The Trypanosoma cruzi dihydroorotate dehydrogenase, a key enzyme in pyrimidine de novo biosynthesis and redox homeostasis, was crystallized in complex with its first reaction product, orotate.
Keywords: dihydroorotate dehydrogenase, pyrimidine biosynthesis, Trypanosoma cruzi, fumarate reductase, redox homeostasis, structure-based drug design
Abstract
Dihydroorotate dehydrogenase (DHOD) catalyzes the oxidation of dihydroorotate to orotate, the fourth step and the only redox reaction in the de novo biosynthesis of pyrimidine. DHOD from Trypanosoma cruzi (TcDHOD) has been expressed as a recombinant protein in Escherichia coli and purified to homogeneity. Crystals of the TcDHOD–orotate complex were grown at 277 K by the sitting-drop vapour-diffusion technique using polyethylene glycol 3350 as a precipitant. The crystals diffract to better than 1.8 Å resolution using synchrotron radiation (λ = 0.900 Å). X-ray diffraction data were collected at 100 K and processed to 1.9 Å resolution with 98.2% completeness and an overall R merge of 7.8%. The TcDHOD crystals belong to the orthorhombic space group P212121, with unit-cell parameters a = 67.87, b = 71.89, c = 123.27 Å. The presence of two molecules in the asymmetric unit (2 × 34 kDa) gives a crystal volume per protein weight (V M) of 2.2 Å3 Da−1 and a solvent content of 44%.
1. Introduction
Chagas disease or American trypanosomiasis is caused by the flagellate protozoan parasite Trypanosoma cruzi and affects approximately 16–18 million people in Central and South America (World Health Organization, 2001 ▶). The progression of this disease can lead to symptoms such as inflammatory cardiomyopathy, digestive injuries and neural disorders. Infection with this protozoan parasite has proven to be extremely difficult to cure, particularly during the chronic phase when most people are diagnosed (Garasia, 2001 ▶). A large percentage of Chagas patients receive no specific antiparasitic therapy because of the ineffectiveness and toxicity of existing pharmacologic agents (Estani et al., 1998 ▶; Urbina, 2001 ▶). Hence, better therapeutic agents are urgently needed.
Dihydroorotate dehydrogenases (DHODs) are flavoenzymes catalyzing the oxidation of l-dihydroorotate to orotate, the fourth step and the only redox reaction in the de novo pyrimidine-biosynthesis pathway. On the basis of amino-acid sequence homology DHODs have been classified into two families, referred to as family 1 and family 2. DHODs of family 1 are cytoplasmic enzymes and are further subdivided into families 1A and 1B. The family 1A enzymes are homodimers and appear to utilize fumarate as their physiological oxidant, whereas the family 1B enzymes (heterotetramers) utilize nicotinamide adenine dinucleotide through the intermediary of a second protein subunit containing an Fe2S2 cluster and a flavin adenine dinucleotide molecule. Family 2 DHODs, which exist as homodimers or monomers, are membrane-bound enzymes that utilize respiratory quinones as their physiological oxidants (Jensen & Bjornberg, 1998 ▶). TcDHOD, belonging to family 1A, exists as a homodimer (MW 2 × 34 kDa) and in addition to DHOD activity also possesses fumarate reductase activity. This suggests that TcDHOD is involved not only in the de novo biosynthesis of pyrimidines but also in the redox homeostasis of the parasite (Gao et al., 1999 ▶; Nara et al., 2000 ▶; Takashima et al., 2002 ▶). In contrast, human DHOD belongs to family 2. This great diversity between parasite and host DHODs makes this enzyme a potential target for new chemotherapeutic drugs.
The crystal structures of human DHOD (Liu et al., 2000 ▶), Lactococcus lactis DHOD A (Rowland et al., 1998 ▶) and Escherichia coli DHOD (Norager et al., 2002 ▶) have been reported. TcDHOD shows a high level of homology and 55% sequence identity with L. lactis DHOD A (Gao et al., 1999 ▶). Determination of the structure of TcDHOD should help in the development of new TcDHOD-specific drugs. This should also help to clarify the catalytic mechanism of dihydroorotate oxidation by family 1A DHODs. Here, we report the overexpression, purification, crystallization and preliminary X-ray diffraction studies of TcDHOD complexed with orotate.
