A new myotoxic Lys49-phospholipase from B. moojeni has been crystallized and X-ray diffraction data were collected to 2.18 Å resolution. Preliminary analysis indicates the presence of four molecules in the asymmetric unit, leading to a possible new oligomeric structure for Lys49-PLA2s.
Keywords: myotoxin-I, Lys49-phospholipase A2
Abstract
A new myotoxic Lys49-phospholipase A2 isolated from Bothrops moojeni snake venom has been crystallized. The crystals diffracted to 2.18 Å resolution using a synchrotron-radiation source and belong to space group C2. The unit-cell parameters are a = 56.8, b = 125.0, c = 64.7 Å, β = 105.5°. Preliminary analysis indicates the presence of four molecules in the asymmetric unit. This may suggest a new quaternary structure for this Lys49-phospholipase A2 in contrast to the dimeric and monomeric structures solved so far for this class of proteins.
1. Introduction
Bothrops moojeni is a snake found in southeastern Brazil that causes an important number of ophidian accidents (Ribeiro et al., 1998 ▶). The acute muscle damage induced by bothropic venoms is mainly caused by one or two basic myotoxic phospholipases A2 (PLA2s; Gutiérrez & Lomonte, 1995 ▶) and may lead to permanent tissue loss, disability and amputation (Rosenfeld, 1971 ▶; Nishioka & Silveira, 1982 ▶). PLA2s are also one of the enzymes involved in the production of eicosanoids. These molecules have physiological effects at very low concentrations, but an increase in their concentration can lead to inflammation (Needleman et al., 1986 ▶). Thus, the study of specific PLA2 inhibitors may be important for the production of structure-based anti-inflammatory agents. Consequently, the study of these proteins is an important issue from scientific, medical and social points of view.
Phospholipases A2 (EC 3.1.1.4) belong to a superfamily of proteins that hydrolyze the sn-2 acyl groups of membrane phospholipids to release arachidonic acid and lysophospholipids. The PLA2 superfamily is divided into 11 classes (Six & Dennis, 2000 ▶) of which five (I, II, III, V and X) are abundant in a variety of biological fluids, particularly pancreatic secretions, inflammatory exudates and reptile and arthropod venoms (Rosenberg, 1990 ▶). PLA2s of groups I and II are the major components of snake venoms, group II being predominant in bothropic venoms. In addition to their catalytic role, snake-venom PLA2s show a broad range of relevant biological effects, including myotoxic, cytotoxic, oedema-inducing, artificial membrane disrupting, anticoagulant, neuromuscular, platelet-aggregation inhibiting, hypotensive, bactericidal, anti-HIV, anti-tumoral, antimalarial and antiparasitic effects (Gutiérrez & Lomonte, 1997 ▶; Ownby, 1998 ▶; Valentin & Lambeau, 2000 ▶).
Myonecrosis (muscle necrosis) may arise indirectly as a consequence of the vessel degeneration and ischaemia caused by haemorrhagic metalloproteases or as a direct effect of myotoxic PLA2 homologues upon the plasma membranes of muscle cells (Rosenfeld, 1971 ▶). It is believed that myotoxins act on the sarcoplasma membrane, thus inducing disorganization of phospholipids, loss of intracellular components and influx of Ca2+ ions (Díaz et al., 1992 ▶). PLA2s with skeletal muscle-damaging (myotoxicity) activity are widely distributed in venomous snakes and can be subdivided into at least three subclasses: (i) the Asp49 enzymes with high catalytic activity, (ii) the Ser49 enzymes with lower catalytic activity and (iii) the Lys49 enzymes, which do not display measurable catalytic activity (Shimohigashi et al., 1995 ▶; Ownby et al., 1999 ▶). The most abundant protein in many bothropic venoms is a natural mutant in which Asp49 is changed to Lys (subclass iii). This Asp49-to-Lys mutation prevents calcium binding and the protein lacks catalytic activity. However, these Lys49-PLA2s are capable of destroying the integrity of membranes and provoking release from liposomes (Rufini et al., 1992 ▶). This process occurs in the absence of calcium ions without detectable lipid hydrolysis. One possibility is that these proteins are catalytically active towards an as yet unidentified phospholipid analogue.
