Abstract
The Helicobacter pylori–produced cytotoxin VacA induces intracellular vacuolation. To elucidate the molecular mechanism of vacuole formation by VacA, we examined the participation of dynamin, a GTPase functioning in intracellular vesicle formation, in human HeLa cells. Immunocytochemistry revealed that endogenous dynamin was localized to vacuoles induced by VacA. In cells transiently transfected with a GTPase-defective (dominant-negative) dynamin mutant, VacA failed to induce vacuolation. In contrast, VacA did induce vacuolation in cells transiently transfected with wild-type dynamin. Furthermore, under VacA treatment, neutral red dye uptake, a parameter of VacA-induced vacuolation, was inhibited in cells stably transfected with the dominant-negative dynamin mutant. In contrast, uptake was markedly enhanced in cells stably transfected with wild-type dynamin. Moreover, VacA cytopathic effects on the viability of HeLa cells were inhibited in cells stably transfected with dominant-negative dynamin-1. Sequential immunocytochemical observation confirmed that expression of dominant-negative dynamin did not affect VacA attachment to or internalization into HeLa cells. We suggest that dynamin is involved in the intracellular vacuolation induced by VacA.
Introduction
Helicobacter pylori, a Gram-negative bacterium colonizing gastric mucosa, is the major cause of chronic gastritis and gastroduodenal ulcers in humans. One important virulence component of H. pylori is the VacA cytotoxin, which is synthesized as a 140-kDa precursor and released from the bacterium as a 95-kDa mature toxin (1). In cells intoxicated with VacA, the multiple vacuoles formed are assumed to represent an early pathophysiological intracellular event leading to the cellular damage resulting from H. pylori infection. The vacuoles induced by VacA contain both a late endosomal marker, Rab7, and a lysosomal marker, Lgp110, suggesting that they are hybrids of late endosomes and lysosomes (2, 3). Papini et al. reported that Rab7, a small molecular weight G-protein, plays an essential role in VacA-induced vacuolation and that other rab proteins might also participate in the vacuolation process (4). Because rab proteins contribute to the regulation of intracellular vesicle traffic in normal cells, the molecular mechanism of VacA-induced vacuolation has been assumed to involve the intracellular vesicle transport machinery. In addition to rab proteins, intracellular vesicle transportation requires mechanoenzymes, which form and move intracellular vesicles directly by generating mechanochemical force. However, the mechanoenzyme which functions in VacA-induced vacuolation remains to be identified.
Dynamin is a large–molecular weight (100 kDa) GTP-binding protein family consisting of three isoforms. Dynamin-1 is neuron-specific, dynamin-2 is ubiquitously expressed, and dynamin-3 is expressed in brain, testes, and lungs. Originally, dynamin was found to function in endocytosis and was assumed to be a mechanoenzyme forming endosomes from the plasma membrane (5). Recently, however, dynamin has been localized to the Golgi apparatus and is assumed to participate in vesicle formation from trans-Golgi networks (6). Dynamin has also been shown to function in apical transport of intracellular vesicles (7). Thus, dynamin is currently thought to catalyze many essential steps in vesicle formation and traffic (8). Given that VacA-induced vacuolation might require the molecular machinery of intracellular vesicle formation and traffic, there is a possibility that dynamin may play a role in that process. We therefore investigated the possible participation of dynamin in VacA-induced vacuolation. We first examined the effect of VacA intoxication on the intracellular distribution of endogenous dynamin in HeLa cells; next we investigated dynamin functions in VacA-induced vacuolation using both transient and stable transfection of GTPase-defective dynamin (dominant-negative dynamin) and wild-type dynamin. Finally, we examined the effect of dominant-negative dynamin transfection on VacA cytotoxicity. We report here that dynamin is indeed involved in the molecular machinery of vacuolation induced by VacA.
Methods
Cell culture, purification and activation of VacA, and intoxication of cells.
HeLa cells, supplied by RIKEN Gene Bank (Tsukuba, Japan), were cultured in DMEM supplemented with 10% FBS and 100 U/ml penicillin in a 5% CO2 atmosphere at 37°C. MKN28, a human gastric mucosal cell line (9), was provided by Toshiyuki Takeuchi (Institute for Molecular and Cellular Regulation, Maebashi, Japan) and was cultured in RPMI with 10% FBS and 100 U/ml penicillin in a 5% CO2 atmosphere at 37°C. VacA cytotoxin was purified from the toxin-producing strain H. pylori ATCC49503 (American Type Culture Collection, Rockville, Maryland, USA), according to reported procedures (10), and was then activated with acid pH treatment, as described (11). For VacA intoxication, control cells or cells transfected with dominant-negative dynamin-1 or wild-type dynamin-1 were treated with 3 μg/ml activated VacA at 37°C. Transiently transfected cells were treated with VacA 24 hours after the transfection. Vacuolation was examined 24 hours after VacA intoxication.