2. Materials and methods
2.1. Overexpression of TcDHOD
The gene coding for TcDHOD was cloned by the polymerase chain reaction (PCR). The template for the PCR was the pyr4 gene (DHOD2; access No. AB122956) from T. cruzi present on plasmid pET28a from BL21(DE3)/pET28aTcDHOD (Takashima et al., 2002 ▶; Annoura et al., 2005 ▶), which expresses His6-tagged TcDHOD. The primers for the PCR were 5′-CAT ATG ATG TGT CTG AAG CTC-3′ and 5′-CGG GAT CCT CAC TCA ATT GTC TTG AC-3′, which were designed to generate BamHI and NdeI sites at the start and the end of the DNA fragment, respectively. The 950 bp PCR product was ligated into the pZErO-2 cloning vector. After transformation of the E. coli strain TOP 10 with the ligation mixture, kanamycin-resistant colonies were selected on agar plates. Plasmids were isolated from ten independent colonies and then sequenced. The correct DNA sequence of the cloned PCR fragment in pZErO-2 was further confirmed by DSQ-2000L (Shimadzu) using the Thermo Sequenase fluorescently labelled primer cycle sequencing kit and 7-deaza-dGTP (Amersham Bioscience). The plasmid pZErO-2-TcDHOD was digested with BamHI and NdeI and the approximately 950 bp cDNA was inserted into the pET3a expression vector (Novagen). The resulting construct was used for transformation of E. coli BL21 (DE3). Single colonies grown on Luria–Bertani (LB) agar containing 100 µg ml−1 ampicillin were selected and grown in LB medium containing the same concentration of antibiotic.
The expression of TcDHOD was induced by the addition of 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) when the cell culture had attained late log phase (optical density at 550 nm of 0.3). Expression conditions were optimized by examining the effect of the IPTG concentration, temperature and time of induction on the expression levels in total cell lysates as determined by SDS–PAGE and the DHOD activity in soluble protein extracts. The best expression was achieved by induction with 1 mM IPTG for 16 h at 298 K.
2.2. Purification of recombinant TcDHOD
All purification steps were conducted at 277 K. The cells were harvested from 10 l of culture by centrifugation for 10 min at 1700g. After three washes in 50 mM Tris–HCl pH 8.0 containing 3 mM ethylenediaminetetraacetic acid (EDTA) and 0.1 mM phenylmethylsulfonyl fluoride, the yellow pellet was resuspended in lysis buffer (50 mM Tris–HCl pH 8.0, 20 mM EDTA, 0.25 mM sodium orotate). After addition of hen egg-white lysozyme to a final concentration of 2 mg ml−1, the mixture was stirred for 10 min. The lysate was then homogenized in a Waring blender to reduce its viscosity and the cells were broken with a French press at 130 MPa. The inclusion-body fraction was removed by centrifugation at 26 000g for 15 min. Streptomycin sulfate was added to the yellow supernatant to a final concentration of 1%(w/v). The solution was stirred for 30 min and the precipitate, primarily consisting of nucleic acids, was removed by centrifugation for 30 min at 26 000g. The soluble protein extract was obtained as a supernatant following centrifugation at 200 000g for 1.5 h.
