Transcriptional regulators of the NF-kB/IkB family promote the expression of well over 100 target genes, the majority of which participate in the host immune response (1). These proteins include a multitude of cytokines and chemokines, receptors required for immune recognition, proteins involved in antigen presentation, and adhesion receptors involved in transmigration across blood vessels walls. Because of this extensive role in immune action, NF-kB has been termed the central mediator of the immune response. Gene knockout and other studies establish roles for NF-kB in the ontogeny of the immune system but also demonstrate that NF-kB participates at multiple steps during oncogenesis (2) and the regulation of programmed cell death (3).
For several reasons, the NF-kB pathway provides an attractive target to viral pathogens. Activation of NF-kB is a rapid, immediate early (IE) event that occurs within minutes after exposure to a relevant inducer, does not require de novo protein synthesis, and results in a strong transcriptional stimulation of several early viral as well as cellular genes. In this review, we will describe strategies that viruses have evolved to modulate the NF-kB pathway, to enhance viral replication, host cell survival, and evasion of immune responses. Activation of NF-kB constitutes an obvious target because many of its target genes — growth factors, cytokines and their receptors, and proto-oncogenes — profoundly influence the host cell cycle. In addition, some viruses exploit the antiapoptotic properties of NF-kB to evade the host defense mechanisms that limit replication by killing infected cells, or conversely to trigger apoptosis as a mechanism to increase virus spread.
Perhaps not surprisingly, the persistent activation of the NF-kB pathway maintained by certain viruses contributes to oncogenic transformation (2). In addition to the classic studies with the avian REV-T retrovirus which contains the v-Rel oncoprotein and induces a rapid and fatal B-cell lymphoma in young birds (4), several lines of evidence demonstrate that NF-kB family members contribute to human oncogenesis. Localization of NF-kB–encoding genes at sites of chromosomal translocations and genomic rearrangements in cancer, high levels of NF-kB activity in many breast cancer cells, and constitutive nuclear NF-kB complexes in Hodgkin’s lymphoma cells all support this view (2). Furthermore, as discussed below, viral oncogene products, including human T-cell leukemia virus type 1 (HTLV-1) Tax protein and Epstein-Barr virus latent infection membrane protein 1 (EBV LMP1), each act by unique mechanisms to disrupt NF-kB regulation and initiate viral transformation.
Biochemistry of NF-kB activation
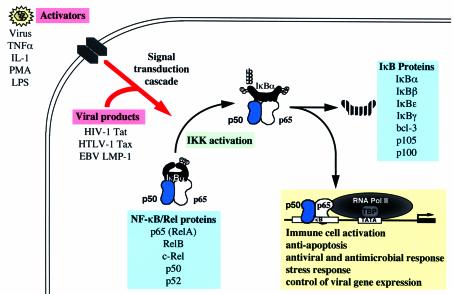
In response to a variety of stimuli, including viral and bacterial pathogens, cytokines, and stress-inducing agents (5), the latent cytoplasmic NF-kB/IkB complex is activated by phosphorylation; in the case of IkBa, this modification occurs at serines 32 and 36 by the IkB kinase (IKK) complex. Phosphorylation targets IkBa for ubiquitination — the covalent attachment of multiple ubiquitin molecules — at IkBa lysine residues 21 and 22, whereupon this inhibitory subunit is degraded by the 26S proteasome, allowing the release of NF-kB proteins. Thus activated, NF-kB translocates to the nucleus, where it stimulates transcription of genes containing the consensus sequence 5¢-GGGRNNYYCC-3¢ (Figure 1). The NF-kB family consists of five structurally related proteins (c-Rel, RelA, RelB, p50/p105, and p52/p100) that share an approximately 300–amino acid NH2-terminal Rel homology domain that contains sequences essential for dimerization, DNA binding, and nuclear transport. Family members c-Rel, RelA, and RelB possess COOH-terminal transactivation domains, while p50/p105 and p52/p100 function as either short DNA binding proteins or larger inactive proteins which contain COOH-terminal inhibitory domains. Because of the potential for generating diverse homo- and heterodimers, these proteins may allow transcriptional specificity by forming combinations that can interact specifically with structural variants of the kB DNA binding site. As shown in Figure 1, several IkB proteins also provide a level of specific regulation by retaining NF-kB complexes in the cytoplasm in a latent inactive state (1).
Figure 1.
The biochemistry of NF-κB activation. NF-κB is sequestered in the cytoplasm by the inhibitory IκB proteins. Stimulation by a diverse array of pathogens and other inducers including viruses, cytokines, and stress-inducing agents leads to the activation of signaling cascades that culminate with the activation of the IKK complex and phosphorylation of the IκB inhibitor. NF-κB DNA binding subunits are released and translocate to the nucleus where they transactivate NF-κB responsive genes containing the decameric sequence (5′-GGGRNNYYCC-3′). Target genes are selectively regulated by the distinct transcriptional activation potential of different NF-κB subunit combinations. TBP, TATA binding protein.
NF-kB activation by the IkB kinase complex
A major advance in the understanding of NF-kB regulation came with the identification of the multisubunit IKK kinase complex, which contains two catalytic subunits, IKK-a and IKK-b, and the regulatory subunit IKK-g (NF-kB essential modulator [NEMO]) (reviewed in ref. 6). The predominant form of the IKK complex is an IKK-a/IKK-b heterodimer associated with IKK-g, and this association is mediated by the interaction of IKK-b and IKK-g. IKK-g is not a kinase per se but is absolutely essential for NF-kB activation by multiple activators. Since the identification of the IKK complex, attention has focused on the upstream kinases of different signal transduction pathways and how these pathways converge on the IKK complex. These kinases include NF-kB–inducing kinase (NIK), mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1 (MEKK1), TGF-b–activated kinase (TAK1), Cot/Tpl-2, protein kinase R (PKR), PKC, Akt (or PKB), and mixed-lineage kinase 3 (MLK3). Once activated, IKK-b becomes autophosphorylated at a carboxy terminal serine cluster, which decreases IKK activity and prevents prolonged NF-kB activation by negative autoregulation.
