Abstract
A thymic epithelial cell line transfected with I-Ek was used in reaggregate cultures to study the role of peptides in positive selection of T cell receptor transgenic thymocytes. In this system, positive selection of CD4 SP cells occurred only after the addition of exogenous peptide. Analysis of antigen analogs indicated an inverse relationship between the antigenicity for peripheral T cells and the concentration of peptide required for positive selection. These data are most consistent with an avidity (rather than an affinity) model of positive selection, in which ligand density and the affinity of T cell receptor act in concert to determine the fate of developing thymocytes.
The mature T cell receptor (TCR) αβ repertoire is determined by positive and negative selection events that take place in the thymus during the course of thymocyte differentiation (1). Although major histocompatibility complex (MHC) recognition is critically involved in both negative and positive selection, the contribution of MHC-associated peptide ligands to this process is not clear. A strong case has been made both conceptually and experimentally that only peptides that are closely related to the structure of the cognate antigen are able to mediate negative selection. Thus, only antigenic and TCR antagonist peptides appear able to support negative selection (2–4). The ability of nonantigenic antagonist peptides to mediate negative selection has led to the view that the affinity threshold for induction of negative selection is lower than that required for stimulation of mature peripheral T cells.
The role of peptides in positive selection has been under intense investigation for the past few years. Analysis of the peptide requirements for positive selection of class I MHC-restricted TCR transgenic thymocytes in fetal thymic organ culture led to the conclusions that cognate antigen (5–7) or closely related TCR antagonist peptides (8–10) were required for positive selection and that structurally unrelated peptides could not induce positive selection. In contrast to these findings, more recent studies of positive selection of class II-restricted responses using systems in which the class II MHC molecules essentially contained one peptide in the antigen binding groove indicated that single peptide–MHC complexes can generate a diverse T cell repertoire capable of generating T cell responses to many foreign antigens (11–14). The use of fetal thymic organ culture to examine the role of peptides in the positive selection of class II MHC-restricted TCR transgenic thymocytes has not been reported as yet.
In the present study, we have reexamined the role of peptides in the positive selection of I-Ek/moth cytochrome c-specific thymocytes by using reaggregate cultures (RCs) consisting of thymocytes from TCR transgenic mice and a thymic epithelial cell (TEC) line that is able to support positive selection in vitro (A.N., C. M. Clegg, and A.G.F., unpublished results). Three important features of this system, in contrast to the fetal thymic organ cultures used previously, were as follows: (i) The use of a stable TEC line allowed for the analysis of positive selection by the epithelial cells in the absence of thymic dendritic cells and macrophages that could efficiently induce negative selection. (ii) Because I-Ek-transfected epithelial cells only induced positive selection after addition of exogenous peptides, the problem of a background of positive selection caused by unknown endogenously generated peptides was eliminated. (iii) Unlike the fetal thymic organ culture systems involved in the study of positive selection of CD8+ cells in which β2-microglobulin- or transporter associated with processing (TAP)-deficient mice (15, 16) that express very low levels of class I MHC were used, the TECs used in this study had amounts of class II MHC similar to that expressed on normal thymic epithelium.
MATERIALS AND METHODS
Mice.
AND transgenic mice on B10.A(4R) background were obtained from S. Hedrick (Univ. of California, San Diego, La Jolla, CA). B10.A and B10.A(4R) mice were obtained from the Jackson Laboratory. The neonatal mice (days 1–5) were obtained from the breeding colony of the La Jolla Institute for Allergy and Immunology.
Antibodies and Cell Surface Staining.
Phycoerythrin-conjugated anti-CD4 (GK1.5; Becton Dickinson); RED613-conjugated anti-CD8 (53-6.72) and fluorescein isothiocyanate (FITC)-conjugated anti-CD8 (53-6.72) (GIBCO/BRL); FITC-conjugated anti-Vα11 (RR8-1), anti-CD3-ɛ (145-2C11), anti-I-Ek (14-4-4S), and anti-I-Ak (10-3.6) (PharMingen) were used for cell surface phenotype analysis. To detect invariant chain, the epithelial cells were grown on glass coverslips, washed in ice-cold PBS, fixed, and permeabilized with precooled (−20°C) methanol for 5 min. The cells were incubated with the anti-invariant chain rat IgG2b mAb In-1 (a gift from Jim Miller, University of Chicago), then stained with FITC-conjugated anti-rat IgG antibody (PharMingen), and analyzed by UV microscopy.
