Abstract
Cardiomyopathy is a multifactorial disease, and the dystrophin-glycoprotein complex has been implicated in the pathogenesis of both hereditary and acquired forms of the disease. Using mouse models of cardiomyopathy made by ablating genes for components of the sarcoglycan complex, we show that long-term treatment with verapamil, a calcium channel blocker with vasodilator properties, can alleviate the severe cardiomyopathic phenotype, restoring normal serum levels for cardiac troponin I and normal cardiac muscle morphology. Interruption of verapamil treatment leads again to vascular dysfunction and acute myocardial necrosis, indicating that predilection for cardiomyopathy is a continuing process. In contrast, verapamil did not prevent cardiac muscle pathology in dystrophin-deficient mdx mice, which neither show a disruption of the sarcoglycan complex in vascular smooth muscle nor vascular dysfunction. Hence, our data strongly suggest that pharmacological intervention with verapamil merits investigation as a potential therapeutic option not only for patients with sarcoglycan mutations, but also for patients with idiopathic cardiomyopathy associated with myocardial ischemia not related to atherosclerotic coronary artery disease.
Introduction
Dilated cardiomyopathy, one of the leading causes of heart failure in the United States, is a multifactorial disease that includes both hereditary and acquired forms (1). Experimental data have demonstrated that several hereditary forms of dilated cardiomyopathy are related to defects of the extrasarcomeric myocyte cytoskeleton (2) and imply that familial cardiomyopathy can result from defective transmission of mechanical force generated in the sarcomere (2). Recent evidence is accumulating that hereditary and acquired forms of cardiomyopathy can also be caused by alterations within the dystrophin-glycoprotein complex (DGC) (3–6). Functional defects of vascular smooth muscle associated with mutations of the β- and δ-sarcoglycan genes have revealed new insights into the pathogenesis of certain DGC-associated cardiomyopathies (5, 6). Moreover, mutations in the human δ-sarcoglycan gene have been characterized in patients with familiar and sporadic cases of dilated cardiomyopathy without significant involvement of the skeletal muscle (7).
The DGC is a multisubunit complex that provides a mechanical link between the extracellular matrix and the cytoskeleton (for review see ref. 8). The DGC is comprised of the cytoskeletal proteins dystrophin, syntrophins, and dystrobrevin; the sarcolemmal localized dystroglycans (α and β subunits); the sarcoglycans (α, β, γ, and δ subunits) and sarcospan. Mutations in any of the subunits (α, β, γ, δ) of the sarcoglycan subcomplex have been shown to be associated with limb-girdle muscular dystrophy (LGMD) types 2C-F, and recently, an increasing number of patients with sarcoglycanopathies have been reported to exhibit cardiomyopathy (refs. 3, 9–12; R.D. Cohn, unpublished observation). Interestingly, in vivo studies of coronary blood flow in human patients with sarcoglycanopathies suggested coronary dysfunction in these patients, which might be related to defects in vascular smooth muscle (13).
We have recently characterized a unique sarcoglycan-sarcospan (SG-SSPN) complex expressed in vascular smooth muscle composed of ε-sarcoglycan (which replaces α-sarcoglycan), β-, γ-, and δ-sarcoglycan, and sarcospan (14–16). The significant impact of the distinct tissue distribution of the sarcoglycans on the pathogenetic mechanisms has been specifically shown in animal models for LGMD type 2E and 2F, which are deficient for β- and δ-sarcoglycan (Sgcb- and Sgcd-null, respectively) (5, 6). Our data suggested that loss of the SG-SSPN complex in vascular smooth muscle of these mice caused perturbation of vascular function with the presence of vascular constrictions leading to intermittent ischemic-like events, which initiated the development of cardiomyopathy. Furthermore, it was shown that acute, treadmill exercise–induced myocardial necrosis was rescued by administration of a vasodilator in Sgcd-null mice (5). These findings prompted us to explore the pathogenetic significance of vascular dysfunction and intermittent ischemia and possible therapeutic options to prevent cardiomyopathy in genetic mouse models with different mutations within the DGC.
Here we demonstrate, for the first time to our knowledge, that long-term treatment with verapamil, a L-type calcium channel blocker with vasodilator properties and clinical relevance, abolishes vascular constrictions and effectively prevents the development of severe cardiomyopathy, as shown by normal serum levels of cardiac troponin I and cardiac muscle morphology in an animal model for the common LGMD type 2E, deficient for β-sarcoglycan. Various early studies have shown beneficial effects of verapamil in the cardiomyopathic hamster that carries a deletion within the δ-sarcoglycan gene (17, 18). Here we also demonstrate beneficial effects of verapamil on the cardiomyopathic phenotype in a more genetically defined mouse model deficient for δ-sarcoglycan. Interruption of verapamil treatment leads again to perturbation of vascular function and myocardial cell necrosis, indicating that intermittent ischemic-like events as a predilection for cardiomyopathy are an ongoing process. Interestingly, verapamil treatment has no beneficial effects on the cardiomyopathic phenotype of dystrophin-deficient mdx mice, which neither show a disruption of the SG-SSPN complex in vascular smooth muscle nor vascular dysfunction.
