Abstract
Serine/threonine phosphorylation of IRS-1 might inhibit insulin signaling, but the relevant phosphorylation sites are difficult to identify in cultured cells and to validate in isolated tissues. Recently, we discovered that recombinant NH2-terminal Jun kinase phosphorylates IRS-1 at Ser307, which inhibits insulin-stimulated tyrosine phosphorylation of IRS-1. To monitor phosphorylation of Ser307 in various cell and tissue backgrounds, we prepared a phosphospecific polyclonal antibody designated αpSer307. This antibody revealed that TNF-α, IGF-1, or insulin stimulated phosphorylation of IRS-1 at Ser307 in 3T3-L1 preadipocytes and adipocytes. Insulin injected into mice or rats also stimulated phosphorylation of Ser307 on IRS-1 immunoprecipitated from muscle; moreover, Ser307 was phosphorylated in human muscle during the hyperinsulinemic euglycemic clamp. Experiments in 3T3-L1 preadipocytes and adipocytes revealed that insulin-stimulated phosphorylation of Ser307 was inhibited by LY294002 or wortmannin, whereas TNF-α–stimulated phosphorylation was inhibited by PD98059. Thus, distinct kinase pathways might converge at Ser307 to mediate feedback or heterologous inhibition of IRS-1 signaling to counterregulate the insulin response.
Introduction
Insulin resistance occurs throughout life; but when combined with impaired insulin secretion, it contributes to type 2 diabetes (1, 2). Initially, β cells of the pancreas increase insulin secretion to compensate for mild hyperglycemia owing to insulin resistance; however, persistent hyperinsulinemia and hyperglycemia might impair insulin action and exacerbate the demand for insulin and promote the development of diabetes (2–4). Recent evidence reveals a close relationship between pancreatic β-cell function and insulin signaling. Disruption of the insulin receptor in pancreatic β cells impairs glucose-stimulated insulin secretion (5). Moreover, mice lacking IRS-2 develop uncompensated peripheral insulin resistance owing at least partially to reduced β-cell mass, suggesting that IRS-2 is a common element for insulin action and β-cell proliferation or survival (6).
Insulin binds to the α subunit of the insulin receptor to promote tyrosine autophosphorylation of the β subunit, which activates the catalytic domain and stimulates phosphorylation of cellular substrates, including the IRS proteins and Shc (7, 8). Phosphorylation of IRS-1 or IRS-2 on multiple tyrosine residues creates an active signaling complex by recruiting various proteins, including the phosphatidylinositol 3 (PI3) kinase, Grb2, Nck, Crk, Fyn, SHP2, and possibly others (9, 10). In humans, mutations of the insulin receptor contribute rarely to type 2 diabetes, and IRS-protein mutations that are directly responsible for diabetes in people are difficult to find (11); however, dysregulation of the insulin receptor and IRS proteins are common occurrences in type 2 diabetes (11–13). Several mechanisms might be involved in the inhibition of insulin-stimulated tyrosine phosphorylation of the insulin receptor and the IRS proteins, including proteasome-mediated degradation (14, 15); phosphatase-mediated dephosphorylation (16, 17), or kinase-mediated serine/threonine phosphorylation (18).
Acute and chronic stress causes insulin resistance, which might be mediated through cytokine-stimulated protein kinase cascades, including those activated by TNF-α or IL1-β (19). TNF-α, a potential mediator of insulin resistance, promotes serine/threonine phosphorylation of IRS-1 and IRS-2 (20–22). Serine/threonine phosphorylation of IRS-1 impairs the ability of IRS-1 to associate with the insulin receptor, which inhibits subsequent insulin-stimulated tyrosine phosphorylation (23). Moreover, during serine phosphorylation, IRS-1 might inhibit insulin-stimulated tyrosine phosphorylation of the insulin receptor itself (20–22, 24, 25). However, the phosphorylation sites in IRS-1 that mediate the inhibitory effects of TNF-α are difficult to determine. The c-Jun NH2-terminal kinase (JNK) is activated by diverse inflammatory stimuli, including TNF-α, IL1-β, double-stranded RNA and lipopolysaccharide. Recently, we reported that JNK-1 forms a stable complex with IRS-1 and phosphorylates Ser307, which inhibits insulin-stimulated tyrosine phosphorylation of IRS-1 (26).
The phosphorylation of Ser307 is difficult to study in intact cells or tissues because the methods used to radioactively label cultured cells induce JNK activity (26). To investigate the mechanism of Ser307 phosphorylation in cells, we developed a phosphospecific polyclonal antibody that specifically immunoblots the phosphorylated Ser307 residue in IRS-1. Using this antibody, we demonstrate that insulin/IGF-1 and TNF-α utilize distinct mechanisms in 3T3-L1 cells to mediate phosphorylation of IRS-1 on Ser307. Moreover, insulin-stimulated phosphorylation of Ser307 is likely to be physiologically important, as it occurs in skeletal muscle of mice, rats, and humans in response to insulin. Given that phosphorylation of Ser307 inhibits insulin-stimulated tyrosyl phosphorylation of IRS-1, we propose that Ser307 integrates feedback and heterologous signals to attenuate IRS-1–mediated signals and contribute to insulin resistance.
Methods
Reagents.