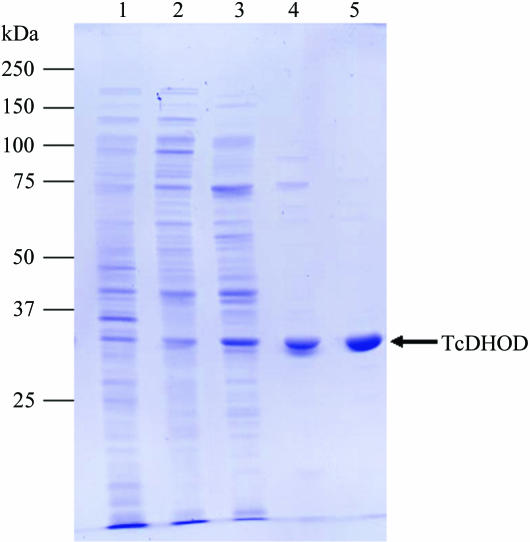
The soluble protein extract was filtered through a 0.22 µm pore-size filter and applied onto a DEAE Sepharose Fast Flow (Amersham Bioscience) column (5.0 × 30 cm) pre-equilibrated in 10 mM Tris–HCl pH 8.0 containing 0.25 mM sodium orotate. The column was washed with 2.5 l of the same buffer and then eluted with a 5 l gradient of 0.0–0.5 M NaCl at a flow rate of 10 ml min−1. The yellow fractions containing TcDHOD, which eluted at 0.25–0.3 M NaCl, were pooled. Ammonium sulfate, sodium orotate and sodium phosphate buffer pH 7.5 were added to final concentrations of 1.2 M, 0.25 mM and 50 mM, respectively. After stirring for 30 min, the supernatant following centrifugation for 30 min at 26 000g was loaded onto a Phenyl Sepharose High Performance (Amersham Bioscience) column (1.6 × 60 cm) pre-equilibrated with 50 mM sodium phosphate buffer pH 7.5 containing 1.2 M ammonium sulfate and 0.25 mM sodium orotate. After washing with 600 ml of the same buffer, TcDHOD was eluted with a 1.5 l gradient of 1.2–0.0 M ammonium sulfate at a flow rate of 5 ml min−1. The active peak fractions were pooled and the buffer was changed to 100 mM sodium phosphate buffer pH 7.5 containing 0.25 mM sodium orotate by repeated dilution and concentration with a Vivaspin centrifugal concentrator tube (Vivaspin 20, 10K MWCO). The concentrated enzyme solution (25–30 mg ml−1) was applied onto a TSK G3000SW (Tosoh) gel-filtration column (7.5 × 600 mm) and the column was run at a flow rate of 1 ml min−1. The active fractions were pooled and concentrated with a Vivaspin 20 (10K MWCO) to 40 mg ml−1. Glycerol was added to a final concentration of 50%(v/v) and the purified protein was stored in small aliquots at 243 K until use. The DHOD activity was assayed as described previously (Takashima et al., 2002 ▶). TcDHOD was purified to apparent homogeneity as shown by SDS–PAGE (Fig. 1 ▶) and was purified 40-fold compared with the cytoplasmic fraction, with a recovery of 10–15 mg from 10 l of culture. Sodium orotate was added to all of the purification steps to stabilize the enzyme activity.
Figure 1.
A Coomassie Brilliant Blue-stained 12.5% SDS–PAGE gel showing the purification of TcDHOD. Lane 1, homogenate fraction (5 µg); lane 2, cytoplasmic fraction (5 µg); lanes 3, 4, and 5, pooled fractions after DEAE Sepharose Fast Flow (5 µg), Phenyl Sepharose High Performance (1 µg) and TSK gel G3000SW (1 µg) columns, respectively.
2.3. Crystallization and X-ray data collection
Crystallization was performed by the sitting-drop vapour-diffusion technique using 96-well CrystalClear Strips (Hampton Research). The experiments were carried out by mixing 1 µl protein solution (10 mg ml−1 in 10 mM Tris–HCl pH 8.0 containing 1 mM orotate) with an equal volume of reservoir solution and allowing the drop to equilibrate with 150 µl reservoir solution. Initial crystallization conditions for TcDHOD were screened at 277 and 293 K using Crystal Screen (Jancarik & Kim, 1991 ▶) and Crystal Screen II (Hampton Research) and Wizard Screens I and II (Emerald BioStructures). Out of 194 conditions, only No. 27 from Wizard Screen II [10%(w/v) PEG 3000, 0.1 M cacodylate pH 6.5 and 0.2 M MgCl2] gave crystals at 277 K. However, these were aggregates of tiny needle-shaped crystals that were not suitable for X-ray diffraction experiments. The crystallization conditions were then optimized using PEGs with different molecular weights and by varying the PEG concentration, the pH and the temperature, but none of the conditions resulted in a marked improvement.
We next tried PEG/Ion Screen and Additive Screen kits (Hampton Research) to examine the effect of adding various salts and additives. Plate-shaped orange–brown single crystals appeared when sodium thiocyanate and hexaamminecobalt (III) chloride were used as additives. The best crystals grew at 277 K from 16%(w/v) PEG 3350 as the main precipitating agent in 100 mM cacodylate pH 6.2, 1 mM sodium orotate, 50 mM hexaamminecobalt (III) chloride and 1 mM sodium thiocyanate. The addition of 50 mM hexaamminecobalt (III) chloride and 1 mM sodium thiocyanate caused no detectable change in the enzymatic activity of TcDHOD, indicating that the effects of these reagents are only on crystallization.