Cells.
CD4+ CD8+ thymocytes were purified from newborn (1–5 days) AND TCR transgenic thymi by positive selection on Ly-2 microbeads (53-6.7; Miltenyi Biotec, Sunnyvale, CA). To a single cell suspension of thymocytes (107 cells per 90 μl of buffer), 10 μl of MACS Ly-2 was added and the mixture incubated for 20 min at 4°C, after which time 400 μl of buffer [Click’s medium (GIBCO/BRL)/5% BSA (ICN Biomedicals)/5 mM EDTA (Sigma)] was added. The cell suspension was applied to mini MACS separation columns. The CD8+ cells were collected and purity was analyzed by flow cytometry with CD4/CD8/Vα11 three-color staining.
The cortical epithelial cell line ANV-41-2 has been described (A.N., C. H. Clegg, and A.G.F., unpublished results). The stable I-Ek-expressing transfectants (ANV/I-Ek) were prepared from this epithelial cell line. The I-Eαk and I-Eβk cDNAs in expression vector pcEXV-3 were obtained from Jim Miller. For transfection of I-Ek genes, 5 μg of DNA for α, 5 μg for β, and 0.5 μg of pcDNA-3 (Invitrogen) for the neoymcin selection marker were cotransfected into ANV-41-2 cells by Lipofectin transfection. Cells were seeded in a tissue culture dish to 40–60% confluency and washed with PBS twice. DNA plus 25 μl of Lipofectin reagent (GIBCO/BRL) in a final volume of 100 μl of serum-free RPMI 1640 medium (GIBCO/BRL) were gently mixed and incubated for 10 min at room temperature, and then 2.8 ml of serum-free medium was added. These Lipofectin reagent–DNA complexes were overlaid onto the cells and incubated for 6 h at 37°C, the DNA-containing medium was replaced with 7 ml of RPMI 1640 medium/10% fetal calf serum, and the cells were incubated for another 48 h. The transfectants were grown in G418-containing medium (0.4 mg/ml, GIBCO/BRL) and enriched for cells expressing I-Ek by positive selection with biotinylated anti-I-Ek (PharMingen) avidin magnetic beads (Dynal, Great Neck, NY). The high-density I-Ek-expressing cell line ANV/I-Ekhi was subsequently obtained by cell sorting with FITC-conjugated anti-I-Ek.
RCs.
Each RC (17–19) was established by centrifuging a mixture of 5 × 105 ANV stromal cells and 10 × 105 DP thymocytes and pipetting the resulting cell pellet onto a Nucleopore filter (Costar) placed on a foam sponge (Upjohn) in RPMI 1640 complete medium containing 10% fetal calf serum, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 μg/ml penicillin, and 100 μg/ml streptomycin (GIBCO/BRL). Normal TECs were prepared from deoxyguanosine-treated day 14–16 B10.A fetal thymic lobes (20) that were depleted of CD45+ cells by using biotinylated anti-CD45 mAbs (PharMingen), avidin micromagnetic beads (Miltenyi Biotec), and passage over MiniMACS magnetic columns (Miltenyi Biotec). For testing the role of peptides during positive selection, the peptides were added to the medium at various concentrations at the start of the culture period, which lasted 4 days. The slurry of cells reaggregated and formed a “lobe” within 12–18 h of culture.
Proliferation Assay.
After 4 days of culture in the reaggregation system, a single cell suspension was prepared. Irradiated B10A spleen cells were distributed in triplicate in wells of a flat-bottom 96-well plate at a concentration of 5 × 105 cells per well. The single cell suspension of thymocytes at a concentration of 5 × 104 cells per well was then added, followed by the addition of antigenic peptide. The cells were cultured at 37°C in a CO2 incubator for 4 days, pulse-labeled with 1 μCi of [3H]thymidine for 16 h, and harvested, and radioactivity was measured.
Peptide Synthesis.
Peptides were synthesized on a Rainin Symphony (Peptide Technologies, Washington, DC) synthesizer as described (21) and purified by reverse-phase HPLC. The identity and purity of the peptides (>90%) was substantiated by mass spectrometry.
RESULTS
Transfection of TEC Line ANV-41-2 with I-Ek.
