Abstract
A recently proposed mechanism of protection for haemoglobin C (HbC; β6Glu→Lys) links an abnormal display of PfEMP1, an antigen involved in malaria pathogenesis, on the surface of HbC infected erythrocytes together with the observation of reduced cytoadhesion of parasitized erythrocytes and impaired rosetting in vitro. We investigated the impact of this hypothesis on the development of acquired immunity against Plasmodium falciparum variant surface antigens (VSA) encoding PfEMP1 in HbC in comparison with HbA and HbS carriers of Burkina Faso. We measured: i) total IgG against a single VSA, A4U, and against a panel of VSA from severe malaria cases in human sera from urban and rural areas of Burkina Faso of different haemoglobin genotypes (CC, AC, AS, SC, SS); ii) total IgG against recombinant proteins of P. falciparum asexual sporozoite, blood stage antigens, and parasite schizont extract; iii) total IgG against tetanus toxoid. Results showed that the reported abnormal cell-surface display of PfEMP1 on HbC infected erythrocytes observed in vitro is not associated to lower anti- PfEMP1 response in vivo. Higher immune response against the VSA panel and malaria antigens were observed in all adaptive genotypes containing at least one allelic variant HbC or HbS in the low transmission urban area whereas no differences were detected in the high transmission rural area. In both contexts the response against tetanus toxoid was not influenced by the β-globin genotype. These findings suggest that both HbC and HbS affect the early development of naturally acquired immunity against malaria. The enhanced immune reactivity in both HbC and HbS carriers supports the hypothesis that the protection against malaria of these adaptive genotypes might be at least partially mediated by acquired immunity against malaria.
Introduction
Red blood cell disorders including haemoglobin variants and thalassaemias provide with their unusually high prevalence and distribution in malaria endemic areas [1]–[3] the most compelling evidence of genetic factors controlling the susceptibility to any infectious disease in humans. Conclusive evidence exists on the protective role of Haemoglobin C (HbC; β6Glu→Lys) against clinical Plasmodium falciparum malaria [4]–[6]. Recently, an abnormal display of PfEMP1, an antigen involved in malaria pathogenesis, was reported [7], [8] on HbAC and HbCC infected erythrocytes that showed reduced cytoadhesion and impaired rosetting in vitro. On this basis it has been proposed that HbC protection might be attributed to the reduced PfEMP1-mediated adherence of parasitized erythrocytes in the microvasculature. Given the reported observations, it could be hypothesized that HbC carriers may develop an altered immune response to PfEMP1. Intriguingly, an enhanced immune recognition of variant surface antigens (VSA) was initially observed in HbAS individuals by Marsh et al., [9] and further demonstrated in a study showing that the presence of HbAS genotype was associated with enhanced recognition of two randomly selected clinical isolates amongst Gabonese children [10]. A cohort study in Kenya lends further support to the hypothesis of an accelerated acquisition of immunity against mild clinical malaria in HbAS children <10 years [11].
The coexistence of HbC and HbS in a hyperendemic malaria context such as Burkina Faso provided a unique opportunity to explore this question in a comparative approach. In the present study, we compared the humoral response to PfEMP1 in individuals belonging to the Mossi ethnic group carrying different β-globin genotypes (AA, AC, CC, AS, SC, SS) living in urban and rural areas of Burkina Faso, West Africa by measuring: i) total IgG against a single VSA (variant surface antigen), A4U, and against a panel of VSA from severe malaria cases; ii) total IgG against recombinant proteins of P. falciparum sporozoite (CSP), blood stage (AMA1, EBA-175, MSP-119, MSP-2, MSP-3) antigens, parasite schizont extract, and iii) total IgG against a non malaria antigen, tetanus toxoid.
Materials and Methods
Study population
Healthy individuals belonging to the Mossi ethnic group of Burkina Faso were recruited after oral informed consent was obtained during the dry seasons 2005/2006 at the “Centre Medical Saint Camille” of the capital city Ouagadougou, and in the villages nearby (Donsin, Kuiti, Nagbagre') of the Kadiogo district, as described in Table 1, within a survey of haemoglobinopathies approved by the Ethics Committee of the Saint Camille Medical Center according to the guidelines of the Ministry of Health of Burkina Faso. Haemoglobin genotype was determined by cellulose acetate electrophoresis (Helena). Parasite genomic DNA was extracted using QIAamp DNA Mini Kit (Qiagen). Plasmodium falciparum genotyping of glurp was carried out according to a standard protocol [12] to evaluate parasite positivity assessed by PCR at the time of sera collection.