Hence, our data reveal new compelling evidence for the pathogenetic mechanism whereby cardiac muscle of Sgcb- and Sgcd-null mice is prone to intermittent ischemic-like events, which consequently leads to cardiomyopathy. Furthermore, pharmacological intervention with verapamil warrants investigation as a potential therapeutic option to prevent severe cardiomyopathy caused by intermittent ischemic damage.
Methods
Study design.
Beginning at the age of 8 weeks, before the existence of any cardiac lesions, Sgcb- and Sgcd-null mice (n = 13 each) received oral supplementation of verapamil (American Regent Laboratories, Shirley, New York, USA) in their drinking water. Treatment in mdx mice started at 16 weeks of age (n = 6 each). Verapamil was dissolved in 10% dextrose solution at a concentration of 1 mg/ml. These mice consumed an average dose of 3.5 mg/day (19). A group of six wild-type mice also received the same amount of verapamil orally in their drinking water. Age-matched groups of Sgcb- and Sgcd-null mice (n = 6 each) were not treated and served as controls for the study. After 16 weeks of oral verapamil treatment, mice were analyzed for morphological alterations and used for perfusion studies. In a subgroup of Sgcb- and Sgcd-null mice (n = 4 each) verapamil administration was stopped at 16 weeks, and 14 days later mice were analyzed for morphological alteration and used for perfusion studies. All studies were performed in accordance with the guidelines of the animal care facility at the University of Iowa (Iowa City, Iowa, USA).
Histopathology studies.
Histopathology studies were performed as described previously (5, 6). After embedding the tissue of treated and untreated mdx, Sgcb-, and Sgcd-null mice (n = 6 each) in paraffin, several hematoxylin and eosin and Masson’s trichrome–stained sections (4 μm) throughout the entire ventricle and lower part of the atrium were prepared to characterize cardiac muscle pathology. All analyses were performed by five different people blinded to the treatment groups of each mouse. The total and damaged myocardial areas of the six different slices of each heart were traced and measured by an image-analyzing system (Scion, Frederick, Maryland, USA). Subsequently, the percentage of these analyses was calculated, and statistical analysis was performed using the unpaired Student’s t test. Immunohistological techniques were performed as described previously (5, 6). Rabbit polyclonal Ab’s against dystrophin (rabbit 31), utrophin (rabbit 56), β-sarcoglycan (goat 26) and δ-sarcoglycan (rabbit 214), β-dystroglycan COOH-terminal peptide (rabbit 83), and sarcospan (rabbit 235) were described previously (5, 6, 16). Microfil (Flowtech Inc., Carver, Massachusetts, USA) perfusion studies were performed as described previously (5, 6).
Determination of cardiac troponin I.
Serum samples from mice were obtained by retro-orbital and/or tail bleeding according to institutional guidelines. Cardiac-specific troponin I (cTnI) was measured by an enzyme sandwich immunoassay (ACS:180; Ciba-Corning Diagnostics Corp., Medfield, Massachusetts, USA) that uses two cTnI-specific mAb’s with independent epitopes for cTnI. All assays were performed by technicians blinded to the source of the serum. The normal range for troponin I levels in the serum is less than 0.2 ng/ml.
Results
Normal serum levels of cTnI in verapamil treated Sgcb- and Sgcd-null mice.