Murine TNF-α was purchased from R&S Systems Inc. (Minneapolis, Minnesota, USA). Human insulin and IGF-1 were a gift from Eli Lilly and Co. (Indianapolis, Indiana, USA). Protein A-agarose was purchased from RepliGen Corp. (Needham, Massachusetts, USA). The 3-isobutylmethylxanthine, dexamethasone, aprotinin, and leupeptin were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA). An enhanced chemiluminescence (ECL) detection system was purchased from Amersham Pharmacia Biotech Inc. (Piscataway, New Jersey, USA). Fugene 6 was purchased from Roche Molecular Biochemicals (Indianapolis, Indiana, USA). Okadaic acid, PD98059, LY294002, wortmannin, and Nonidet P-40 were purchased from Calbiochem-Novabiochem Corp. (La Jolla, California, USA). Monoclonal anti-phosphotyrosine antibody (PY20) was purchased from Upstate Biotechnology Inc. (Lake Placid, New York, USA). Polyclonal anti-active MAPK (αpMAPK) was purchased from Promega Corp. (Madison, Wisconsin, USA). Polyclonal anti-phospho-Akt (Ser473) was purchased from New England Biolabs Inc.(Beverly, Massachusetts, USA). Monoclonal anti-hemagglutinin (HA) antibody and anti–JNK-1 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA). Polyclonal anti-IRS-1 antibodies were raised against the PTB domain (JD no. 228, used at a dilution of 1:100 for immunoprecipitation) or the full length (JD no.159, used at a dilution of 1:15,000 for immunoblotting) of rat IRS-1. Polyclonal anti-phospho-Ser307 (αpS307) was raised against a synthetic peptide (ESITATpS307PASMVGGK) flanking Ser307in IRS-1 that is conserved among mouse, rat, and human.
Plasmid construction.
Ser307 in rat IRS-1 was mutated to Ala (IRS-1S307A) with the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, California, USA) using the primer 5′-GCATCACTGCCACCGCCCCTGCCAGTA-3′. IRS-1 and IRS-1S307A were tagged with HA at the COOH-terminus via the PCR-based mutagenesis; the mutations were verified by DNA sequencing. The cytomegalovirus promoter controlled the expression of HA-tagged IRS-1 and IRS-1S307A.
Cell culture, transfection, and differentiation.
Human embryonic kidney 293 cells were grown at 37°C in 5% CO2 in DMEM supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FBS. Chinese hamster ovary (CHO) cells were grown at 37°C in 5% CO2 in F12 medium supplemented with 100 U/ml penicillin, 100-μg/ml streptomycin, and 10% FBS. Confluent CHO cells were split at the ratio of 1:10 (100-mm cell culture dish) 24 hours before transfection. The cells were transfected with 6 μg plasmid per dish, using Fugene 6 according to the protocol recommended by the manufacturer, and used for experiments 24 hours after transfection.
3T3-L1 preadipocytes were grown at 37°C in 10% CO2 in DMEM containing 25 mM glucose, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% calf serum. For adipocyte differentiation, confluent preadipocytes were cultured for three days in differentiation medium (DMEM supplemented with 25-mM glucose, 1 μM insulin, 0.5 mM 3-isobutylmethylxanthine, 1-μM dexamethasone, and 10% FBS), and 3 days in DMEM supplemented with 1 μM insulin and 10% FBS. The cells were then grown for an additional 4–9 days in DMEM containing 25 mM glucose and 10% FBS without any other additives (>90% cells are adipocytes).
Immunoprecipitation and immunoblotting.
Confluent cells were deprived of serum overnight in DMEM (for 3T3-L1 and 293 cells) or F12 (for CHO cells) containing 1% BSA and treated with different ligands at 37°C. The cells were rinsed three times with ice-cold PBSV (10 mM sodium phosphate [pH 7.4], 150 mM NaCl, and 1 mM Na3VO4), solubilized in lysis buffer (50 mM Tris [pH 7.5], 1% Nonidet P-40, 150 mM NaCl, 2 mM EGTA, 1 mM Na3VO4, 100 mM NaF, 10 mM Na4P2O7, 50 nM okadaic acid, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin), and centrifuged at 14,000 g for 10 minutes at 4°C. The supernatant (cell lysates) was incubated with the indicated antibody on ice for 2 hours. The immune complexes were collected on protein A-agarose during 1-hour incubation at 4°C. The beads were washed three times with washing buffer (50 mM Tris [pH 7.5], 1% Nonidet P-40, 150 mM NaCl, and 2 mM EGTA) and boiled for 5 minutes in a mixture (80:20) of lysis buffer and SDS-PAGE sample buffer (250 mM Tris-HCl [pH 6.8], 10% SDS, 10% β-mercaptoethanol, 40% glycerol, and 0.01% bromophenol blue). The solubilized proteins were separated by SDS-PAGE (7% gels). Proteins on the gel were transferred to nitrocellulose membrane (Amersham Pharmacia Biotech Inc.) and detected by immunoblotting with the indicated antibody using ECL. Some membranes were subsequently incubated at 55°C for 30 minutes in stripping buffer (100 mM β-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl [pH 6.7]) to prepare them for a second round of immunoblotting.
JNK in vitro kinase assay.
Cells were treated and lysed as already described here. JNK was immunopurified using anti–JNK-1, and incubated with 2 μg GST-cJun (amino acids 1-79) at 30°C for 30 minutes in the kinase reaction buffer (25 mM HEPES [pH 7.4], 50 nM okadaic acid, 25 mM MgCl2, 0.1 mM Na3VO4, 0.5 mM DTT, 10 μM ATP, and 2 μCi [γ-32P]-ATP). The reaction was stopped by boiling for 5 minutes in SDS-PAGE sample buffer. GST-cJun was then resolved by 12% SDS-PAGE, and subjected to autoradiography.
Preparation of muscle samples of mice, rats, and humans.
Human insulin was injected as a bolus into the inferior vena cava of anesthetized mice or into rats fasted overnight. Soleus muscles were isolated and homogenized in lysis buffer (50 mM Tris [pH 7.5], 1% Nonidet P-40, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 2 mM EGTA, 1 mM Na3VO4, 100 mM NaF, 10 mM Na4P2O7, 50 nM okadaic acid, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin). Human vastus lateralis muscle biopsies were obtained at baseline and then at 15 minutes during a hyperinsulinemic euglycemic clamp (insulin dose, 400 mU/m2/min). Approximately 50 mg muscle was pulverized and homogenized in ice-cold buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 2 mM EGTA, 1% IGEPAL CA-630, 10 mM Na4P2O7, 100 mM NaF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 2 mM Na3VO4, 1 mM PMSF, and 50 μM okadaic acid). One milligram of total protein in cell lysates was used for immunoprecipitation.