Table 1. Study samples of Mossi ethnic group of Burkina Faso.
| Geographic Origin | Period of blood collection | Haemoglobin genotype | ||||||
| Rural samples (EIR>100) | AA | AC | CC | AS | SC | SS | Total | |
| Donsin | Dry season (April 2006 ) | 46 (16.0) | 16 (11.7) | 4 (9.0) | 2 (8.5) | 2 (13.0) | - | 70 |
| Kuiti | Dry season (April 2006 ) | 20 (10.7) | 11 (10.2) | 8 (9.2) | 5 (10.2) | 2 (9.0) | 2 (9.5) | 48 |
| Nagbagré | Dry season (April 2006 ) | - | - | 2+1* (15.0) | - | - | - | 3 |
| Urban samples (EIR:1-10) | ||||||||
| Ouagadougou | Dry season (January–May 2005) | - | 80 (30.0)** | 10 (27.4)*** | 40 (29.5)§ | 8 (19.2)§§ | 2 (8.0) | 140 |
| Ouagadougou | Dry season (January–May 2006) | 86 (29.0)& | 26 (24.5)&& | 3 | 8 (32.5)&&& | 3 (18.3) | - | 126 |
| Total | 152 | 133 | 28 | 55 | 15 | 4 | 387 | |
Individuals are indicated for each genotype and origin; age is given in brackets.
recruited in December 2004;
age available for 50 subjects;
age available only for 5 subjects;
age available only for 29 subjects;
age available only for 6 subjects;
age available only for 63 subjects;
age available only for 22 subjects;
age available only for 6 subjects.
Antibody Assays
Antibody responses against the parasite infected-erythrocyte surface antigens were tested using the method with modifications of Williams et al. [13] on 85 urban and 85 rural individuals including all haemoglobin genotypes which were processed in a single assay against i) a reference isolate A4U, derived by sequential selection for binding to the monoclonal antibody BC6, which is specific to the expressed A4U var gene, resulting in a population of infected erythrocytes expressing predominantly one specific PfEMP1 variant on their surface, A4U PfEMP1 [14] and ii) a composite isolate (CI) derived by pooling a panel of wild isolates obtained from children presenting to the wards of Kilifi district hospital in Kenya with malaria at high parasitemia (T.H and T.N.W unpublished data).
FACS analysis
Mature trophozoite stage parasitized RBC (pRBC) at between 3–5% parasitaemia were thawed from frozen culture using saline solutions according to a gradient from 12% to 0.9% in a suspension at 50% haematocrit with buffer (0.5% BSA/PBS). 1 µl of serum was pipetted into separate wells of a 96-well U-bottomed plate (Falcon, Becton Dickinson, USA) to which was added 11.5 µl of the infected pRBC cell suspension in 0.5%BSA/PBS and 10 µg/ml of ethidium bromide. The mixture was incubated at room temperature for 30 min, following which the cells were washed three times with 0.5%BSA/PBS, centrifuging at 1000 rpm for 3 min per wash. The cells were then re-suspended in 50 µl 0.5%BSA/PBS containing a 1:50 dilution of sheep anti-human γ chain (FITC) fluorescein isothiocyanate-conjugated antibody (The Binding Site) was added to each well. A further incubation at room temperature in darkness, for 30 min was carried out, after which, following a further series of washes, at least 1000 pRBC were counted on an EPIC/XL flow cytometer (Coulter, UK). The Mean Fluorescence Intensity (MFI) was defined as the difference between the geometric mean of the fluorescence emitted by trophozoite pRBC and the geometric mean of the fluorescence emitted by the non pRBC. Non-specific recognition as measured by European negative controls was subtracted by the MFI of tested individuals.
