicated instructions for the drug-discrimination task. Second, the more rapid onset of action for sublingual estradiol allowed for peak estradiol levels to occur approximately 1 hour prior to the peak onset of d-amphetamine effects.
Subjects initially underwent two separate training sessions in which they practiced the study tasks until performance was consistent and accurate across consecutive trials. No drugs were administered during these training sessions. The experiment proper consisted of three phases: sampling, control and test. Experimental sessions that comprised the sampling and control phases were conducted without regard to menstrual cycle phase, and during these phases the pretreatment dose was always placebo sublingual estradiol. Experimental sessions that comprised the test phase were conducted during the placebo days of the oral contraception regimen when systemic levels of both estradiol and progesterone were at their lowest levels. The interval between experimental sessions varied according to study phase and subject availability. During the sampling and control phases, sessions occurred every 3 days on average (range = 1–28, mode = 1). During test phase, sessions were usually conducted daily (range = 1–4), within the placebo phase of the oral contraception regimen.
Sampling Phase
The first two sessions of the experiment proper comprised the Sampling Phase, in which the subject was informed that the medication she received was “Drug A” (15 mg/70 kg oral d-amphetamine) and that she should pay close attention to how “Drug A” made her feel, because in future sessions, she would not be told what drug she would receive. Prior to and every half hour after drug administration for three hours, subjects completed assessments consisting of the Addiction Research Center Inventory (ARCI), the Stimulant Items from the Adjective-Rating Scale, Visual Analog Scale (VAS) ratings, and the Digit Symbol Substitution Task (DSST). During assessments completed after drug administration, subjects also completed a Drug Discrimination Task. Blood pressure and heart rate were recorded at the end of each assessment.
Control Phase
The Control Phase determined whether subjects acquired the discrimination between 15 mg/70 kg of d-amphetamine (i.e., “Drug A”) and placebo (“Not Drug A”). Control Phase sessions were identical to those of the Sampling Phase, except that subjects were not told which drug was administered and bonus money could be earned based on the accuracy of their performance on the Drug Discrimination Task. Each subject received 15 mg/70 kg of d-amphetamine at least twice and placebo at least twice. The order of drug administration was random. The criteria for having acquired the discrimination was 80% correct responding at the final, 3-h post-drug time point on the Drug Discrimination Task for five consecutive sessions. When a subject did not meet the criteria during the first five sessions of the Control Phase, training was extended up to a maximum of 12 sessions. When a subject met the acquisition criteria, she progressed to the Testing Phase. If a subject did not meet the acquisition criteria after 12 Control Phase sessions, she was dismissed from the study.
Test Phase
During the Test Phase dose-response functions were determined for d-amphetamine (0, 3.125, 7.5, and 15 mg/70 kg) alone and in combination with 0.25 mg estradiol. Each dose combination was tested once for a total of eight sessions. Up to four sessions of the Test Phase were conducted during the placebo days of each oral contraception cycle; therefore, the Test Phase typically required two or three menstrual cycles to complete. The placebo days correspond to the onset of menses and the early follicular phase of the menstrual cycle, during which estrogen and progesterone are at their lowest levels. During sessions in which subjects received the training dose condition (i.e., placebo pretreatment and 0 or 15 mg/70 kg d-amphetamine), subjects received feedback on the accuracy of their performance at the end of the session, and payment was contingent upon accuracy, as during the Control Phase. During sessions in which a novel dose condition was administered (Test Session), subjects did not receive any feedback regarding the accuracy of their discrimination performance. Instead, a subject was told that the condition she received that day would not be disclosed. Prior to the test phase, subjects were informed that payment for Test Sessions was determined based upon the average earnings from all other sessions.
Daily Schedule
Session length was approximately 3.5 h and start times were fixed for each subject. Subjects were instructed to abstain from illicit drugs for the duration of the study, medications and alcohol for 24 h prior to all scheduled sessions, and from eating or consuming caffeine for 4 h prior to the start of each session.