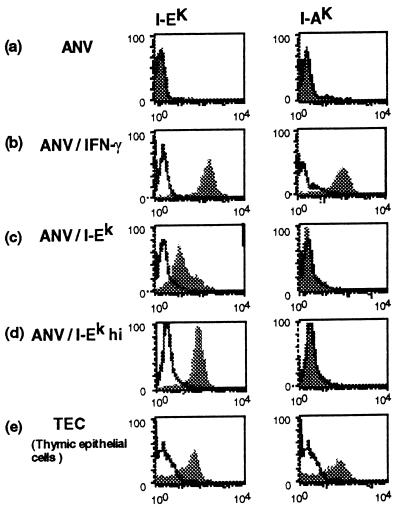
The ANV-41-2 cell line (ANV) is capable of inducing positive selection in the RC system (A.N., C. H. Clegg, and A.G.F., unpublished results). However, it does so only after stimulation with γ-interferon (IFN-γ) to induce expression of class II MHC. To generate a cell line that constitutively expresses class II MHC, we transfected the ANV cell line with the α and β genes for I-Ek. Stable transfectants analyzed for I-Ek by immunofluorescence indicated that the majority of cells expressed relatively low levels of I-Ek. After cell sorting for high I-Ek expression, a higher expressing variant could be selected. Fig. 1 illustrates the relative expression of I-E in the unselected population and the population selected for high expression (ANV/I-Ekhi). Also shown are the I-E and I-A expression levels of untransfected ANV cells that were or were not treated with IFN-γ, as well as the expression levels of normal TECs isolated from deoxyguanosine-treated day 14–16 embryonic thymi. As shown in Fig. 1, although the ANV/I-Ek transfected cells clearly expressed significant levels of I-Ek, the expression was about 20% that of freshly isolated TECs. However, after selection for the high I-E expressing cells, the level was similar to that of TECs and somewhat lower than INF-γ-stimulated ANV cells. As expected, positive staining for I-Ak was only observed on ANV/IFN-γ-treated cells and normal TECs, confirming that the I-Ek expressed by the transfected cells was a product of the transfected genes and not a product of the endogenous class II MHC genes.
Figure 1.
Expression of class II MHC on transfected ANV cortical epithelial cell lines. I-Ek and I-Ak expression levels on the following cells are shown: (a) ANV cell line. (b) ANV stimulated with 100 units of IFN-γ (ANV/IFN-γ) for 4 days. (c) I-Ek-transfected ANV (ANV/I-Ek). (d) ANV/I-Ek selected for high expression of I-Ek by cell sorting (ANV/I-Ekhi). (e) Normal TECs.
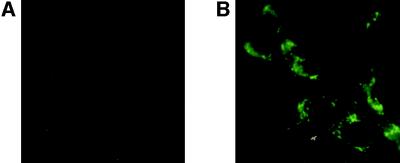
We also examined the same ANV populations for the expression of invariant chain. As shown in Fig. 2A, undetectable levels of invariant chain were found in intracellular compartments of I-Ek transfected ANV cells or untransfected ANV (data not shown). However, after stimulation with IFN-γ, ANV cells that were either untransfected (Fig. 2B) or I-Ek-transfected (data not shown) contained readily detectable levels of invariant chain.
Figure 2.
Expression of invariant chain by ANV cell lines. Fixed and permeabilized ANV cells were stained with the invariant-chain-specific antibody IN-1. (A) ANV/I-Ek. (B) ANV/IFN-γ.
Capacity of I-Ek Transfected ANV Cells to Support Thymic Differentiation.