ELISA analysis
Enzyme linked immunosorbant assays (ELISA) were performed according to well established protocols [15], [16]. Assays were performed in duplicate for each serum sample (N = 387) against the circumsporozoite protein (CSP) of P. falciparum (NANP16 CDC reagent gift from Patrick Corran, LSHTM, London, UK) and several blood stage antigens: full length 3D7 allele of AMA-1 (gift from David Lanar, Walter Reed Army Institute for Research, Washington, DC), Wellcome allele of MSP-119 (gift from Patrick Corran, LSHTM, with permission of Tony Holder, London, UK), Dd2 allele of MSP-2 (gift from David Cavanagh, Institute of Immunology and Infection Research, Edinburgh, UK), 3D7 allele of MSP-3 (gift from Spencer Polley, LSHTM, London, UK), Camp allele F2 domain of EBA-175 region II (gift from Chetan Chitnis, ICGEB, New Delhi, India), and the Wellcome strain parasite schizont extract (gift from David Walliker, Institute of Cell, Animal and Population Biology, Edinburgh prepared by Lindsay Stewart, LSTHM, London, UK). When antigens were conjugated to GST or MBP, the optical density (OD) of GST or MBP for each sample was subtracted from that of the antigen to obtain the final OD. All assays were performed in Dynex Immunolon 4HBX ELISA plates (Dynex Technologies Inc). Wells were coated with 50 ng for all blood stage antigens (1 µg for CSP and PSE) in 100 µl of carbonate buffer (15 mM Na2CO3, 35 mM NAHCO3, pH 9.3), and incubated overnight at 4°C before washing four times in PBS/Tween (Phosphate Buffered Saline/0.05% Tween 20). The plates were then incubated in 200 µl/well of blocking buffer (1% skimmed milk in PBS/Tween) for 5 hours at room temperature. Antigen-coated wells were washed and incubated overnight at 4°C with test sera (1/500 dilution) at 100 µl/well. Unbound antibody was washed off, and 100 µl of secondary antibody (HRP-conjugated rabbit anti-human IgG, Dako Ltd.) diluted to 1/5000 in blocking buffer was added into each well and incubated for 3 hours at room temperature before detection with O-phenylenediamine (OPD, Sigma). The reaction was stopped with 25 µl/well of 2M H2SO4. The absorbance was read at OD 492nm using the SPECTRAFluor program, (XFLUOR4 Version: V 4.11) and analysed using Excel (Microsoft). The coefficient of variation (CV) between duplicate wells was calculated in Excel (Microsoft) using the formula below to ensure accuracy:
The mean OD of each duplicate was used as the final read-out. The cut-off for positive samples was determined by taking the mean plus 3 standard deviations of twenty (20) non-immune sera for all antigens. All samples were tested for each antigen and its conjugated molecule in a single assay to avoid any daily variation. OD values were log-transformed for normal distribution and differences in means of antibody levels and MFIs according to the different genotypes were tested by Kruskal-Wallis and pairwise comparisons by Two Sample T test. For all tests, P values of less than 0.05 were considered significant. Logistic regression was used to examine the relationship between antibody levels and the chosen variables: Hb genotype, age categories (1/9, 10/max years), gender and parasite positivity assessed by PCR. All analyses were carried out in STATA (StataCorp.1999, Release (9.2)).
Results
Serological reactivities to P. falciparum VSA
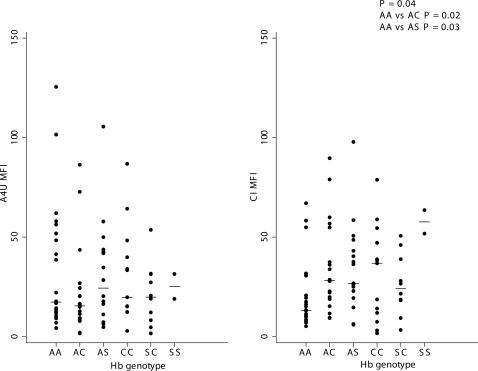
No differences in the Mean Fluorescence Intensity (MFI) according to the haemoglobin genotype were detected when looking at urban and rural samples all together. Further comparisons were carried out separately with the urban and the rural samples due to the different EIR (entomological inoculation rate) and mean age of the two subsets whose characteristics are described in Table 1. No differences were observed in the MFI of the different haemoglobin genotypes against the A4U isolate in both subsets, whereas we found significant differences in the urban sera (P = 0.04 amongst all adaptive genotypes and HbAA; P = 0.02 between HbAA and HbAC; P = 0.03 between HbAA and HbAS) when testing the panel of composite isolates (CI) of severe malaria VSA from Kilifi (Figure 1).