Mice deficient in IKK-b and IKK-a have recently been created; ikk–/–mice die at about 14 days of gestation due to massive hepatic cell apoptosis, a phenotype remarkably similar to that seen in mice deficient in the RelA(p65) subunit of NF-kB. This phenotype may be due to the loss of the antiapoptotic effects of NF-kB, since NF-kB activation is decreased after TNF-a or IL-1 treatment of knockout fibroblasts. In contrast, mice lacking IKK-a maintain functional NF-kB activation but exhibit profound epidermal and morphogenic abnormalities and die in utero or soon after birth (6). These perplexing phenotypes hint that the IKK subunits are associated with still-undefined functions.
Viral activation of NF-kB
NF-kB is activated by multiple families of viruses, including HIV-1, HTLV-1, hepatitis B virus (HBV), hepatitis C virus (HCV), EBV, and influenza virus. This activation may serve several functions: to promote viral replication, prevent virus-induced apoptosis, and mediate the immune response to the invading pathogen. Although the molecular details remain unclear, viral products that activate NF-kB (the HTLV-1 Tax protein, the HIV-1 Tat protein, and the EBV LMP1, among others; ref. 5) appear to act through several distinct mechanisms to enhance virus replication. With HIV, viral and cellular membrane fusion activates NF-kB, and this process requires CD4; other viruses may activate similar membrane proximal signaling cascades that activate NF-kB. NF-kB is also activated by synthetic dsRNA, suggesting that viruses that generate dsRNA replicative intermediates employ a common mechanism to enhance viral replication. Influenza virus hemagglutinin (HA), matrix protein (MA), and nucleoprotein (NP), as well as HBV HBx (x protein of hepatitis B) protein and HCV core protein, can also induce NF-kB.
NF-kB activation in HIV-1 infection
The intracellular efficiency of HIV-1 gene expression and replication is due in part to the ability of HIV-1 to co-opt host signaling pathways to activate viral transcription (7). The promoter-proximal (enhancer) region of the HIV-1 long terminal repeat (LTR) contains two adjacent NF-kB binding sites (–109 to –79) that play a central role in mediating inducible HIV-1 gene expression. In fact, transdominant mutants of IkBa that block NF-kB induction also inhibit de novo HIV-1 infection in T cells by interfering with viral transcription (8, 9).
The molecular organization of the LTR in different naturally occurring HIV-1 subtypes appears to have coevolved with variations in the HIV-1 enhancer that influence viral expression and replication. For example, HIV-1 clade E (the predominant subtype in Asia) contains a single NF-kB binding site in the enhancer region and has reduced TNF-a responsiveness (10), whereas the HIV-1 LTR from subtype C (the predominant subtype in Africa) contains three NF-kB binding sites and has higher transactivational activity than the HIV-1 LTR from the other subtypes. Unexpectedly, the subtype E, with one NF-kB binding site, displays a higher transmission efficiency than the North American subtype B, which carries two NF-kB binding sites. The explanation for this discrepancy appears to lie with a single nucleotide deletion in the upstream copy of the NF-kB binding sites in the clade E LTR, which creates a binding site for the GABPa and GABPb1 transcription factor. When the GABP binding site is introduced into the LTR of the clade B virus, the resulting virus replicates better than the wild type (11).
NF-kB activity is further enhanced through synergism with Sp-1, which binds to three sites adjacent to NF-kB binding sites. In addition, NFAT1 (NFATp) negatively regulates the LTR by competing with NF-kB for its binding site, whereas NFAT2 (NFATc) positively regulates HIV-1 LTR through the NF-kB binding sites (12). Together, these studies illustrate the complexity of NF-kB control of the HIV LTR and suggest that these transcriptional modifications alter viral replication and perhaps pathogenesis (13).
The role of NF-kB in activating HIV transcription has been extensively analyzed. In normal human CD4+ T lymphocytes, NF-kB binding activity is low and consists predominantly of p50, but not p65, DNA binding. T-cell activation results in the formation of p50-p65 NF-kB complexes and enhancer-dependent HIV LTR transactivation, indicating that unstimulated CD4+ T lymphocytes offer a cellular environment of low permissiveness to HIV LTR function. In HIV-infected T cells, NF-kB–dependent transactivation is essential for HIV-LTR induction; interestingly, even the function of HIV Tat in resting CD4 T lymphocytes depends on kB responsive elements in the LTR. Furthermore, CD4+ T lymphocytes carrying an infectious HIV provirus with point mutations in these elements fail to transcribe viral RNA upon cell activation (14). However, other studies indicate that NF-kB sites are not absolutely required for viral growth, since HIV-1 will grow, albeit slowly, in the absence of NF-kB domains (15).
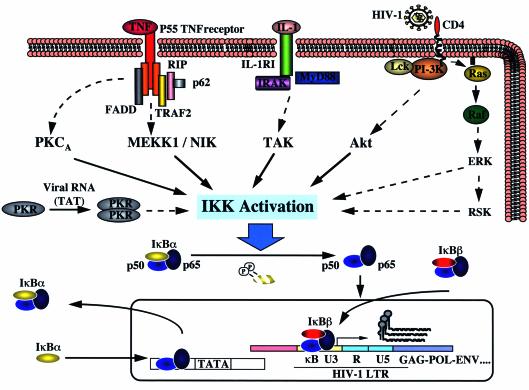
NF-kB is constitutively activated in primary monocytes and myeloid cell lines chronically infected with HIV-1 (7). Increasing the intracellular levels of NF-kB may permit high levels of HIV-1 gene expression and could thus provide a favorable environment for HIV replication. Upstream of NF-kB activity, the IKK complex is constitutively activated in chronically HIV-1–infected myeloid cells (16, 17). How IKK is activated by HIV-1 infection is currently unclear, although multiple mechanisms appear to be operating, and several HIV-1 proteins can activate NF-kB. Envelope glycoprotein gp120 can signal NF-kB by engaging the CD4 receptor in a pathway that involves p56lck and activates NF-kB and HIV-1 LTR transcription (18). Ras, an upstream signaling molecule of Raf, is also activated by CD4 crosslinking and can activate NF-kB (19, 20). Binding of HIV-1 to CD4 also activates the phosphatidylinositol 3-kinase (PI-3K), which functions upstream of Akt to stimulate IKK (21, 22, 23). Therefore, CD4 signaling upon HIV-1 binding or gp120 ligation may activate NF-kB via two different but closely related pathways, one through p56lck and Raf, and a second through PI-3K, Akt, and IKK (Figure 2).