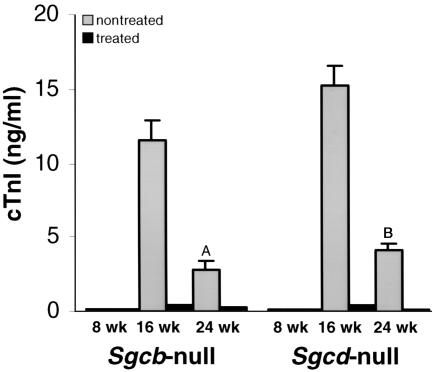
Treatment of Sgcb- and Sgcd-null mice was started at 8 weeks of age, before the existence of any cardiac lesions. To monitor cardiac muscle damage during verapamil treatment we measured cTnI levels in the sera of our mice at 8, 16, and 24 weeks of age (Figure 1). Throughout ontogeny, cTnI is not expressed in skeletal muscle, and unlike creatine kinase isoenzyme MB, it has not been detected in other tissues apart from the heart at any developmental stage or in diseased skeletal muscle (20). Thus, the presence of cTnI in the circulation above the reference limit is highly specific for myocardial injury. At 8 weeks of age cTnI values were normal in both verapamil-treated and nontreated Sgcb- and Sgcd-null mice, which is in accordance with histopathological observations (data not shown). At 16 weeks of age, cTnI levels were significantly elevated in untreated Sgcb- and Sgcd-null mice (11.5 ± 1.4 and 15.3 ± 1.3 ng/ml, respectively). These values correlate with the histological findings of severe ongoing focal myocytolytic cell damage observed in 16-week-old Sgcb- and Sgcd-null mice (5, 6). At 24 weeks of age, cTnI levels decreased to 2.8 ± 0.6 and 4.2 ± 0.41 ng/ml in untreated Sgcb-null and Sgcd-null mice, respectively. Again, these values are in accordance with previous histological findings of few areas of acute ongoing necrosis at this stage of the disease, where changes like fibrosis and calcification become more prominent (5, 6). In marked contrast, cTnI values were normal or only slightly elevated in the treated group of Sgcb- and Sgcd-null mice at 16 and 24 weeks of age (0.4 ± 0.09 and 0.53 ± 0.05 ng/ml; 0.3 ± 0.06 and 0.2 ± 0.04), respectively (Figure 1). Evaluation of cTnI level in wild-type mice revealed normal values at different ages (<0.2 ng/ml), but elevated in dystrophin-utrophin–deficient mice, which develop a severe cardiomyopathy (9.8 ± 0.05 ng/ml; n = 3). Our data thus indicated that no significant cardiac muscle pathology occurred during verapamil treatment in Sgcb- and Sgcd-null mice.
Figure 1.
Levels of cTnI in verapamil-treated versus untreated Sgcb- and Sgcd-null mice. Note the significant elevation of cTnI in the untreated group of Sgcb- and Sgcd-null mice. Data are represented as mean plus or minus SD. ASgcb-null treated versus untreated P < 0.002; BSgcd-null treated versus untreated P < 0.001.
Verapamil prevents morphological alterations of cardiac muscle in Sgcb- and Sgcd-null mice.
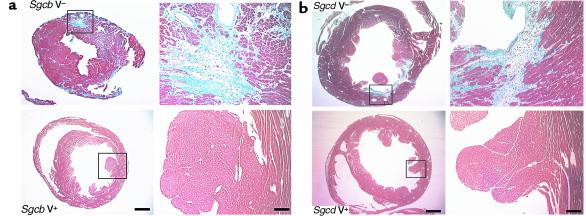
Evaluation of multiple histological cross sections from different levels of the entire heart showed at the end of the verapamil-treatment trial that the cardiomyopathic phenotype was effectively prevented in 24-week-old Sgcb- and Sgcd-null mice. No regions of acute or remote necrosis were observed. In contrast, histological evaluation of cardiac muscle from age-matched, untreated Sgcb- and Sgcd-null mice showed the expected severe morphological signs of cardiomyopathy with extensive fibrosis, scarring, and focal deposition of calcium (Figure 2, a and b). However, no significant beneficial effect of verapamil treatment was observed in Sgcb- and Sgcd-null mice when treatment was started in the case of already existing severe cardiac muscle fibrosis, indicating the potential preventative effect of verapamil (data not shown). Morphometric analysis of cardiac muscle, determining total and damaged area of treated versus untreated mice, confirmed the significant beneficial effect of verapamil on the cardiac muscle of the treated mice (data not shown). Verapamil did not have any deleterious effects in wild-type mice, and no serious side effects were observed in any of the treated mice. Histological analysis of skeletal muscle (diaphragm and quadriceps) did not reveal any significant differences between treated and untreated mice (data not shown). This is most likely due to the fact that treatment with verapamil was started at 8 weeks of age. Since the severe alterations of muscular dystrophy start as early as 2 weeks of age, it is not surprising that verapamil had no preventative effect in skeletal muscle damage.
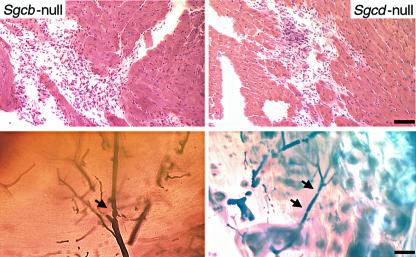
Figure 2.
