Abstract
The lipid mediator prostaglandin E2 (PGE2) has diverse biological activity in a variety of tissues. Four different receptor subtypes (EP1–4) mediate these wide-ranging effects. The EP-receptor subtypes differ in tissue distribution, ligand-binding affinity, and coupling to intracellular signaling pathways. To identify the physiological roles for one of these receptors, the EP1 receptor, we generated EP1-deficient (EP1–/–) mice using homologous recombination in embryonic stem cells derived from the DBA/1lacJ strain of mice. The EP1–/– mice are healthy and fertile, without any overt physical defects. However, their pain-sensitivity responses, tested in two acute prostaglandin-dependent models, were reduced by approximately 50%. This reduction in the perception of pain was virtually identical to that achieved through pharmacological inhibition of prostaglandin synthesis in wild-type mice using a cyclooxygenase inhibitor. In addition, systolic blood pressure is significantly reduced in EP1 receptor–deficient mice and accompanied by increased renin-angiotensin activity, especially in males, suggesting a role for this receptor in cardiovascular homeostasis. Thus, the EP1 receptor for PGE2 plays a direct role in mediating algesia and in regulation of blood pressure.
Introduction
Prostaglandin E2 (PGE2) is produced during inflammatory responses, and increased levels of PGE2 help mediate some of the cardinal features of inflammation, including pain, edema, and fever (1, 2). PGE2 exerts its effects through interactions with EP receptors, termed EP1–4 (3). Nonsteroidal anti-inflammatory drugs (NSAIDs) act by inhibiting cyclooxygenase (COX) enzymes and thereby inhibiting prostaglandin production. In the context of this putative mechanism of action, direct cause-and-effect relationships between interruption of specific receptor-mediated signaling pathways and therapeutic actions have not been firmly established. While NSAIDs are effective analgesic agents, certain NSAIDs have a number of troublesome side effects that are due in part to their broad inhibition of a variety of COX products (4, 5).
Defining the molecular mechanisms underlying both the therapeutic and adverse actions of NSAIDs should provide useful targets for new, more specific therapeutic strategies. Therefore, we focused on a receptor for one of the prostaglandins (PGE2), the EP1 receptor (6). We generated EP1-deficient mice by gene targeting and compared their physiological responses to genetically matched wild-type controls. We find that EP1–/– animals have reduced nociceptive pain perception as well as altered cardiovascular homeostasis. These results demonstrate the critical actions of EP1 receptors in two physiological functions: pain perception and blood pressure regulation.
Methods
EP1 targeting vector construction and production of EP1–/– mice.
Mouse genomic clones containing Ptgerep1, mouse gene symbol for EP1 receptor, were isolated from a DBA/1lacJ genomic λ-phage library (Stratagene, La Jolla, California, USA). Long-template PCR was used to amplify 5′and 3′ fragments of the clone using T3 or T7 and EP1-specific primers. A 4.5-kb 5′ fragment and 6.0-kb 3′ fragment were cloned into pCRII vector (Invitrogen Corp., San Diego, California, USA). These fragments were sequence confirmed and subcloned into pHok, a plasmid containing PGK-neo and PGK-thymidine kinase cassettes. The EP1 targeting vector was designed to replace 671 bp of coding sequence with the PGK-neo cassette. This 671-bp coding region was cloned into pCRII for subsequent expression analysis (see below). DBA1/lacJ embryonic stem cells (ES cells) were grown, transformed, and screened using standard techniques (7). Colonies in which the plasmid had integrated by homologous recombination were identified using Southern blot analysis with a 2.2-kb XbaI/SacI probe that was external to the targeting construct. Targeted ES cells were introduced into blastocysts from C57BL/6 mice using established techniques (8). Male chimeras were mated with DBA/1lacJ females, and the targeted EP1 allele was detected in offspring of these crosses using Southern blot analysis of genomic DNA isolated from tail biopsies. Offspring carrying the mutant allele were intercrossed to obtain inbred DBA/1lacJ-strain mice that were homozygous for the targeted mutation (EP1–/–). Animals were housed in microisolator cages in a pathogen-free barrier facility. All experimentation was performed under approved institutional guidelines.
RNA expression analysis.
Kidneys, lungs, and uteri were harvested from EP1+/+ and EP1–/– mice, and total RNA was extracted from the tissues using TRIzol reagent (Life Technologies Inc., Rockville, Maryland, USA). The SuperScript Preamplification System kit (Life Technologies Inc.) was used to generate first-strand cDNA from each tissue by using 1 μg of RNA and oligonucleotide dT in the presence of reverse transcriptase at 42°C for 1 hour. EP1 PCR was performed using oligos EP1-90F (5′ AACCTGAGCCTAGCGGATGAGG 3′) and EP1-807R (5′ TTCGGAATCGTCGAGAGCGACG 3′) and 2 μl of the RT reaction as template. EP1 PCR cycling conditions were: 94°C for 3 minutes for 1 cycle; 94°C for 15 seconds, 61°C for 15 seconds, 72°C for 2 minutes for 40 cycles; 72°C 10 minutes for 1 cycle. The expected fragment size for the EP1-90F and EP1-807R oligonucleotide set was 717 bp. RNA integrity for each sample was checked using a mouse β-actin oligonucleotide set (CLONTECH Laboratories Inc., Palo Alto, California, USA), following manufacturer’s recommendations. There was no detectable product in the absence of reverse transcriptase followed by PCR (data not shown). In situ hybridization was performed on kidney sections as described previously using the 671-bp murine EP1 digoxigenin probe (9).
