Abstract
There is considerable confusion concerning the mechanism of lymphocyte death during HIV infection. During the course of HIV infection, M-tropic viruses (R5) that use CCR5 chemokine coreceptors frequently evolve to T-tropic viruses (X4) that use CXCR4 receptors. In this study we show that activation of the CD4 or CCR5 receptor by R5 HIVenv causes a caspase 8–dependent death of both uninfected and infected CD4 T cells. In contrast, CXCR4 activation by X4 HIVenv induces a caspase-independent death of both uninfected CD4 and CD8 T cells and infected CD4 cells. These results suggest that activation of the chemokine receptor by HIVenv determines the mechanism of death for both infected and uninfected T lymphocytes.
Introduction
Enhanced depletion of mature peripheral T cells is in part responsible for the progressive decline of CD4 and, in the late stages of HIV infection, of CD8 T cells. Multiple mechanisms have been proposed to explain the depletion of peripheral T cells that fall into two broad categories overall: “indirect” death of uninfected T cells by apoptosis secondary to their aberrant activation and “direct” killing of HIV-infected T cells. Furthermore, it has been suggested that these two forms of T-cell death are mediated by different molecular mechanisms. Uninfected CD4 T cells may undergo apoptosis after their aberrant activation by soluble or membrane-bound HIVenv by CD4 (1–5). This env/CD4–dependent activation of the T lymphocyte by HIVenv sensitizes uninfected cells to a Fas/FasL–mediated apoptosis, which is a caspase-dependent process (6, 7). HIVenv also mediates apoptosis of active HIV-infected T cells (4, 8). In contrast, this direct HIV-mediated T-cell death is independent of caspase activation (9). Furthermore, HIV-induced cell death of the infected cell is not antagonized by antiapoptotic molecules such as bcl-2 (10).
During the early stages of HIV infection, M-tropic or R5 strains predominate. These strains use both the CCR and CD4 receptors for viral entry and are clinically associated with a decline in CD4, but not CD8 T cells. In later stages of infection, HIV evolves into the T-tropic or X4 strain, which requires CXCR4 as its coreceptor for viral entry. This stage is clinically characterized by depletion of CD4 T cells and, importantly, depletion of CD8 T cells (11–16). The apparent different behavior of R5 and X4 strains with regard to CD4 T-cell depletion has been confirmed recently in SHIV-infected macaques (17). While the underlying molecular basis for this observation is unknown, it suggests that the chemokine HIV coreceptor, and hence HIVenv, may play a major role in T-cell death.
In this study we have investigated whether HIVenv is responsible for both the death of primary-uninfected and HIV-infected T cells by studying the role of the HIVenv/chemokine coreceptor interaction in mediating T-cell death.
Methods
Cells, cell-receptor stimulation, and reagents.
PBMCs were isolated by Ficoll-Hypaque from healthy blood donors, and CD4 and CD8 cells were purified by negative depletion using magnetic columns (Stem Cell Technologies Inc., Vancouver, British Columbia, Canada) as described previously (6), yielding CD4 (99% CD3+ and 98% CD4) and CD8 (98% CD3+ and 91% CD8) T-lymphocyte populations, as determined by using flow cytometry. Whole blood from CCR5 Δ32-mutant donors was a generous gift from Ronald Collman (University of Pennsylvania, Philadelphia, Pennsylvania, USA).
Lymphocyte receptors were cross-linked with Ab’s as described previously (6). Primary Ab was used at 5 μg/2 × 106 cell/ml for 1 hour at 4°C. Anti-CD4 Ab (Leu-3a) and isotype IgG controls were purchased from Becton Dickinson Immunocytometry Systems (San Diego, California, USA). Anti-CXCR4 12G5 and anti-CCR5 MAB183 Ab’s were obtained form the NIH AIDS Research and Reference Program (Bethesda, Maryland, USA). Recombinant soluble(s) X4 (HIVLAI) gp120 was purchased from Protein Sciences Corp. (Bethesda, Maryland, USA) and incubated with lymphocytes at a concentration of 5 μg/2 × 106 cell/ml for 1 hour. Primary 92Ug20.9 X4 env was generously donated by Claudia Cicola (NIH) and used at 20 nM. In some experiments, results were confirmed using 0.5 μg/2 × 106 cells. Ab- or gp120-treated primary T cells were then counted and transferred to 96-well plates in triplicate at a concentration of 105 cells/well. Cells assessed for Fas-mediated apoptosis were further treated with anti-Fas cross-linking IgM Ab (CH-11) (Upstate Biotechnology Inc., Lake Placid, New York, USA) for 18 hours at 37°C. In some experiments, CD4 and CD8 T cells were pretreated with the pancaspase inhibitor Z-VAD (Calbiochem-Novabiochem Corp., San Diego, California, USA), the caspase 8 inhibitor Z-IETD (Calbiochem-Novabiochem Corp.), or caspase 9 inhibitor Z-LEHD (Calbiochem-Novabiochem Corp.) at a concentration of 20 μM at 37°C for1 hour. Pertussis toxin (Calbiochem-Novabiochem Corp.) was used at a concentration of 1 μg/mL at 37°C for 1 hour. SDF1α (Becton Dickinson Immunocytometry Systems) was used at 250 nM when incubated with primary lymphocytes for 1 hour at 37°C. MIP1β (Becton Dickinson Immunocytometry Systems) was incubated with lymphocytes for 1 hour at 37°C at 1 μg/ml. Soluble sCD4 (NIH AIDS Research and Reference Program) was used at 2–100 μg/ml for 30 minutes at 4°C when incubated with soluble gp120 or 293T-env–expressing cells. IL-4 (R&D Systems Inc., Minneapolis, Minnesota, USA) was used at 10 ng for 48 hours at 37°C. Azidothymidine (AZT) was purchased from Sigma Chemical Co. (St. Louis, Missouri, USA).
Cell-mixing experiments, cell-death analysis, and flow cytometry.
293T cells were transiently transfected by Fugene (Roche Molecular Biochemicals, Indianapolis, Indiana, USA) according to manufacturer’s specification, with an HIV-rev–expressing plasmid (pREV) and an empty vector (SFFV), or SFFV plasmid expressing the envelope cDNA of the R5 strain HIVJRFL, or the X4 strain HIVHXB2 (provided by D. Littman, Skirball Institute, New York University Medical Center, New York, New York, USA). After 48 hours, the 293T cells were harvested and mixed in suspension with CD4 or CD8 T cells at 106 293T cells/2 × 106 lymphocytes in duplicate in a 24-well plate for 2 hours. T cells were then harvested, counted, and plated in triplicate in 96-well plates at 105 T cells/well for 18 hours. To determine expression of env, transfected 293T cells were lysed, separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with anti-gp120 Ab’s (NIH AIDS Research and Reference Program).