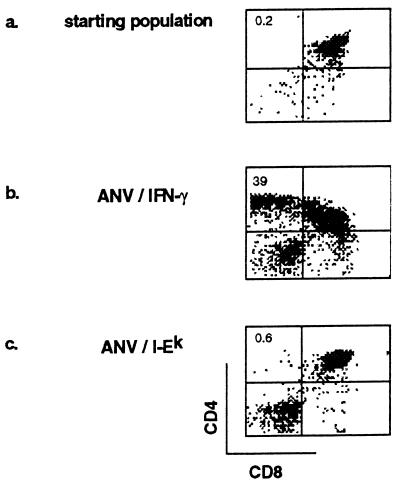
We next studied the capacity of ANV/I-Ek to induce positive selection of TCR transgenic thymocytes expressing a moth cytochrome c-specific TCR. The TCR transgenic animals used were bred on to a B10.A(4R) background that, due to the absence of I-Ek, cannot positively select for CD4 SP transgenic thymocytes. The small population of CD4 SP cells present in the newborn thymus (presumably expressing endogenous TCR α genes and positively selected by I-Ak) was depleted by anti-CD8 magnetic bead selection, which further enriched the DP population before the establishment of the reaggregation cultures. As shown in Fig. 3a, the starting population of thymocytes contained very few CD4 SP cells, routinely 0.1–0.4% of the total cell number. As reported (A.N., C. H. Clegg, and A.G.F., unpublished results), when ANV/IFN-γ was used as the source of cortical epithelial cells in RCs, a dramatic increase in CD4 single positive cells was observed (Fig. 3b). This was in striking contrast to the data obtained when ANV/I-Ek-transfected TECs were used in the RCs, where no significant increase in CD4 SP cells was observed (Fig. 3c). It was considered that the inability of ANV/I-Ek cells to induce positive selection was most likely related to their lack of expression of invariant chain, because it has been shown that (i) the invariant chain is important in directing the MHC to the peptide-loading compartment and (ii) invariant-chain-deficient mice have a profound defect in the positive selection of an MCC 88-103 reactive repertoire (22).
Figure 3.
Capacity of ANV cell lines to induce positive selection of MCC 88-103-specific TCR transgenic thymocytes. RCs were established with anti-CD8 purified DP thymocytes obtained from B10A(4R) TCR transgenic mice and analyzed for CD4 and CD8 expression. (a) Starting population of thymocytes before RC. (b) Four-day RCs that used ANV/IFN-γ as the source of cortical epithelial cells. (c) Four-day RCs that used ANV/I-Ek as the source of cortical epithelial cells. Positive selection of CD4 SP thymocytes occurred when ANV/IFN-γ were used as cortical epithelial cells but not when ANV/I-Ek were used.
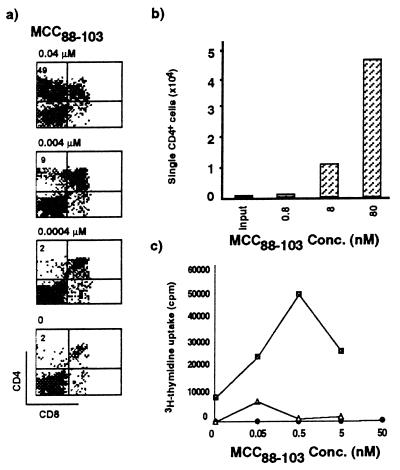
Because ANV/I-Ek-transfected cells were unable to process and present endogenous peptides capable of inducing positive selection, we could assess the impact of exogenous peptides on the differentiation of the MCC 88-103-specific TCR transgenic thymocytes in vitro. Initially, the cognate antigen recognized by the AND TCR, MCC 88-103, was tested for its capacity to induce positive selection in the RC system. Fig. 4a illustrates the effect of various concentrations of MCC 88-103 on the RC. A dose-dependent increase in single positive cells after culture with this peptide for 4 days was observed, with a maximum of 49% of single positive CD4 thymocytes being recovered from the cultures containing 0.04 μM antigen. Significant positive selection was obtained with as little as 0.004 μM MCC 88-103. Calculations of the absolute numbers of recovered CD4 SP cells demonstrated a large increase in the number of cells (up to 24-fold) over the starting population of CD4 SP cells (Fig. 4b). This increase in absolute numbers of SP cells demonstrates that preferential survival of preexisting SP cells over other thymocyte populations does not play a significant role in the increase of SP cells in the reaggregation system. Furthermore, experiments with BrdUrd indicate that the few SP cells present at the start of culture do not undergo extensive proliferation (data not shown), further supporting the conclusion that the increase in SP cells upon exposure to antigen was due to differentiation of DP cells.
Figure 4.
MCC 88-103 can induce positive selection of TCR transgenic thymocytes. RCs were established with ANV/I-Ek as the source of cortical epithelial cells, and MCC 88-103 was added at various concentrations at the start of the 4-day culture period. (a) Dose–response experiment of positive selection. At the optimal dose of 0.04 μM MCC 88-103, 49% of thymocytes were CD4 SP. (b) Recovery of CD4 SP thymocytes after RC in presence of MCC 88-103. (c) Antigen-specific proliferative capacity of thymocytes recovered from RCs. ▪, Thymocytes from RCs with 0.04 μM MCC 88-103; ▵, thymocytes from RCs with no peptide added; •, starting population of DP thymocytes before RC.