At the beginning of each session, subjects answered open-ended questions regarding sleep, drug and medication use, eating behavior and health status during the preceding 24 h, and completed field-sobriety, breath and urine tests to assess drug use and possible pregnancy. Subjects then consumed a low-fat snack. Assessments were completed before (i.e., time 0) and at half-hourly intervals for 3 h after drug administration. Each assessment was approximately 4–6 minutes in duration. During each assessment, activities were presented in the following order:
Visual-Analog Scales (VAS)
Subjects rated items (I like the drug effect; I feel high, stimulated, sedated, anxious, hungry, thirsty, a drug effect and confident) presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not At All” and “Extremely”.
Addiction Research Center Inventory (ARCI)
The 49-item short form of the true-false inventory (Martin et al., 1971) yielded information on five dimensions: LSD scale, Amphetamine (A) Scale, Benzedrine-Group (BG) Scale, Morphine-Benzedrine Group (MBG) Scale and the Pentobarbital, Chlorpromazine, Alcohol Group (PCAG) Scale.
Adjective-Rating Scale
The Adjective-Rating Scale consists of 32 items and contains two subscales: Sedative and Stimulant (Oliveto et al., 1992). In the present study, only the 16 items from the Stimulant subscale were presented. Subjects rated each item using a numeric keypad to select among one of five response options: “Not at All”, “A Little Bit”, “Moderately”, “Quite a Bit”, and “Extremely” (scored numerically from 0 to 4, respectively; maximum score = 64).
Drug-Discrimination Task
This task was completed during assessments occurring after drug administration. Two circles labeled “Drug A” and “Not Drug A” were displayed on the computer screen, each associated with a training dose condition. Counters were displayed directly below the circles. A cursor was displayed on the screen, which could be moved using a mouse. Mouse button presses increased the counter associated with the circle where the cursor was located according to a fixed-interval 1-sec schedule. The cursor could be moved between the circles without any consequence for the fixed-interval schedule (i.e., no change-over-delay). Up to 60 points could be allocated across the two options. A monetary bonus of $0.04 per point was earned for points accumulated on the circle associated with the dose condition on training dose sessions, as described above. The dependent variable for this task was the percent responding on the “Drug A” circle (i.e., drug-appropriate responding).
Digit-Symbol Substitution Task (DSST)
Subjects completed a 2-min computerized version of the DSST adopted from McLeod et al., 1982. Subjects earned $0.02 for each correct symbol completed. The dependent variables for this psychomotor task were symbol completion rate and accuracy.
Cardiovascular Assessment
Oscillometric heart rate and systolic and diastolic blood pressure measures were obtained (Sentry II, NBS Medical, Costa Mesa, CA).
Drug
Doses of d-amphetamine (0, 3.125, 7.5, 15 mg/70 kg; Mallinckrodt, St. Louis, MO) were prepared by the University of Kentucky Investigational Pharmacy in size 0 opaque capsules with cornstarch filler. Placebo capsules contained only cornstarch. One capsule containing the appropriate dose of d-amphetamine was taken orally with approximately 150 mL of water. Commercially available estradiol tablets (0 [Necon 1/35-28 placebo] and 0.25 mg [Estrace, Bristol-Myers Squibb, Princeton, NJ]) were administered sublingually and allowed to dissolve under the tongue over a period of approximately one to two minutes as described by Price et al. (1997). Estradiol doses were not adjusted for body weight because the sublingual route of administration was used.
Data Analysis
All results were considered significant at p<0.05. Data were analyzed as raw scores using a linear repeated-measures model with d-amphetamine dose (0.0, 3.125, 7.5 and 15.0 mg/70 kg), estradiol dose (0 and 0.25 mg) and time (0, 30, 60, 90, 120, 150 and 180 minutes post dose) as factors (JMP, SAS Institute Inc., Cary, NC). If a significant interaction of any of the factors was observed, simple effects models were used to interpret the results; multiple comparisons were not conducted. Effects reported here were limited to those measures for which a significant main effect of d-amphetamine or estradiol, or an interaction of d-amphetamine, estradiol and/or time was obtained.