Several crystal structures of Lys49-PLA2s from the genus Bothrops have already been solved, revealing very similar fold patterns (Arni et al., 1995 ▶, 1999 ▶; de Azevedo et al., 1997 ▶, 1999 ▶; da Silva-Giotto et al., 1998 ▶; Lee et al., 2001 ▶; Magro et al., 2003 ▶). However, new insights into the quaternary structure changes and the lack of phospholipase activity have recently been reported (Magro et al., 2003 ▶; Soares et al., 2004 ▶). The lack of catalytic activity of myotoxic Lys49-PLA2s, which was first related solely to the fact that Lys49 occupies the position of the calcium ion in the catalytically active site of Asp49-PLA2s, has also been attributed to Lys122, which interacts with the carbonyl of Cys29, hyperpolarizing the peptide bond between Cys29 and Gly30 (Lee et al., 2001 ▶; Soares et al., 2004 ▶). It has been observed that Lys122 is present in a conformation interacting with Cys29 for both monomers in those structures bound with a ligand (Watanabe et al., 2005 ▶). However, considering the high sequential and structural homology of this class of proteins, further structural studies seem to be essential in order to obtain a deeper understanding of their lack of phospholipase activity and their pharmacological effects.
The isolation, biochemical/pharmacological characterization and amino-acid sequence of myotoxin I from B. moojeni (MjTX-I) has been reported (Soares et al., 2000 ▶). Protein sequencing indicated that MjTX-I is a Lys49-PLA2 and consists of 121 amino acids (MW = 13 669 Da; SWISS-PROT database code No. P82114). The protein showed local myotoxic and oedema-inducing activities in mice and is lethal by intraperitoneal injection. In addition, it is cytotoxic to myoblasts/myotubes in culture and disrupts negatively charged liposomes. MjTX-I does not present measurable enzymatic and anticoagulant activities (Soares et al., 2000 ▶).
In the present paper, we describe the crystallization and X-ray diffraction data collection of MjTX-I, aiming to solve the structure and gain insights into functional aspects of this protein.
2. Experimental procedures
2.1. Purification
MjTX-I was isolated from B. moojeni snake venom by ion-exchange chromatography on CM-Sepharose (Soares et al., 2000 ▶). The homogeneity of the toxin was assayed by SDS–PAGE, cathodic PAGE, isoelectric focusing and immunoelectrophoresis (Soares et al., 2000 ▶).
2.2. Crystallization
A lyophilized sample of MjTX-I was dissolved in ultrapure water at a concentration of 12 mg ml−1. The sparse-matrix method (Jancarik & Kim, 1991 ▶) was used to perform initial screening of the crystallization conditions (Crystal Screens I and II, Hampton Research).
Small polycrystals of MjTX-I were obtained by the conventional hanging-drop vapour-diffusion method (McPherson, 1982 ▶), in which the protein solution was equilibrated against a reservoir containing 0.2 M magnesium chloride, 30%(w/v) polyethylene glycol 4000 and 0.1 M Tris–HCl pH 8.5 (Hampton Research Crystal Screen I, condition No. 6) after two months at 291 K. In order to improve the crystal quality, we made some modifications to the original reservoir solution. Better monocrystals were obtained with 0.15 M magnesium chloride, 32%(w/v) polyethylene glycol 4000 and 0.1 M Tris–HCl pH 8.5 and measured approximately 0.20 × 0.15 × 0.10 mm after approximately 12 months (Fig. 1 ▶).
Figure 1.
Crystals of MjTX-I from B. moojeni.
2.3. X-ray data collection and processing
X-ray diffraction data from a single MjTX-I crystal were collected at a wavelength of 1.421 Å (at 100 K) using a synchrotron-radiation source (Laboratório Nacional de Luz Sincrotron, LNLS, Campinas, Brazil) and a MAR CCD imaging-plate detector (MAR Research). The crystal was mounted in a nylon loop and flash-frozen in a steam of nitrogen at 100 K using no cryoprotectant. The crystal-to-detector distance was 80 mm and an oscillation range of 1° was used; 146 images were collected. The data were processed to 2.18 Å resolution using the HKL program package (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
The data-collection statistics are shown in Table 1 ▶. The data set is 97.0% complete at 2.18 Å resolution with R merge = 6.0%. The crystals belong to space group C2, with unit-cell parameters a = 56.8, b = 125.0, c = 64.7 Å, β = 105.5°.