Results
Phosphorylation of Ser307 mediates the inhibitory effect of anisomycin on IRS-1 tyrosyl phosphorylation.
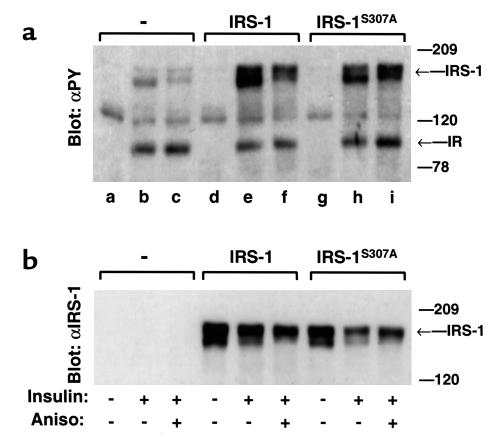
We reported previously that anisomycin activates JNK and inhibits insulin action (26). To confirm that anisomycin inhibits insulin-stimulated tyrosine phosphorylation of IRS-1 by promoting phosphorylation of Ser307, we prepared CHO cells expressing wild-type IRS-1 or IRS-1 containing the Ser307→Ala substitution (IRS-1S307A). IRS-1 or IRS-1S307A were expressed transiently in CHOIR cells that stably overexpress the insulin receptor. Anisomycin did not alter tyrosyl phosphorylation of the insulin receptor during insulin stimulation; however, it inhibited insulin-stimulated tyrosyl phosphorylation of both endogenous and recombinant IRS-1 (Figure 1). In contrast, anisomycin did not inhibit insulin-stimulated tyrosyl phosphorylation of IRS-1S307A, confirming that Ser307 mediates the inhibitory action of anisomycin, as shown previously (26).
Figure 1.
Ser307 in IRS-1 mediates the inhibitory effect of anisomycin on insulin-induced tyrosyl phosphorylation of IRS-1. CHO cells were transiently transfected with plasmids (10 μg) encoding IRS-1 or IRS-1S307A. Twenty-four hours later, cells were deprived of serum overnight and preincubated for 30 minutes with 5 μg/ml anisomycin before 50 nM insulin stimulation for an additional 5 minutes. (a) Proteins (30 μg) in cell lysates were resolved by SDS-PAGE and immunoblotted with anti-phosphotyrosine. (b) The same blot was reprobed with αIRS-1. The migration of molecular weight standards, IRS-1, and IR is indicated.
Phosphospecific antibodies reveal anisomycin-stimulated Ser307 phosphorylation.
Polyclonal antibodies against a synthetic phospho-Ser307 peptide based on the amino acid sequence around Ser307 in IRS-1 were prepared in rabbits. The ability of this antibody (αpS307) specifically to recognize IRS-1 phosphorylated on Ser307 was evaluated in CHO and HEK293 cells stimulated with anisomycin for 30 minutes. αIRS-1 immunoprecipitates from untreated CHO cells did not react with αpS307 during immunoblotting. However, after incubation with 5-μg/ml anisomycin for 30 minutes, IRS-1 from both CHO and 293 cells was strongly immunoblotted with αpS307 (Figure 2, a and b); dephosphorylation with alkaline phosphatase of IRS-1 from anisomycin-stimulated cells prevented αpS307 immunoblotting (data not shown). Moreover, incubation of αpS307 with the phosphorylated antigen used to generate αpS307 blocked immunoblotting, whereas incubation with the unphosphorylated peptide did not inhibit immunoblotting (Figure 2c). To verify that αpS307 only immunoblots phospho-Ser307, HA-tagged IRS-1 or IRS-1S307A was transiently expressed to the same level in CHO cells (data not shown). After 30 minutes of stimulation with 5 μg/ml anisomycin, recombinant IRS-1 or IRS-1S307A was immunoprecipitated with HA-specific antibodies and immunoblotted with αpS307. Under these experimental conditions, IRS-1 was strongly immunoblotted with αpS307, whereas IRS-1S307A was not recognized (Figure 2d). These results suggest that αpS307 binds specifically to phospho-Ser307 and does not cross-react with other phosphoserine residues in IRS-1.
Figure 2.
Anisomycin stimulates phosphorylation of IRS-1 on Ser307. (a and b) CHO (a) or 293 (b) cells were treated with 5 μg/ml anisomycin (aniso) for 30 minutes. IRS-1 was immunoprecipitated (IP) with αIRS-1 and immunoblotted with αpS307. The same blot was stripped and reprobed with αIRS-1. (c) αpS307 was preincubated on ice for 15 minutes with 5-μg phospho-Ser307 peptide (pS307) or non-phospho-Ser307 (S307) peptide. αIRS-1 immunoprecipitates from control or anisomycin-stimulated CHO cells were immunoblotted with αpS307 in the presence of pS307 or S307, respectively. (d) CHO cells were transfected transiently with 5-μg plasmids encoding HA-tagged IRS-1 or IRS-1S307A. Cells were treated with 5 μg/ml anisomycin for 30 minutes. HA-tagged IRS-1 and IRS-1S307A in cell lysates were immunoprecipitated with αHA. The αHA immunoprecipitates were divided evenly into two and immunoblotted with αpS307 and αIRS-1, respectively.
TNF-α stimulates phosphorylation of IRS-1 on Ser307 and inhibits insulin-stimulated tyrosyl phosphorylation.
TNF-α promotes insulin resistance during trauma and chronic obesity, and several reports suggest that this occurs through serine phosphorylation of IRS proteins (18, 21). In agreement with these reports, TNF-α inhibited insulin-stimulated tyrosyl phosphorylation of IRS-1 and insulin-stimulated activation of ERK1/2 in 3T3-L1 adipocytes (Figure 3a). However, TNF-α–stimulated serine phosphorylation of IRS-1 has never been observed directly, leaving open the possibility that other mechanisms are involved. Because TNF-α activates JNK, Ser307 might be an important site in the inhibition of IRS-1 signaling. Therefore, we used αpS307 to determine whether Ser307 is phosphorylated in TNF-α–stimulated cells.