Figure 2.
Proposed mechanisms of NF-κB activation in HIV-1–infected cells. Several mechanisms may regulate NF-κB activity in HIV-1–infected cells. Binding of HIV-1 to CD4 leads to NF-κB activation via Lck/Raf and PI-3K/Akt. CD4 crosslinking induces Ras activation, which also leads to NF-κB activation. PKR, induced by low level IFN and HIV-1 transcripts, may induce IKK activation. Autocrine release of cytokines such as TNF-α and IL-1 may constitutively stimulate the signaling pathways leading to NF-κB activation. Activation of the IKK complex either directly by HIV-1 regulatory proteins or by cytokine release leads to the phosphorylation and degradation of IκBα and IκBβ, thus releasing NF-κB to translocate to the nucleus and transactivate responsive genes. Newly synthesized IκBβ enters the nucleus and prevents IκBα-mediated termination of the NF-κB response, thus maintaining constitutive NF-κB activity at the protein-DNA level and creating an intracellular environment conducive to viral replication.
HIV regulatory or accessory proteins may also stimulate NF-kB activity. HIV Tat protein stimulates a wide variety of cellular responses, including NF-kB induction. Manna and Aggerwal have compared the role of the T cell–specific tyrosine kinase p56lck in HIV-tat mediated cellular responses in Jurkat T cells and JCaM1 cells, an isogeneic lck-deficient T-cell line. They find that treatment with HIV-tat protein activates NF-kB and induces NF-kB–dependent reporter gene expression in a time-dependent manner in Jurkat cells but not in JCaM1 cells, suggesting the critical role of p56lck kinase. HIV-tat also induces cytotoxicity, activates caspases, and promotes the accumulation of reactive oxygen intermediates in Jurkat cells, but not in JCaM1 cells, unless the latter cells are reconstituted with p56lck tyrosine kinase. Overall, these results demonstrate that p56lck plays a critical role in the activation of NF-kB and apoptosis by Tat protein (24). A recent report also ascribes a similar role to the virion-derived accessory protein Vpr, which activates IL-8 expression in T cells and macrophages through a mechanism dependent on NF-kB and NF–IL-6. Elevated Vpr expression also increases the activity of transcription of viral promoters for HIV, cytomegalovirus (CMV), and SV40, as well as for several proinflammatory cytokine genes (25). Finally, HIV-1 Nef protein, which associates with membrane microdomains known as rafts, can increase IL-2 secretion from T cells stimulated via CD3 or CD28. The Nef-mediated superinduction of IL-2 reflects activation of both NFAT and NF-kB. By this mechanism, Nef enhances HIV-1 transcription in response to CD3 or CD28 stimulation, thus promoting HIV-1 replication and viral spread (26).
HIV infection of immune system cells is itself a potent and persistent cause of immune activation. Several endogenous cytokines released by activated cells profoundly affect HIV disease progression. Expression of these cytokines is dysregulated in HIV-infected individuals (7). Many of these factors, including IL-1a, IL-1b, IL-2, -3, -6, -7, -15, -18, TNF-a, TNF-b, and growth factors M-CSF and GM-CSF, induce HIV expression. Conversely, IL-13, IL-15, IFN-a, and IFN-b suppress viral replication, and TGF-b, IL-4, IL-10, IL-13, and IFN-g can either induce or suppress HIV replication, depending on the duration of treatment and the cell system. Studies in PBMCs and lymph node mononuclear cells indicate that HIV transcription is intimately related to increased expression of TNF-a, IL-1b, IL-6, TGF-b, and IFN-g, some of which activate NF-kB via IKK. Activation of this pathway will, in turn, further augment cytokine and LTR-directed gene expression. dsRNA generated during HIV-1 replication can interact in infected cells with endogenous IFN to activate dsRNA-activated kinase PKR and thereby induce IKK activity and degradation of IkBa and IkBb. Recently, Zamanian-Daryoush and colleagues (27) showed that PKR is involved in dsRNA-induced NF-kB activation, which is mediated by NIK and IKK.
In addition to the various pathways illustrated in Figure 2 by which HIV proteins can activate NF-kB, this virus can act through IkBb to maintain NF-kB in a persistently activated state. Interestingly, in HIV-infected myeloid cells, IkBb is found in NF-kB/DNA binding complexes that are resistant to IkBa-mediated dissociation (16). Association of IkBb with DNA-bound NF-kB apparently protects NF-kB–DNA complexes from IkBa-mediated dissociation and helps maintain NF-kB activity in these cells (Figure 2).
Hijacking of IkB kinases by the Tax transforming protein of HTLV-1
HTLV-1 is the etiologic agent of adult T-cell leukemia (ATL), an acute malignancy of CD4+ T lymphocytes. The HTLV-1 Tax oncoprotein is required for gene transcription directed from the 5¢ LTR from the HTLV proviral genome and mediates the oncogenic activity of HTLV-1. Tax immortalizes primary human T lymphocytes, and transgenic mice expressing Tax develop mesenchymal tumors or large granular lymphocytic leukemia. Thus, Tax appears both necessary and sufficient for the induction of neoplastic transformation. Tax transactivates not only the HTLV-1 LTR but also interacts with NF-kB, CREB/ATF, SRF, and NF-AT and other transcription factors to transactivate a wide range of cellular promoters. The existence of specific Tax mutants that differentially affect NF-kB and CREB/ATF functions suggests that Tax acts on these two host signaling pathways by distinct mechanisms (28).