The percentage of cell mortality was calculated using Trypan blue dye exclusion as follows: 1 – (total number of cells viable on day 2/total number of cells viable on day 1 immediately after stimulation) × 100. Cell death was also confirmed in each experiment using two flow-cytometry–based methods: cells with reduced FSC and increased hypodiploid DNA content following propidium iodide staining (18). For the flow-cytometric methods, the percentage of cell death is presented as: percent of death in infected sample minus percent of baseline death in noninfected sample. For terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assays, 2 × 106 CD4 T cells were incubated with 106 293 T cells at 37°C for 24 hours. Cells were treated with terminal deoxynucleotidyl transferase and strained with FITC-labeled UTP according to manufacturer’s instructions (Coulter Beckman Electronics Ltd., Miami, Florida, USA). In these experiments, lymphocytes were isolated by selectively gating on CD4+ cells in which cell death was subsequently analyzed.
To determine the expression of CD4 and chemokine receptor, 106 T cells were stained with anti-CXCR4 (12G5)–FITC (Becton Dickinson Immunocytometry Systems), anti-CCR5 (2D7)–FITC (PharMingen, San Diego, California, USA), anti–CD4-phycoerythrin (anti–CD4-PE) (Caltag Laboratories Inc., Burlingame, California, USA), or mouse isotype controls (Becton Dickinson Immunocytometry) as previously described (6). Flow cytometry was performed using a FACScan (Becton Dickinson Immunocytometry Systems), and analysis was done using CellQuest software. Laser-scanning confocal microscopy was performed using a Zeiss LSM-510 (Carl Zeiss Inc., Thornwood, New York, USA). Images were saved at eight bits per channel and were analyzed for fluorescent interacting using a computer analysis package ANALYZE (Mayo Foundation, Rochester, Minnesota, USA).
HIV infection.
Five-day-old phytohemagglutinin (PHA) (5 μg/ml; Roche Molecular Biochemicals) and 200 IU of IL-2 (Chiron Therapeutics, Emeryville, California, USA) treated 5 × 106 CD4 T cells were infected with the X4 HIV strain(LavBru) and the R5 HIV strain(JR-FL) (NIH AIDS Research Reference Program) (360 ng of p24) or mock infected. Cells were thereafter passed every 3 days at a concentration of 106 cells/ml. For some experiments (see Figure 5b), 4- to 6-day-old HIV-infected CD4 T cells were washed and treated with 5 μM of AZT for 6 hours, followed or not followed by the addition of 20 μM of the various caspase inhibitors. Eighteen hours later cells were fixed and permeabilized (Fix and Perm; Caltag Laboratories) and stained for intracytoplasmic HIV p24 using anti–p24-FITC Ab’s (Coulter Beckman Electronics Ltd.). Flow cytometry was performed using FACScan, and analysis was done using CellQuest software.
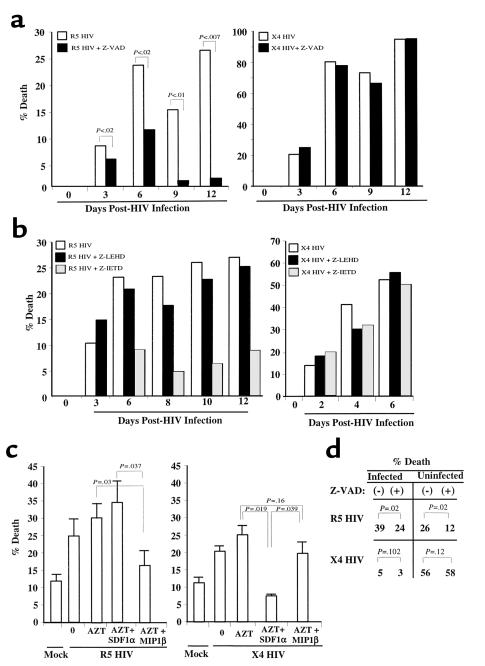
Figure 5.
Infected CD4 T-cell blasts die by chemokine receptor–dependent mechanisms. (a) PHA and IL-2–stimulated cells were infected with X4 HIV(Lav-Bru), or R5 HIV(JRFL), or mock (NI) virus. Cells were counted every 2–3 days and analyzed for cell death or by using flow cytometry changes in FSC consistent with apoptosis and for PI cell cycle. On day 6 of infection the percentage of cells infected with the R5 HIV strain shown to be apoptotic by using PI staining were 25% (– Z-VAD) and 15% (+ Z-VAD) or by FSC were 29% (– Z-VAD) and 19% (+ Z-VAD). On day 9 of infection the percentage of cells infected with X4 HIV shown to be apoptotic by PI staining were 71% (– Z-VAD) and 69% (+ Z-VAD) or by FSC were 68% (– Z-VAD) and 64% (+ Z-VAD). p24 values from the supernatants were: R5, 293(+)Z-VAD and 178(–)Z-VAD (P < 0.1); X4, 41(+)Z-VAD and 40(–) Z-VAD. SD = <10% at each data point. (b) The procedure was the same as in a, except cells were incubated in the presence or absence of caspase 8 inhibitor (Z-IETD) or caspase 9 inhibitor (Z-LEHD). (c) PHA and IL-2 stimulated and infected with R5 HIV(JR-FL), or X4 HIV(Lav-Bru), or mock infected were treated with AZT for 6 hours on day 3 of X4 HIV infection and on day 6 of R5 HIV infection. Thereafter, cells were extensively washed and incubated in the presence or absence of SDF1α or MIP1β, and cell death was analyzed 24 hours later. (d) Chemokine-receptor activation mediates death of directly HIV-infected and -uninfected CD4 T cells. PHA and IL-2–stimulated cells were infected with R5 HIV(JR-FL), or X4 HIV(Lav-Bru), or mock infected (NI). On day 4 for X4 HIV infection and day 6 for R5 HIV infection, cells were treated with AZT, and half the population was treated with Z-VAD; 18 hours later cells were stained for intracytoplasmic p24. The percentage of p24+ cells and apoptotic cells, as determined changes by FSC, were analyzed by using flow cytometry. The percentage of p24+ in the R5 HIV infection of T cells was 11% and 15% in the X4 HIV infection (+). SD = <10% at each data point.
Statistical analysis.