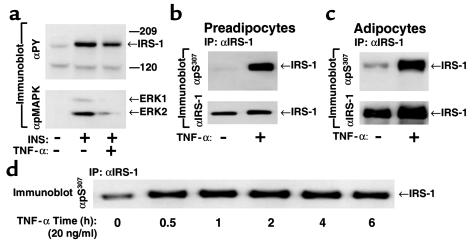
Figure 3.
TNF-α stimulates phosphorylation of IRS-1 on Ser307 and inhibits insulin-promoted tyrosyl phosphorylation of IRS-1 and activation of ERK1/2. (a) 3T3-L1 adipocytes were pretreated with 20 ng/ml TNF-α for 5 hours before 50 nM insulin stimulation for 10 minutes. Proteins (40 μg) in cell lysates were immunoblotted with anti-phosphotyrosine and anti–phospho-MAPK, respectively. (b and c) 3T3-L1 preadipocytes (b) and adipocytes (c) were stimulated with 20 ng/ml TNF-α for 30 minutes. IRS-1 in cell lysates was immunoprecipitated with αIRS-1 and immunoblotted with αpS307. The same blots were stripped and reprobed with αIRS-1. (d) The 3T3-L1 adipocytes were stimulated with 20 ng/ml TNF-α for the indicated time. IRS-1 immunoprecipitates were immunoblotted with αpS307.
3T3-L1 preadipocytes were treated for 30 minutes with TNF-α (20 ng/ml), and IRS-1 in cell lysates was immunoprecipitated with αIRS-1 and immunoblotted with αpS307. Before TNF-α stimulation, IRS-1 was not immunoblotted with αpS307, whereas after TNF-α stimulation, IRS-1 reacted strongly with αpS307 (Figure 3b), indicating that TNF-α stimulates Ser307 phosphorylation. Similarly, TNF-α stimulated phosphorylation of Ser307 in 3T3-L1 adipocytes (Figure 3c). The effect of TNF-α (20 ng/ml) on the phosphorylation of Ser307 was detected after 5 minutes, reached a maximum within the first 15 minutes, and remained elevated during 6 hours of TNF-α treatment (Figure 3d). Interestingly, compared with fibroblasts, the basal phosphorylation of Ser307 was slightly elevated in 3T3-L1 adipocytes before TNF-α stimulation (Figure 3, c and d). Because adipocytes produce TNF-α, the increase in the basal phosphorylation of Ser307 might represent autocrine action, as suggested previously (27–29).
Insulin and IGF-1 stimulated phosphorylation of IRS-1 on Ser307.
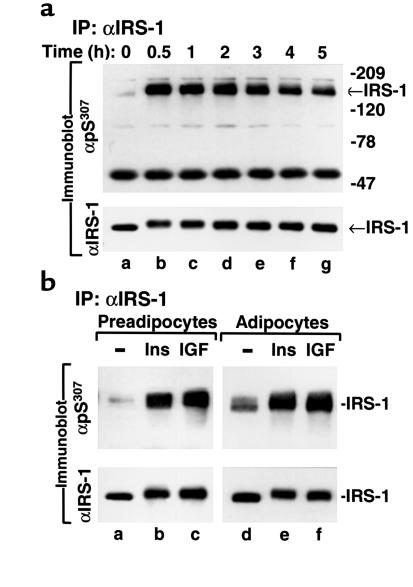
Chronic insulin stimulation inhibits insulin signal transduction in many experimental systems, which might exacerbate insulin resistance during compensatory hyperinsulinemia (2, 4, 23, 30). To determine whether insulin stimulates phosphorylation of IRS-1 on Ser307, 3T3-L1 preadipocytes or adipocytes were treated with insulin (100 nM) for various time intervals, and immunopurified IRS-1 was immunoblotted with αpS307. Insulin strongly stimulated phosphorylation of IRS-1 on Ser307, which reached maximal levels within 30 minutes (Figure 4a). Ser307 phosphorylation remained elevated during 3 hours of insulin stimulation and then decreased slightly, even though the level of IRS-1 was unchanged during the 5-hour experiment (Figure 4a). Similarly, IGF-1 strongly stimulated phosphorylation of IRS-1 on Ser307 in both 3T3-L1 preadipocytes and adipocytes (Figure 4b). By contrast, EGF did not stimulate phosphorylation of IRS-1 on Ser307 (data not shown).
Figure 4.
Insulin and IGF-1 stimulate phosphorylation of IRS-1 on Ser307 in both 3T3-L1 preadipocytes and adipocytes. (a) 3T3-L1 preadipocytes were stimulated with 100 nM insulin for the indicated time. IRS-1 in cell lysates was immunoprecipitated with αIRS-1 and immunoblotted with αpS307. The same blot was stripped and reprobed with αIRS-1. (b) 3T3-L1 preadipocytes and adipocytes were stimulated for 30 minutes with 100 nM insulin (Ins) and 100 ng/ml IGF-1 (IGF), respectively. IRS-1 immunoprecipitates were immunoblotted with αpS307. The same blot was stripped and reprobed with αIRS-1.
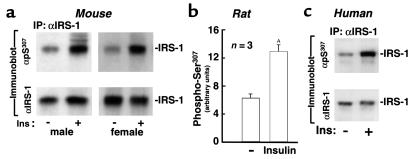
Phosphorylation of Ser307 during prolonged insulin stimulation might contribute to desensitization of the insulin response or cause insulin resistance especially during compensatory hyperinsulinemia. To determine whether insulin-stimulated phosphorylation of IRS-1 on Ser307 occurs in the whole organism, soleus muscle from male and female mice was isolated and homogenized and IRS-1 was immunopurified with αIRS-1. Immunoblotting with αpS307 revealed insulin-stimulated phosphorylation of Ser307 in skeletal muscle from both male and female mice (Figure 5a). Similarly, insulin increased phosphorylation of IRS-1 on Ser307 in muscle of rat more than twofold (Figure 5b).
Figure 5.