Morphological analysis of cardiac muscle of Sgcb- and Sgcd-null mice. The untreated mice (V–) show extensive areas of fibrosis in the heart (left panels). In contrast, most of the cardiac muscle of verapamil-treated mice (V+) is free of any morphological alterations (right panels). Bar, 1.5 mm and 75 μm, respectively. (a) Sgcb-null mice. (b) Sgcd-null mice.
Verapamil has no beneficial effect on cardiomyopathy in mdx mice.
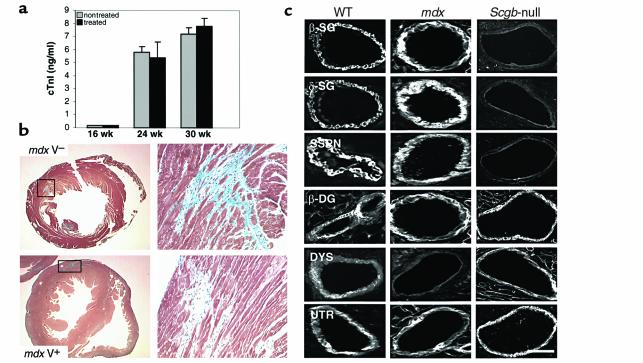
Preliminary morphological observations of the cardiomyopathic phenotype in dystrophin-deficient mdx mice suggested that the cardiomyopathy in these mice is not related to vascular dysfunction and intermittent ischemic-like events. To test this we decided to study the effects of verapamil supplementation on the development of cardiomyopathy in mdx mice. At the beginning of treatment at 16 weeks of age, no significant elevations of cTnI level were detected in treated and untreated mdx mice. However, at 24 weeks (5.8 ± 0.4 ng/ml and 5.4 ± 1.2 ng/ml, respectively) and 30 weeks (7.2 ± 0.5 ng/ml and 7.8 ± 0.6 ng/ml, respectively) of age, both treated and untreated mdx mice showed elevation of cTnI levels, suggesting that cardiac muscle damage was present in both groups of mice (Figure 3a). Histological analysis of cardiac muscle from treated and untreated mdx mice displayed severe fibrotic alterations and areas of acute ongoing necrosis, despite supplementation of verapamil (Figure 3b). Evaluation of cTnI levels in mice at 36 weeks of age, where only a few areas of acute necrosis can be observed, showed only mild elevations of cTnI levels. The lack of beneficial effects of verapamil on the cardiomyopathic phenotype in dystrophin-deficient mdx mice prompted us to study the expression of the SG-SSPN complex in vascular smooth muscle of coronary arteries in these mice. Interestingly, immunohistochemical analysis revealed that despite the loss of dystrophin in vascular smooth muscle, no alteration in the expression pattern of the SG-SSPN complex was observed (Figure 3c). Our data strongly suggest that the molecular mechanism responsible for the development of cardiomyopathy in mdx mice is different from the mechanism of vascular dysfunction demonstrated in Sgcb- and Sgcd-null mice. Furthermore, the data substantiate the specificity of the beneficial effects of verapamil on the cardiomyopathic phenotype in Sgcb- and Sgcd-null mice.
Figure 3.
Verapamil does not prevent cardiomyopathy in dystrophin-deficient mdx mice. (a) Levels of cTnI in verapamil-treated versus untreated mdx mice. Both groups of mice show elevations of cTnI levels in the serum. (b) Histological analysis of hearts from mdx mice after treatment with verapamil reveals areas of fibrosis in both groups of mice. (c) Immunohistochemical expression of β-sarcoglycan, δ-sarcoglycan, sarcospan, and β-dystroglycan is preserved in vascular smooth muscle of coronary arteries of mdx mice. In contrast, the SG-SSPN complex is absent in vascular smooth muscle of Sgcb-null mice. Note that utrophin is expressed at similar levels in wild-type, mdx, and Sgcb-null mice. β-SG, β-sarcoglycan; δ-SG, δ-sarcoglycan; β-DG, β-dystroglycan; DYS, dystrophin; and UTR, utrophin. Bar, 20 μm.
Abolition of vascular dysfunction in verapamil-treated Sgcb- and Sgcd-null mice.