Results
Drug-Discrimination Task
d-Amphetamine functioned as a discriminative stimulus, and the 10 subjects met the discrimination criteria in 5–12 Control Phase sessions (median = 5). When placebo (i.e., 0 mg oral d-amphetamine administered in combination with 0 mg sublingual estradiol) was administered during the final five sessions of the Control Phase, subjects reported an average of 1.0 (SEM = 0.19) percent drug-appropriate responding on the Drug-Discrimination Task during the final post-drug assessment. When the training dose of d-amphetamine (i.e., 15 mg/70 kg) was administered during the final five sessions of the Control Phase, it engendered an average of 100.0 (SEM = 0.0) percent drug-appropriate responding on the Drug-Discrimination Task during the final post-drug assessment. The top panel of Figure 1 demonstrates the time course for discriminative-stimulus effects of the training conditions administered during the final five sessions of the Control Phase.
Figure 1.
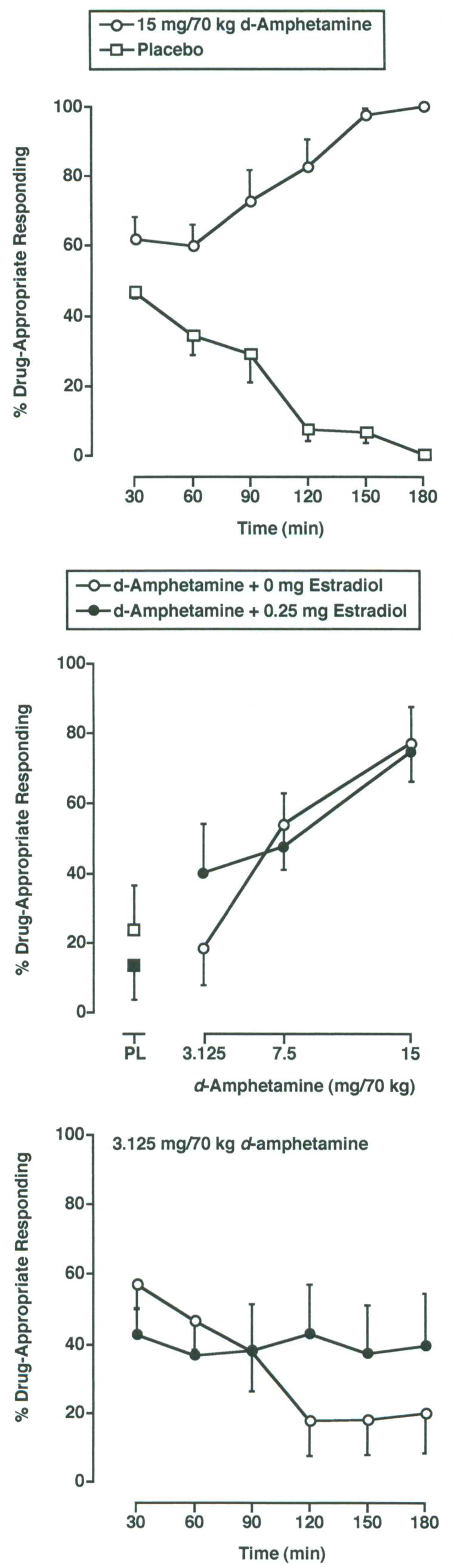
Top panel: Time course for the discriminative-stimulus effects of the training conditions (i.e., 0 mg/70 kg d-amphetamine [open squares] and 15 mg/70 kg d-amphetamine [open circles]). Data are from the final five sessions of the Control Phase. The x-axis represents experimental session time following drug administration in minutes. The y-axis represents the percentage of d-amphetamine-appropriate responding. Data points show means (± SEM) of 10 subjects. Middle panel: Dose-effect function for the discriminative-stimulus effects of d-amphetamine administered alone (open circles) and in combination with 0.25 mg estradiol (filled circles) during the Test Phase. Data are the average percent drug-appropriate responding for the 120–180 min time points based on the time course data for the 3.125 mg/70 kg dose of d-amphetamine (see bottom panel). The x-axis represents d-amphetamine dose; PL denotes placebo d-amphetamine (open square) and estradiol alone (i.e., estradiol + placebo d-amphetamine; filled square). Other details are as in top panel. Bottom panel: Time course for the discriminative-stimulus effects of 3.125 mg/70 kg d-amphetamine administered alone (open circles) and in combination with 0.25 mg estradiol (filled circles) during the test phase. Other details are as in top panel.