Insulin promotes phosphorylation of IRS-1 on Ser307in mouse, rat, and human skeletal muscle. (a) Saline or 5 U human insulin was injected as a bolus into the inferior vena cava of anesthetized mice. Soleus muscle was isolated 5 minutes later and homogenized. IRS-1 was immunoprecipitated with αIRS-1 and immunoblotted with αpS307. The same blots were stripped and reprobed with αIRS-1. (b) Insulin (5 U) was injected intraperitoneally into anesthetized rat. Saline was used as control. Soleus muscle was isolated 5 minutes later and homogenized. IRS-1 immunoprecipitates were immunoblotted with αpS307 or αIRS-1. Phospho-Ser307 was quantitated by phosphoimaging and normalized to IRS-1 level. The data were presented as mean ± SEM (three rats for each group; AP < 0.02). (c) Normal volunteer subjects were subjected to hyperinsulinemic euglycemic clamp. Muscle biopsies were obtained after insulin-clamp for 0 or 15 minutes and homogenized. IRS-1 immunoprecipitates were immunoblotted with αpS307. The data represent one of three independent experiments.
To determine whether insulin also promotes phosphorylation of IRS-1 on Ser307 in human skeletal muscle, normal volunteers were subjected to hyperinsulinemic euglycemic clamps for 0 and 15 minutes. IRS-1 was immunopurified from homogenized vastus lateralis muscle biopsy samples and immunoblotted with αpS307. Under these conditions, insulin strongly stimulated phosphorylation of IRS-1 on Ser307 (Figure 5c). Interestingly, phosphorylation of IRS-1 on Ser307 increased after prolonged insulin treatment (data not shown).
Insulin and TNF-α stimulate distinct pathways that converge at Ser307.
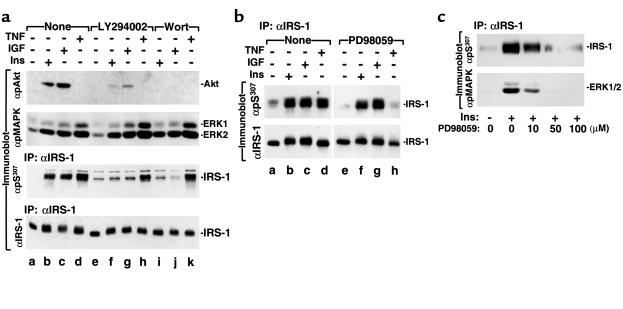
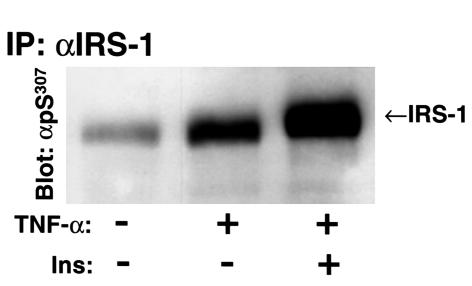
Distinct signaling pathways mediate insulin and TNF-α signal transduction, but both ligands promote Ser307 phosphorylation. LY294002 or wortmannin were used to determine whether PI 3-kinase–dependent pathways mediate Ser307 phosphorylation during insulin/IGF-1 or TNF-α stimulation. As expected, the PI-3 kinase inhibitors LY294002 or wortmannin inhibited activation of PKB/Akt in insulin or IGF-1 stimulated 3T3-L1 preadipocytes, but were without effect on ERK1/2. LY294002 or wortmannin abolished the phosphorylation of Ser307 induced by insulin/IGF-1 but not by TNF-α (Figure 6a). Both TNF-α and insulin/IGF-1 stimulated phosphorylation and activation of ERK1/2, which was blocked by PD98059 (Figure 6a, and data not shown). TNF-α–stimulated activation of ERK1/2 was reduced to the basal level within 2 hours (data not shown). However, PD98059 inhibited Ser307 phosphorylation induced by TNF-α, but not by insulin/IGF-1 (Figure 6b). The inhibition of TNF-α–induced Ser307 phosphorylation by PD98059 was dose-dependent and was correlated with the inhibition of ERK1/2 (Figure 6c). These results suggest that elements of the PI 3-kinase cascade mediate insulin/IGF-1–stimulated phosphorylation of Ser307, whereas MEK1 or another PD98059-sensitive kinase might mediate the effects of TNF-α. Consistent with the idea that two distinct signaling pathways mediate the phosphorylation of Ser307 in IRS-1 by insulin and TNF-α, treatment of cells simultaneously with both TNF-α and insulin stimulates much stronger phosphorylation of Ser307 than does treatment with either insulin or TNF-α alone (Figure 7).
Figure 6.
Two distinct pathways mediate Ser307 phosphorylation on IRS-1 induced by insulin/IGF-1 and TNF-α. (a) The 3T3-L1 preadipocytes were preincubated for 30 minutes with 20 μM LY294002 or 100 nM wortmannin (Wort) before stimulation for an additional 30 minutes with 100 nM insulin, 100 ng/ml IGF-1, or 20 ng/ml TNF-α. Proteins (50 μg) in the cell lysates were immunoblotted directly with antibodies against phospho-Akt or phospho-MAPK; or IRS-1 was immunoprecipitated from the lysates with αIRS-1 and immunoblotted with αpS307 or with αIRS-1. (b) The 3T3-L1 preadipocytes were preincubated for 30 minutes with 100 μM PD98059 before stimulation for an additional 30 minutes with 100 nM insulin, 100 ng/ml IGF-1, or 20 ng/ml TNF-α. IRS-1 in cell lysates was immunoprecipitated with αIRS-1. Half of the αIRS-1 immunoprecipitates were immunoblotted with αpS307, and half were immunoblotted with αIRS-1. (c) The 3T3-L1 preadipocytes were preincubated for 30 minutes with the indicated concentration of PD98059 before stimulation for an additional 30 minutes with 20 ng/ml TNF-α. Immunopurified IRS-1 was immunoblotted with αpS307. Cell extracts were immunoblotted with anti–phospho-MAPK.