Expression of PKN in EP1–/– animals.
Brain lysates (10 μg) were prepared from EP1-deficient animals and EP1+/+ controls, suspended in RIPA buffer, resolved by 10- to 20%-gradient SDS-PAGE, and transferred onto PVDF membrane. The primary and secondary Ab’s were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA). Western blot analysis was performed according to the manufacturer’s protocol using PKN C-19 rabbit polyclonal Ab (1 μg/ml dilution) followed by a goat anti-rabbit horseradish peroxidase–conjugated (HRP-conjugated) secondary Ab (1:1000 dilution) and processing of the blot using chemiluminescence.
Nociception assays.
In a series of assays testing sensitivity to inflammatory pain, we compared nociceptive responses in EP1–/– mice and controls consisting of EP1+/+ or EP1+/– mice. All mice were fasted overnight before the experiments, and assays performed were blind to EP1 genotypes.
For the acetic acid experiments, 16 μl/g body weight of 0.7% acetic acid in water was administered by intraperitoneal injection. The mice were placed in 1 L (inner volume) clear glass beakers, and the number of stretches was counted for 20 minutes beginning immediately after acetic acid treatment. In separate groups of EP1+/+ and EP1–/– mice, the COX inhibitor, piroxicam (5 mg/kg), was administered 30 minutes before acetic acid injection. The piroxicam was dissolved in 0.1% methylcellulose and 0.9% NaCl and was administered by subcutaneous injection.
For the 2-phenyl-1, 4-benzoquinone–induced (PBQ-induced) stretch assays, 0.2% 2-phenyl-1, 4-benzoquinone was dissolved in 5% ethanol, and 15 μl/g body weight was injected intraperitoneally. Beginning 5 minutes after PBQ injection, stretching responses were assessed for 10 minutes.
Measurement of PGE2 and 6-keto-PGF1α in peritoneal fluid.
After acetic treatment, prostaglandin concentrations were derived from peritoneal fluid after lavaging the peritoneal cavity with 900 μl sterile PBS immediately after behavioral observations. PGE2 and 6-keto-PGF1α (PGI2 stable metabolite) levels were quantified using the scintillation proximity assay method (Amersham Pharmacia Biotech Inc., Piscataway, New Jersey, USA), according to the manufacturer’s instructions.
Systolic blood pressure measurements in conscious mice.
Systolic blood pressure was measured in conscious mice as described previously with a computerized tail-cuff system (Visitech Systems, Apex, North Carolina, USA) that determines systolic blood pressure using a photoelectric sensor (10). This system allows pressures to be measured in four mice simultaneously and minimizes the potential for observer bias. Before the study was initiated, mice were adapted to the apparatus for at least 5 days. The validity of this system has been established previously, and we have demonstrated its correlation with intra-arterial pressure measurements in several experimental systems (11). To determine the effects of the EP1 gene disruption on the adaptation to changes in dietary salt intake, we measured systolic blood pressures in mice that were sequentially fed diets of different sodium chloride content (Harlan-Teklad Laboratory, Madison, Wisconsin, USA). EP1+/+ and EP1–/– mice were first fed a control diet containing 0.4% sodium chloride. This was followed by a 10-day period in which the animals were fed a low-salt diet containing less than 0.02% sodium. Throughout the period of study, mice were allowed free access to water, and systolic blood pressures were measured at least five times per week.
Renin mRNA expression.
To examine the effects of the EP1 mutation on the renin-angiotensin system, we analyzed kidney renin mRNA expression in whole kidney using a ribonuclease assay as described previously (12). To estimate the concentration of renin mRNA in each sample, a standard curve was constructed by hybridizing a renin probe with known quantities of renin cDNA that had been transcribed in vitro. Renin mRNA levels are expressed in picograms of renin mRNA per microgram of total kidney RNA.
Plasma renin activity.
Following exposure of mice to CO2 vapors, blood was drawn from the renal artery and collected in EDTA-coated tubes in less than 30 seconds. Plasma was isolated and stored at –70°C until the assay was performed. Plasma renin activity was determined using radioimmunoassay, according to the manufacturer’s protocol (NEN Life Science Products Inc., Boston, Massachusetts, USA).
Data analysis.
The values for each parameter within a group are expressed as the mean ± SEM. For comparisons between EP1–/– and control groups statistical significance was assessed using Student’s t tests.
Animal handling.
Animals were housed in microisolator cages in a pathogen-free barrier facility. All experimentation was performed under approved protocols by the Pfizer Global Research and Development Institutional Animal Care and Use Committees.
Results
Generation of EP1–/– mice.