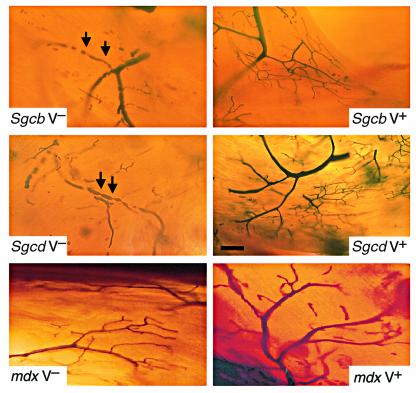
One rationale for using verapamil as a potential therapeutic agent to prevent cardiomyopathy in our animal models was given primarily by its potential to act as a vasodilator. Therefore, we analyzed the vascular function of verapamil-treated mdx, Sgcb-, and Sgcd-null mice by using Microfil perfusion as an in vivo approach and compared it with age-matched untreated mice (Figure 4). As suggested by the immunohistochemical results obtained in coronary arteries of mdx mice, even in the presence of cardiac muscle damage, neither treated nor untreated mdx mice exhibited any signs of vascular perfusion abnormalities, demonstrating that the observed vascular dysfunction is not secondarily related to preexisting cardiac muscle damage. In contrast, numerous microvascular constrictions associated with aneurysms were detected in coronary arteries of untreated Sgcb- and Sgcd-null mice. Remarkably, long-term oral administration of verapamil not only prevented morphological alterations of cardiac muscle in Sgcb- and Sgcd-null mice, but also abolished the observed vascular dysfunction in coronary arteries. The coronary artery bed of treated mice was comprised of smoothly tapered lumens without any evidence of vascular constrictions (Figure 4).
Figure 4.
Perfusion of coronary arteries of verapamil-treated and untreated mice. Transillumination of Microfil-perfused coronary arteries in untreated mice shows multiple constrictions (arrows) with pre- and poststenotic dilations, as well as narrow vessels with a serrated, rather than a smooth, contour (upper and middle left panels). In contrast, coronary vessels of treated Sgcb- and Sgcd-null mice (upper and middle right panels) reveal smoothly tapered vessels without signs of constriction and/or focal narrowing. Note the normal vascular perfusion of treated and untreated mdx mice (lower panel). Bar, 40 μm.
Predilection for cardiomyopathy is an ongoing process in Sgcb- and Sgcd-null mice.
Next we tested whether the potential for vasoconstriction as a predisposition for cardiomyopathy was an ongoing process in Sgcb- and Sgcd-null mice. We interrupted administration of verapamil in Sgcb- and Sgcd-null mice after 16 weeks of treatment. Two weeks later we analyzed these mice for cardiac muscle morphology and vascular function. All mice developed focal myocytolytic lesions in the heart, a phenomenon that is usually observed around 14–18 weeks of age in untreated mice. Furthermore, in vivo perfusion revealed numerous microvascular constrictions and vessel walls with a serrated contour (Figure 5). Determination of cTnI in the serum of these mice revealed elevated levels after interruption of verapamil treatment (8.5 ± 0.1 ng/ml and 11.2 ± 0.1 ng/ml, respectively), which confirmed the histopathological findings. These data indicate that vascular dysfunction and the necrotizing process as a predilection for cardiomyopathy is an ongoing process that most likely continues for the life of Sgcb- and Sgcd-null mice. Thus, a long-term treatment with verapamil would be necessary to prevent heart disease in these mice.
Figure 5.
Interruption of verapamil treatment leads to vascular dysfunction and myocardial necrosis. Analysis of cardiac muscle morphology and vascular perfusion 10 days after interruption of 16 weeks of verapamil therapy exhibits the development of focal necrotic lesions and microvascular constrictions in Sgcb- and Sgcd-null mice. Bar, 75 μm and 40 μm, respectively.
Discussion
An increasing number of patients have been reported to develop cardiomyopathy due to mutations of either β-, γ-, or δ-sarcoglycan (9–12). In our study we monitored cardiac muscle damage in vivo via determination of cTnI. So far, measurement of cTnI has been used exclusively as a marker for myocardial infarcts in adult patients with ischemic heart disease (20). Here, we were able to demonstrate for the first time to our knowledge in genetic mouse models with muscular dystrophy and cardiomyopathy, that evaluation of cTnI could indeed serve as an excellent marker to determine cardiac muscle damage in patients with muscular dystrophy due to its high specificity as an indicator for myocardial necrosis. Evaluation of cTnI levels in patients with different forms of muscular dystrophy could potentially reveal further insights into the onset and extent of cardiac muscle lesions at different stages of the disease. Because cTnI is a marker of acute myocardial damage, this would be particularly important for early stages of cardiomyopathy where only small lesions in the heart might be present. Studies from our laboratory showed that even in the presence of rather severe morphological damage in cardiac muscle of Sgcd-null mice, only about one-third of mice demonstrated significant physiological dysfunction as observed by transthoracic echocardiography (K.P. Campbell et al., unpublished observations). Although a direct physiological comparison between mice and humans is difficult to make, it should be taken into account that elevation of cTnI might indicate early and minor cardiac muscle damage that would precede the onset of physiological abnormalities of cardiac muscle function.