Figure 7.
TNF-α and insulin act synergistically to promote phosphorylation of Ser307 in IRS-1. The 3T3-L1 preadipocytes were treated for 30 minutes with 20 ng/ml TNF-α and 100 nM insulin either separately or simultaneously. IRS-1 in cell lysates was immunoprecipitated with αIRS-1 and immunoblotted with αpS307.
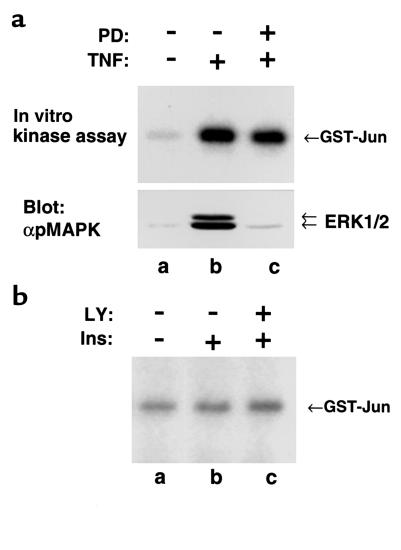
The close relation between MEK1 activity and Ser307 phosphorylation was unexpected, because JNK-1 was thought to mediate Ser307 phosphorylation during TNF-α stimulation (26). To determine whether JNK is involved in phosphorylation of Ser307 in response to TNF-α, 3T3-L1 preadipocytes were treated with 20 ng/ml TNF-α in the presence or absence of PD98059. JNK-1 was immunoprecipitated with anti–JNK-1, and subjected to an in vitro kinase assay using GST-cJun as a substrate. TNF-α stimulated robust activation of both JNK-1 and ERK1/2. PD98059 inhibited the activation of ERK1/2, but not JNK (Figure 8a). Because PD98059 inhibits Ser307 phosphorylation without any effect on JNK-1 activation, JNK-1 is unlikely to mediate TNF-α–induced Ser307 phosphorylation. Insulin only slightly stimulated JNK-1 activation, and the PI 3-kinase inhibitor LY294002 did not inhibit JNK-1, suggesting that JNK-1 is not the kinase to phosphorylate Ser307 in response to insulin (Figure 8b).
Figure 8.
Kinase(s) other than JNK phosphorylate Ser307 in IRS-1. (a) The 3T3-L1 preadipocytes were preincubated for 30 minutes with or without 100 μM PD98059 before stimulation for an additional 15 minutes with 20 ng/ml TNF-α. JNK-1 was immunoprecipitated with αJNK-1, and subjected to an in vitro kinase assay using GST-cJun as a substrate (top). Cell lysates were immunoblotted with anti–phospho-MAPK (bottom). (b) The 3T3-L1 preadipocytes were preincubated for 30 minutes with or without 20 μM LY294002 before stimulation for an additional 10 minutes with 100 nM insulin. JNK-1 was immunoprecipitated with αJNK1, and subjected to an in vitro kinase assay using GST-cJun as a substrate.
Discussion
In addition to type 2 diabetes, insulin resistance is associated with a variety of physiological states, including trauma and infection, hypertension, glucose intolerance, and obesity; however, the molecular mechanisms that modulate insulin signaling under these conditions are difficult to resolve. Many studies suggest that serine phosphorylation of the IRS proteins might inhibit insulin signaling, but the identification of physiologically relevant sites and the regulation of their phosphorylation and dephosphorylation has progressed slowly (26, 32–34). Recently, we discovered that JNK-1 associates with IRS-1 and promotes phosphorylation of Ser307. Although our current results show that JNK is not the only kinase that phosphorylates Ser307, several results including a Ser307→Ala substitution reveals that the phosphorylation of Ser307 inhibits insulin-stimulated tyrosine phosphorylation of IRS-1 in cultured cells (26).
The phosphorylation of specific residues in IRS-1 is an attractive regulatory mechanism because it provides specificity at multiple levels, including the interaction between specific serine kinases and IRS-1, regulation of the associated kinase activity, and selection of specific phosphorylation sites. However, it is difficult to demonstrate that serine phosphorylation of IRS-1 changes under specific physiological conditions, because IRS-1 contains nearly 100 potential serine phosphorylation sites that complicate ordinary biochemical analysis. Immunodetection of specific serine or threonine phosphorylation sites is successful in many cases. Applying this strategy to IRS-1, we prepared αpS307, a polyclonal antiserum generated in rabbits against the peptide sequence around phosphorylated Ser307 in IRS-1. Several experiments demonstrate that αpS307 recognizes specifically the phosphorylated Ser307 residue: αpS307 fails to immunoblot IRS-1 immunoprecipitated from quiescent cells; αpS307 strongly immunoblots IRS-1 immunoprecipitated from cells stimulated with anisomycin, TNF-α, IGF-1, or insulin; under stimulated conditions, dephosphorylation of IRS-1 immunoprecipitates with alkaline phosphatase inhibits immunoreactivity of αpS307; and phosphorylated peptides used to generate αpS307 inhibit immunoblotting, whereas unphosphorylated peptides have no inhibitory effect. Finally, αpS307 fails to recognize the mutant IRS-1 molecule containing the Ser307→Ala substitution. Together these results demonstrate that αpS307 reacts specifically with phosphorylated Ser307 in IRS-1 from mouse, rat, and human tissues; it is unreactive with other IRS proteins because the peptide sequence around Ser307 is unique to IRS-1.
The use of αpS307 provides clear evidence that Ser307 phosphorylation is regulated by several mechanisms. Although previous work suggested indirectly that anisomycin and TNF-α stimulate Ser307 phosphorylation, immunoblots with αpS307 confirm this conclusion for the first time. Moreover, these results support the hypothesis that the inhibitory effect of anisomycin and TNF-α on insulin-stimulated tyrosine phosphorylation is mediated by the phosphorylation of Ser307 (26). Moreover, immunoblotting with αpS307 can help establish biologic relevance, by assessing Ser307 phosphorylation in IRS-1 isolated from tissues. For example, Ser307 is phosphorylated in liver, fat, and muscle isolated from rats subjected to thermal injury, suggesting that TNF-α or other inflammatory cytokines might also have effects in animals (data not shown).