The lack of potent and specific agonists and antagonists of the individual EP-receptor subtypes that are suitable for in vivo experiments has been an impediment to defining their distinct functions. To directly examine the role of PGE2 acting through the EP1 receptor in physiological responses, including pain perception, we generated EP1 receptor–deficient mice by gene targeting in ES cells from DBA/1lacJ inbred mice (13, 14). To produce a null mutation in the EP1-receptor gene, a replacement construct was designed that deletes 671 bp of the EP1-coding sequence from exon 2 (Figure 1a). The construct was introduced into the DBA/1lacJ ES cell line by electroporation, and six correctly targeted cell lines were identified by using Southern blot analysis. ES cells from two EP1-targeted lines were microinjected into C57BL/6J blastocysts, and three DBA/1lacJ-C57BL/6 chimeric male mice were generated. The chimeras were crossed with DBA/1lacJ females to establish germline transmission and to maintain the DBA/1lacJ inbred background of the mice bearing the EP1 mutation.
Figure 1.
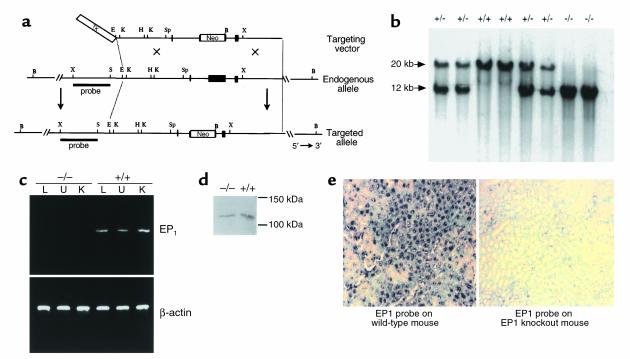
Generation of PGE2 EP1 receptor–deficient DBA/1lacJ mice. (a) Strategy to inactivate EP1 by homologous recombination in DBA/1lacJ ES cells. Restriction maps depicting EP1 gene-targeting vector (top), endogenous EP1 gene (middle), and the targeted EP1 allele (bottom) are shown. Restriction enzymes used for mapping were: B, BamH1; E, EcoR1; H, HindIII; K, Kpn1; S, SacI; Sp, Spe1; and X, XbaI. PGK-tk and PGK-neo were inserted in opposite orientation to the EP1 gene as depicted in targeting vector (top). (b) Southern blot analysis of EP1+/+ (wild-type), EP1+/– (heterozygotes), and EP1–/– (homozygous mutants) genotypes. Genomic DNA from mouse tail was isolated and digested with BamH1 and hybridized with the external XbaI/SacI probe. The insertion of the neomycin gene into the EP1 locus produces a shift from the approximately 20-kb endogenous BamH1 fragment to 12 kb. (c) Expression of EP1 mRNA in EP1–/– and EP1+/+ mice by RT-PCR analysis of lung (L), uterus (U), and kidney (K) RNA. Positive PCR product was observed in tissues from EP1+/+ mice; however, none was present in EP1–/– mouse tissues, confirming loss of EP1 expression. β-actin controls were performed confirming presence of intact RNA in all samples. (d) Western blot analysis of PKN expression in brain lysates isolated from EP1–/– (left) and EP1+/+ (right) mice demonstrates that brain PKN protein levels are not affected by the targeting of ptgerep1. (e) In situ hybridization of kidney sections from EP1+/+ (left) and EP1–/– (right) mice demonstrates EP1-specific (not PKN) tubular expression.
Homozygous EP1–/– mice were generated by intercrossing EP1+/– heterozygotes (Figure 1b). Among the first 95 progeny of these heterozygous crosses, only 13 of 95 (13.7%) were EP1–/–, significantly fewer than the 25% predicted by simple Mendelian inheritance. However, when EP1-deficient mice were produced by crossing EP1–/– and EP1+/– animals, the expected 50:50 ratio of EP1+/– and EP1–/– progeny was observed (56 and 52, respectively). Thus, unlike other mouse lines with targeted mutations in genes in the COX pathway, such as the FP receptor (15), the EP2 receptor (16, 17), or COX-2 (18, 19), the absence of the EP1 receptor does not cause marked alteration in reproductive functions. The EP1–/– animals were normal in appearance and could not be distinguished from their wild-type littermates by simple observation. Furthermore, no histopathological changes were observed in 39 tissues from EP1–/– male and female mice, including the adrenal gland, brain (cerebrum, cerebellum, pons), bone marrow, cecum, colon, duodenum, epididymis, esophagus, eyes, femur, harderian gland, heart, gall bladder, ileum, jejunum, liver, lung, mammary gland, mesenteric lymph nodes, ovaries, pancreas, pituitary gland, prostate, salivary gland, seminal vesicle, skin, spinal cord, spleen, sternum, stomach, testes, thymus, thyroid gland, trachea, urinary bladder, uterus, and vagina. Importantly, kidneys from EP1-deficient mice appeared structurally normal with no evidence of the glomerular and renal cortical abnormalities that were described previously in COX-2–deficient mice (18, 19).