In contrast to its transient mode of action during a physiologic T-cell response, NF-kB is chronically activated in HTLV-1–infected T cells. Activation of NF-kB by Tax results in the upregulation of cellular genes that govern normal growth-signal transduction, such as cytokines and growth factors (IL-2, IL-6, IL-15, TNF, and GM-CSF), cytokine receptors (IL-2 and IL-15 receptor a chains), proto-oncogenes (c-Myc), and antiapoptotic proteins (bcl-xl). In particular, upregulation of IL-2 and IL-2 receptor a chain expression, as well as IL-15 and IL-15 receptor a, initiates an autostimulatory polyclonal expansion of T cells during the early phase of HTLV-1 infection. The persistent activation of NF-kB by Tax contributes to the initiation and/or maintenance of the malignant phenotype. In transgenic mice expressing Tax, elevated levels of NF-kB activity are absolutely required for the continued growth of tumor cells both in vivo and in vitro. NF-kB activation thus likely links the expression of Tax to T-cell transformation in HTLV-1 infected cells (28).
Three recent observations indicate that the cytokine-responsive IKKs are the primary cellular targets of Tax in the NF-kB pathway. First, kinase-inactive forms of IKK-a and IKK-b interfere with Tax-mediated NF-kB activation. Second, mutants of Tax that are unable to interact with IKKs also prevent Tax-mediated activation of IKK and NF-kB. Third, Tax associates with constitutively active IKKs to form higher order complexes that are resistant to dissociation in vitro (29). Thus, Tax interferes with the NF-kB pathway via direct Tax/IKK interaction, which leads sequentially to chronic IKK activation, continuous IkB turnover, and persistent NF-kB expression.
This association of IKK with Tax appears to be mediated by the IKK-g component of the IKK complex (29–31). Genetic complementation analyses with IKK-g–deficient rat fibroblast cells that fail to activate NF-kB in the presence of Tax (32) suggest that IKK-g is required for the assembly of the Tax-responsive IKK complex. In human T cells, interference with IKK-g expression by antisense expression inhibits Tax-mediated activation of NF-kB but not of CREB/ATF (29). Harhaj et al. isolated mutant T-cell lines deficient in IKK-g expression that are blocked in NF-kB activation when stimulated by both T-cell costimulatory signals and HTLV-1 Tax. This signaling defect can be rescued by expression of exogenous IKK-g (33). Although significant IkB kinase activity can be reconstituted in vivo by overexpressing Tax, IKK-g, and either IKK-a or IKK-b, interaction of Tax with IKK-a and IKK-b does not induce kinase activity in the absence of IKK-g expression, demonstrating that IKK-g functions as a molecular adaptor and provides a site for Tax binding in the assembly of Tax/IKK complexes (31) (Figure 3). Interactions with Tax require sequences at the COOH-terminal region of IKK-g; by contrast, the NH2-terminal region of this protein (amino acids 1–120) is required for the formation of a stable and active IKK complex (29, 30).
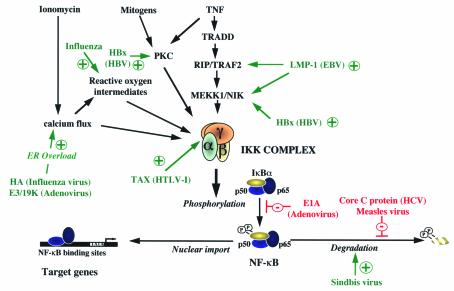
Figure 3.
Modulation of the NF-κB pathway by viruses. Mechanisms of activation of the NF-κB pathway by HTLV-1–Tax protein include direct association of Tax and IKK-γ or latent NF-κB/IκBα complexes. EBV LMP1–mediated signaling results in the recruitment of TRADD, which interacts with TRAF2 and RIP kinase to activate NF-κB. TRAF2 potentially activates NIK and MEKK1, which phosphorylate and activate the IKK complex. Other mechanisms of activation of the NF-κB pathway by viruses include direct activation of MEKK1 and PKC by HBx protein from HBV, persistent degradation of IκBα by SV, ER overload by influenza and adenovirus, and generation of reactive oxygen intermediates by influenza virus. NF-κB activation is inhibited at either the IKK activation step by E1A of adenovirus or at the IκBα degradation step by MV and HCV core C protein. Lines in green with a + indicate activation of NF-κB, while lines in red with a – indicate inhibition of NF-κB.
Another mechanism by which Tax may contribute to T-cell transformation and ATL development involves the inactivation of the p53 tumor suppressor. Inactivation of p53 function correlates with the ability of Tax to induce NF-kB and can be circumvented by overexpressing a dominant IkB mutant. Furthermore, Tax-induced p53 inactivation is blocked in p65-deficient fibroblasts. In wild-type cells, this inactivation correlates with the phosphorylation of p53 at serines 15 and 392, thus supporting the provocative idea that divergent NF-kB proliferative and p53 cell-cycle arrest pathways are crossregulated at several levels, including posttranslational modification of p53 (34).
EBV LMP1–mediated activation of NF-kB
EBV is a human g herpesvirus that causes lymphoproliferative disease and is associated with a number of human malignancies. EBV primarily targets B lymphocytes with the potential to produce indefinitely proliferating lymphocytes. Although primary infection is controlled principally by an antiviral T-cell response, EBV persists by establishing a latent infection in memory B lymphocytes; genetic analyses combined with in vitro B lymphocyte transformation assays demonstrate that the viral oncoprotein LMP1 is essential for EBV-mediated lymphocyte transformation. LMP1 is a transmembrane protein, expressed in most EBV-associated carcinomas and in Hodgkin’s lymphoma, that constitutively transmits signals that activate NF-kB and stimulate cell proliferation. LMP1 confers anchorage- and growth factor–independent growth and loss of contact inhibition to fibroblast cell lines, and these changes in cellular phenotype correlate with enhanced tumorigenicity in a nude mouse assay. Focus formation and tumorigenicity can be blocked by expressing a mutated form of LMP1 defective in NF-kB activation or a dominant-negative IkBa. LMP1 is also associated with the upregulation seen in EBV-transformed B lymphocytes of activation markers (CD23 and CD40), and cell adhesion molecules (LFA1, ICAM1, and LFA3).