In experiments using an in vitro system to study positive selection of class I MHC-restricted thymocytes, it was reported (8) that antigenic peptides could only support the development of nonfunctional single positive thymocytes that were unresponsive to stimulation with antigenic peptide. Therefore, it was important to determine the functional status of CD4 cells generated in RCs containing MCC 88-103. A representative experiment is shown in Fig. 4c. Sources of thymocytes included the double-positive input thymocytes for the RCs, thymocytes obtained from RCs established with optimal concentrations of antigen (0.04 μM), and thymocytes recovered from RCs set up in the absence of peptide. When established in secondary cultures containing B10A splenic antigen-presenting cells, only the thymocytes from RCs containing antigen were able to proliferate in response to antigen. Thus, the CD4 SP thymocytes generated in the RCs as a consequence of exposure to antigen have acquired the hallmark functional property of positively selected thymocytes, namely, antigen responsiveness.
Further evidence for the functional maturation of these single positive cells was obtained by the analysis of surface antigens. Thus, along with their differentiation to single positive cells, they also expressed increased levels of TCR, expressed CD69, and expressed increased levels of L selectin, all characteristics of normally differentiating CD4 SP thymocytes (data not shown).
Capacity of Antigen Analogs to Induce Positive Selection.
Having established that the cognate antigen was capable of inducing positive selection of TCR transgenic thymocytes in the RC system, we next wished to determine the relationship between the antigenicity of peptides for mature T cells and their capacity to induce positive selection. For this purpose, we selected a panel of cytochrome c analogs that had been assayed for their capacity to stimulate proliferation of a T cell line derived from an AND transgenic animal. The following three categories of peptides were selected: relatively strong antigens capable of stimulating a proliferative response at a concentration of <1 μM, weak antigens that required concentrations between 1 and 100 μM to stimulate T cells, and nonstimulatory analogs. The latter peptides were classified as either TCR antagonists or nonantagonists, as measured in the prepulse assay (23). The peptides chosen for study are listed in Table 1, along with the concentration of peptide required to stimulate a proliferative response from the AND cell line.
Table 1.
Correlation between antigenicity and positive selecting capacity of antigen analogs
| No. | Sequence | Substitution | Antigenicity, μM | Positive selection, μM |
|---|---|---|---|---|
| 1 | KAERADLIAYLKQATAK | PCC | 0.015 | 0.0002 |
| 2 | ANERADLIAYLKQAGK | 102T-G | 0.038 | 0.0005 |
| 3 | ANERADLIAYLKQATK | MCC | 0.05 | 0.006 |
| 4 | ANERADLIAYLKNATK | 100Q-N | 0.5 | 0.09 |
| 5 | KAERADLIAYLRQATAK | 99K-R | 1.5 | 0.9 |
| 6 | ANERADLIAILKQATK | 97Y-I | 4 | 2 |
| 7 | ANERADLINYLKQATK | 96A-N | 11 | 6 |
| 8 | ANERADLIAYLOQATK | 99K-Q | 16 | 1 |
| 9 | ANERADLIAYLKQAOK | 102T-Q | 50 | 59 |
| 10 | ANERADLIAYLKQALK | 102T-L | —A* | — |
| 11 | ANERADLIVYLKQATK | 96A-V | —A | — |
| 12 | ANERADLIGYLKQATK | 96A-G | —† | — |
| 13 | ANERADLIAYLKQITK | 101A-I | — | 45 |
| 14 | ANERADLIAYLKQARK | 102T-R | — | — |
| 15 | ANERADLIAYLKQAEK | 102T-E | — | — |
Antigenicity was measured as the concentration of peptide required to stimulate 40% of the maximum proliferative response from the AND T cell line. Positive selection was measured as the concentration of peptide required to induce 20% of CD4 SP cells in RC.
Antagonist analogs.
No response at 40 μM.