Figure 1. Mean Fluorescence Intensity (MFI) of total IgG against Plasmodium falciparum A4 Ultra parasite isolate (A4U) and a panel of Composite Isolates (CI) expressing VSA in urban samples of Mossi from Ouagadougou, Burkina Faso according to the different haemoglobin genotypes.
Serological reactivities to P. falciparum antigens and parasite schizont extract
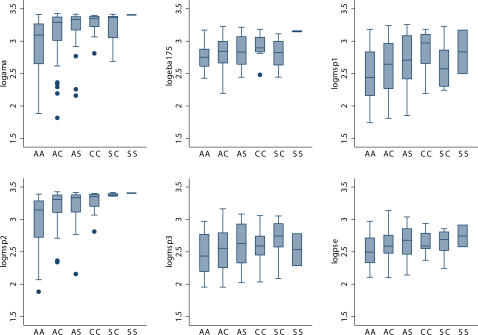
Prevalence and levels of antibodies tested against all antigens was consistently higher in the villages due to higher exposure to malaria of the rural subset despite the relatively younger age (Table 2, Table 3). Within the urban samples higher levels of total IgG amongst genotypes containing at least one adaptive haemoglobin allele compared to HbAA were observed for most of the P. falciparum antigens tested by ELISA, with some degree of statistical significance as measured by Kruskal-Wallis test and pairwise T-test (Table 2, Figure 2). Remarkably, the same observation of higher levels of IgG in all genotypes including HbC and HbS alleles was confirmed by CSP and schizont extract, both considered markers of exposure (Table 2, Figure 2). However, significantly higher levels of total IgG against P. falciparum antigens were observed in the rural samples only against EBA-175 in the overall comparisons, and against AMA1 in the comparisons between HbAA versus HbAC, and HbAA versus HbCC (Table 2). Differences observed in the primary analysis were tested by logistic regression including Hb genotype, age categories (1/9, 10/max years), gender and parasite status confirming the role of adaptive genotypes for most of the tested antigens (Table 3). Finally, a non malaria antigen, tetanus toxoid, was tested revealing no differences in any of the subsets (data not shown).
Table 2. Total IgG means of log transformed OD values against several blood stage antigens (AMA1, EBA-175, MSP-119, MSP-2, MSP-3), circumsporozoite protein (CSP), and parasite schizont extract (PSE) of Plasmodium falciparum in urban and rural sera of Burkina Faso.
| Antigen | Prevalence | Pairwise comparisons | ||||
| Urban samples (N = 266) | AA vs AC | AC vs CC | AA vs CC | AA vs AS | Overall | |
| AMA1 | 78% | P = 0.001 | NS | P = 0.01 | P = 0.001 | P = 0.0001 |
| EBA-175 | 73% | P = 0.04 | NS | P = 0.01 | P = 0.03 | P = 0.02 |
| MSP-119 | 67% | NS | P = 0.03 | P = 0.01 | P = 0.01 | NS (P = 0.06) |
| MSP-2 | 64% | P = 0.001 | NS | P = 0.03 | P = 0.004 | P = 0.0001 |
| MSP-3 | 70% | NS | NS | NS | P = 0.02 | NS |
| CSP | 65% | P = 0.05 | NS | P = 0.03 | P = 0.001 | P = 0.03 |
| PSE | 74% | P = 0.01 | NS | P = 0.04 | P = 0.01 | P = 0.05 |
| Rural samples (N = 121) | ||||||
| AMA1 | 90% | P = 0.03 | NS | P = 0.03 | NS | NS |
| EBA-175 | 76% | NS (P = 0.07) | NS | NS | NS (P = 0.06) | P = 0.05 |
| MSP-119 | 70% | NS | NS | NS | NS | NS |
| MSP-2 | 82% | NS | NS | NS | NS | NS |
| MSP-3 | 77% | NS | NS | NS | NS | NS |
| CSP | 68% | NS | NS | NS | NS | NS |
| PSE | 78% | NS | NS | NS | NS | NS |
Table 3. Geometric means and 95% Confidence intervals in brackets of log transformed OD values against several blood stage antigens (AMA1, EBA-175, MSP-119, MSP-2, MSP-3), circumsporozoite protein (CSP), and parasite schizont extract (PSE) of Plasmodium falciparum in urban and rural sera of Burkina Faso.