In addition to its effects on cell proliferation, LMP1 prevents apoptosis in a variety of settings. In resting B lymphocytes, LMP1 provides a trophic signal (otherwise supplied only by CD40 costimulation), thus blocking cell death following B-cell receptor engagement. LMP1 also induces antiapoptotic proteins such as Bcl-2, Mcl-1, A20, and TRAF1 (35) and can protect cells from serum starvation–, etoposide-, and p53-induced apoptosis.
LMP1 activates NF-kB by usurping the cytoplasmic TNF signaling pathway (Figure 3). TNF-a activates NF-kB within minutes of ligand binding and induces apoptosis upon prolonged stimulation. TNF-a binding induces receptor oligomerization; the cytoplasmic tail of TNF receptor 1 (TNFR1) contains an 80 amino acid death domain motif which interacts with the TNFR-associated death domain (TRADD) protein, a signaling molecule with a similar death domain motif. Overexpression of TRADD activates NF-kB and induces apoptosis, implicating it as an important mediator of TNF-a signaling. TRADD also interacts with the TNFR-associated factor 2 (TRAF2) and with receptor-interacting kinase (RIP), both of which activate NF-kB. LMP1 interacts with TRAF2 and its relatives through a COOH-terminal domain called Transformation Effector Site 1 (TES1) and with TRADD and RIP through a second domain called TES2. Mutagenesis of the COOH-terminal domain of LMP1 shows that the TRAF interaction site and the TRADD/RIP interaction site lead to NF-kB activation and are critical for efficient outgrowth of transformed B lymphocytes. Mutations in either TES1 or TES2 diminish NF-kB activation by LMP1 and prevents lymphoblast immortalization. However, LMP1 signaling through TRADD and RIP activates NF-kB without inducing apoptosis, since blocking NF-kB–induced gene expression sensitizes cells to TNF-a–mediated apoptosis but does not induce apoptosis when LMP1 or the LMP1-TES2 domain is expressed. As with TNF-a, NF-kB activation by the LMP1 signaling cascade is mediated through IkBa phosphorylation and degradation (6). The interactions of LMP1 with TRAFs, TRADD, or RIP activate a kinase cascade that includes NIK and the IKK complex; overexpression of dominant-negative mutants of TRAF2, NIK, IKK-a, and IKK-b can interfere with LMP1-induced activation of NF-kB (36). Thus, like HTLV-1, EBV has appropriated the NF-kB pathway into its mechanism of B-lymphocyte transformation (Figure 3). Remarkably, a similar pathway involving TNF signaling and NF-kB activation is also key to the development of EBV-negative Hodgkin’s disease.
NF-kB activation by hepatitis virus
HBx is a 17 kDa polypeptide encoded by HBV that promotes the hepatocellular carcinoma seen in chronic HBV infection. Interestingly, transgenic expression of HBx also causes spontaneous, p53-independent cell death in the mouse liver. HBx modulates host cellular gene transcription by activating the transcription factors AP-1, AP-2, and C/EBP, as well as NF-kB, suggesting that HBx works through the Ras and PKC pathways to activate NF-kB and its target genes. The ability of HBx to induce IkBa degradation and to target p105 for proteolytic processing appears linked to the MAPK pathway via MEKK-1 activation. These events increase nuclear transport of RelA and c-Rel–related complexes and transactivation of NF-kB–responsive genes. Weil et al. (37) recently demonstrated that Hbx interacts directly with the second ankyrin repeat of IkBa and is transported to the nucleus by a piggyback mechanism. The overlap of this small region with one of the domains of IkBa mediating interaction with the p50 and p65, as well as its proximity to the nuclear export sequence of IkBa, may help explain the nuclear accumulation of IkBa and HBx. It is thus likely that induction of NF-kB–regulated genes by HBx establishes an activated cellular phenotype preceding tumor development. As with Tax and LMP1, HBx acts as a tumor promoter but, given the long latency before the development of hepatocarcinoma, it is probably insufficient to establish the malignant phenotype. It has yet to be determined whether direct or indirect interaction between HBx and components of the IKK complex are key to HBV-mediated NF-kB activation.
The HCV core protein is a multifunctional protein that binds to the death domain of the TNFR1 and to the cytoplasmic tail of the lymphotoxin receptor. These protein-protein interactions result in NF-kB activation (38) and indicate that the HCV core protein may modulate apoptotic and antiapoptotic pathways during HCV infection. NF-kB is activated in HCV-infected liver tissues and in HCV–core transfected cells and confers resistance to TNF-induced apoptosis in HCV–core transfected cells (39). Hence, HCV infection stimulates an antiapoptotic response through NF-kB activation, which may in turn contribute to a chronically activated, persistent state and possibly to hepatocarcinogenesis in HCV-infected cells.