Experiments from every figure were performed in duplicate and repeated at least three times. All measurements are presented as means and SD’s with statistical comparisons made between conditions using the Student’s t test for paired observations.
Results
Individual activation of CCR5 and CD4 in uninfected CD4 T cells leads to a caspase 8–dependent death.
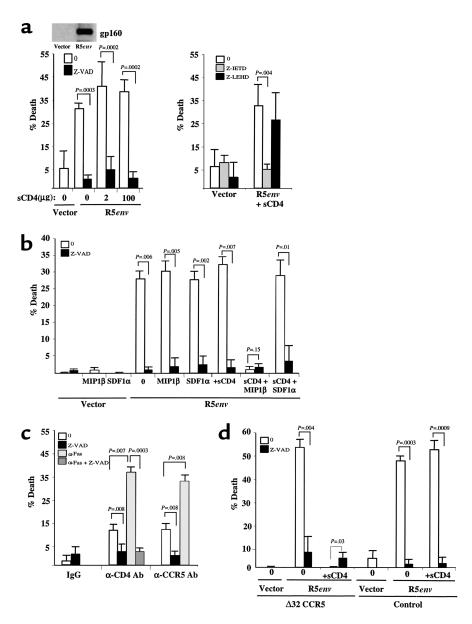
To investigate how HIVenv/chemokine coreceptor interactions may mediate CD4 T-cell death, we first investigated whether R5env mediates death of uninfected T cells. Purified resting CD4 T lymphocytes were coincubated with 293T cells expressing R5env or empty vector. R5env, as compared with empty vector, caused CD4 T-cell death, which was blocked by the pancaspase inhibitor Z-VAD (Figure 1a). Because R5env interacts with both CD4 and CCR5 receptors, the function of each receptor in mediating R5env-triggered death was investigated individually. To study the R5env-triggered death by CCR5, 293T cells expressing R5env or empty vector were preincubated with soluble CD4 (sCD4). This protein blocks the interaction of HIVenv with membrane CD4 and also causes a conformational change that enhances its binding to the chemokine receptor (19–22). When 293T cells expressing R5env were treated with different concentrations of sCD4, a caspase-dependent CD4 T-cell death continued to be observed (Figure 1a), suggesting that CCR5 mediates a caspase-dependent death of uninfected CD4 T cells. To identify the caspase mediating the R5env/CCR5–induced death, CD4 T cells were pretreated with caspase 8 (Z-IETD) or caspase 9 (Z-LEHD) inhibitors immediately before their coincubation with R5env-expressing 293T cells (preincubated with sCD4). The fact that Z-IETD, but not Z-LEHD, reversed the R5env/CCR5–induced death (Figure 1a, right panel) suggests that caspase 8, but not 9, is involved in the R5env/CCR5-induced T-cell death.
Figure 1.
CCR5 activation induces a caspase-dependent death of CD4 T cells. (a) CD4 T cells were incubated with 293T cells transfected with empty vector or 293T cells expressing the HIVJRFL-env (R5env), either untreated or treated with soluble CD4 at different concentrations (sCD4). 293T cells were lysed and blotted by duo, tropic anti-gp160 to verify envelope expression (left panel). CD4 T cells were left untreated, pretreated with Z-IETD (a caspase 8 inhibitor) or Z-LHED (a caspase 9 inhibitor) before incubation with 293T cells expressing empty vector or R5env (right panel). (b) CD4 T cells treated or not treated with Z-VAD, SDF1α, or MIP1β were incubated with 293T cells expressing R5env or empty vector pretreated or not pretreated with sCD4, respectively. (c) CD4 T cells were treated with IgG-matched isotype control, anti-CD4 Leu-3a (αCD4 Ab), or anti-CCR5 MAB183 (αCCR5 Ab), followed by their cross-linking with goat anti-mouse Ab’s. Each point was assessed for caspase-dependent death (Z-VAD) as well as for Fas susceptibility (αFas). (d) CD4 T cells from a healthy donor with the CCR5 Δ32 mutation or from a control donor lacking the Δ32 CCR5 deletion were pretreated or not pretreated with Z-VAD and coincubated with 293T cells expressing R5env or empty vector, pretreated or not pretreated with sCD4.
To confirm that CCR5 mediates R5env-triggered death of CD4 T cells, the cells were treated with the natural ligand of CCR5 (MIP1β) and, as control, with the natural ligand of CXCR4 (SDF1α) before their coculture with R5env-expressing 293T cells in the presence or absence of sCD4. As shown in Figure 1b, MIP1β, but not SDF1α, abrogated the caspase-dependent death triggered by R5env in the presence of sCD4. Both MIP1β and SDF1α treatment completely abrogated the expression of CCR5 and CXCR4 in these cells, respectively, as determined by using flow-cytometric analysis (not shown) and did not cause death (Figure 2b).
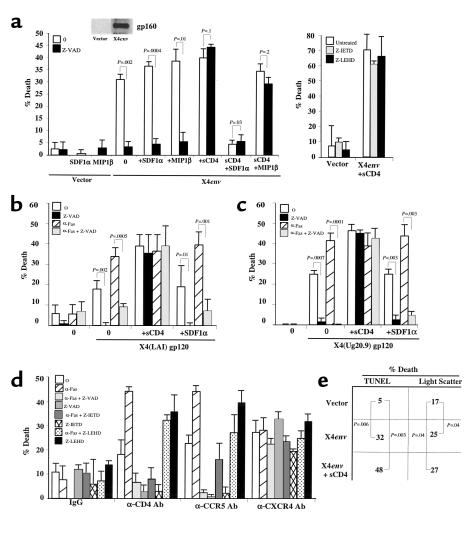
Figure 2.