| Antigen | AA | AC | AS | CC | SC | SS |
| Urban Samples (N = 266) | ||||||
| AMA1 | 2.90 (2.78–3.03) | 3.14 (3.06–3.22) | 3.20 (3.10–3.31) | 3.27 (3.16–3.38) | 3.23 (3.05–3.41) | 3.40 (3.35–3.46) |
| EBA-175 | 2.75 (2.70–2.81) | 2.82 (2.78–2.87) | 2.84 (2.77–2.92) | 2.91 (2.79–3.04) | 2.82 (2.65–2.99) | 3.15 (3.05–3.25) |
| MSP-119 | 2.45 (2.34–2.58) | 2.56 (2.46–2.66) | 2.67 (2.54–2.81) | 2.83 (2.60–3.08) | 2.60 (2.38–2.85) | 2.81 (2.58–3.05) |
| MSP-2 | 2.97 (2.84–3.10) | 3.19 (3.13–3.26) | 3.22 (3.12–3.32) | 3.26 (3.15–3.38) | 3.38 (3.36–3.40) | 3.40 (3.35–3.46) |
| MSP-3 | 2.44 (2.35–2.53) | 2.50 (2.43–2.58) | 2.60 (2.50–2.71) | 2.55 (2.37–2.74) | 2.66 (2.38–2.97) | 2.52 (2.45–2.61) |
| CSP | 2.74 (2.69–2.80) | 2.82 (2.77–2.87) | 2.88 (2.82–2.95) | 2.88 (2.75–3.01) | 2.85 (2.70–3.01) | __ |
| PSE | 2.50 (2.44–2.57) | 2.60 (2.56–2.65) | 2.64 (2.56–2.72) | 2.66 (2.55–2.77) | 2.63 (2.46–2.82) | 2.74 (1.26–3.95) |
| Rural samples (N = 121) | ||||||
| AMA1 | 3.12 (3.04–3.20) | 3.27 (3.18–3.36) | 3.09 (2.54–3.76) | 3.31 (3.23–3.40) | 3.06 (2.59–3.62) | 3.39* |
| EBA-175 | 2.82 (2.74–2.90) | 2.94 (2.85–3.03) | 3.03 (2.76–3.34) | 2.95 (2.81–3.08) | 2.70 (2.28–3.20) | 3.16* |
| MSP-119 | 2.82 (2.60–3.06) | 2.54 (1.31–4.92) | 1.92* | __ | __ | __ |
| MSP-2 | 2.82 (2.70–2.94) | 2.87 (2.75–2.99) | 2.82 (2.37–3.35) | 2.89 (2.57–3.25) | 2.88 (2.41–3.45) | 3.24* |
| MSP-3 | 2.57 (2.48–2.67) | 2.71 (2.58–2.84) | 2.52 (2.11–3.01) | 2.69 (2.41–3.01) | 2.86 (1.03–5.97) | 2.55* |
| CSP | 2.80 (2.75–2.85) | 2.82 (2.77–2.86) | 2.88 (2.82–2.94) | 2.76 (2.73–2.79) | 2.77 (2.68–2.85) | __ |
| PSE | 2.55 (2.46–2.65) | 2.61 (2.49–2.74) | 2.72 (2.55–2.90) | 2.59 (2.48–2.70) | 2.56 (0.83–7.89) | 2.62* |
single individual
Figure 2. Means levels of total IgG against several blood stage antigens of Plasmodium falciparum (AMA1, EBA-175, MSP-119, MSP-2, MSP-3) and parasite schizont extract in urban samples of Mossi from Ouagadougou, Burkina Faso according to the different haemoglobin genotypes.
The horizontal lines in each box correspond to the median values, the lower edge of each box is the 25% ile and the upper edge is the 75% ile. The whiskers represent the range of the data beyond these percentiles, excluding outliers represented by dots.