As shown in the middle panel of Figure 1, the discriminative-stimulus effects of d-amphetamine were a function of d-amphetamine dose, estradiol dose, and time (i.e., 3-way interaction; F18,162 = 2.1; p ≤ 0.01). d-Amphetamine dose dependently increased drug-appropriate responding on the Drug-Discrimination Task compared to placebo during the test phase. Responding came under control of the discriminative-stimulus effects of d-amphetamine at the 90 min time point and continued through 180 min. Figure 1 also shows that estradiol enhanced the discriminative-stimulus effects of the 3.125 mg/70 kg dose of d-amphetamine (middle panel) at the 120–180 min time points (bottom panel). In addition, estradiol appeared to have reduced the latency in the onset of the discriminative cue at the 15 mg/70 kg dose (data not shown).
Visual-Analog Scales
For three of the VAS items, Stimulated, Thirsty and Feel Drug, subject ratings were a function of d-ampheta mine dose and time (i.e., 2-factor interaction; F’s18,62 ≥ 1.8; p’s ≤ 0.05). For the VAS item Like Drug, subject ratings were a function of d-amphetamine dose, estradiol dose and time (i.e., 3-factor interaction; F18,162 = 1.9; p ≤ 0.01). d-Amphetamine dose dependently increased subject ratings on these VAS items, and these effects were apparent through the 90–180 min time points. In addition, estradiol increased the magnitude, and decreased the latency of the subject-rated effects for the item Like Drug at the 15 mg/70 kg dose of d-amphetamine. The dose-effect function for this item following administration of d-amphetamine, alone and in combination with estradiol, is presented in the left panel of Figure 2. Data are presented as area-under-the-time-effect curve (AUC) to incorporate the time-dependent enhancement of the discriminative-stimulus effects of d-amphetamine in combination with estradiol.
Figure 2.
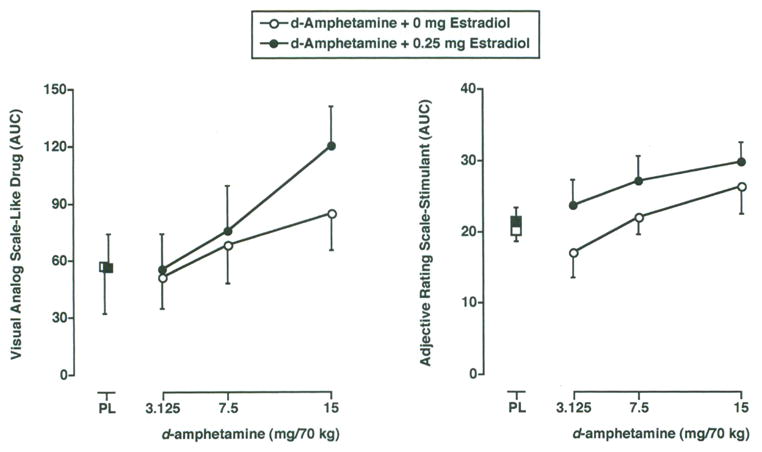
Dose-effect function for the subject-rated effects of d-amphetamine administered alone (open circles) and in combination with 0.25 mg estradiol (filled circles) during the Test Phase on the VAS item Like Drug (Left Panel) and the Stimulant subscale of the Adjective-Rating Scale (Right Panel). Data are presented as area-under-the-time-effect curve (AUC) to incorporate the time-dependent enhancement of the subject-rated effects of d-amphetamine in combination with estradiol. All other details are as in Figure 1, middle panel.