Table 1. Data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| Unit-cell parameters (Å) | a = 56.8, b = 125.0, c = 64.7, β = 105.5 |
| Space group | C2 |
| Resolution (Å) | 40–2.18 (2.28–2.18) |
| Unique reflections | 22875 (2843) |
| Completeness (%) | 97.0 (96.8) |
| Rmerge† (%) | 6.0 (52.5) |
| Radiation source | Synchrotron (LNLS-CPr) |
| Data-collection temperature (K) | 100 |
| σ(I) cutoff for data processing‡ | −3 |
| I/σ(I) | 19.2 (2.0) |
| Redundancy | 3.8 (3.7) |
R
merge = 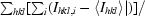
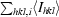 , where I
hkl,i is the intensity of an individual measurement of the reflection with Miller indices h, k and l and 〈I
hkl〉 is the mean intensity of that reflection. Calculated for I > −3σ(I).
, where I
hkl,i is the intensity of an individual measurement of the reflection with Miller indices h, k and l and 〈I
hkl〉 is the mean intensity of that reflection. Calculated for I > −3σ(I).
Data processing used the HKL suite (Otwinowski & Minor, 1997 ▶).
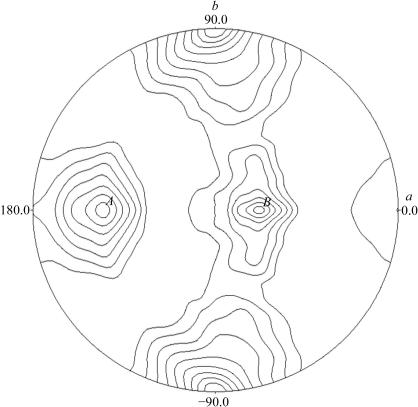
Packing-parameter calculations based on the protein molecular weight indicate the presence of three or four molecules in the asymmetric unit. This corresponds to a Matthews coefficient (Matthews, 1968 ▶) of 2.0 Å3 Da−1 with a solvent content of 54.5% or 2.7 Å3 Da−1 with a solvent content of 39.3% for three and four molecules in the asymmetric unit, respectively. To evaluate the local symmetry of MjTX-I, the self-rotation function was calculated between 15 and 3.5 Å resolution applying an integration radius of 20 Å with the program POLARRFN (Collaborative Computational Project, Number 4, 1994 ▶). The κ = 180° self-rotation function projection in the ab plane (Fig. 2 ▶) reveals a peak at ϕ = 90 and −90° on the circumference arising from the crystallographic twofold axis. The presence of two peaks with similar height (A and B) suggests the presence of two twofold non-crystallographic symmetry (NCS) axes, which is compatible with 222 symmetry and a tetramer in the asymmetric unit. This leads to a new quaternary structure for MjTX-I in contrast to the dimeric (Arni et al., 1995 ▶; de Azevedo et al., 1997 ▶, 1999 ▶; da Silva-Giotto et al., 1998 ▶; Lee et al., 2001 ▶; Magro et al., 2003 ▶) and monomeric (Arni et al., 1999 ▶) Lys49-phospholipase A2 structures solved to date.
Figure 2.
Self-rotation function projection of the κ = 180° section for MjTX-I calculated using POLARRFN (Collaborative Computational Project, Number 4, 1994 ▶). Spherical polar angles are defined as follows: ϕ, the angle from the Cartesian x axis (a) on the xy plane (ab); ω, the angle from z axis (c); κ, the rotation around the axis defined by ϕω.
Efforts are being made towards molecular-replacement solution using the AMoRe program (Navaza, 1994 ▶) with the coordinates of several Lys49-PLA2s.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação para o Desenvolvimento da UNESP (FUNDUNESP) and Laboratório Nacional de Luz Síncrotron (LNLS). We thank Dr G. Oliva (IFSC-USP) for useful discussions.
References
- Arni, R. K., Fontes, M. R. M., Barberato, C., Gutiérrez, J. M., Díaz-Oreiro, C. & Ward, R. J. (1999). Arch. Biochem. Biophys.366, 177–182. [DOI] [PubMed] [Google Scholar]
- Arni, R. K., Ward, R. J. & Gutiérrez, J. M. (1995). Acta Cryst. D51, 311–317. [DOI] [PubMed] [Google Scholar]
- Azevedo, W. F. de Jr, Ward, R. J., Gutiérrez, J. M. & Arni, R. K. (1999). Toxicon, 37, 371–384. [DOI] [PubMed] [Google Scholar]
- Azevedo, W. F. de Jr, Ward, R. J., Lombardi, F. R., Giglio, J. R., Soares, A. M., Fontes, M. R. M. & Arni, R. K. (1997). Protein Pept. Lett.4, 329–334.