Antibodies.
Anti–dynamin-1 and anti–dynamin-2 goat polyclonal antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA). Anti-clathrin mouse monoclonal antibody was from Affinity BioReagents Inc. (Golden, Colorado, USA). Cy3-conjugated and horseradish peroxidase–conjugated donkey anti-goat IgG, FITC-conjugated donkey anti-rabbit IgG, and FITC-conjugated donkey anti-mouse IgG were from Jackson ImmunoResearch Laboratories Inc. (West Grove, Pennsylvania, USA). Anti-VacA rabbit polyclonal antibody was generated against purified VacA, as described (11).
Plasmid and transfection.
Either wild-type or dominant-negative (GTPase-defective mutant K44E) rat dynamin-1 cDNA (from R.B. Vallee, Worcester Foundation for Experimental Biology, Shrewsbury, Massachusetts, USA) was subcloned into the pCXN2 expression vector, as described (12), and transfected into HeLa cells using the LipofectAMINE reagent (Life Technologies Inc., Rockville, Maryland, USA), according to the manufacturer’s instructions. HeLa cells, seeded 24 hours previously at a density of 105/cm2, were transiently transfected with either wild-type or dominant-negative dynamin-1 (K44E) cDNA for 48 hours. For stable transfection of HeLa cells, positive clones were selected and cultured in the presence of 2 mg/ml geneticin (Sigma Chemical Co., St. Louis, Missouri, USA). Geneticin-resistant clones were screened for their potential to express transfected wild-type or dominant-negative dynamin-1 by Western blotting with anti–dynamin-1 antibody, as described below.
Transient transfection of MKN28 was carried out using the adenovirus-mediated technique described previously (13). Briefly, MKN28 cells seeded at a density of 105/cm2 in a culture plate were incubated in RPMI supplemented with 80 μg/ml DEAE-dextran and 0.2 μg/ml dominant-negative dynamin-1–pCXN2 plasmid and adenovirus stock solution for 2 hours. After the incubation, cells were washed twice with 10% DMSO in PBS, and then incubated for a further 48 hours in RPMI with 10% FBS.
Immunofluorescence microscopy.
Cells were fixed with 2% formaldehyde in PBS, treated with Triton X-100 in PBS for 5 minutes, and incubated sequentially with Blocking Ace (Snow Brand Milk Products Co., Tokyo, Japan), first antibodies, and secondary antibodies. For staining using anti–dynamin-1 or anti–dynamin-2 antibody, Cy3-conjugated donkey anti-goat IgG was used as the secondary antibody. For staining using anti-clathrin antibody, FITC-conjugated donkey anti-mouse IgG was used as the secondary antibody. For double staining with anti–dynamin-1 and anti-VacA antibodies, Cy3-conjugated donkey anti-goat IgG and FITC-conjugated donkey anti-rabbit IgG were used as the secondary antibodies. Samples were examined under a Nikon E-600 microscope (Nikon Co., Tokyo, Japan). Images were captured and digitized using a Spot charged coupled device camera (Diagnostic Instruments Inc., Sterling Heights, Michigan, USA), then edited using Adobe Photoshop 5.0 (Adobe Systems Inc., Mountain View, California, USA) software.
Western blotting.
For electrophoresis, 30 μg protein from each sample per lane was loaded onto 7.5% SDS-polyacrylamide electrophoresis gels and run at 200 V. Proteins were then transferred to nitrocellulose membranes at 30 V for 3 hours. Western blotting was done as described previously (14), using the enhanced chemiluminescence reagent to visualize the secondary antibody.
Neutral red uptake assay.
HeLa cells were seeded into 96-well plates and cultured for 24 hours. Cells were then treated with VacA and incubated for a further 24 hours. After the incubation, the vacuolation rate induced by VacA was examined by the method described (11), in which uptake of neutral red dye into intracellular acidic compartments was determined by measuring the absorbance at 540 nm. The potential differences in cell numbers of each preparation were corrected by measuring the protein concentration of each sample.
MTT assay.
To examine cellular viability, we used the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay described previously (15). Cells seeded into a 96-well plate were incubated in PBS with 0.5 mg/ml MTT (Sigma Chemical Co.) for 2 hours at 37°C. After the incubation, cells were dissolved in 0.04 N HCl in isopropanol to solubilize the dye, which had been converted to purple. Absorbance of converted dye was measured at a wavelength of 570 nm. The potential differences in cell numbers of each preparation were corrected by measuring the protein concentration of each sample.