Surprisingly, insulin and IGF-1 stimulate phosphorylation of Ser307 in 3T3-L1 fibroblasts and adipocytes. This unexpected result appears to be physiologically important, as injection of insulin into mice or rats promotes Ser307 phosphorylation in muscle. In humans, pharmacological insulin levels achieved during a hyperinsulinemic euglycemic clamp stimulate Ser307 phosphorylation on IRS-1 immunopurified from muscle biopsies. Thus, the regulated phosphorylation of Ser307 might be a common pathway to counterregulate the insulin signal during traumatic stress or chronic hyperinsulinemia.
The phosphorylation of Ser307 in IRS-1 was first observed during in vitro phosphorylation experiments with recombinant IRS-1 and JNK-1. JNK-1 associates specifically with IRS-1 and, during this association, mediates phosphorylation of Ser307 (26). Because JNK-1 is activated by TNF-α or anisomycin, these results reveal a pathway to regulate IRS-1 serine phosphorylation. However, the finding that insulin stimulates Ser307 phosphorylation of IRS-1 suggests that other kinases might also be involved. Interestingly, inhibition of PI 3-kinases by LY294002 and wortmannin abrogates phosphorylation of Ser307 induced by insulin and IGF-1, but not by TNF-α. In contrast, inhibition of MEK1 by PD98059 abolishes phosphorylation of Ser307 induced by TNF-α, but not by insulin or IGF-1; this finding is unexpected because PD98059 is not reported to inhibit JNK or kinases upstream of JNK activation and suggests that another kinase might mediate the effect of TNF-α on IRS-1. Thus, at least three kinases mediate phosphorylation of Ser307, including JNK, serine kinases in the PI 3-kinase cascade that are activated by insulin or IGF-1, and MEK1-sensitive kinase cascades during TNF-α stimulation. Thus, Ser307 phosphorylation might integrate signals mediated through multiple serine kinases, including those promoted by heterologous ligands as well as insulin itself.
Interestingly, although PD98059 blocks the MEK/ERK pathway activated by both insulin/IGF-1 and TNF-α, it only inhibits TNF-α-induced Ser307 phosphorylation. These observations suggest that the MEK/ERK cascade might be required, but is not sufficient for Ser307 phosphorylation. Perhaps other signals that are specific to TNF-α but not insulin/IGF-1 act together with the MEK/ERK pathway to induce Ser307 phosphorylation. In this scenario, the MEK/ERK pathway is not involved in phosphorylation of Ser307 in response to insulin and IGF-1. Alternatively, an unidentified, PD98059-sensitive kinase(s) that is activated by TNF-α but not insulin/IGF-1 mediate TNF-α-stimulated Ser307 phosphorylation.
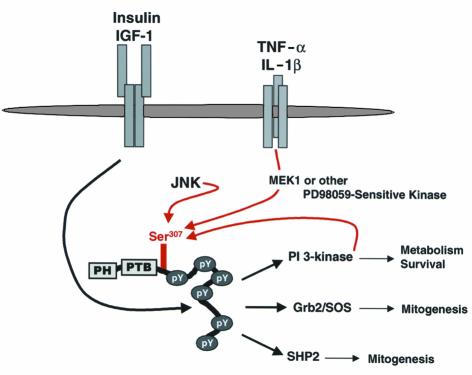
Our results suggest that phosphorylation of Ser307 in IRS-1 might be a common site integrating heterologous inhibition of the insulin signal by certain cytokines with feedback inhibition during chronic hyperinsulinemia (Figure 9). Consistent with this conclusion, insulin and IGF-1 act synergistically with TNF-α to promote phosphorylation of Ser307 to a much higher extent. A potential mechanism for peripheral insulin resistance emerges from these results. During the early stages of glucose intolerance, moderate hyperinsulinemia compensates for peripheral insulin resistance. Although this strategy promotes adequate metabolic regulation in moderate cases, chronic hyperinsulinemia might increase the steady-state level of Ser307 phosphorylation, eventually reducing the available pool of active IRS-1 molecules. Compensation might continue to adjust to this dynamic interaction until heterologous mechanisms, such as cytokine-stimulated phosphorylation of Ser307, exacerbate the situation and diabetes ensues because β cells fail to produce sufficient insulin (Figure 9).
Figure 9.
Models for the function of Ser307 phosphorylation. Upon insulin stimulation, IRS-1 is tyrosyl-phosphorylated by the IR, resulting in the activation of PI 3-kinase that mediates Ser307 phosphorylation. Ser307 phosphorylation subsequently inhibits the ability of IRS-1 to be further tyrosyl phosphorylated by the IR and to propagate insulin signaling. An increase in Ser307 phosphorylation, such as that possibly trigged by TNF-α in chronic obesity, also induces insulin resistance. As β cells compensate for insulin insensitivity with compensatory hyperinsulinemia, chronic insulin stimulation induces more Ser307 phosphorylation through the PI 3-kinase pathway, thus further increasing the pool of inactive, Ser307 phosphorylated IRS-1. This vicious cycle might continue until hyperinsulinemia fails to compensate for insulin resistance.
At the molecular level, the mechanism of inhibition of IRS-1 by Ser307 phosphorylation is an important question, but the answer is unclear. Previous reports suggest that serine or threonine phosphorylation of IRS-1 impairs its association with the insulin receptor (23, 35, 36). This inhibition might result from dysregulation of PTB domain. Previous reports indicate that TNF-α might inhibit the ability of the PTB domain in IRS-1 to interact with the phosphorylated NPEY-motif in the activated insulin receptor (23). Given that Ser307 is adjacent to the PTB domain in IRS-1, this mechanism must be directly tested. Alternatively, phosphorylation of Ser307 might create a binding site for inhibitory molecules, including the binding of protein tyrosine phosphatases.