Pleiotropic use of NF-kB by viruses
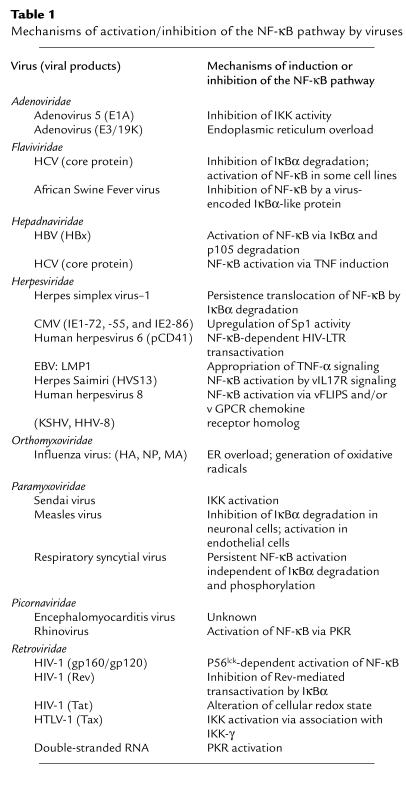
A range of other viruses have evolved mechanisms to exploit aspects of NF-kB biology to facilitate their replication, host cell survival, and evasion of immune responses (Figure 3; Table 1). Many viruses that induce NF-kB activity also harbor NF-kB binding sites in their viral promoters, thus providing a potential selective replicative advantage for the virus. Activation of NF-kB, either directly through viral infection or indirectly by stimulation of the immune response (inflammatory cytokines, for example), results in the transactivation of the NF-kB site–containing viral promoter, further enhancing viral transcription. NF-kB is involved in the gene expression of retroviruses (HIV), adenoviruses, papova viruses (JC virus) and herpes viruses (herpes simplex virus–1 [HSV-1] and CMV) (5). A low level of NF-kB activation likely permits some viruses to maintain their chronic infections as exemplified by constitutive NF-kB activation in HIV-1 chronically infected cells (16).
Table 1.
Mechanisms of activation/inhibition of the NF-κB pathway by viruses
As seen with HTLV-1 Tax, and HBx of HBV, the expression of a single viral protein can be sufficient to activate NF-kB. The E3/19k protein of adenovirus is another such factor. Using the adenovirus model, Pahl and Baeuerle demonstrated that overexpression of E3/19K, a viral protein that resides in the (endoplasmic reticulum (ER), potently activates NF-kB (40). The inducing signal was triggered by the accumulation of proteins in the ER membrane, a condition termed “ER overload” that causes Ca2+ release from the ER. Because NF-kB plays a key role in mounting an immune response, ER overload caused by viral proteins may constitute a simple antiviral response with broad specificity.
Human CMV also uses NF-kB to enhance expression of its IE genes. In this case, not only is the CMV IE promoter responsive to NF-kB, but the IE gene product IE1 is also involved in NF-kB induction, thus creating an autoregulatory loop that sustains productive human cytomegalovirus (HCMV) infection. HCMV IE gene products (IE1-72, IE2-55, and IE2-86) upregulate p65 and p105/p50 promoter activity by increasing Sp1 expression (41). Similarly, HSV induces a persistent translocation of NF-kB in a process that depends on the HSV ICP24 or ICP27 IE gene products (42). Although these experiments may indicate that NF-kB activation is involved in HSV replication, no NF-KB–dependent gene expression has been identified in HSV-infected cells.
Although little is known about the intracellular signaling pathways that are activated after influenza virus infection, numerous downstream target genes, encoding ILs, TNF-a, IFNs, chemokines, proapoptotic molecules, and adhesion molecules, are deregulated upon infection — even in cells that are nonpermissive for viral replication. Transactivation of NF-kB by the HA product from a highly pathogenic avian influenza virus occurs via an ER-overload mechanism (40), mediated by viral NP, MA, and HA proteins. The mechanism involves the generation of oxidative radicals and integrates activation of IKK-b as a signal intermediate. Considering the ability of influenza A/B virus infection to induce apoptotic death of HeLa cells, NF-kB activation is likely to play a role in influenza pathogenesis.
Induction of the IKK activity by Sendai virus, a member of the paramyxovirus family, precedes the phosphorylation and degradation of IkB (43). Infection of airway epithelial cells by another family member — respiratory syncytial virus — results in persistent NF-kB activation and NF-kB–mediated IL-8 production, independently of IkBa phosphorylation and degradation (44), through a mechanism that is not understood.
Virus-mediated blockade of the immune response via NF-kB
Several viruses other than HIV have evolved strategies to block apoptosis via NF-kB activation (45). For example, NF-kB blocks apoptosis induced by the HCV core protein or by encephalomyocarditis virus (EMCV). p50 knockout mice infected with EMCV display increased apoptosis but survive EMCV infection, whereas normal mice die, thus directly linking NF-kB–mediated suppression of apoptosis to EMCV-associated virulence. In contrast, activation of NF-kB by Dengue and Sindbis virus (SV) is associated with the induction of apoptosis, which can be blocked by proteasome inhibitors such as MG132. Use of chimeric SV that expresses a super-repressor form of IkBa indicates that steady-state NF-kB activity regulates expression of an inactive death protein that is posttranscriptionally modified by SV, leading to apoptosis and viral spread.
In still other cases, viral proteins inhibit NF-kB activity and either enhance replication or contribute to viral pathogenicity. The E1A protein of adenovirus sensitizes cells to g-irradiation and TNF-a by inhibiting NF-kB through suppression of IKK activity and IkBa phosphorylation; this inhibition requires the p300 and Rb binding domains of E1A (46). The core protein of HCV can suppress TNF-a–, okadaic acid-, phorbol ester–, and hydrogen peroxide–induced NF-kB activation through inhibition of IkBa degradation. However, in different cells, the HCV core protein can promote cell death via the TNF signaling pathway, as a result of its interaction with the cytoplasmic tail of TNFR1 and activation of NF-kB. The African swine fever virus encodes an IkB-like protein lacking the classic serine residues involved in signal-induced phosphorylation; this virus-derived IkB can still interact with p65 and inhibit NF-kB activation by TNF-a, IFN-g, and PMA. Measles virus (MV) infection is characterized by its failure to induce HLA class I or IFN-b in neuronal cells; this block is associated with the inability of MV to induce phosphorylation and degradation of IkBa. Like MV, mumps virus and dsRNA fail to induce IkBa degradation in neuronal cells, suggesting that this defect provides a mechanism of viral persistence in neurons and a means to escape immune surveillance.