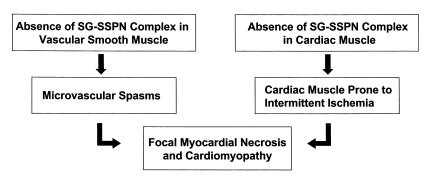
The current study and our data reported previously suggest a novel pathogenetic mechanism for cardiomyopathy associated with mutations within the β- or δ-sarcoglycan gene (Figure 6). Disruption of the SG-SSPN complex in vascular smooth muscle leads to perturbation of vascular function as demonstrated by multiple vascular constrictions. In addition, loss of the SG-SSPN complex and a unique ε-sarcoglycan–containing complex in cardiomyocytes (see below) causes the cardiac muscle to be prone to intermittent ischemic-like events that eventually lead to focal myocardial necrosis and cardiomyopathy. Identification of this novel mechanism prompted us to explore the possibility of pharmacological intervention as a treatment option for cardiomyopathy in these genetic mouse models. Here we demonstrate, we believe for the first time, that pharmacological intervention using long-term oral treatment with verapamil is able to prevent severe cardiomyopathy in mice with disruption of the smooth muscle sarcoglycan complex by alleviation of vascular constrictions and protection from intermittent ischemic damage. Treatment with verapamil in older mice did not have beneficial effects on cardiac muscle pathology, indicating that verapamil in fact serves as a preventative treatment option. Interestingly, verapamil did not have any beneficial effects on the cardiomyopathic phenotype in dystrophin-deficient mdx mice, which do not display vascular dysfunction. Hence, our data substantiate the concept of intermittent ischemic-like events as the pathogenetic mechanism for the development of cardiomyopathy in genetic mouse models with targeted ablation of the β- or δ-sarcoglycan gene.
Figure 6.
Pathogenesis of cardiomyopathy in LGMD 2E/2F. The flow chart represents the current understanding of the pathogenesis of cardiomyopathy due to primary mutations within the β- or δ-sarcoglycan gene. Absence of SG-SSPN complex in vascular smooth muscle leads to vascular dysfunction in the form of microvascular spasms. In addition, loss of the SG-SSPN complex in cardiac muscle renders the heart susceptible to intermittent ischemic-like events, which eventually leads to the development of focal myocardial necrosis and severe cardiomyopathy.
Vascular smooth muscle tone and myocardial contractility both depend on calcium entry. Verapamil, a first-generation voltage-gated L-type calcium channel antagonist, has been shown to be nearly equipotent in reducing vascular smooth muscle tone and inhibiting the membrane calcium influx into cardiomyocytes (for review see ref. 21). The beneficial use of verapamil has been shown extensively in a variety of diseases such as hypertension and cardiac arrhythmias, as well as in humans and animal models with cardiomyopathy (17, 18, 22, 23). Interestingly, the cardioprotective efficacy of verapamil was attributed to increased transmural blood flow related to vasodilation of the coronary artery bed (17, 22) as well as to the potential of verapamil to inhibit membrane calcium influx into cardiomyocytes (18). The current data reveal new compelling evidence that perturbed vascular function and intermittent ischemic-like events play a significant role in the pathogenesis of cardiomyopathy in Sgcb- and Sgcd-null mice. Abolition of microvascular constrictions over an extended time period is able to protect the cardiac muscle of Sgcb- and Sgcd-null mice from ischemic damage, which would consequently lead to the development of cardiomyopathy. Interestingly, histological examination of coronary arteries in recently reported patients with cardiomyopathy due to mutations in the δ-sarcoglycan gene did not reveal any obvious coronary artery dysfunction (7). Future studies will be needed to more carefully evaluate (e.g., functional studies) the possible involvement of coronary arteries in patients with mutations within the δ-sarcoglycan and/or β-sarcoglycan gene. Furthermore, it will be interesting to study the molecular basis of how the absence of the SG-SSPN complex in vascular smooth muscle affects vascular function and whether the beneficial effect of verapamil on the vasculature can be interpreted as a specific dysfunction of calcium metabolism in vascular smooth muscle.
The pathogenetic mechanism and the specificity of the verapamil action in these mice was further supported by the lack of beneficial effects of verapamil in dystrophin-deficient mdx mice, which neither show perturbation of the SG-SSPN complex in vascular smooth muscle nor any signs of vascular dysfunction. Moreover, mdx mice did not develop the characteristically focal myocytolytic lesions observed in the sarcoglycan-deficient mice (5, 6) but showed a rather diffuse distribution pattern of histopathological abnormalities that further support the hypothesis that that cardiac muscle pathology of mdx mice is not related to a primary perturbation of vascular function. However, biochemical isolation of the DGC of cardiac muscle from mdx mice revealed a dissociation of the sarcoglycan and dystroglycan complexes (M. Durbeej and K.P. Campbell, unpublished observations), suggesting that destabilization of the DGC due to loss of dystrophin within the cardiac muscle per se predisposes mdx mice to development of cardiomyopathy.