Discussion
Despite substantial evidence of protection against clinical malaria given by the haemoglobin variants HbC and HbS, the precise mechanism(s) are still under debate. A recently proposed mechanism suggested that the protection conferred by HbC may be attributed to the reduction of cytoadherence and impaired rosetting observed in relation to the altered display of PfEMP1 on HbCC erythrocytes [7], [8] which raises the question of whether this would affect VSA recognition, and ultimately the immune reactivity against VSA. Taking on from this hypothesis we anticipated a difference in antibody levels of HbCC homozygous, possibly a reduction due to their impaired VSA expression. Also, an “immunological hypothesis” initially proposed for HbS has been supported by a number of studies in various epidemiological contexts although the functional mechanisms involved in the enhanced acquisition of natural immunity observed in HbAS remain elusive [9], [10]. Therefore, we investigated haemoglobin phenotype-specific immune reactivity to a composite isolate expressing a wide range of P. falciparum VSAs in subjects resident in two different malaria endemic contexts of Burkina Faso. Results showed that the reported abnormal cell-surface display of PfEMP1 on HbC infected erythrocytes observed in vitro is not associated to lower anti- PfEMP1 humoral response in vivo. In fact, higher immune response against the P. falciparum VSA panel and several antigens were observed in all adaptive genotypes containing at least one allelic variant HbC or HbS in the low transmission urban area (Figure 1). Interestingly, cross-reactive antibody responses, i.e. those directed against erythrocyte surface expressed parasite antigens from heterologous parasite isolates are an important component of acquired immunity against malaria [17]–[19]. However, no differences were detected in the high transmission rural areas. Significantly higher levels of antibodies at several P. falciparum antigens were also observed in the same urban samples (Table 2, Table 3, Figure 2). These differences were not explained by age or parasite status at that time point as confounders for most of the antigens examined (Table 4), rather reflected the cumulative effect of a life long exposure to P. falciparum malaria in the presence of a protective factor such as HbC and/or HbS. In both contexts the response against tetanus toxoid was not influenced by the β-globin genotype. Thus, these findings suggest that both HbC and HbS could promote the early development of naturally acquired immunity against malaria. The enhanced immune reactivity in both HbC and HbS carriers supports the hypothesis that the protection against malaria of these adaptive genotypes might be at least partially mediated by acquired immunity against malaria.
Table 4. Logistic regression analysis of antibody levels against all Plasmodium falciparum tested antigens.
| Antigen | Variable | |||
| Hb genotype | Age | Gender | Parasite + | |
| AMA1 | P = 0.001 | NS | NS | NS |
| EBA-175 | NS | NS | NS | NS |
| MSP-119 | NS (P = 0.06) | NS | NS | NS |
| MSP-2 | P = 0.003 | NS | NS | NS |
| MSP-3 | NS (P = 0.08) | NS | NS | NS |
| CSP | P = 0.004 | NS | NS | NS |
| PSE | P = 0.01 | P = 0.01 | NS | NS |
| A4 MFI | NS | NS | NS | NS |
| CI MFI | P = 0.05 | NS | NS | NS |
We believe that the discrepancy between results from urban and rural settings could be the consequence of saturated immunity in high transmission contexts. These observations emphasize the need, when studying candidates of genetic resistance/susceptibility to malaria and their underlying hypothesized mechanisms, to take into full account the epidemiological context as a potential confounder.
Although this study can not conclusively validate any of the different functional mechanisms proposed to explain the protection of haemoglobin variants, a consistently enhanced immune reactivity in both HbC and HbS adaptive genotypes suggests the idea of a convergence in terms of their impact on the acquisition of immunity against malaria. It has been already shown that the model initially proposed for explaining G6PD protection, by enhanced phagocytosis of ring- parasitized altered erythrocytes, fits well also in the case of HbS and β-thalassemia [20]. Thus, it may be possible that this mechanism may also be extended to the case of HbC. Further investigation in the rural areas so far examined, focusing on young children, especially below five years, is undergoing to unravel the modulation exerted by HbC (and HbS) on the onset of naturally acquired immunity to malaria.
Acknowledgments
This work is dedicated to the memory of our colleague, Gaia Luoni (1970–2004). We are grateful to the study participants in Burkina Faso for their cooperation, to the laboratory staff at the Centre Medical Saint Camille of Ouagadougou, Burkina Faso and at KEMRI, Kenya for excellent technical support. We thank all the colleagues who kindly provided us with recombinant proteins of Plasmodium falciparum. Also, we are grateful to Lars Hviid and colleagues at CMP for stimulating discussions, and to prof. Coluzzi for his advice and encouragement. This article is published with the permission of the Director of KEMRI.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This project was supported by the Wellcome Trust grant n° 071626 (Advanced Training Fellowship in Tropical Medicine to FV). This work is also part of the activities of the BioMalPar European Network of Excellence supported by a European grant (LSH-CT-2004-503578) from the Priority 1 Life Sciences, Genomics and Biotechnology for Health in the 6th Framework Programme. FV is currently funded by the malaria Network sponsored by the Compagnia di San Paolo, Turin, Italy. The above mentioned funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation, review, or approval of the manuscript.