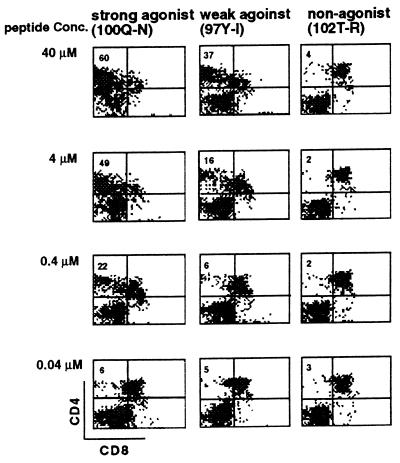
Each of the analogs was tested over a wide range of concentrations for the capacity to induce CD4 SP thymocyte differentiation in RCs. A striking correlation was observed between the concentration of antigen required to stimulate peripheral T cells and the capacity to stimulate positive selection. Representative data from a strong, weak, and nonantigenic peptide are shown in Fig. 5. As was observed with the cognate antigen, the capacity for antigen analogs to induce positive selection was dose-dependent. The relatively potent antigen analog Q100N was required at 0.4 μM to induce approximately 20% CD4 SP cells. In contrast, between 4 and 40 μM of the weak antigen analog Y97I was required to achieve a similar degree of positive selection. The nonstimulatory analog T102R was incapable of producing any detectable positive selection at the highest dose tested, 40 μM. As shown in Table 1, all peptides that were detectably antigenic for the AND T cell line were also capable of inducing positive selection. On the other hand, only one of the six nonantigenic peptides analyzed was consistently capable of inducing positive selection. This peptide, 101A-I, was not a TCR antagonist.
Figure 5.
Capacity of MCC 88-103 analogs to cause positive selection. Strong (100Q–N), weak (101A–V), or non- (102T–R) antigenic peptides were added at various concentrations at the initiation of RC and the generation of CD4 SP cells was measured on day 4.
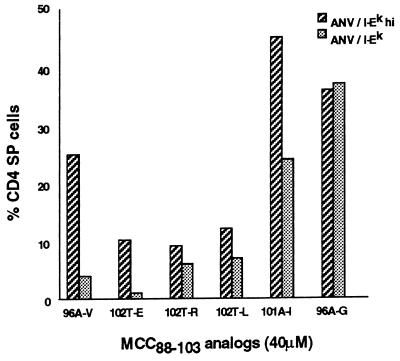
Because the ANV/I-Ek cells used in these experiments were unselected for high I-Ek expression, we repeated the RC in which nonantigenic peptides were added, with the ANV/I-Ekhi cells that expressed quantities of I-Ek similar to that of normal TECs. Fig. 6 shows the percent CD4 SP cells obtained from RCs at the highest concentration of peptide tested (40 μM) when ANV/I-Ek and ANV/I-Ekhi cells were used as the source of TECs. In most cases, an increase in CD4 SP cells was observed when ANV/I-Ekhi cells were used as the source of thymic stromal cells, with the 20% endpoint being achieved by two of the six peptides (101A-I and 96A-G), and a marginally positive result with a third peptide (96A-V). Again, there was no correlation between the capacity of these nonantigenic peptides to induce positive selection and their TCR antagonist properties.
Figure 6.
Capacity of nonantigenic peptides to cause positive selection. The six nonantigenic analogs from Table 1 were added at a concentration of 40 μM to RCs established with ANV/I-Ek or ANV/I-Ekhi as epithelial cells. The percentage of CD4 SP cells present in cultures with no added antigen has been subtracted (ANV/I-Ek, 0.4%; ANV/I-Ekhi, 6%).
Finally, to obtain further information on the structural characteristics of peptides capable of inducing positive selection, we studied a panel of polyalanine-containing analogs of MCC 88-103, all of which had a high binding capacity for I-Ek due to the presence of the major MHC contact residues Ile-95 and Lys-103 but differed in the number of TCR contact residues. Previous studies with single amino acid substitution analogs of MCC 88-103 had indicated that Lys-99 and Thr-102 were the major TCR contact residues, whereas Ala-96, Tyr-97, Gln-100, and Ala-101 played less important roles in recognition by AND T cells. A set of peptides with various numbers of major and minor TCR contact residues was constructed. This set of peptides allowed us to evaluate how many and which TCR contact residues were required for induction of positive selection. The data summarized in Table 2 indicate that the minimum requirement for induction of positive selection was the presence of three minor and one major TCR contact residues (AYAT, AYKA), whereas the peptide with four minor TCR contacts (AYQA) or two minor and one major contact residues (AAT) failed to induce positive selection. As was the case with the single amino acid substituted analogs of MCC 88-103, there was a strong correlation between antigenicity for the AND T cell line and the capacity of the polyalanine-containing peptides to induce positive selection.