In summary, TNF-α, insulin, and IGF-1 stimulate phosphorylation of Ser307 in IRS-1 in cultured cells. Insulin also stimulates phosphorylation of Ser307 in skeletal muscle of mice, rats and humans. Insulin– and IGF-1–induced phosphorylation of Ser307 depends on PI 3-kinase, whereas phosphorylation of Ser307 induced by TNF-α requires the activation of MEK1 or an unknown PD98059-sensitive kinase. Phosphorylation of Ser307 inhibits subsequent tyrosyl phosphorylation of IRS-1 and activation of IRS-1–mediated signaling pathways in response to insulin, which might contribute to insulin resistance.
Acknowledgments
We thank Tohru Uchida and Tracy Fisher for their technical support, and Lauren Kelly for her assistance with manuscript preparation.
References
- 1.Withers DJ, White MF. Insulin action and type 2 diabetes: lessons from knockout mice. Curr Opin Endocrinol Diab. 1999;6:141–145. [Google Scholar]
- 2.DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Review. 1997;5:177–269. [Google Scholar]
- 3.DeFronzo RA, Prato SD. Insulin resistance and diabetes mellitus. J Diabetes Complications. 1996;10:243–245. doi: 10.1016/1056-8727(96)00046-3. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1997;73:1294–1301. doi: 10.1210/jcem-73-6-1294. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni RN, et al. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 6.Withers DJ, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 7.Yenush L, White MF. The IRS-signaling system during insulin and cytokine action. Bioessays. 1997;19:491–500. doi: 10.1002/bies.950190608. [DOI] [PubMed] [Google Scholar]
- 8.Pronk GJ, et al. Interaction between the p21ras GTPase activating protein and the insulin receptor. J Biol Chem. 1993;267:24058–24063. [PubMed] [Google Scholar]
- 9.Myers MG, Jr, White MF. Insulin signal transduction and the IRS proteins. Annu Rev Pharmacol Toxicol. 1996;36:615–658. doi: 10.1146/annurev.pa.36.040196.003151. [DOI] [PubMed] [Google Scholar]
- 10.Lee CH, et al. Nck associates with the SH2 domain docking proteins IRS-1 in insulin stimulated cells. Proc Natl Acad Sci USA. 1993;90:11713–11717. doi: 10.1073/pnas.90.24.11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maezono K, et al. PI 3-kinase but not map kinase signaling is insulin resistant in obesity and niddm. Diabetes. 1998;47:A333. (Abstr.) [Google Scholar]
- 13.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-α−induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–976. doi: 10.1074/jbc.272.2.971. [DOI] [PubMed] [Google Scholar]
- 15.Egawa K, et al. Persistent activation of phosphatidylinositol 3-kinase causes insulin resistance due to accelerated insulin-induced insulin receptor substrate-1 degradation in 3T3-L1 adipocytes. Endocrinology. 2000;141:1930–1935. doi: 10.1210/endo.141.6.7516. [DOI] [PubMed] [Google Scholar]
- 16.Elchebly M, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein BJ, Ahmad F, Ding W, Li PM, Zhang WR. Regulation of the insulin signalling pathway by cellular protein-tyrosine phosphatases. Mol Cell Biochem. 1998;182:91–99. [PubMed] [Google Scholar]
- 18.Hotamisligil GS. Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 19.Carter EA. Insulin resistance in burns and trauma. Nutr Rev. 1998;56:S170–S176. doi: 10.1111/j.1753-4887.1998.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 20.Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor α-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. 1995;270:23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- 21.Hotamisligil GS, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 22.Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-α inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem. 1996;271:13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- 23.Paz K, et al. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem. 1997;272:29911–29918. doi: 10.1074/jbc.272.47.29911. [DOI] [PubMed] [Google Scholar]
- 24.Tanti JF, Gremeaux T, Van Obberghen E, Le Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem. 1994;269:6051–6057. [PubMed] [Google Scholar]
- 25.Mothe I, Van Obberghen E. Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J Biol Chem. 1996;271:11222–11227. doi: 10.1074/jbc.271.19.11222. [DOI] [PubMed] [Google Scholar]
- 26.Aguirre V, Uchida T, Yenush L, Davis RJ, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 27.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotamisligil GS. The role of TNF-alpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo, R.A. 1995. Insulin resistance and hyperinsulinemia: the link between NIDDM, CAD, hypertension and dyslipidemia. In New horizons in diabetes mellitus and cardiovascular disease. C.J. Schwartz and G.V.R. Born, editors. Science Press. London, United Kingdom. 11–27.
- 31.Sun XJ, et al. The structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 32.De Fea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. J Biol Chem. 1997;272:31400–31406. doi: 10.1074/jbc.272.50.31400. [DOI] [PubMed] [Google Scholar]
- 33.Barthel A, Nakatani K, Dandekar AA, Roth RA. Protein kinase C modulates the insulin-stimulated increase in Akt1 and Akt3 activity in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1998;243:509–513. doi: 10.1006/bbrc.1998.8134. [DOI] [PubMed] [Google Scholar]
- 34.Lam K, Carpenter CL, Ruderman NB, Friel JC, Kelly KL. The phosphatidylinositol 3-kinase serine kinase phosphorylates IRS-1: stimulation by insulin and inhibition by wortmannin. J Biol Chem. 1994;269:20648–20652. [PubMed] [Google Scholar]
- 35.Tanti JF, Gremeaux T, Van Obberghen E, Le Marchand-Brustel Y. Insulin receptor substrate 1 is phosphorylated by the serine kinase activity of phosphatidylinositol 3-kinase. Biochem J. 1994;304:17–21. doi: 10.1042/bj3040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staubs PA, Nelson JG, Reichart DR, Olefsky JM. Platelet-derived growth factor inhibits insulin stimulation of insulin receptor substrate-1 associated phosphatidylinositol 3-kinase in 3T3-L1 adipocytes without affecting glucose transport. J Biol Chem. 1998;273:25139–25147. doi: 10.1074/jbc.273.39.25139. [DOI] [PubMed] [Google Scholar]