References
- 1.Haldane JBS. Disease and evolution. Ric Sci Suppl. 1949;A 19:68–76. [Google Scholar]
- 2.Allison AC. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans R Soc Trop Med Hyg. 1954;48(4):312–318. doi: 10.1016/0035-9203(54)90101-7. [DOI] [PubMed] [Google Scholar]
- 3.Weatherall DJ. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat Rev Genet. 2001;2(4):245–255. doi: 10.1038/35066048. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Guindo A, Cissoko Y, Taylor JG, Coulibaly D, et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood. 2000;96(7):2358–2363. [PubMed] [Google Scholar]
- 5.Modiano D, Luoni G, Sirima BS, Simpore J, Verra F, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414(6861):305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 6.Mockenhaupt FP, Ehrhardt S, Cramer JP, Otchwemah RN, Anemana SD, et al. Hemoglobin C and resistance to severe malaria in Ghanaian children. J Infect Dis. 2004;190(5):1006–1009. doi: 10.1086/422847. [DOI] [PubMed] [Google Scholar]
- 7.Fairhurst RM, Fujioka H, Hayton K, Collins KF, Wellems TE. Aberrant development of Plasmodium falciparum in hemoglobin CC red cells: implications for the malaria protective effect of the homozygous state. Blood. 2003;101(8):3309–3315. doi: 10.1182/blood-2002-10-3105. [DOI] [PubMed] [Google Scholar]
- 8.Fairhurst RM, Baruch DI, Brittain NJ, Ostera GR, Wallach JS, et al. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005;435(7045):1117–1121. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 9.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83(3):293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 10.Cabrera G, Cot M, Migot-Nabias F, Kremsner PG, Deloron P, et al. The sickle cell trait is associated with enhanced immunoglobulin G antibody responses to Plasmodium falciparum variant surface antigens. J Infect Dis. 2005;191(10):1631–1638. doi: 10.1086/429832. [DOI] [PubMed] [Google Scholar]
- 11.Williams TN, Mwangi TW, Roberts DJ, Alexander ND, Weatherall DJ, et al. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005;2(5):e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viriyakosol S, Siripoon N, Petcharapirat C, Petcharapirat P, Jarra W et al. Genotyping of Plasmodium falciparum isolates by the polymerase chain reaction and potential uses in epidemiological studies. Bull World Health Organ. 1995;73(1):85–95. [PMC free article] [PubMed] [Google Scholar]
- 13.Williams TN, Newbold CI. Reevaluation of flow cytometry for investigating antibody binding to the surface of Plasmodium falciparum trophozoite-infected red blood cells. Cytometry A. 2003;56(2):96–103. doi: 10.1002/cyto.a.10088. [DOI] [PubMed] [Google Scholar]
- 14.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavanagh DR, McBride JS. Antigenicity of recombinant proteins derived from Plasmodium falciparum merozoite surface protein 1. Mol Biochem Parasitol. 1997;85(2):197–211. doi: 10.1016/s0166-6851(96)02826-5. [DOI] [PubMed] [Google Scholar]
- 16.Cavanagh DR, Elhassan IM, Roper C, Robinson VJ, Giha H, et al. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]
- 17.Giha HA, Staalsoe T, Dodoo D, Elhassan IM, Roper C, et al. Nine-Year Longitudinal Study of Antibodies to Variant Antigens on the Surface of Plasmodium falciparum-Infected Erythrocytes. Infect Immun. 1999;67(8):4092–4098. doi: 10.1128/iai.67.8.4092-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bull PC, Lowe BS, Kaleli N, Njuga F, Kortok M, et al. Plasmodium falciparum Infections Are Associated with Agglutinating Antibodies to Parasite-Infected Erythrocyte Surface Antigens among Healthy Kenyan Children. J Infect Dis. 2002;185(11):1688–1691. doi: 10.1086/340420. [DOI] [PubMed] [Google Scholar]
- 19.Kinyanjui SM, Mwangi TW, Bull PC, Newbold CI, Marsh K, et al. Protection against clinical malaria by heterologous immunoglobulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. . J Infect Dis. 2004;190(9):1527–1533. doi: 10.1086/424675. [DOI] [PubMed] [Google Scholar]
- 20.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–3371. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]