Table 2.
Correlation between peptide structure and positive selection
| No. | Sequence | TCR contact residues | Antigenicity, μM | Positive selection, μM |
|---|---|---|---|---|
| 1 | ANERADLIAYLKOATK | AYKQAT | 0.05 | 0.006 |
| 2 | AAAAAAAIAYAAAATK | AYAT | 1.0 | 1.0 |
| 3 | AAAAAAAIAYAKAAAK | AYKA | —* | 14.0 |
| 4 | AAAAAAAIAYAAOAAK | AYQA | — | — |
| 5 | AAAAAAAIAAAAAATK | AAT | — | — |
| 6 | AAAAAAAIAAAAAAAK | AA | — | — |
Antigenicity was measured as the concentration of peptide required to stimulate 40% of the maximum proliferative response from the AND T cell line. Positive selection was measured as the concentration of peptide required to induce 20% of CD4 SP cells in RC.
No response at 40 μM.
DISCUSSION
Transfection of the cortical epithelial cell line ANV-41-2 with I-Ek resulted in the generation of class II MHC-expressing cells that lacked the expression of invariant chain. Consequently, they were deficient in the capacity to process and present many endogenous protein-derived peptides but could efficiently bind and present exogenously added peptides. Consistent with the previous finding of Mathis and colleagues (22) using invariant-chain-deficient mice, these invariant-chain-negative I-Ek-positive cells could not support positive selection of thymocytes expressing an MCC 88-103/I-Ek-specific TCR transgene. These cells allowed us to examine the properties of peptides able to mediate positive selection in an RC system, in which the epithelial cells expressed a near normal density of the class II MHC restriction element. Another important aspect of this system was that because the source of epithelial cells was a cell line and the source of thymocytes were mice [B10A(4R)] that lacked the MHC restriction element I-Ek, there was a complete lack of bone-marrow-derived cells capable of mediating negative selection. This allowed us to evaluate the characteristics of positive selection in the absence of the confounding feature of negative selection that is present in other in vitro systems.
Previous reports have analyzed the nature of peptides capable of selecting CD8+ TCR transgenic thymocytes specific for class I MHC epitopes in fetal thymic organ cultures. Conflicting data were obtained, in that some groups reported that the cognate antigen at low concentrations was capable of inducing positive selection (5–7) and others reported that the cognate antigen was incapable of inducing positive selection and that only nonantigenic TCR antagonist peptides were able to induce differentiation into functional CD8 single positive cells (8–10). This latter result is most compatible with an affinity model of positive selection, in which there is some upper and lower limit of affinity that is compatible with positive selection, regardless of the concentration of MHC–peptide ligand complex present. The reports that showed that cognate antigen was very effective at causing positive selection, but less antigenic analogs were less efficient and required higher concentrations, were interpreted as favoring an avidity model in which affinity and ligand concentration work in concert in determining the capacity to induce positive selection. The current study systematically examined the role of peptides in positive selection of class II MHC-restricted CD4+ cells in a reaggregation culture. Our results strongly support the differential avidity model, in that there was a very strong correlation between TCR affinity (as estimated by the concentration of peptide required to induce proliferation of mature T cells) and the capacity to induce positive selection, and there was no upper limit of affinity/antigenicity above which positive selection did not occur.
The critical question left unanswered by all studies performed to date is: What is the nature of the naturally processed peptides in the thymus that induce positive selection? Are they low-affinity ligands expressed at high concentration or high-affinity ligands expressed at low concentration? Recent studies that have explored the capacity of a single peptide ligand to positively select CD4+ cells indicate that a large and diverse TCR repertoire can be generated by high concentrations of a single peptide ligand (11–14). This result and experiments that indicate certain highly expressed naturally processed self-peptides when used at high concentrations can cause positive selection of TCR transgenic thymocytes whose cognate antigen bears little structural similarity to the selecting peptide strongly support the idea that high concentrations of low-affinity ligands can cause positive selection. Whether the concentrations of peptides used to demonstrate this result are physiologically relevant is in question, and if not, what is the structure of the higher-affinity peptides expressed at lower, more relevant, concentrations that are functional in the thymus?