CXCR4 activation induces a caspase-independent death of CD4 T cells. (a) CD4 T cells treated or not pretreated with Z-VAD, SDF1α, or MIP1β were incubated with 293T cells transfected with empty vector (vector) or HIVHXB2-env (X4env), and untreated or pretreated soluble CD4 (sCD4) (left panel). CD4 T cells were untreated or pretreated with Z-IETD (a caspase 8 inhibitor) or Z-LEHD (a caspase 9 inhibitor) before incubation with 293T cells expressing empty vector or X4env (right panel). (b) CD4 T cells were untreated or treated with soluble gp120 (X4 gp120) alone or in combination with soluble CD4 (sCD4). Some cells were pretreated with SDF1α to block CXCR4 receptors. Caspase-dependent death was analyzed using Z-VAD (Z-VAD), and Fas susceptibility was analyzed using treatment with anti-Fas Ab’s (αFas). (c) CD4 T cells were preincubated or not preincubated with SDF1α and treated or not treated with soluble primary (92Ug20.9) X4 gp120 that was preincubated or not preincubated with sCD4. (d) CD4 T cells were cross-linked with anti-CD4 Leu-3a (αCD4 Ab), anti-CCR5 MAB 183 (αCCR5 Ab), anti-CXCR4 12G5 (αCXCR4 Ab), or matched isotype control (IgG) and assessed for death and/or Fas susceptibility as in b in the presence or absence of the pancaspase inhibitor (Z-VAD), caspase 8 inhibitor (Z-IETD), and caspase 9 inhibitor (Z-LEHD). (e) CD4 T cells were preincubated with 293T expressing or not expressing X4env preincubated or not preincubated with sCD4. Cell death was analyzed using changes in the light scatter or by TUNEL analysis using FACS.
When these experiments are performed in the absence of sCD4, thus allowing for the interaction of R5env with both CD4 and CCR5, neither MIP1β nor SDF1α blocked the R5env-mediated caspase-dependent death. This suggests that both CD4 and CCR5 individually and independently mediate the caspase-dependent death when activated by R5env. Additional experiments confirmed that the CD4-mediated death also requires caspase 8, but not caspase 9 (data not shown and Figure 2d). The role of CCR5 and CD4 in individually mediating caspase-dependent death was further confirmed using cross-linking Ab’s against each receptor (Figure 1c).
The CCR5 Δ32 deletion present in CD4 T cells from HIV-seropositive individuals is associated with low or absent HIV replication and CD4 T-cell depletion. Based on this, we argued that CD4 T cells carrying such homozygous deletion would be refractory to R5env-mediated death by CCR5 but not by CD4. To test this, CD4 T cells from a healthy donor with a CCR5 Δ32 deletion were compared with those from a control individual lacking such deletion with regard to the ability of R5env to mediate CD4 T-cell death by CCR5. As shown in Figure 1d, CCR5 Δ32 CD4 T cells were killed by R5env in the absence of sCD4. However, preincubation of R5env-expressing 293T cells with sCD4 completely abrogated CD4 T-cell death from the Δ32 CCR5 donor, but not from the control individual. Taken together these data indicate that CCR5 is functional in mediating the R5env-triggered, caspase 8–dependent, CD4 T-cell death independently of CD4.
CXCR4 activation causes a caspase-independent death of uninfected CD4 T cells.
Based on our observation that CCR5 can mediate R5env death, we questioned whether the other HIV coreceptor (CXCR4) could exert a similar function. The 293T cells expressing the X4 HIVenv(HXB2) also triggered death of CD4 T cells in a caspase-dependent manner (Figure 2a, left panel). Because the X4env interacts with both CD4 and CXCR4, we further investigated the individual role of each receptor. Surprisingly, when X4env-expressing 293T cells were preincubated with sCD4 (allowing the interaction between X4env and CXCR4), the death incurred in CD4 T cells was caspase independent.
To confirm that CXCR4 can mediate a caspase-independent death when activated by X4env, CD4 T cells were preincubated with the CXCR4 ligand (SDF1α) and, as control, with the CCR5 ligand (MIP1β) (23–25) before their coculture with 293T cells expressing X4env in the presence or absence of sCD4. SDF1α, but not MIP1β, blocked the X4env-triggered (preincubated with sCD4) death of CD4 T cells (Figure 2a). However, in the absence of sCD4, neither SDF1α nor MIP1β inhibited the death of CD4 T cells triggered by X4env, once more highlighting that CD4 alone can trigger a caspase-dependent death when activated in isolation by either R5env or X4env. As expected, neither the caspase 8 nor caspase 9 inhibitor blocked the X4env/sCD4 death of CD4 T cells (Figure 2a, right panel), and SDF1α or MIP1β treatment did not cause CD4 T-cell death (Figure 2a) (26). Therefore, X4env can trigger a caspase-dependent death when interacting with CD4 but a caspase-independent one when interacting with CXCR4. This is in striking contrast with the R5env-mediated death, which is caspase dependent regardless of its interaction with CD4 or CCR5.
The ability of X4env/CXCR4 interactions to cause a caspase-independent CD4 T-cell death was further confirmed by activating CXCR4 through other ligands, such as soluble X4env and anti-CXCR4 Ab’s. As shown in Figure 2, b and c, two different soluble X4 gp120 (a laboratory-adapted strain, or LAI, and a patient-derived primary X4 HIV strain, 92Ug20.9) killed CD4 T cells in a caspase-independent manner in the presence of sCD4; an effect that was reversed in the presence of SDF1α. Likewise, when CD4 T cells were incubated with anti-CXCR4 cross-linked Ab’s, a caspase-independent death was observed (Figure 2d). This contrasts with the caspase 8-dependent death that ensues when CD4 T cells are activated by CD4 or CCR5 (Figure 2d).
Both the CD4- and CXCR4-mediated CD4 T-cell death were also evident by using TUNEL and changes in FSC were evident by using flow cytometry, suggesting both CD4- and CXCR4-triggered T-cell death are a form of an apoptotic process, even though it is mediated by different molecular mechanisms.
The relationship between CXCR4 and CD4 expression influences the mechanism of uninfected CD4 T-cell death.
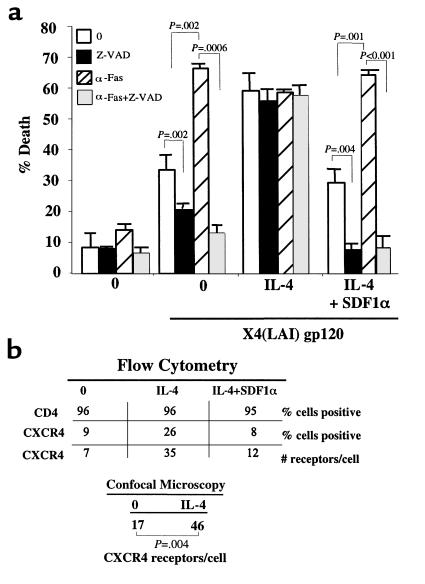
Although X4env binds to both CD4 and CXCR4, only a CD4 caspase-dependent death is observed when X4env is incubated with primary resting CD4 T cells, suggesting that the CD4 signal overrides the CXCR4 signal. This could be due to cross-talk between both receptors resulting in, possibly, the desensitization of CXCR4 signaling by CD4 (27, 28) and/or to the relative expression level of each receptor. Recent studies in HIV-infected patients demonstrate that lymphocytes in the periphery and lymphoid tissue have a decreased CD4/CXCR4 ratio resulting from higher expression of CXCR4 when compared with noninfected patients (29). This prompted us to investigate whether upregulation of CXCR4 expression in primary CD4 T cells would favor a X4env-mediated, caspase-independent death. For this purpose we used IL-4 to increase the surface expression of CXCR4 on T lymphocytes, and high levels of this cytokine are present in patients with late-stage HIV infection when a predominance of X4 HIV strains is noted (30, 31). In the absence of IL-4, X4 gp120 triggered a caspase-dependent cell death and further rendered the cell susceptible to Fas-mediated apoptosis (Figure 3a). However, in the IL-4 treated CD4 T cells, X4 gp120 induced a CXCR4, caspase-independent death (Figure 3a). Addition of SDF1α to IL-4–treated CD4 T cells reverted the X4 gp120-mediated death to a CD4 caspase-dependent death (Figure 3a). Therefore, X4env favors the CXCR4, caspase-independent mode of death when CXCR4 expression is increased relative to CD4.