Addiction Research Center Inventory
Subject ratings for four of the ARCI scales varied as a function of d-amphetamine dose and time (i.e., 2-factor interaction; F’s18,62 ≥ 3.0; p’s < 0.001). d-Amphetamine dose dependently increased subject ratings on the BG, MBG and A scales of the ARCI compared to placebo. In addition, d-amphetamine dose dependently decreased subject ratings on the PCAG scale compared to placebo. The time of onset for the effects of d-amphetamine varied according to scale. Estradiol had no effect on ARCI scale ratings.
Adjective-Rating Scale (Stimulant subscale)
Subject ratings for the Stimulant subscale of the Adjective-Rating Scale were a function of d-amphetamine dose, estradiol dose and time (i.e., 2-factor interaction of d-amphetamine dose and time; F18,62 = 5.5; p < 0.001, and significant main effect of estradiol; F1,9 = 6.8; p ≤ 0.05). As shown in the right panel of Figure 2, d-amphetamine dose dependently increased composite score on this scale, and estradiol increased this score at every active dose of d-amphetamine. Data are presented as AUC to incorporate the time-dependent effects of d-amphetamine.
Digit-Symbol Substitution Task
DSST rate was a function of d-amphetamine dose and time (i.e., 2-factor interaction; F18,162 = 1.8; p < 0.05). d-Amphetamine increased DSST rate, and these effects were apparent at the 60–180 min time points. d-Amphetamine had no effect on the accuracy on DSST performance. No significant estradiol effects were detected on DSST performance.
Cardiovascular Assessment
Heart rate and blood pressure changed as a function of d-amphetamine dose and time (i.e., 2-factor interaction; F’s18,62 ≥ 3.0; p’s < 0.001). d-Amphetamine dose dependently increased heart rate and systolic and diastolic blood pressure, and these effects were apparent at the 90–180 min time points. No significant estradiol effects were observed.
Discussion
The present study used a drug-discrimination methodology in an effort to detect interactions between d-amphetamine and exogenously administered estradiol in women. In addition, a battery of self-report questionnaires, as well as a performance task and cardiovascular measures of physiological activity, were incorporated to more fully characterize the effects of the combination of d-amphetamine and estradiol. Briefly, estradiol enhanced the effects of certain doses of d-amphetamine on the drug-discrimination task, one VAS item (i.e., Like Drug) and on the composite score of the Stimulant subscale of the Adjective-Rating Scale. That only a subset of measures was sensitive to the combined effects of d-amphetamine and estradiol is concordant with previous research that has found limited effects of estradiol on the subject-rated effects of d-amphetamine (Justice and de Wit, 2000a,b).
Consistent with prior research, d-amphetamine alone functioned as a discriminative-stimulus, dose dependently increased drug-appropriate responding, produced positive subject-rated effects (e.g., increased ratings of Like Drug and scores on the MBG, BG and A scales of the ARCI), enhanced performance on the DSST and elevated cardiovascular measures (Chait et al., 1985; Heishman and Henningfield, 1991; Rush et al., 1998, 2003, 2004; Lile et al., 2005a,b;). The behavioral effects of d-amphetamine peaked at approximately 2 h, in agreement with previous human laboratory research (e.g., Chait et al., 1985; Kelly et al., 1993; Rush et al., 1998) and the time course for peak plasma levels following oral administration (Angrist et al., 1987).
Estradiol alone (i.e., 0.25 mg estradiol and 0 mg/70 kg d-amphetamine) did not occasion d-amphetamine-appropriate responding, produce subject-rated effects, impact psychomotor performance or alter heart rate or blood pressure. In a previous study in which 0.8 mg estradiol was administered transdermally in combination with d-amphetamine (0 and 10 mg, p.o.), no significant main effects of estradiol were observed, but post-hoc analysis revealed that estradiol alone did increase subject ratings of Pleasant Stimulation compared to placebo (Justice and deWit, 2000b). In that study, plasma estradiol levels were 760 pg/mL on average when measured 2 and 6 h after patch application. For reference, estradiol peaks at around 200 pg/mL during the late follicular phase of the menstrual cycle (Thorneycroft et al., 1971). In the present study, a dose of 0.25 mg estradiol was administered sublingually. Although blood levels were not measured here, a previous study indicated that sublingual administration of this estradiol dose resulted in serum estradiol levels that reached 300 pg/mL 1 hour after administration, and declined to 120 and 60 pg/mL 2 and 3 h, respectively, following administration (Price et al., 1997). These data suggest that higher estradiol doses might be necessary for the effects of estradiol alone to become apparent.