To verify that the targeted mutation introduced into the EP1 gene locus (ptgerep1) resulted in complete inactivation of the gene, we examined expression of EP1-receptor mRNA by RT-PCR and in situ hybridization in tissues known to express EP1 receptors. EP1 mRNA was not detected in the kidney, lung, or uterus by RT-PCR analysis in tissues isolated for EP1 receptor–deficient mice (Figure 1c). It has been suggested that the ptgerep1 locus may overlap with a gene encoding protein kinase N, PKN (20). Based on the deduced sequences, the mutation induced in the ptgerep1 locus lies within the 3′ untranslated region of the PKN gene. To determine whether the targeted mutation altered expression of PKN, we measured PKN protein levels using Western blot analysis. Because previous studies have documented expression of PKN in the brain and kidney, protein lysates of these tissues were prepared from EP1–/– and EP1+/+ animals. PKN protein was not detected in kidney lysates from either wild-type or EP1-deficient animals (data not shown). However, as shown in Figure 1d, PKN protein was easily detected in brain lysates from wild-type animals. Abundant PKN expression was also seen in the EP1-deficient animals, and there was no difference in the levels of PKN expression between EP1+/+ and EP1–/– animals. In situ hybridization using an EP1 receptor–specific probe showed a complete absence of detectable signals in EP1–/– kidneys (Figure 1e) consistent with the results obtained from the PKN protein analysis in EP1–/– and EP1+/+ tissues.
Reduced nociceptive perception in EP1-deficient animals (acetic acid–induced stretch model).
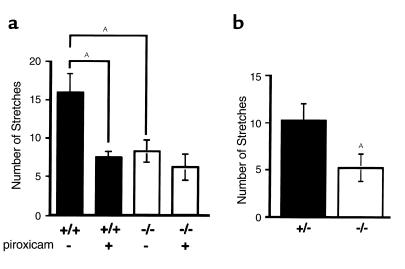
During an inflammatory response, pain is produced through complex interactions between a number of inflammatory mediators that are released at the site of injury or insult (21, 22). To understand the specific role of the EP1 receptor in mediating pain responses, we tested EP1–/– mice using established models of algesia (23). We first compared acetic acid–induced stretching in inbred DBA/1lacJ EP1+/+ and EP1–/– mice. As depicted in Figure 2a, injection of acetic acid into the peritoneum of wild-type mice induced significant stretching behavior with a mean of 16 stretches over the first 20 minutes after injection (median = 17). In EP1-deficient mice, acetic acid–induced stretching was significantly reduced to a mean of eight stretches over 20 minutes (median = 9; P < 0.01 versus EP1+/+).
Figure 2.
Nociceptive responses in EP1 receptor–deficient mice. (a) Acetic acid–induced stretching. The number of stretches was counted for 20 minutes immediately after 0.7% acetic acid injection intraperitoneally. Piroxicam (5 mg/kg subcutaneously) or vehicle was administered 30 minutes before acetic acid treatment. n = 8 for each group. AP < 0.01. (b) PBQ-induced stretching. Stretching behaviors were induced by injection of 0.02% PBQ intraperitoneally. The number of stretches was counted from 5 to 15 minutes after PBQ treatment. n = 9 EP1+/– (filled bar) and n = 11 EP1–/– (open bar) mice. AP < 0.05.
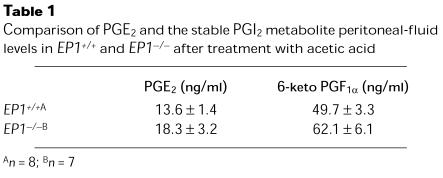
To determine whether differences in prostanoid production might explain the amelioration of pain in the EP1–/– animals, we compared the concentrations of PGE2 and prostacyclin (PGI2) in peritoneal fluid from wild-type and EP1-deficient mice. There were no differences in the concentration of PGE2 or PGI2 in peritoneal lavage fluid from EP1–/– and EP1+/+ mice after administration of acetic acid (Table 1).
Table 1.
Comparison of PGE2 and the stable PGI2 metabolite peritoneal-fluid levels in EP1+/+ and EP1–/– after treatment with acetic acid
To test the relative activities of other prostaglandins in this model, separate groups of wild-type and EP1-deficient mice were treated with the COX inhibitor, piroxicam (24), before administration of acetic acid. In wild-type mice, pretreatment with the NSAID piroxicam reduced acetic acid–induced stretching to a mean of seven stretches in 20 minutes (P < 0.01 versus EP1+/+ without piroxicam); this inhibition of pain sensation is virtually identical to that of EP1–/– mice that did not receive piroxicam (Figure 2a). In contrast, piroxicam treatment did not significantly alter acetic acid–induced stretching in the EP1–/– mice (P = 0.15 versus EP1–/– without piroxicam) (Figure 2a). Thus, the absence of the EP1 receptor was associated with a 50% reduction in pain sensitivity compared with controls. In this model, the lowering of pain responses in the EP1-deficient mice was equivalent to that achieved by COX inhibition in wild-type mice, suggesting that a significant portion of the actions of NSAIDs in reducing pain responses involves inhibition of EP1 signaling. Despite the significant diminution of pain sensitivity with EP1 deficiency or piroxicam administration, there is still a persistent response confirming the presence of other, nonprostaglandin, mediators of pain in this model.