Figure 3.
CXCR4/CD4 receptor ratio determines the mode of CD4 T-cell death. (a) Untreated, IL-4 alone, or IL-4– and SDF1α-treated CD4 T cells were incubated or not incubated (0) with soluble X4 gp120. Cells were assessed for Fas (αFas) susceptibility and caspase-dependent death (Z-VAD). (b) CD4 T cells from a were simultaneously stained for CD4 and CXCR4 and analyzed using flow cytometry. CD4 T cells stained for CXCR4 were also analyzed using confocal microscopy.
Chemokine receptors are constantly cycling to the cell surface; therefore, the percentage of CXCR4-expressing cells at any time point may underestimate the overall level of surface expression that is present during a 24-hour period in which the CD4 T cells are exposed to the different ligands. This could account for potential differences in the degree of death observed with respect to the chemokine receptor expression (32, 33). Using FACS and confocal-microscopy analysis, we demonstrated that IL-4 resulted in CXCR4 surface-expression upregulation per individual cell, which was suppressed by SDF1α, while not modifying CD4 surface expression (Figure 3b). While we can not rule out the possibility that IL-4 may directly enhance CXCR4 activity or interfere with the potential desensitization of CXCR4 by CD4, we confirmed that IL-4–treated or –untreated CD4 T cells remained equally susceptible to Fas-mediated apoptosis after cross-linking of CD4 with anti-CD4 Ab’s followed or not followed by anti-Fas Ab treatment (data not shown).
X4env/CXCR4 interaction causes a direct caspase-independent death of CD8 T cells.
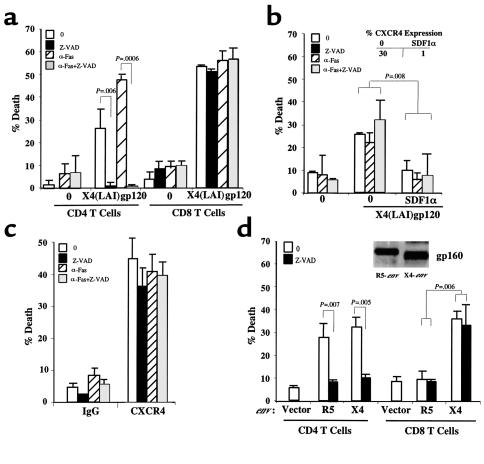
The observation that X4env, but not R5env, triggered caspase-independent CD4 T lymphocyte death by CXCR4 raised the possibility that CD8 T cells may also be direct targets for X4env. This scenario is clinically relevant because CD8 T cells, which are important in the control of HIV infection, are known to be progressively depleted in the late stages of HIV infection, especially when X4 HIV strains predominate (15, 16). Purified resting CD4 or CD8 T cells from the same donor were separately coincubated with soluble recombinant X4 (LAI) gp120. As expected, X4 gp120 triggered death of resting, non–IL-4-treated, CD4 T cells by a caspase-dependent mechanism and further sensitized CD4 T cells to Fas-dependent apoptosis (Figure 4a). However, X4 gp120 triggered CD8 T-cell death that was not blocked by caspase inhibitors and did not sensitize the CD8 T cell to Fas-mediated apoptosis (Figure 4a). CD8 T-cell death by apoptosis was again confirmed by changes in FSC/SSC by the use of flow cytometry (see Figure 4a legend). In this experiment, the surface expression of CXCR4 was similar in both CD4 and CD8 T cells (17% and 18%, respectively).
Figure 4.
CD8 T cells die by HIVenv-CXCR4 infection. (a) Purified CD4 and CD8 T cells were untreated (0) or treated with soluble X4 (LAI) gp120 and assessed for Fas susceptibility (αFas) or caspase-dependent death (Z-VAD). Ten percent of untreated cells, 23% of cells treated with X4 gp120, and 24% of cells treated with Z-VAD and X4 gp120 (P < 0.006) demonstrated changes in FSC compatible with apoptosis in flow cytometry. (b) CD8 T cells were untreated (0) or treated with SDF1α before incubating with X4 (LAI) gp120 and analyzed for caspase-dependent death (Z-VAD) or susceptibility to Fas-mediated apoptosis (αFas). CD8 T cells were simultaneously analyzed using flow cytometry for CXCR4 expression with and without SDF1α treatment (inset). (c) CD8 T cells were cross-linked with anti-CXCR4 12G5 Ab (αCXCR4 Ab) or matched isotype control (IgG) and assessed for Fas susceptibility (αFas) or caspase-dependent death (Z-VAD). (d) CD4 and CD8 T cells were mixed with 293T cells expressing empty vector (vector), HIVJRFL-env (R5env), or HIVHXB2-env (X4env). Cells were assessed for caspase-dependent death (Z-VAD). Transfected 293T cells were lysed and blotted by duotropic anti-gp160 Ab to verify envelope expression (inset).
To demonstrate that the X4 gp120–mediated death of primary CD8 T cells was triggered by CXCR4, purified CD8 T cells were treated or not treated with SDF1α before the addition of X4 gp120. As shown in Figure 4b, the caspase-independent death of CD8 T cells triggered by X4 gp120 was significantly decreased in the presence of SDF1α, which concurrently decreased CXCR4 expression in the same cells (Figure 4b, inset). The role of X4env/CXCR4 interactions in mediating CD8 T-cell death through caspase-independent mechanisms was again confirmed using cross-linked anti-CXCR4 Ab’s (Figure 4c).