Acknowledgments
We thank Karen Anderson for her technical support and Joyce Joseph for assistance in preparation of the manuscript. This work was supported by Grants AI-24137 and AG-04360 from the National Institutes of Health (A.G.F.) and Grant AI18634 from the National Institutes of Health (H.M.G.). This is publication 197 of the La Jolla Institute for Allergy and Immunology.
ABBREVIATIONS
- RC
reaggregate culture
- TEC
thymic epithelial cell
- TCR
T cell receptor
- MHC
major histocompatibility complex
- FITC
fluorescein isothiocyanate
- IFN-γ
interferon γ
References
- 1.Jameson S C, Hogquist K A, Bevan M J. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 2.Murphy K M, Helmberger A B, Loh D Y. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 3.Pircher H, Rohrer U H, Moskophidis D, Zinkernagel R M, Hengartner H. Nature (London) 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 4.Page D M, Alexander J, Snoke K, Appella E, Sette A, Hedrick S M, Grey H M. Proc Natl Acad Sci USA. 1994;91:4057–4061. doi: 10.1073/pnas.91.9.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashton-Rickardt P G, Bandeira A, Delaney J R, Van-Kaer L, Pircher H P, Zinkernagel R M, Tonegawa S. Cell. 1994;76:593–596. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 6.Sebzda E, Wallace V A, Meyer J, Yeung R S, Mak T W, Ohashi P S. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 7.Sebzda E, Kundig T M, Thomson C T, Aoki K, Mak S-Y, Mayer J P, Zamborelli Y, Nathenson S G, Ohashi P S. J Exp Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogquist K A, Jameson S C, Heath W R, Howard J L, Bevan M J, Carbone F R. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 9.Barnden M J, Heath W R, Rodda S, Carbone F R. Eur J Immunol. 1994;24:2452–2456. doi: 10.1002/eji.1830241029. [DOI] [PubMed] [Google Scholar]
- 10.Hogquist K A, Jameson S C, Bevan M J. Immunity. 1995;3:79–86. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 11.Ignatowicz L, Kappler J, Parker D C, Marrack P. J Immunol. 1996;157:1827–1831. [PubMed] [Google Scholar]
- 12.Ignatowicz L, Rees W, Pacholczyk R, Ignatowicz H, Kushnir E, Kappler J, Marrack P. Immunity. 1997;7:179–186. doi: 10.1016/s1074-7613(00)80521-x. [DOI] [PubMed] [Google Scholar]
- 13.Tourne S, Miyazaki T, Oxenius A, Klein L, Fehr T, Kyewski B, Benoist C, Mathis D. Immunity. 1997;7:187–196. doi: 10.1016/s1074-7613(00)80522-1. [DOI] [PubMed] [Google Scholar]
- 14.Surh C D, Lee D-S, Fung-Leung Wp, Karlsson L, Sprent J. Immunity. 1997;7:209–220. doi: 10.1016/s1074-7613(00)80524-5. [DOI] [PubMed] [Google Scholar]
- 15.Ashton-Rickardt P G, Van-Kaer L, Schumacher T N, Ploegh H L, Tonegawa S. Cell. 1993;73:1041–1049. doi: 10.1016/0092-8674(93)90281-t. [DOI] [PubMed] [Google Scholar]
- 16.Hogquist K A, Gavin M A, Bevan M J. J Exp Med. 1993;177:1469–1473. doi: 10.1084/jem.177.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkinson E J, Anderson G, Owen J J. J Exp Med. 1992;176:845–853. doi: 10.1084/jem.176.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson G, Jenkinson E J, Moore N C, Owen J J. Nature (London) 1993;362:70–73. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- 19.Anderson G, Owen J J, Moore N C, Jenkinson E J. J Immunol. 1994;153:1915–1920. [PubMed] [Google Scholar]
- 20.Ernst B, Surh C D, Sprent J. J Exp Med. 1995;182:961–971. doi: 10.1084/jem.182.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaFace D M, Couture C, Anderson K, Shih G, Alexander J, Sette A, Mustelin T, Altman A, Grey H M. J Immunol. 1997;158:2057–2064. [PubMed] [Google Scholar]
- 22.Tourne S, Nakano N, Viville S, Benoist C, Mathis D. Eur J Immunol. 1995;25:1851–1856. doi: 10.1002/eji.1830250709. [DOI] [PubMed] [Google Scholar]
- 23.De Magistris M T, Alexander J, Coggeshall M, Altman A, Gaeta F C A, Grey H M, Sette A. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]