Reduced nociceptive perception in EP1 receptor–deficient mice (PBQ-induced stretch model).
As a second measure of inflammatory nociception, we evaluated responses in the PBQ-induced stretching assay (23) (Figure 2b). In control animals, injection of PBQ induced a mean of 10 stretches in 10 minutes (median = 11). In contrast, the mean number of PBQ-induced stretches was only five in EP1–/– mice (P < 0.05 versus controls). Thus, the absence of the EP1 receptor was associated with a similar degree of amelioration of PBQ-induced stretching (50%), as was observed in the acetic acid model.
Systolic blood pressure is significantly reduced in EP1 receptor–deficient mice.
Along with its role as an inflammatory mediator, PGE2 has vascular actions that may contribute to the control of both systemic and regional hemodynamics (25). Based on its effects on vascular resistance and renal sodium excretion, a role for PGE2 in blood pressure homeostasis has been suggested (26). Furthermore, aggravation of blood pressure control has been reported as a complication of chronic treatment with NSAIDs (27).
To assess the role of the EP1 receptor in the regulation of blood pressure, we examined the functional consequences of EP1-receptor inactivation on blood pressure. Systolic blood pressure was measured in conscious mice using an automated tail-cuff manometer system. Resting blood pressure was significantly lower in a mixed group of male and female EP1-deficient mice (114 ± 3 mmHg; n = 18) compared with controls (122 ± 2 mmHg; n = 16, P < 0.05). Because we had found previously that the contribution of individual EP-receptor subtypes to the regulation of vascular tone differs dramatically between males and females (28), we next analyzed blood pressure in separate groups of male and female EP1–/– mice compared with male and female wild-type controls. Systolic blood pressures were more than 10 mmHg lower in male EP1–/– mice compared with male controls (Figure 3). While blood pressures also tended to be lower in female EP1–/– mice (105 ± 3 mmHg; n = 6) than controls (112 ± 4 mmHg; n = 6), this difference did not achieve statistical significance (P = 0.19).
Figure 3.
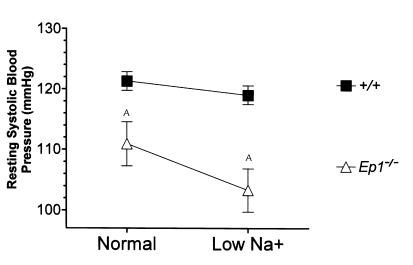
The absence of EP1 receptors causes significant changes in cardiovascular homeostasis. Resting systolic blood pressure was measured by tail-cuff manometry as described in Methods. Data shown are the average ± SEM (millimeters of mercury) of 5 consecutive days of training followed by 5 consecutive days of measurements. Ten males of each genotype were used for the recordings. (Normal sodium diet: EP1–/–, 111 ± 4 mmHg; EP1+/+, 122 ± 2 mmHg. Low-sodium diet: EP1–/–, 103 ± 4 mmHg; EP1+/+, 111 ± 4 mmHg.) AP < 0.05 Student’s unpaired t test.
Cardiovascular regulation in EP1–/– mice.
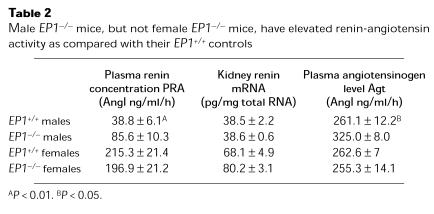
The low blood pressures in male EP1–/– mice triggered physiological compensatory responses. First, pulse rates were significantly increased in male EP1–/– (732 ± 7 beats/minute; n = 10) mice compared with male EP1+/+ animals (694 ± 9 beats/minute; n = 10, P < 0.01). Second, kidney renin mRNA levels and plasma renin activity were significantly elevated in male EP1–/– mice compared with male wild-type controls, but not females (Table 2). These are typical responses to reductions in blood pressure and/or extracellular fluid volume. To explore this issue further, we monitored blood pressures in male EP1–/– and EP1+/+ mice during feeding of a standard diet containing 0.4% (wt/wt) sodium chloride followed by a diet with a very low-sodium content (<0.02% wt/wt). Systolic blood pressures were virtually identical in the wild-type animals on the control (122 ± 2 mmHg; n = 10) and low-sodium diets (119 ± 2 mmHg; n = 10). In contrast, mean systolic blood pressures decreased by an additional 8 mmHg in the male EP1–/– mice (n = 10) on the low-salt diet (Figure 3) (P = 0.062 versus EP1–/– on control diet, using paired Student’s t test; P = 0.008 versus male EP1+/+ on low-salt diet using unpaired Student’s t test). Thus, the EP1 receptor plays a key role in regulating blood pressure, especially in males.
Table 2.