We next investigated whether CD8 T-cell death could result from interactions with cells expressing membrane-bound X4 gp160 and whether R5env played a role in killing CD8 T cells. For this, X4env- or R5env-expressing 293T cells were incubated with either purified CD4 or CD8 T cells from the same donor in the presence or absence of caspase inhibitors. While R5env caused a caspase-dependent cell death of CD4 T cells, it did not induce death of purified CD8 T cells (Figure 4d). Interestingly, X4env-expressing cells caused a caspase-dependent death of resting, untreated CD4 T cells and a caspase-independent death of CD8 T cells (Figure 4d). The expression of CCR5 in this experiment was higher in CD8 than CD4 T cells (15% and 5%, respectively), while CXCR4 expression was the same (15% and 14%, respectively). As seen in Figure 3 for CXCR4, the expression level of CCR5 may underestimate the real expression present during the duration of the experiment due to the rapid cycling of these receptors to the cell surface. Therefore, the inability of R5env to cause CD8 T-cell death was not due to lack of CCR5 expression.
In conclusion, these findings highlight the different molecular mechanisms whereby death of uninfected primary T cells can be triggered by HIVenv through its specific interaction with chemokine receptors. Furthermore, our results confirm that both soluble and membrane-bound env mediate the same cell-death mechanisms and that X4, but not R5env, can directly kill CD8 T cells by CXCR4.
HIV-infected CD4 T cells die via chemokine receptor–mediated mechanisms.
Direct killing of HIV-infected CD4 T cells has been proposed by some groups as a major cause of CD4 T-cell depletion in HIV-infected patients. This death is thought to be mediated by caspase-independent mechanisms (9, 10). Interestingly, such conclusions have been determined through the use of X4 HIV strains. Our observation that HIVenv can signal different modes of death to uninfected T cells, depending upon which chemokine receptor is activated, led us to question whether the HIV coreceptors would also mediate the so-called direct death of the HIV-infected cell. If so, we would predict that R5 HIV strains would kill CD4 T cells via caspase-dependent mechanisms, an effect that would be reversed in the presence of the CCR5 ligand, MIP1β. On the contrary, we would predict that X4 HIV strains could kill via caspase-independent mechanisms and that the death would be blocked by SDF1α, if X4 HIV-mediated death is dependent upon the isolated interaction of X4env with CXCR4. Such observations would bridge the two schools of thought arguing for direct or indirect death as being caused by separate molecular mechanisms.
CD4 T cells were infected in parallel with R5 HIV(JR-FL) or X4 HIV(Lav-Bru) strains and treated or not treated with the pancaspase inhibitor Z-VAD immediately after infection. We observed that CD4 T cells infected with R5 HIV died via a caspase-dependent mechanism, similar to the mechanism of death observed in uninfected cells after R5env interactions with CD4 and CCR5. In contrast, CD4 T cells infected with X4-HIV died via a caspase-independent mechanism (Figure 5a), resembling the X4env/CXCR4-mediated death of uninfected IL-4–treated cells. HIV p24 values were higher in the culture supernatants of the R5 HIV-infected Z-VAD–treated samples, than the R5 HIV–infected non–Z-VAD-treated samples (Figure 5a legend), indirectly reflecting the higher viability of infected cells and hence resulting in overall higher viral load. The known downregulation of CD4 and potential upregulation of CXCR4 during HIV infection (29, 34–36) may shift the CD4/CXCR4 ratio, thus favoring a predominant interaction between X4env and CXCR4, ultimately resulting in a caspase-independent death. This would be similar to the pattern observed with IL-4 treatment in the uninfected CD4 T cells (Figure 3). The direct HIV-mediated cell death was further confirmed by FACS analysis (see Figure 5a legend). In addition, this experiment was repeated using two other R5 (SF162) and X4 (IIIB) HIV strains, yielding the same results.
To further characterize the specific caspase involved in the R5env-mediated death, additional experiments, similar to those described in Figure 5a, were performed, except that the HIV-infected cultures were incubated in the presence of the selective caspase 8 inhibitor (Z-IETD) or the caspase 9 inhibitor (Z-LEHD). As shown in Figure 5b, only Z-IETD inhibited the R5 HIV-mediated death. As expected from results shown in Figure 5a, X4 HIV–induced CD4 T-cell death was not blocked by either Z-IETD or Z-LEHD. This further suggests that the death of uninfected and HIV-infected cells by R5env and R5 HIV, respectively, is mediated by similar mechanisms.
To address formally whether it is the HIVenv interaction with the corresponding chemokine coreceptor that ultimately triggers death of the infected cells, we investigated whether blocking such interaction would result in decreased HIV-mediated death. Such observation would strongly argue for the hypothesis that it is the HIVenv viral protein that is ultimately responsible for the death of both uninfected and HIV-infected CD4 T cells. To test this hypothesis, we used the natural ligand of each chemokine receptor (MIP1β for CCR5 and SDF1α for CCR4) as inhibitors of the HIVenv/chemokine receptor interaction. This approach was proven to be feasible for the study of uninfected CD4 T cells (Figure 1b and 2b). To avoid the interference of the corresponding chemokine with new cycles of infection, HIV-infected CD4 T cells were first treated with the reverse transcriptase inhibitor, AZT. As shown in Figure 5c, the death observed in R5 HIV-infected, AZT-treated cells was not blocked by SDF1α but was significantly reduced by MIP1β. Conversely, the death observed in X4 HIV, AZT-treated cells was significantly reduced by SDF1α, but not by MIP1β. The number of CD4 T cells expressing intracytoplasmic p24 (Figure 5d and data not shown) was not different in the AZT-treated cells incubated or not incubated with the various chemokines, suggesting that the chemokine effect was exerted by interfering with the env/chemokine receptor interaction rather than by reducing HIV infection.
To investigate whether env expressed in the HIV-infected CD4 T cells mediates the death of both the infected and uninfected CD4 T cells present in the HIV-infected culture, primary CD4 T-cell blasts were infected with X4(Lav-Bru) or R5(JR-FL) HIV strains. On days 4–6 after infection the cells were treated with AZT to block any further infection. This was done in a similar manner as for Figure 5c when 15–20% of cells were determined to be HIV infected by intracytoplasmic p24 staining, because infection rates beyond these levels resulted in very rapid cell death that precluded adequate analysis using flow cytometry. After AZT treatment, half of the culture was treated with the pancaspase inhibitor Z-VAD, and after 18 hours, cells were analyzed for intracytoplasmic p24 and apoptosis using flow cytometry. Results from these experiments indicated that CD4 T-cell death was present in both the uninfected (p24–) and HIV-infected (p24+) cells (Figure 5d). As expected, the R5 HIV-mediated death of both infected and uninfected CD4 T cells was mediated via a caspase-dependent mechanism, whereas the X4 HIV–mediated death was not. The higher percentage of death observed in the uninfected versus infected cells within the X4 HIV–infected cultures is a function of the experimental design, because in later, postinfection times a higher degree of death is observed in the p24+ cells. Therefore, HIVenv activation of the chemokine receptors mediates the death of both infected as well as uninfected CD4 T cells present within the same HIV infected culture.