The equipotent potential of verapamil to improve calcium metabolism of cardiomyocytes raises the possibility that a direct interaction of verapamil on the calcium metabolism of cardiomyocytes had some additional beneficial effect in Sgcb- and Sgcd-null mice. Calcium overload causes myocardial cell death and various mechanisms such as ischemia, catecholamine exposure, and membrane defects of cardiomyocytes may give rise to increased cellular calcium concentrations. In fact, recent biochemical data from our laboratory obtained from Sgcb- and Sgcd-null mice suggest defects of the cardiac membrane in addition to the observed vascular dysfunction, due to disruption of a second ε-sarcoglycan–containing complex in skeletal and cardiac muscle of Sgcb- and Sgcd-null mice (ref. 6; M. Durbeej and K.P. Campbell, unpublished observations). It is therefore possible that a membrane abnormality renders the cardiomyocytes more susceptible to intermittent ischemia related to microvascular constrictions. Prevention of vascular constrictions and possible intervention with calcium homeostasis by verapamil may lead to myocyte protection and the absence of cardiomyopathy.
Taken together, the successful treatment of cardiomyopathy with verapamil in mouse models with mutations in the β- or δ-sarcoglycan gene implies that efforts toward drug therapy can be of tremendous benefit in preventing certain forms of hereditary cardiomyopathy. This particularly accounts for cardiomyopathy as a serious complication in patients with LGMD types 2E and 2F, where no treatment options are currently available. Because of the preventative beneficial effects of verapamil, screening for mutations within the β- and δ-sarcoglycan genes should be extended to a wider group of patients with inherited cardiomyopathy. This is further supported by recent studies, which showed that familiar and sporadic cases of dilated cardiomyopathy caused by mutations within the δ-sarcoglycan gene can occur even in the absence of significant skeletal muscle problems (7). Therefore, screening for mutations should also include patients without a primary skeletal muscle involvement but who have been reported to have an idiopathic cardiomyopathy associated with myocardial ischemia in the absence of atherosclerotic coronary artery disease (24–27). Identification of novel mutations and characterization of the associated phenotypes would eventually warrant treatment trials with verapamil in these patients.
Acknowledgments
We would like to thank all members of the Campbell Laboratory for the critical reading of the manuscript, fruitful discussions, and supply of critical reagents. R.D. Cohn was supported by the Deutsche Forschungsgemeinschaft (Co 241-1). This work was also supported by the Muscular Dystrophy Association (K.P. Campbell). K.P. Campbell is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Chien KR. Complexity in simplicity: monogenic disorders and complex cardiomyopathies. J Clin Invest. 1999;103:1483–1485. doi: 10.1172/JCI7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–277. doi: 10.1016/0167-5273(90)90082-g. [DOI] [PubMed] [Google Scholar]
- 4.Badorff C, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5:320–326. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 5.Coral-Vazquez R, et al. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98:465–474. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- 6.Durbeej M, et al. Disruption of the β-sarcoglycan gene reveals a complex pathogenetic mechanism for LGMD 2E. Mol Cell. 2000;5:141–151. doi: 10.1016/s1097-2765(00)80410-4. [DOI] [PubMed] [Google Scholar]
- 7.Tsubata S, et al. Mutations in the human delta-sarcoglycan gene in familial and sporadic dilated cardiomyopathy. J Clin Invest. 2000;106:655–662. doi: 10.1172/JCI9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn RD, Campbell KP. The molecular basis of muscular dystrophy. Muscle Nerve. 2000;23:1456–1471. doi: 10.1002/1097-4598(200010)23:10<1456::aid-mus2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Nigro V, et al. Autosomal recessive limb-girdle muscular dystrophy, LGMD2F, is caused by a mutation in the delta-sarcoglycan gene. Nat Genet. 1996;14:195–198. doi: 10.1038/ng1096-195. [DOI] [PubMed] [Google Scholar]