Male EP1–/– mice, but not female EP1–/– mice, have elevated renin-angiotensin activity as compared with their EP1+/+ controls
Discussion
Our experiments identify critical actions of the EP1 receptor in acute, prostaglandin-dependent pain responses. In the acetic acid model, our studies indicate that inhibition of signaling through the EP1 receptor accounts for a major component of the effect of NSAIDs to ameliorate pain. Studies by Murata and colleagues using mice lacking prostacyclin receptors (IP) have also suggested that IP receptors play a large role in this response (29). While the reasons for these differing results are not clear, variation in the genetic background of the mice is one factor that may contribute. Background genes have profound effects on the phenotype of other EP receptor–deficient mice (9). However, if genetic background affects pain responses to prostaglandins, one must be concerned about potentially confounding effects caused by the mixed genetic background of the IP-receptor knockouts. By contrast, the EP1-deficient mice were produced on an inbred DBA/1lacJ background. Recent studies in which PGE2 and PGE analogues were microinjected into the rat ventromedial hypothalamus are also consistent with a role for EP1 receptors in the antinociceptive actions of PGE2 (30). Alternatively, it is possible that signals from both the EP1 and IP receptors are involved in the acetic acid response and that the absence of either receptor alone is sufficient to attenuate the response. The observation that IP and EP1 receptors are coexpressed in dorsal root ganglia provides theoretical support for this possibility (31). Taken together, these data indicate that the EP1 receptor, along with IP receptors, are potential therapeutic targets for ameliorating inflammatory pain. There is a substantial body of evidence that supports a role for PGE2 in the regulation of blood pressure and vascular tone. Acute administration of PGE2 causes marked vasodilation, and these hemodynamic actions of PGE2 are probably most important for short-term regulation of blood flow. We and others have recently used gene targeting to define the relative roles of EP receptors in mediating the acute vascular actions of PGE2 (28, 32). In these studies, the EP2 and EP4 receptors appeared to be the dominant mediators of vasodilation. However, there were substantial differences between males and females in the contributions of individual EP receptors to the vasodilatory response. In particular, the EP1 receptor appears to mediate vasodilation in males.
The chronic effects of PGE2 to influence blood pressure seem to be variable. Depending on the circumstance and mode of administration, chronic administration of PGE2 has been reported to either increase (33, 34) or reduce blood pressure in vivo (28, 35). However, over the long term, actions of PGE2 to influence sodium excretory mechanisms in the kidney should be a critical determinant in its actions to regulate blood pressure. In this regard, the EP1-receptor expression has been demonstrated in mouse, rat, rabbit, and human kidneys (36–39). Our studies demonstrate a unique role for the EP1 receptor in blood pressure homeostasis since the absence of EP1 receptors is associated with significant reductions in blood pressure, especially in males. Furthermore, our data suggest that the absence of the EP1 receptor is associated with an inability to maintain normal extracellular fluid volume since pulse and plasma renin activity are elevated when the animals are fed a “normal” 0.4% sodium diet. The observation that there is an additional fall in blood pressure when the animals are fed a sodium-deficient diet is also consistent with actions of the EP1 receptor to promote sodium reabsorption in the mouse.
Evidence from a number of sources has suggested that mechanisms of blood pressure homeostasis might be quite different for males and females (40, 41). For example, in several models of hypertension, elevated blood pressure develops sooner and is more severe in males than females (42). In addition, experimental studies in humans have identified significant differences between males and females in the relationship of sodium chloride intake to alterations in blood pressure (43–45). Our findings suggest that the EP1 receptor may contribute to the sexual dimorphism in blood pressure regulation. This finding is also consistent with our previous studies demonstrating that the contribution of various EP receptors to the vasodilator actions of PGE2 differ between sexes (28).
Inhibition of the COX pathway using gene targeting (18, 46, 47) or pharmacological inhibitors have demonstrated the importance of prostaglandins, and specifically PGE2, in mediating pain and/or inflammation (48–50). We provide evidence supporting the role of the EP1 receptor in mediating pain and inflammation. The role of PGE2 on cardiovascular homeostasis is more complex and is still debated as reports suggest that it can act both as an antihypertensive or prohypertensive hormone. This is due in part to the different EP receptor–mediated signal-transduction pathways. We show that the absence of the EP1 receptor causes a significant decrease in systolic blood pressure in males, but not females, and that dietary sodium restriction exacerbates this male-specific hypotension. The reduction in blood pressure is accompanied by a compensatory increase in activity of the renin-angiotensin system. In conclusion, our data suggest that selective inhibition of the EP1 receptor might inhibit pain responses while also providing favorable cardiovascular effects.
In summary, the findings reported here suggest that the analgesic actions of NSAIDs in inflammatory pain, especially visceral stimuli, are mediated to a significant degree by inhibition of signaling through the EP1 receptor. Moreover, the absence of EP1 receptors does not cause abnormalities in kidney structure and results in a lowering of resting blood pressure. Accordingly, these data identify the EP1 receptor as a selective target for therapies that would possess the analgesic effects of NSAIDs without adverse effects on the kidney.