Diffraction data were collected under liquid-nitrogen-cooled conditions at 100 K. A crystal mounted in a nylon loop was transferred and soaked briefly in reservoir solution supplemented with 20%(v/v) glycerol and then frozen by rapidly submerging it in liquid nitrogen. X-ray diffraction data were collected by the rotation method at the BL44XU beamline of SPring-8 using a DIP6040 detector at a wavelength of 0.900 Å. A total of 180 frames were recorded with an oscillation angle of 1°, an exposure time of 5 s per frame and a crystal-to-detector distance of 250 mm. The intensities were integrated with MOSFLM (Leslie, 1992 ▶) and scaled with SCALA (Evans, 1993 ▶) from the CCP4 suite (Collaborative Computational Project, Number 4, 1994 ▶).
3. Results and discussion
The first crystallization trials of TcDHOD were carried out using His6-tagged TcDHOD, but we were unable to obtain crystals. As found when expressing the recombinant SH3 domain of chicken tyrosine kinase in E. coli (Kim et al., 2001 ▶), this problem can occur owing to spontaneous α-N-6-phosphogluconoylation at the His6 tag site even if SDS–PAGE and dynamic light scattering show the protein to be homogeneous (Geoghegan et al., 1999 ▶). For this reason, we removed the His6 tag from His6-TcDHOD using thrombin, but this resulted in complete loss of enzymatic activity. As a final attempt, we used an alternative expression system to produce native TcDHOD without the His6 tag. Although the expressed enzyme gradually lost its enzymatic activity during purification, the addition of 0.25 mM sodium orotate stabilized the enzyme for more than several weeks. The enzyme could be purified to homogeneity (>95%) using sequential steps of ion-exchange, hydrophobic interaction and gel-filtration chromatography.
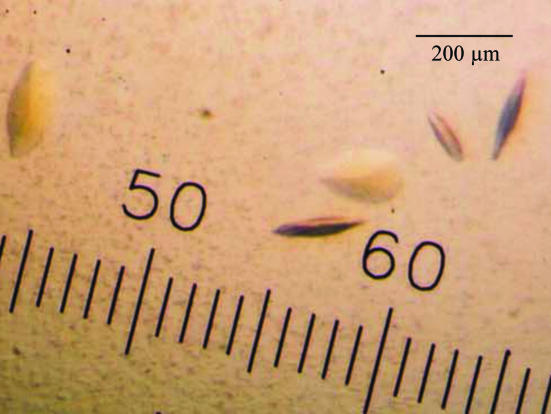
The best crystals were obtained at 277 K in the presence of 16%(w/v) PEG 3350, 100 mM cacodylate pH 6.2, 1 mM sodium orotate, 50 mM hexaamminecobalt (III) chloride and 1 mM sodium thiocyanate. The crystals usually appeared within 1 d and reached maximum dimensions of 0.15 × 0.10 × 0.03 mm after a week (Fig. 2 ▶). The crystals are stable and can be kept for several months. Although significant reflections were observed beyond 1.8 Å resolution at the beginning of data collection, radiation damage gradually diminished the reflections at higher resolution. A good-quality data set to 1.9 Å resolution was obtained after scaling and merging the 180 images. The data-collection and processing statistics are summarized in Table 1 ▶.
Figure 2.
Crystals of T. cruzi dihydroorotate dehydrogenase obtained by sitting-drop vapour diffusion in the presence of 16%(w/v) PEG 3350 with 50 mM hexaamminecobalt (III) chloride as an additive.
Table 1. Statistics of data collection and processing.
Values for the highest resolution shell are given in parentheses.