When estradiol and d-amphetamine were administered in combination, they were well tolerated by all subjects and no adverse events were reported. Concurrent administration of estradiol significantly enhanced the discriminative-stimulus effects of the lowest d-amphetamine dose (i.e., 3.125 mg/70 kg). In addition, a significant interaction of estradiol and time was observed at the 15 mg/70 kg dose of d-amphetamine, reflecting a reduced latency of onset of the discriminative cue at this dose. However, visual comparison of the time course data from the control phase and the test phase for this dose suggested that the interaction could be interpreted as a decrease in the discriminative-stimulus effects of d-amphetamine at the 90-min time point only, rather than an enhancement of this dose of d-amphetamine by estradiol, per se, and should therefore be interpreted with caution. It is not clear why estradiol effects were not apparent at the moderate dose (7.5 mg/70 kg). One possibility is that the ability of estradiol to enhance the discriminative-stimulus effects of d-amphetamine might be limited to lower doses. Consistent with this notion, preclinical studies that have compared sex and/or manipulated estrogen levels and studied a range of self-administered drug doses have found greater differences in the reinforcing effects at lower doses of psychostimulants (Hu et al., 2004; Jackson et al., 2006). Although no change in the group mean of the magnitude of the discriminative-stimulus effects of the higher d-amphetamine doses was observed following estradiol pretreatment, inspection of individual subject data indicated that the effects the 7.5 mg/70 kg dose were enhanced in four of the ten subjects, consistent with a decreased ability of estradiol to modulate the behavioral effects of higher d-amphetamine doses.
A limited number of the subject-rated drug effects of d-amphetamine were also enhanced by estradiol. Both the magnitude and the rate of onset for ratings of Like Drug Effect were increased at the 15 mg/70 kg dose of d-amphetamine. In addition, estradiol increased the magnitude and rate of onset for composite score on the Stimulant subscale of the Adjective-Rating Scale at all d-amphetamine doses. However, these were the only two subject-rated measures for which estradiol enhanced d-amphetamine effects. That many of the subject-rated effects of d-amphetamine were unaffected by estradiol is consistent with some of the previous human laboratory research that has compared the effects of d-amphetamine during the early versus the late follicular phase of the menstrual cycle (Justice and de Wit, 2000a) and in combination with exogenously administered estradiol (Justice and de Wit, 2000b).
The present study appears to be the first to have used drug-discrimination procedures to assess the influence of estradiol on the discriminative-stimulus effects of a psychostimulant. Because drug-discrimination procedures require extended periods of training and testing, and the purpose of the study was to examine the interaction of estradiol and d-amphetamine, a unique experimental design was developed. In order to evaluate the effects of estradiol under conditions in which basal levels of sex hormones were low, Test Phase experimental sessions were conducted during the placebo days of oral contraceptive cycles, which corresponded to the onset of menstruation and the early follicular phase. Typically only four experimental sessions could be conducted per menstrual cycle (i.e., per month). It would have been difficult to bring a subject’s behavior under the stimulus control of the training drug if the Control Phase was limited to only four days per month. Therefore, control phase sessions were conducted without regard menstrual cycle phase. One concern associated with this design was that as hormone levels fluctuated across Control Phase sessions, the discriminative-stimulus effects of the training dose of d-amphetamine might have varied as well, making it difficult to acquire the discrimination, or leading to greater variability in the discriminative cue learned by the subjects. As described above, some of the subject-rated effects of d-amphetamine are menstrual cycle phase dependent (Justice and de Wit, 1999; White et al., 2002), which might translate to the discriminative-stimulus effects as well (Preston and Bigelow, 1991; reviewed in Kelly et al., 2003). Despite this concern, this approach appeared successful. Subjects learned to discriminate d-amphetamine from placebo and concurrent estradiol administration enhanced its discriminative-stimulus effects at the lowest dose.