- 10.Moreira ES, et al. A first missense mutation in the delta sarcoglycan gene associated with a severe phenotype and frequency of limb-girdle muscular dystrophy type 2F (LGMD2F) in Brazilian sarcoglycanopathies. J Med Genet. 1998;35:951–953. doi: 10.1136/jmg.35.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melacini P, et al. Heart involvement in muscular dystrophy due to sarcoglycan gene mutations. Muscle Nerve. 1999;22:473–479. doi: 10.1002/(sici)1097-4598(199904)22:4<473::aid-mus8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Barresi R, et al. Disruption of heart sarcoglycan complex and severe cardiomyopathy caused by beta sarcoglycan mutations. J Med Genet. 2000;37:102–107. doi: 10.1136/jmg.37.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnecchi-Ruscone T, et al. Cardiomyopathy in Duchenne, Becker and sarcoglycanopathies: a role for coronary dysfunction? Muscle Nerve. 1999;22:1549–1556. doi: 10.1002/(sici)1097-4598(199911)22:11<1549::aid-mus10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 14.Durbeej M, Campbell KP. Biochemical characterization of the epithelial dystroglycan complex. J Biol Chem. 1999;274:26609–26616. doi: 10.1074/jbc.274.37.26609. [DOI] [PubMed] [Google Scholar]
- 15.Straub V, et al. epsilon-sarcoglycan replaces alpha-sarcoglycan in smooth muscle to form a unique dystrophin-glycoprotein complex. J Biol Chem. 1999;274:27989–27996. doi: 10.1074/jbc.274.39.27989. [DOI] [PubMed] [Google Scholar]
- 16.Barresi R, Moore SA, Stolle CA, Mendell JR, Campbell KP. Expression of γ-sarcoglycan in smooth muscle and its interaction with the sarcoglycan-sarcospan complex. J Biol Chem. 2000;275:38554–38560. doi: 10.1074/jbc.M007799200. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi A, et al. Effects of verapamil on experimental cardiomyopathy in the Bio 14.6 Syrian hamster. J Am Coll Cardiol. 1987;10:1128–1138. doi: 10.1016/s0735-1097(87)80356-x. [DOI] [PubMed] [Google Scholar]
- 18.Factor SM, Cho SH, Scheuer J, Sonnenblick EH, Malhotra A. Prevention of hereditary cardiomyopathy in the Syrian hamster with chronic verapamil therapy. J Am Coll Cardiol. 1988;12:1599–1604. doi: 10.1016/s0735-1097(88)80031-7. [DOI] [PubMed] [Google Scholar]
- 19.Dong R, Liu P, Wee L, Butany J, Sole MJ. Verapamil ameliorates the clinical and pathological course of murine myocarditis. J Clin Invest. 1992;90:2022–2030. doi: 10.1172/JCI116082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodor GS, Porterfield D, Voss EM, Smith S, Apple FS. Cardiac troponin-I is not expressed in fetal and healthy or diseased adult human skeletal muscle tissue. Clin Chem. 1995;41:1710–1715. [PubMed] [Google Scholar]
- 21.Little WC, Cheng CP. Vascular versus myocardial effects of calcium antagonists. Drugs. 1994;47(Suppl.):41–45. doi: 10.2165/00003495-199400474-00007. [DOI] [PubMed] [Google Scholar]
- 22.Udelson JE, et al. Verapamil prevents silent myocardial perfusion abnormalities during exercise in asymptomatic patients with hypertrophic cardiomyopathy. Circulation. 1989;79:1052–1060. doi: 10.1161/01.cir.79.5.1052. [DOI] [PubMed] [Google Scholar]
- 23.Gistri R, et al. Effect of verapamil on absolute myocardial blood flow in hypertrophic cardiomyopathy. Am J Cardiol. 1994;74:363–368. doi: 10.1016/0002-9149(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 24.Cannon RO, III, et al. Myocardial ischemia in patients with hypertrophic cardiomyopathy: contribution of inadequate vasodilator reserve and elevated left ventricular filling pressures. Circulation. 1985;71:234–243. doi: 10.1161/01.cir.71.2.234. [DOI] [PubMed] [Google Scholar]
- 25.Dunn RF, et al. Comparison of thallium-201 scanning in idiopathic dilated cardiomyopathy and severe coronary artery disease. Circulation. 1982;66:804–810. doi: 10.1161/01.cir.66.4.804. [DOI] [PubMed] [Google Scholar]
- 26.Opherk D, et al. Coronary dilatory capacity in idiopathic dilated cardiomyopathy: analysis of 16 patients. Am J Cardiol. 1983;51:1657–1662. doi: 10.1016/0002-9149(83)90205-9. [DOI] [PubMed] [Google Scholar]
- 27.Pasternac A, Bourassa MG. Pathogenesis of chest pain in patients with cardiomyopathies and normal coronary arteries. Int J Cardiol. 1983;3:273–280. doi: 10.1016/0167-5273(83)90168-7. [DOI] [PubMed] [Google Scholar]