| Wavelength (Å) | 0.900 |
| Space group | P212121 |
| Unit-cell parameters (Å) | a = 67.87, b = 71.89, c = 123.27 |
| Resolution range (Å) | 28.3–1.9 (2.0–1.9) |
| No. of reflections | 196908 |
| Unique reflections | 47235 |
| Completeness (%) | 98.2 (99.4) |
| Rmerge (%) | 7.8 (20.1) |
| I/σ(I) | 6.5 (3.2) |
Analysis of the symmetry and systematic absences in the recorded diffraction pattern indicates that the crystals belong to the orthorhombic space group P212121, with unit-cell parameters a = 67.87, b = 71.89, c = 123.27 Å. Assuming the presence of two TcDHOD molecules in the asymmetric unit, the calculated Matthews coefficient (Matthews, 1968 ▶) is 2.2 Å3 Da−1, which corresponds to a solvent content of 44%. An attempt to solve the structure using molecular replacement with the MOLREP program (Navaza, 1994 ▶) as implemented within the CCP4 package (Collaborative Computational Project, Number 4, 1994 ▶) was carried out using the refined coordinates of DHOD A from L. lactis (PDB code 1ovd), with which TcDHOD shows 55% sequence identity. X-ray diffraction data in the resolution range 8.0–4.0 Å were used. The best solution had a correlation coefficient (CC) of 0.654 and an R factor of 51.4%, while for the second-best solution the CC was 0.412 and the R factor was 58.0%. The model subsequently subjected to rigid-body refinement gave an R factor of 45.5% and further refinement of the dimer structure of TcDHOD complexed with orotate is in progress. The current R factor is 21.5% (R free = 25.5%) and the electron-density map clearly shows the orotate molecule as stacking with the FMN. In parallel with the refinement, we are also trying to obtain crystals of TcDHOD complexed with a variety of other ligands, such as substrate analogues and effector-site inhibitors.
Since TcDHOD is essential for the survival and growth of T. cruzi (Annoura et al., 2005 ▶), this enzyme is a promising target for chemotherapy. It is hoped that the details of the interactions between TcDHOD and ligands will help elucidate the catalytic mechanism of TcDHOD and advance structure-based drug design aimed at Chagas disease.
References
- Annoura, T., Nara, T., Makiuchi, T., Hashimoto, T. & Aoki, T. (2005). J. Mol. Evol.60, 113–127. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Estani, S. S., Segura, E. L., Ruiz, A. M., Valazquez, E., Porcel, B. M. & Yampotis, C. (1998). Am. J. Trop. Med. Hyg.55, 586–588. [DOI] [PubMed]
- Evans, P. R. (1993). Proceedings of the CCP4 Study Weekend. Data Collection and Processing, edited by L. Sawyer, N. Isaacs & S. Bailey, pp. 114–122. Warrington: Daresbury Laboratory.
- Gao, G., Nara, T., Nakajima-Shimada, J. & Aoki, T. (1999). J. Mol. Biol.285, 149–161. [DOI] [PubMed] [Google Scholar]
- Garasia, L. S. (2001). Diagnostic Medical Parasitology, 4th ed. Washington, DC: ASM Press.
- Geoghegan, K. F., Dixon, H. B. F., Rosner, P. J., Hoth, L. R., Lanzetti, A. J., Borzilleri, K. A., Marr, E. S., Pezzulo, L. H., Martin, L. B., Lemotte, P. K., McColl, A. S., Kamath, A. V. & Stroh, J. G. (1999). Anal. Biochem.267, 169–184. [DOI] [PubMed] [Google Scholar]
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411. [Google Scholar]
- Jensen, K. F. & Bjornberg, O. (1998). Paths Pyrimidines, 6, 20–28.
- Kim, K. M., Yi, E. C., Baker, D. & Zhang, K. Y. J. (2001). Acta Cryst. D57, 759–762. [DOI] [PubMed] [Google Scholar]
- Leslie, A. G. W. (1992). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr.26
- Liu, S., Neidhardt, E. A., Grossman, T. H., Ocain, T. & Clardy, J. (2000). Structure Fold. Des.8, 25–33. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Nara, T., Hashimoto, T. & Aoki, T. (2000). Gene, 256, 209–222. [DOI] [PubMed]
- Navaza, J. (1994). Acta Cryst. A50, 157–163. [Google Scholar]
- Norager, S., Jensen, K. F., Bjornberg, O. & Larsen, S. (2002). Structure, 10, 1211–1223. [DOI] [PubMed] [Google Scholar]
- Rowland, P., Bjornberg, O., Nielsen, F. S., Jensen, K. F. & Larsen, S. (1998). Protein Sci.7, 1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima, E., Inaoka, D. K., Osanai, A., Nara, T., Odaka, M., Aoki, T., Inaka, K., Harada, S. & Kita, K. (2002). Mol. Biochem. Parasitol.122, 189–200. [DOI] [PubMed] [Google Scholar]
- Urbina, J. A. (2001). Curr. Opin. Infect. Dis.6, 733–741. [DOI] [PubMed]
- World Health Organization (2001). Chagas. http://www.who.int/ctd/chagas/disease.htm.