Two limitations of the present study warrant mention. First, only a single estradiol dose was tested. As noted above, the dose of estradiol (0.25 mg) tested should have produced serum levels around 120–60 pg/mL (Price et al., 1997) during peak d-amphetamine effects (i.e., 2–3 h following administration), whereas maximum estradiol levels during the late follicular phase of the menstrual cycle are typically 200 pg/mL. Therefore, higher estradiol doses might have been required to more closely approximate the d-amphetamine and estradiol interactions that could occur during the late follicular phase, and for a more pronounced enhancement of d-amphetamine’s effects to have become apparent. Another limitation is that serum levels of d-amphetamine, estradiol and progesterone were not determined. Measurement of serum d-amphetamine levels would have provided valuable information about whether estradiol enhancement of the effects of d-amphetamine could be explained by a change in the pharmacokinetic profile of d-amphetamine. Presently, there do not appear to be any studies that have addressed that possibility. In addition, monitoring estradiol and progesterone levels would have allowed us to correlate plasma hormone levels with the effects of d-amphetamine, as has been done previously (e.g., White et al., 2002). Blood samples were not obtained for several reasons, including cost and time associated with sampling and analysis, as well as the concern for subject recruitment and retention. That is, subjects might have been reluctant to participate in a relatively long study that required daily catheter insertion. Importantly, this experimental design (i.e., the use of exogenously administered estradiol in combination with various doses of d-amphetamine in the same subjects) precluded the need to measure estradiol levels to obtain useful data regarding interactions with d-amphetamine.
As noted above there is evidence that progesterone opposes the influence of estradiol on, or directly reduces, psychostimulant effects (e.g., Sofuoglu et al., 1999; White et al., 2002; Sofuoglu et al., 2004; Evans and Foltin, 2006). One limitation to comparing the effects of psychostimulants across the menstrual cycle is that the levels of estradiol increase across the follicular phase, whereas levels of estrogen and progesterone remain at relatively consistent levels for a longer period of time during the luteal phase. Therefore, it would have been useful to test the effects of d-amphetamine in these subjects during the luteal phase of their menstrual cycle, which would have allowed for a direct comparison of the influence of both hormones relative to estradiol alone during relatively consistent hormone levels. Another interesting experimental manipulation would have been to include male subjects. Although animal studies have identified conditions under which females are more sensitive to the behavioral effects of psychostimulants, human laboratory studies have been less consistent, and most studies fail to find an increased sensitivity in women. For example, studies that have controlled for menstrual cycle phase have either not revealed any sex or menstrual cycle differences (Mendelson et al., 1999), have found that women and men report similar subject ratings following psychostimulant administration during the follicular phase, but women report decreased subject-rated effects during the luteal phase (Sofuoglu et al., 1999; White et al., 2002; Evans and Foltin, 2006), or have found that men are more sensitive to psychostimulant effects (Lukas et al., 1996). Clearly, additional research is needed to reconcile discordant animal and human laboratory findings.
Experimental data from animal studies indicate that there are interactions between gonadal sex hormones and psychoactive drugs that result in hormone cycle differences in the behavioral effects of drugs. Much of this research has focused on the interaction between estradiol and psychostimulants, and some findings suggest that there is an enhancement of psychostimulant effects by estradiol that occurs via CNS dopamine systems. The present results provide limited support for this notion and extend the results of previous studies to the discriminative-stimulus effects of d-amphetamine in humans. Together, this body of research indicates that women might be differentially susceptible to the abuse-related effects of psychostimulants depending on menstrual cycle phase.