Taken together, these results indicate that the interaction of the HIVenv with the corresponding chemokine coreceptor mandates the molecular mechanism of T-cell death in both the uninfected bystander and HIV-infected T cells.
Discussion
Nearly a decade ago, HIVenv was proposed and confirmed as an HIV protein responsible for the direct killing of HIV-infected CD4 T cells (4). Soon after, it became clear that the env, either in a soluble or membrane-bound form, could also mediate death of uninfected bystander T cells (2–6). The recent identification of the HIV coreceptors has presented the opportunity to study their role in the HIVenv-mediated death. Our results have highlighted that the specific HIVenv-coreceptor interaction dictates the molecular mechanisms of T-cell death. Moreover, we have found a striking similarity between how HIV-infected cells and uninfected T cells die. Specifically, CCR5 activation induced a caspase-dependent death of both infected and uninfected CD4 T cells, and CXCR4 activation by X4env caused a caspase-independent death of both infected and uninfected CD4 T cells and uninfected CD8 T cells. This may explain the conclusions drawn by several studies arguing for a lack of caspase-mediated death of HIV-infected cells when X4 HIV strains were used. The bifunctional role of X4 env in causing a caspase-dependent death when interacting with the CD4 receptor, but a caspase-independent death when interacting with the CXCR4 receptor, highlights the separate roles of these two receptors in mediating lymphocyte death. Furthermore, these results are consistent with recent observations arguing that X4env is capable of mediating caspase-dependent death (37). Also, our results could explain the differences in rate and location of CD4 T-cell depletion by R5 or X4 SHIV-infected macaques, reported by Harouse et al. (17).
The peripheral T-cell depletion characteristic of HIV infection is thought to be due, at least in part, to death of uninfected T cells. The death of these uninfected T cells has been shown to occur in lymphoid tissue from HIV-infected patients when contacted by an HIV-infected cell (5). Our results suggest this may be explained by membrane-env–triggered death. In addition, soluble gp120 produced within the infected lymphoid tissue could also directly kill or sensitize the T cell to subsequent death. We have demonstrated that CD4 T-cell death occurs in both the HIV-infected and uninfected cells within the HIV-infected culture. Independently of the exact proportion of infected versus uninfected cell dying, our results clearly support that env-mediated killing by the corresponding chemokine receptor mandates the form of death for both infected and uninfected cells present in the HIV-infected culture. From previous studies that calculated the amount of viral proteins present in lymphoid tissue, concentrations of soluble gp120 ranging between 120 and 960 ng/mL may exist in the lymph nodes of HIV-infected individuals (38–40). In fact, we performed dose-response studies of soluble gp120 and found that concentrations as low as 500 ng/mL were sufficient to mediate significant T-cell death (P < 0.006).
Our results also raise the possibility that CD8 T cells may be depleted in vivo if enough X4env-expressing infected T cells or soluble X4env are present within lymphoid tissue. Previous studies have demonstrated that CD8 T cells, in addition to CD4 T cells, undergo apoptosis in lymphoid tissue from HIV-infected patients (5, 41, 42). The conclusion of such studies has been generally that CD8 T-cell death is secondary to their aberrant activation. An alternative explanation is that they are actively dying by X4env/CXCR4 interactions. Supportive evidence for this line of reasoning could result from determining whether R5 HIV-infected lymphoid tissue does not contain CD8 T-cell death, whereas X4 HIV does. However, our results contradict, in part, other studies that report that X4env indirectly triggers death of CD8 T cells by macrophage production of TNF (16). Using highly purified CD8 T cells in which every effort was made to limit monocyte contamination, we have shown that caspase inhibitors did not block X4env/CXCR4-triggered CD4 T-cell death, arguing against a role for TNF-mediated death in our studies, and hence of any effector monocyte contamination, because TNF-mediated death is caspase dependent. We believe that definitive proof that X4env mediates CD8 T-cell death will require analysis of lymphoid tissue of X4 HIV-infected patients because in vitro cultures of primary T cells, or even ex vivo infection of lymphoid tissue, may not ultimately reflect the true in vivo situation. This is important when considering AIDS pathogenesis, because CD8 T cells are key in the control of this chronic viral infection. The depletion and increased uncompensated turnover of CD8 T cells observed in the late stages of HIV infection, when more X4 strains may be present, will hinder the immune clearance of the virus.
The ratio of CD4 to chemokine-receptor expression may also be an important factor regulating T-cell death, especially in infected cells or those contacted by X4 HIV or X4env, respectively. Recently, several groups have described cell death by this CXCR4 mechanism in neurons and T cells (26, 43, 44). Therefore, this X4env/CXCR4 mechanism of death has profound implications for not only peripheral T-cell depletion, but also neuronal loss and AIDS-related dementia during the late stages of HIV infection. Another novel aspect of our results is the apparent protective role that CD4 may play in CXCR4-mediated death. The well-documented downregulation of CD4 in HIV-infected cells may explain why membrane-bound X4env can interact easily with CXCR4 on the surface of the infected T cell. In uninfected T cells, the upregulation of CXCR4, as shown recently to be the case in T cells from lymphoid tissue in HIV-infected patients, may suffice to alter the CD4/CXCR4 ratio to favor X4env/CXCR4 interactions.
The results of this study improves our understanding of the pathogenesis of HIV-mediated T-cell death. Specifically, we have shown that different death-prone signaling pathways are triggered by HIVenv interacting with its receptors and thereby clarifying the significant confusion existing as to how T cells die in the context of HIV infection.
Acknowledgments
This work was supported by NIH grant R01 AI40384. The authors would like to thank N.E. Vlahakis for technical support with confocal microscopy, C. Cicala and A. Fauci for providing us with 92Ug20.9 envelope protein, D. Littman for providing the HXB2 and JRFL gp160 plasmids, members of the Paya Laboratory for helpful discussions, and Teresa Hoff for manuscript preparation.