Death effector domains (DEDs), originally identified in TNFR molecules and now recognized in many proapoptotic signaling molecules, recruit caspase 8 and its homologs to a region of the cell surface known as the death-inducing signaling complex, which consists of the death domain–containing receptors and various signaling adaptors. Certain pathogenic viruses, including human herpesvirus 8 and the Kaposi’s sarcoma herpes virus, encode proteins with such DEDs, which block cell death induced by DED-containing receptors. These proteins (called FLICE inhibitory proteins or vFLIPs) also physically interact with several proteins in the NF-kB pathway, such as the TRAFs, RIP, NIK, and the IKKs, suggesting that this pathway affects the natural history of infection by herpes viruses as well.
Conclusions
As viruses evolve under the highly selective pressures of the immune system, they acquire the capacity to target critical steps in the host cell life, hijacking vital cellular functions to promote viral pathogenesis. Many viruses have evolved mechanisms to target the NF-kB pathway to facilitate their replication, cell survival, and evasion of immune responses. In addition, some viruses use the NF-kB pathway either for its antiapoptotic properties to evade the host defense mechanisms or to trigger apoptosis as a mechanism of virus spread. Not surprisingly, recent studies focusing on the interrelations between NF-kB and virus pathogenesis reveal that many viral products bypass signal-induced stimulation and/or receptor-proximal steps to directly interface with the IKK complex. Continuous activation of NF-kB as a consequence of viral persistence in some instances leads to oncogenic transformation, an area of increasing scientific study and fascination. Ongoing research will undoubtedly continue to reveal other novel interactions between viruses and the NF-kB pathway that will permit a more precise molecular dissection of this parasitic fatal attraction.
Supplementary Material
Acknowledgments
The authors thank members of the Molecular Oncology Group and the McGill AIDS Center at the Lady Davis Institute, McGill University for helpful discussions and comments during the preparation of this review. The authors would like to apologize to the many researchers who have contributed to this area of research but have not been acknowledged in this review because of space limitations. Interested readers can find a supplementary reading list at www.jci.org/cgi/content/full/107/2/143/DC1. This research program is supported by research grants and training fellowships from the Canadian Institutes of Health Research, the National Cancer Institute of Canada, Fonds de la Recherche en Sante du Quebec, the Canadian Foundation for AIDS Research and the CANVAC Network Centers of Excellence.
References
- 1.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 2.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 3.Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 4.Gilmore TD. Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene. 1999;18:6925–6937. doi: 10.1038/sj.onc.1203222. [DOI] [PubMed] [Google Scholar]
- 5.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 6.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 7.Roulston A, Lin R, Beauparlant P, Wainberg MA, Hiscott J. Regulation of HIV-1 and cytokine gene expression in myeloid cells by NF-kB/Rel transcription factors. Microbiol Rev. 1995;59:481–505. doi: 10.1128/mr.59.3.481-505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon H, et al. Inducible expression of IkBa repressor mutants interferes with NF-kB activity and HIV-1 replication in Jurkat T cells. J Biol Chem. 1998;273:7431–7440. doi: 10.1074/jbc.273.13.7431. [DOI] [PubMed] [Google Scholar]
- 9.Quinto I, et al. Potent and stable attenuation of live HIV-1 by gain of a proteolysis-resistant inhibitor of NF-kB (IkBa S32/36A) and the implications for vaccine development. J Biol Chem. 1999;274:17567–17572. doi: 10.1074/jbc.274.25.17567. [DOI] [PubMed] [Google Scholar]
- 10.Montano MA, Nixon CP, Essex M. Dysregulation through the NF-kappaB enhancer and TATA box of the human immunodeficiency virus type 1 subtype E promoter. J Virol. 1998;72:8446–8452. doi: 10.1128/jvi.72.10.8446-8452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhoef K, Sanders RW, Fontaine V, Kitajima S, Berkhout B. Evolution of the human immunodeficiency virus type 1 long terminal repeat promoter by conversion of an NF-kappaB enhancer element into a GABP binding site. J Virol. 1999;73:1331–1340. doi: 10.1128/jvi.73.2.1331-1340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita S, et al. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 13.Jeeninga RE, et al. Functional differences between the long terminal repeat transcriptional promoters of human immunodeficiency virus type 1 subtypes A through G. J Virol. 2000;74:3740–3751. doi: 10.1128/jvi.74.8.3740-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alcami J, et al. Absolute dependence on kappa B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 1995;14:1552–1560. doi: 10.1002/j.1460-2075.1995.tb07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen BJ, Feinberg MB, Baltimore D. The kB sites in the human immunodeficiency virus type 1 long terminal repeat enhance virus replication yet are not absolutely required for virus growth. J Virol. 1997;71:5495–5504. doi: 10.1128/jvi.71.7.5495-5504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLuca C, Petropoulos L, Zmeureanu D, Hiscott J. Nuclear IkBb maintains persistent NF-kB activation in HIV-1-infected myeloid cells. J Biol Chem. 1999;274:13010–13016. doi: 10.1074/jbc.274.19.13010. [DOI] [PubMed] [Google Scholar]
- 17.Asin S, Taylor JA, Trushin S, Bren G, Paya CV. IKK mediates NF-kB activation in HIV-infected cells. J Virol. 1999;73:3893–3903. doi: 10.1128/jvi.73.5.3893-3903.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flory E, et al. Plasma membrane-targeted Raf kinase activates NF-kappaB and human immunodeficiency virus type 1 replication in T lymphocytes. J Virol. 1998;72:2788–2794. doi: 10.1128/jvi.72.4.2788-2794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamma SM, et al. CD4 cross-linking (CD4XL) induces RAS activation and tumor necrosis factor-alpha secretion in CD4+ T cells. Blood. 1997;90:1588–1593. [PubMed] [Google Scholar]