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Díaz, C., Gutiérrez, J. M. & Lomonte, B. (1992). Arch. Biochem. Biophys.298, 135–142. [DOI] [PubMed] [Google Scholar]
- Gutiérrez, J. M. & Lomonte, B. (1995). Toxicon, 33, 1405–1424. [DOI] [PubMed] [Google Scholar]
- Gutiérrez, J. M. & Lomonte, B. (1997). Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism, edited by R. M. Kini, pp. 321–352. Chichester: John Wiley & Sons.
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411. [Google Scholar]
- Lee, W. H., da Silva-Giotto, M. T., Marangoni, S., Toyama, M. H., Polikarpov, I. & Garratt, R. C. (2001). Biochemistry, 40, 28–36. [DOI] [PubMed] [Google Scholar]
- McPherson, A. (1982). Preparation and Analysis of Protein Crystals. New York: Wiley.
- Magro, A. J., Soares, A. M., Giglio, J. R. & Fontes, M. R. M. (2003). Biochem. Biophys. Res. Commun.311, 713–720. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Navaza, J. (1994). Acta Cryst. A50, 157–163. [Google Scholar]
- Needleman, P., Turk, J., Jakschik, B. A., Morrison, A. R. & Lefkowith, J. B. (1986). Annu. Rev. Biochem.55, 69–102. [DOI] [PubMed] [Google Scholar]
- Nishioka, S. A. & Silveira, P. V. P. (1982). Am. J. Trop. Med. Hyg.47, 805–810. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Ownby, C. L. (1998). J. Toxicol. Toxin Rev.17, 1003–1009.
- Ownby, C. L. H., Selistre de Araujo, S., White, S. P. & Fletcher, J. E. (1999). Toxicon, 37, 411–445. [DOI] [PubMed] [Google Scholar]
- Ribeiro, L. A., Albuquerque, M. J. & Pires de Campos, V. A. F. (1998). Rev. Assoc. Med. Bras.44, 312–318. [DOI] [PubMed] [Google Scholar]
- Rosenberg, P. (1990). Handbook of Toxinology, edited by W. Shier & D. Mebs, pp. 67–277. New York: Marcel Dekker.
- Rosenfeld, G. (1971). Venomous Animals and their Venoms II, edited by W. Bucherl, E. Buckley & V. Deulofeu, pp. 345–384. New York: Academic Press.
- Rufini, S., Cesaroni, P., Desideri, R. F., Gubensek, F., Gutiérrez, J. M., Luly, P., Maassoud, R., Morero, R. & Pedersen, J. Z. (1992). Biochemistry, 31, 12424–12430. [DOI] [PubMed] [Google Scholar]
- Shimohigashi, Y., Tani, A., Matsumoto, H., Nakashima, K. & Yamaguchi, Y. (1995). J. Biochem.118, 1037–1044. [DOI] [PubMed] [Google Scholar]
- Silva-Giotto, M. T. da, Garratt, R. C., Oliva, G., Mascarenhas, Y. P., Giglio, J. R., Cintra, A. C. O., de Azevedo, W. F. Jr, Arni, R. K. & Ward, R. J. (1998). Proteins, 30, 442–454. [DOI] [PubMed] [Google Scholar]
- Six, D. A. & Dennis, E. A. (2000). Biochim. Biophys. Acta, 1488, 1–19. [DOI] [PubMed] [Google Scholar]
- Soares, A. M., Andrião-Escarso, S. H., Angulo, Y., Lomonte, B., Gutiérrez, J. M., Marangoni, S., Toyama, M. H., Arni, R. K. & Giglio, J. R. (2000). Arch. Biochem. Biophys.373, 7–15. [DOI] [PubMed] [Google Scholar]
- Soares, A. M., Fontes, M. R. M. & Giglio, J. R. (2004). Curr. Org. Chem.8, 1677–1690.
- Valentin, E. & Lambeau, G. (2000). Biochimie, 82, 815–831. [DOI] [PubMed] [Google Scholar]
- Watanabe, L., Soares, A. M., Ward, R J., Fontes, M. R. M. & Arni, R. K. (2005). Biochimie, 87, 161–167. [DOI] [PubMed] [Google Scholar]