References
- 1.Cohen J. What causes the immune system collapse seen in AIDS? Science. 1993;260:1256. doi: 10.1126/science.8098551. [DOI] [PubMed] [Google Scholar]
- 2.Zarling JM, et al. HIV-infected humans, but not chimpanzees, have circulating cytotoxic T lymphocytes that lyse uninfected CD4+ cells. J Immunol. 1990;144:2992–2998. [PubMed] [Google Scholar]
- 3.Nardelli B, Gonzalez CJ, Schechter M, Valentine FT. CD4+ blood lymphocytes are rapidly killed in vitro by contact with autologous human immunodeficiency virus-infected cells. Proc Natl Acad Sci USA. 1995;92:7312–7316. doi: 10.1073/pnas.92.16.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurent-Crawford AG, et al. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retroviruses. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 5.Finkel TH, et al. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 6.Algeciras A, Dockrell DH, Lynch DH, Paya CV. CD4 regulates susceptibility to Fas ligand- and tumor necrosis factor-mediated apoptosis. J Exp Med. 1998;187:711–720. doi: 10.1084/jem.187.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westendorp MO, et al. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 8.Corbeil J, Richman DD. Productive infection and subsequent interaction of CD4-gp120 at the cellular membrane is required for HIV-induced apoptosis of CD4+ T cells. J Gen Virol. 1995;76:681–690. doi: 10.1099/0022-1317-76-3-681. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi RT, et al. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med. 1998;187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolesnitchenko V, et al. A major human immunodeficiency virus type 1-initiated killing pathway distinct from apoptosis. J Virol. 1997;71:9753–9763. doi: 10.1128/jvi.71.12.9753-9763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penn ML, Grivel JC, Schramm B, Goldsmith MA, Margolis L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc Natl Acad Sci USA. 1999;96:663–668. doi: 10.1073/pnas.96.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asjo B, et al. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986;2:660–662. [PubMed] [Google Scholar]
- 13.Tersmette M, et al. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989;63:2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng-Mayer C, Seto D, Tateno M, Levy JA. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 15.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbein G, et al. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 17.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 18.Algeciras-Schimnich A, Griffith TS, Lynch DH, Paya CV. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J Immunol. 1999;162:5205–5211. [PubMed] [Google Scholar]
- 19.Wu L, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 20.Bandres JC, et al. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J Virol. 1998;72:2500–2504. doi: 10.1128/jvi.72.3.2500-2504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill CM, et al. Envelope glycoproteins from human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus can use human CCR5 as a coreceptor for viral entry and make direct CD4-dependent interactions with this chemokine receptor. J Virol. 1997;71:6296–6304. doi: 10.1128/jvi.71.9.6296-6304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan N, et al. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleul CC, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 24.Oberlin E, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1 [erratum 1996, 384:288] Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 25.Amara A, et al. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berndt C, Mopps B, Angermuller S, Gierschik P, Krammer PH. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4(+) T cells. Proc Natl Acad Sci USA. 1998;95:12556–12561. doi: 10.1073/pnas.95.21.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su SB, et al. Inhibition of tyrosine kinase activation blocks the down-regulation of CXC chemokine receptor 4 by HIV-1 gp120 in CD4+ T cells. J Immunol. 1999;162:7128–7132. [PubMed] [Google Scholar]
- 28.Madani N, Kozak SL, Kavanaugh MP, Kabat D. gp120 envelope glycoproteins of human immunodeficiency viruses competitively antagonize signaling by coreceptors CXCR4 and CCR5. Proc Natl Acad Sci USA. 1998;95:8005–8010. doi: 10.1073/pnas.95.14.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson J, et al. Early reduction of immune activation in lymphoid tissue following highly active HIV therapy. AIDS. 1998;12:F123–F129. doi: 10.1097/00002030-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, et al. Determinant in human immunodeficiency virus type 1 for efficient replication under cytokine-induced CD4(+) T-helper 1 (Th1)- and Th2-type conditions. J Virol. 1999;73:316–324. doi: 10.1128/jvi.73.1.316-324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jourdan P, et al. IL-4 induces functional cell-surface expression of CXCR4 on human T cells [erratum 1999, 162:3105] J Immunol. 1998;160:4153–4157. [PubMed] [Google Scholar]
- 32.Orsini MJ, Parent JL, Mundell SJ, Benovic JL. Trafficking of the HIV coreceptor CXCR4. Role of arrestins and identification of residues in the c-terminal tail that mediate receptor internalization. J Biol Chem. 1999;274:31076–31086. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- 33.Haribabu B, et al. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J Biol Chem. 1997;272:28726–28731. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- 34.Hoxie JA, et al. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986;234:1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- 35.Littman DR. The CD4 molecule. Roles in T lymphocytes and in HIV disease. Introduction. Curr Top Microbiol Immunol. 1996;205:v–x. [PubMed] [Google Scholar]
- 36.Garcia JV, Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 37.Cicala C, et al. HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4(+) T cells. Proc Natl Acad Sci USA. 2000;97:1178–1183. doi: 10.1073/pnas.97.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantaleo G, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 39.Fox CH, et al. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J Infect Dis. 1991;164:1051–1057. doi: 10.1093/infdis/164.6.1051. [DOI] [PubMed] [Google Scholar]
- 40.Sunila I, Vaccarezza M, Pantaleo G, Fauci AS, Orenstein JM. gp120 is present on the plasma membrane of apoptotic CD4 cells prepared from lymph nodes of HIV-1-infected individuals: an immunoelectron microscopic study. AIDS. 1997;11:27–32. doi: 10.1097/00002030-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Rosok BI, et al. Correlates of apoptosis of CD4+ and CD8+ T cells in tonsillar tissue in HIV type 1 infection. AIDS Res Hum Retroviruses. 1998;14:1635–1643. doi: 10.1089/aid.1998.14.1635. [DOI] [PubMed] [Google Scholar]
- 42.Cotton MF, et al. Apoptosis of CD4+ and CD8+ T cells isolated immediately ex vivo correlates with disease severity in human immunodeficiency virus type 1 infection. Pediatr Res. 1997;42:656–664. doi: 10.1203/00006450-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Hesselgesser J, et al. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 44.Baird-Piechaczyk M, Robert-Hebmann V, Roland J, Coudronniere N, Devaux C. Role of CXCR4 in HIV-1-induced apoptosis of cells with a CD4+, CXCR4+ phenotype. Immunol Lett. 1999;70:1–3. doi: 10.1016/s0165-2478(99)00124-8. [DOI] [PubMed] [Google Scholar]