- 20.Norris JL, Baldwin AS., Jr Oncogenic Ras enhances NF-kappaB transcriptional activity through Raf-dependent and Raf-independent mitogen-activated protein kinase signaling pathways. J Biol Chem. 1999;274:13841–13846. doi: 10.1074/jbc.274.20.13841. [DOI] [PubMed] [Google Scholar]
- 21.Briand G, Barbeau B, Tremblay M. Binding of HIV-1 to its receptor induces tyrosine phosphorylation of several CD4-associated proteins, including the phosphatidylinositol 3-kinase. Virology. 1997;228:171–179. doi: 10.1006/viro.1996.8399. [DOI] [PubMed] [Google Scholar]
- 22.Ozes ON, et al. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 23.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 24.Manna SK, Aggarwal BB. Differential requirement for p56lck in HIV-tat versus TNF-induced cellular responses: effects on NF-kappaB, activator protein-1, c-Jun N-terminal kinase, and apoptosis. J Immunol. 2000;164:5156–5166. doi: 10.4049/jimmunol.164.10.5156. [DOI] [PubMed] [Google Scholar]
- 25.Roux P, Alfieri C, Hrimech M, Cohen EA, Tanner JE. Activation of transcription factors NF-kappaB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J Virol. 2000;74:4658–4665. doi: 10.1128/jvi.74.10.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JK, Kiyokawa E, Verdin E, Trono D. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc Natl Acad Sci USA. 2000;97:394–399. doi: 10.1073/pnas.97.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BR. NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappaB-inducing kinase and IkappaB kinase. Mol Cell Biol. 2000;20:1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun SC, Ballard DW. Persistent activation of NF-kappaB by the Tax transforming protein of HTLV-1: hijacking cellular IkappaB kinases. Oncogene. 1999;18:6948–6958. doi: 10.1038/sj.onc.1203220. [DOI] [PubMed] [Google Scholar]
- 29.Chu ZL, Shin YA, Yang JM, DiDonato JA, Ballard DW. IKKg mediates the interaction of cellular IkB kinases with the tax transforming protein of HTLV-1. J Biol Chem. 1999;274:15297–15300. doi: 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- 30.Harhaj EW, Sun SC. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem. 1999;274:22911–22914. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- 31.Jin DY, Giordano V, Kibler KV, Nakano H, Jeang KT. Role of adaptor function in oncoprotein-mediated activation of NF-kB. HTLV-1 Tax interacts directly with IKKg. J Biol Chem. 1999;274:17402–17405. doi: 10.1074/jbc.274.25.17402. [DOI] [PubMed] [Google Scholar]
- 32.Yamaoka S, et al. Complementation cloning of NEMO, a component of the IkB kinase complex essential for NF-kB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 33.Harhaj EW, et al. Somatic mutagenesis studies of NF-kappa B signaling in human T cells: evidence for an essential role of IKK gamma in NF-kappa B activation by T-cell costimulatory signals and HTLV-I Tax protein. Oncogene. 2000;19:1448–1456. doi: 10.1038/sj.onc.1203445. [DOI] [PubMed] [Google Scholar]
- 34.Pise-Masison CA, et al. Inactivation of p53 by human T-cell lymphotropic virus type 1 tax requires activation of the NF-kappaB pathway and is dependent on p53 phosphorylation. Mol Cell Biol. 2000;20:3377–3386. doi: 10.1128/mcb.20.10.3377-3386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cahir McFarland ED, Izumi KM, Mosialos G. Epstein-Barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-kappaB. Oncogene. 1999;18:6959–6964. doi: 10.1038/sj.onc.1203217. [DOI] [PubMed] [Google Scholar]
- 36.Sylla BS, et al. Epstein-Barr virus-transforming protein latent infection membrane protein 1 activates transcription factor NF-kB through a pathway that includes the NF-kB-inducing kinase and the IkB kinases IKKa and IKKb. Proc Natl Acad Sci USA. 1998;95:10106–10111. doi: 10.1073/pnas.95.17.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weil R, et al. Direct association and nuclear import of the hepatitis B virus X protein with the NF-kappaB inhibitor IkappaBalpha. Mol Cell Biol. 1999;19:6345–6354. doi: 10.1128/mcb.19.9.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You LR, Chen CM, Lee YHW. Hepatitis C virus core protein enhances NF-kappaB signal pathway triggering by lymphotoxin-beta receptor ligand and tumor necrosis factor alpha. J Virol. 1999;73:1672–1681. doi: 10.1128/jvi.73.2.1672-1681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai DI, et al. Activation of nuclear factor kappaB in hepatitis C virus infection: implications for pathogenesis and hepatocarcinogenesis. Hepatology. 2000;31:656–664. doi: 10.1002/hep.510310316. [DOI] [PubMed] [Google Scholar]
- 40.Pahl HL, Baeuerle PA. The ER-overload response: activation of NF-kB. Trends Biochem Sci. 1997;22:63–67. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- 41.Yurochko AD, Mayo MW, Poma EE, Baldwin AS, Jr, Huang ES. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-kappaB promoters. J Virol. 1997;71:4638–4648. doi: 10.1128/jvi.71.6.4638-4648.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel A, et al. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology. 1998;247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 43.Algarté M, Nguyen H, Heylbroeck C, Lin R, Hiscott J. IkB mediated inhibition of virus-induced interferon b transcription. J Virol. 1999;73:2694–2702. doi: 10.1128/jvi.73.4.2694-2702.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiedler MA, Wernke-Dollries K. Incomplete regulation of NF-kappaB by IkappaBalpha during respiratory syncytial virus infection in A549 cells. J Virol. 1999;73:4502–4507. doi: 10.1128/jvi.73.5.4502-4507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 46.Shao R, et al. E1A sensitizes cells to tumor necrosis factor-induced apoptosis through inhibition of IkappaB kinases and nuclear factor kappaB activities. J Biol Chem. 1999;274:21495–21498. doi: 10.1074/jbc.274.31.21495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.