Acknowledgments
The project described was made possible by grant P20 RR 15592 from the National Center for Research Resources (NCRR, THK). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. This research project was also supported by K01 DA 018772 from the National Institute on Drug Abuse (NIDA, JAL) and a grant from the University of Kentucky Research Foundation (SLK). The authors wish to thank Cleeve Emurian, Glenn Robbins, Beth Eaves, Susan Holliday, Kathryn Bylica and Oriaku Njoku for help in executing the study and preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angrist B, Corwin J, Bartlik B, Cooper T. Early pharmacokinetics and clinical effects of oral d-amphetamine in normal subjects. Biol Psychiatry. 1987;22:1357–68. doi: 10.1016/0006-3223(87)90070-9. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–9. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effects of 17β-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–64. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981;204:361–72. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29:929–42. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–9. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Chait LD, Uhlenhuth EH, Johanson CE. The discriminative stimulus and subjective effects of d-amphetamine in humans. Psychopharmacology. 1985;86:307–12. doi: 10.1007/BF00432219. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–74. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Henningfield JE. Discriminative stimulus effects of d-amphetamine, methylphenidate, and diazepam in humans. Psychopharmacology. 1991;103:436–42. doi: 10.1007/BF02244241. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, Locke KW. Neural mechanisms of drug stimuli: experimental approaches. Psychopharmacol Ser. 1988;4:138–53. [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–38. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav. 2000a;66:509–15. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000b;71:51–9. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore M, Lane SD, Harrington NG, et al. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology. 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: dose and time profiles of marijuana, amphetamine, alcohol and diazepam. J Anal Toxicol. 1993;17:264–72. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Stoops WW, Perry AS, Prendergast MA, Rush CR. Clinical neuropharmacology of drugs of abuse: a comparison of drug discrimination and self-report measures. Behav Cogn Neurosci Rev. 2003;2:227–60. doi: 10.1177/1534582303262095. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Vansickel AR, Glaser PE, Hays LR, Rush CR. Aripiprazole attenuates the discriminative-stimulus and subject-rated effects of d-amphetamine in humans. Neuropsychopharmacology. 2005a;30:2103–14. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Wagner FP, Glaser PE, Rush CR. Oxazepam does not modulate the behavioral effects of d-amphetamine in humans. Neuropsychopharmacology. 2005b;30:2103–14. doi: 10.1016/j.pbb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, et al. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology. 1996;125:346–54. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–58. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling JE. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instr. 1982;14:463–6. [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–94. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Subjective and discriminative effects of drugs. Behav Pharmacol. 1991;2:293–313. [PubMed] [Google Scholar]
- Price TM, Blauer KL, Hansen M, Stanczyk F, Lobo R, Bates GW. Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17β-estradiol. Obstet Gynecol. 1997;89:340–5. doi: 10.1016/S0029-7844(96)00513-3. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, Pazzaglia PJ. Discriminative-stimulus and participant-rated effects of methylphenidate, bupropion, and triazolam in d-amphetamine-trained humans. Exp Clin Psychopharmacol. 1998;6:32–44. doi: 10.1037//1064-1297.6.1.32. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PEA. Alprazolam attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Clin Psychopharmacol. 2004;24:410–20. doi: 10.1097/01.jcp.0000130553.55630.ad. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PEA, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacology. 1999;7:274–83. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2004 National Survey on Drug Use and Health: National findings. Rockville, MD: Office of Applied Studies Publications and Data Dissemination; 2005. [Google Scholar]
- Thorneycroft IH, Mishell DR, Jr, Stone SC, Kharma KM, Nakamura RM. The relation of serum 17- hydroxyprogesterone and estradiol-17β levels during the human menstrual cycle. Am J Obstet Gynecol. 1971;111:947–51. doi: 10.1016/0002-9378(71)90951-3. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Neumark YD, Anthony JC. Male-female differences in the earliest stages of drug involvement. Addiction. 1999;94:1413–9. doi: 10.1046/j.1360-0443.1999.949141312.x. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–41. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Mol Brain Res. 2002;100:75–83. doi: 10.1016/s0169-328x(02)00134-1. [DOI] [PubMed] [Google Scholar]