Acknowledgments
We thank Melanie Allen, Jane Bennett, Kim Kowsz, and Karen Steeber for excellent technical assistance. The authors also thank Richard Griffiths and Ivan Otterness for critical reading of the manuscript. Finally, we are indebted for the excellent animal care provided by the Pfizer Comparative Medicine and Biology Support staff, especially Gary Barrie, Frank Mercado, and Pete Silva. Laurent P. Audoly was a recipient of a fellowship from the American Heart Association.
Footnotes
Laurent P. Audoly’s present address is: Department of Inflammation, Pfizer Global Research and Development, Groton, Connecticut, USA.
Jeffrey L. Stock and Katsuhiro Shinjo contributed equally to this work.
References
- 1.Griffiths, R. 1999. Prostaglandins and inflammation. In Inflammation: basic principles and clinical correlates. J. Gallin and R. Snyderman, editors. Lippincott Williams and Wilkins. Philadelphia, Pennsylvania, USA. 349–360.
- 2.Coleman R, Smith W, Narumiya S. Pharmacological classification of prostanoid receptors: properties, distribution, and structure of receptors and their subtypes. Pharmacol Rev. 1994; 46:205–229. [PubMed] [Google Scholar]
- 3.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999; 79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 4.Zambraski E. The effects of nonsteroidal anti-inflammatory drugs on renal function: experimental studies in animals. Semin Nephrol. 1995; 15:205–213. [PubMed] [Google Scholar]
- 5.Johnson A. NSAIDs and increased in blood pressure. What is the clinical significance? Drug Safety. 1997; 17:277–289. doi: 10.2165/00002018-199717050-00001. [DOI] [PubMed] [Google Scholar]
- 6.Funk C, et al. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J Biol Chem. 1993; 268:26767–26772. [PubMed] [Google Scholar]
- 7.Hogan, B., Beddington, R., Costantini, F., and Lacy, E. 1994. Manipulating the mouse embryo, a laboratory manual. 2nd edition. Cold Spring Harbor Press. Cold Spring Harbor, New York, USA. 253–290.
- 8.Stewert C. Production of chimeras between embryonic stem cells and embryos. Methods Enzymol. 1993; 225:823–854. doi: 10.1016/0076-6879(93)25053-5. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen M, et al. The prostaglandin receptor EP4 triggers remodeling of the cardiovascular system at birth. Nature. 1997; 390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 10.Krege J, Hodgin J, Hagaman J, Smithies O. A computerized system for measuring blood pressure in mice. Hypertension. 1995; 25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, et al. Regulation of blood pressure by the type 1A receptor for angiotensin II. Proc Natl Acad Sci USA. 1995; 92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliverio MI, et al. Angiotensin II responses in AT1a receptor-deficient mice: a role for AT1b receptors in blood pressure regulation. Am J Physiol. 1997; 272:F515–F520. doi: 10.1152/ajprenal.1997.272.4.F515. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths R, et al. Collagen-induced arthritis is reduced in 5-lipoxygenase-activating protein-deficient mice. J Exp Med. 1997; 185:1123–1129. doi: 10.1084/jem.185.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roach M, Stock J, Byrum R, Koller B, McNeish J. A new embryonic stem cell line from DBA/1lacJ mice allows genetic modification in a murine model of human inflammation. Exp Cell Res. 1995; 221:520–525. doi: 10.1006/excr.1995.1403. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto Y, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997; 277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 16.Tilley S, et al. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest. 1999; 103:1539–1545. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy C, et al. Salt sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999; 5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 18.Morham S, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995; 83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 19.Dinchuk J, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995; 378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 20.Batshake B, Sundelin J. The mouse genes for the EP1 prostanoid receptor and the PKN protein kinase overlap. Biochem Biophys Res Commun. 1996; 227:70–76. doi: 10.1006/bbrc.1996.1469. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira S, Nakamura M, AbreuCastro M. The hyperalgesic effects of prostacyclin and PGE2. Prostaglandins. 1978; 16:31–38. doi: 10.1016/0090-6980(78)90199-5. [DOI] [PubMed] [Google Scholar]
- 22.Davies P, Bailey P, Goldenberg M, Ford-Hutchinson A. The role of arachidonic acid oxygenation products in pain and inflammation. Annu Rev Immunol. 1984; 2:335–357. doi: 10.1146/annurev.iy.02.040184.002003. [DOI] [PubMed] [Google Scholar]
- 23.Otterness, I., and Bliven, M. 1985. Laboratory models for testing nonsteroidal antiinflammatory drugs. In Nonsteroidal antiinflammatory drugs. J. Lombardino, editor. John Wiley & Sons Inc. New York, New York, USA. 112–251.
- 24.Milne G, Twomey T. The analgesic properties of piroxicam in animals in correlation with experimentally determined plasma levels. Agents Actions. 1980; 10:31–37. doi: 10.1007/BF02024176. [DOI] [PubMed] [Google Scholar]
- 25.McGiff J, Malik K, Terragno N. Prostaglandins as determinants of vascular reactivity. Fed Proc. 1976; 35:2382–2387. [PubMed] [Google Scholar]
- 26.Dunn M, Grone H. The relevance of prostaglandins in human hypertension. Adv Prostaglandin Thromboxane Leukot Res. 1985; 13:179–187. [PubMed] [Google Scholar]
- 27.Brooks P, Day R. Nonsteroidal antiinflammatory drugs: differences and similarities. N Engl J Med. 1991; 324:1716–1725. doi: 10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- 28.Audoly L, et al. Identification of specific EP receptors responsible for the hemodynamic effects of PGE2. Am J Physiol. 1999; 277:H924–H930. doi: 10.1152/ajpheart.1999.277.3.H924. [DOI] [PubMed] [Google Scholar]
- 29.Murata T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997; 388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 30.Hosoi M, et al. Prostaglandin E(2) has antinociceptive effects through EP(1) receptor in the ventromedial hypothalamus in rats. Pain. 1999; 83:221–227. doi: 10.1016/s0304-3959(99)00105-0. [DOI] [PubMed] [Google Scholar]
- 31.Oida H, et al. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol. 1995; 116:2828–2837. doi: 10.1111/j.1476-5381.1995.tb15933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, et al. Characterization of murine vasopressor and vasodepressor prostaglandin E2 receptors. Hypertension. 2000; 35:1129–1134. doi: 10.1161/01.hyp.35.5.1129. [DOI] [PubMed] [Google Scholar]
- 33.Gerber J, Nies R. The hemodynamic effects of prostaglandins in the rat. Evidence for important species variation in renovascular responses. Circ Res. 1979; 44:406–410. doi: 10.1161/01.res.44.3.406. [DOI] [PubMed] [Google Scholar]
- 34.Hockel G, Cowley A. Role of the renin-angiotensin system in prostaglandin E2-induced hypertension. Hypertension. 1980; 2:529–537. doi: 10.1161/01.hyp.2.4.529. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong J, Boura A, Hamberg M, Samuelsson B. A comparison of the vasodepressor effects of the cyclic effects of the cyclic endoperoxides PGG and PGH2 with those of PGD2 and PGE2 in hypertensive and normotensive rats. Eur J Pharmacol. 1976; 39:251–258. doi: 10.1016/0014-2999(76)90133-3. [DOI] [PubMed] [Google Scholar]
- 36.Boye Y, et al. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol. 1997; 340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- 37.Sugimoto Y, et al. Distinct cellular localization of mRNAs for three subtypes of prostaglandin E receptor in kidney. Am J Physiol. 1994; 35:F823–F828. doi: 10.1152/ajprenal.1994.266.5.F823. [DOI] [PubMed] [Google Scholar]
- 38.Guan Y, et al. Prostaglandin E2 inhibits renal collecting duct Na+ absorption by activating the EP1 receptor. J Clin Invest. 1998; 102:194–201. doi: 10.1172/JCI2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morath R, Klein T, Seyberth HW, Nusing RM. Immunolocalization of the four prostaglandin E2 receptor proteins EP1, EP2, EP3, and EP4 in human kidney. J Am Soc Nephrol. 1999; 10:1851–1860. doi: 10.1681/ASN.V1091851. [DOI] [PubMed] [Google Scholar]
- 40.Haywood J, Hinojosa-Laborde C. Sexual dimorphism of sodium-sensitive renal-wrap hypertension. Hypertension. 1997; 30:667–671. doi: 10.1161/01.hyp.30.3.667. [DOI] [PubMed] [Google Scholar]
- 41.von Eiff AW, Gogolin E, Jacobs U, Neus H. Ambulatory blood pressure in children followed for 3 years. Influence of sex and family history of hypertension. Clin Exp Hypertens A. 1986; 8:577–581. doi: 10.3109/10641968609046575. [DOI] [PubMed] [Google Scholar]
- 42.Dahl L, Knudsen K, Ohanian E, Muirhead M, Tuthill R. Role of gonads in hypertension-prone rats. J Exp Med. 1975; 142:748–759. doi: 10.1084/jem.142.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kojima S, et al. A gender difference in the association between salt sensitivity and family history of hypertension. Am J Hypertens. 1992; 5:1–7. [PubMed] [Google Scholar]
- 44.Elliott P, Dyer A, Stamler R. INTERSALT study: results for 24 hour sodium and potassium, by age and sex. J Hum Hypertens. 1989; 3:323–330. [PubMed] [Google Scholar]
- 45.Myers J, Morgan T. The effect of sodium intake on the blood pressure related to age and sex. Clin Exp Hypertens A. 1983; 5:99–118. doi: 10.3109/10641968309048813. [DOI] [PubMed] [Google Scholar]
- 46.Ballou L, Botting R, Goorha S, Zhang J, Vane J. Nociception in cyclooxygenase isozyme-deficient mice. Proc Natl Acad Sci USA. 2000; 97:10272–10276. doi: 10.1073/pnas.180319297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langenbach R, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. . Cell. 1995; 83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 48.Portanova JP, et al. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996; 184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seibert K, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA. 1994; 91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith CJ, et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci USA. 1998; 95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]