Transferrin uptake assay.
HeLa cells were allowed to attach to a glass slide and incubated in DMEM supplemented with 20 μg/ml Texas red–conjugated transferrin (Sigma Chemical Co.) and 100 mM sucrose for 1 hour at 37°C. Controls were prepared under the same conditions, but without the sucrose. The cells were then fixed and examined under a Nikon E-600 microscope (Nikon Corp.). Vacuolation induced by VacA under hypertonic conditions was examined by incubating HeLa cells in DMEM supplemented with 100 mM sucrose and 3 μg/ml activated VacA for 24 hours in a 5% CO2 atmosphere at 37°C.
Statistical analysis.
ANOVA was used to determine statistical significance, unless otherwise indicated. P < 0.05 was considered significant.
Results
Localization of endogenous dynamin-2 to VacA-induced vacuoles in HeLa cells.
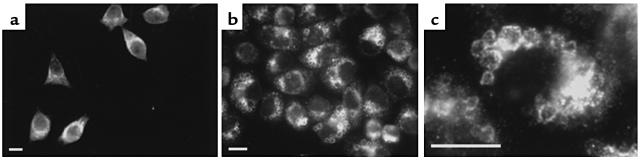
In the first set of experiments, we examined the intracellular localization of endogenous dynamin in control and VacA-treated HeLa cells using immunocytochemistry. Of the three isoforms of the dynamin family, dynamin-2, a ubiquitous isoform, had been found to be present in human epithelial cells (16). We therefore investigated the intracellular localization of dynamin-2 in HeLa cells, using a specific antibody. As shown in Figure 1a, staining of untreated HeLa cells revealed a perinuclear distribution of dynamin-2. In contrast, in VacA-treated HeLa cells, dynamin-2 was localized to vacuoles induced by VacA (Figure 1, b and c). These data suggest that dynamin participates in vacuole formation induced by VacA.
Figure 1.
Immunocytochemical localization of endogenous dynamin-2 to VacA-induced vacuoles in HeLa cells. Naive HeLa cells (a) and VacA-treated HeLa cells (b and c) were fixed, stained with anti–dynamin-2 antibody, and visualized with Cy3-conjugated anti-goat IgG. (a) Perinuclear distribution of endogenous dynamin-2 in naive HeLa cells. (b) Localization of endogenous dynamin-2 on VacA-induced vacuoles in intoxicated HeLa cells. (c) A higher-magnification image showing the localization of endogenous dynamin-2 to VacA-induced vacuoles. Bars, 10 μm. ×400.
Dominant-negative dynamin-1 inhibits VacA-induced vacuolation in HeLa cells.
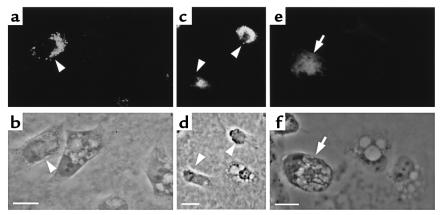
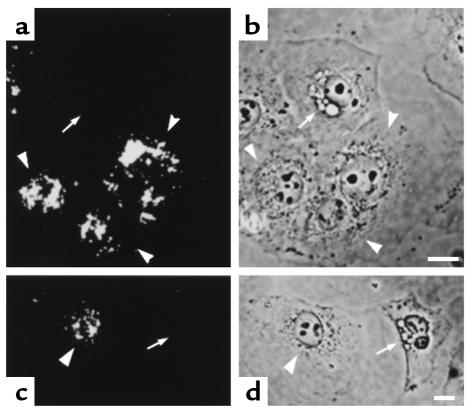
To investigate the participation of dynamin in VacA-induced vacuolation, we generated HeLa cells transiently overexpressing either wild-type or GTPase-defective mutant-type (dominant-negative) dynamin-1, designated K44E. We then examined the effects of VacA on vacuolation in these transfected cells. For easy distinction from endogenous dynamin-2, we used dynamin-1 to construct transfected cells. Although dynamin-1 is a neuron-specific isoform, transfection of dominant-negative dynamin-1 (K44E) had been reported to inhibit endogenous dynamin–2 function in HeLa cells (17). VacA failed to induce vacuolation in HeLa cells transiently overexpressing this dominant-negative dynamin-1, whereas it did induce vacuolation in nontransfected HeLa cells (Figure 2, a–d). In contrast, overexpression of wild-type dynamin-1 did not attenuate the vacuolation induced by VacA in HeLa cells. Multiple vacuoles were observed in both wild-type dynamin-transfected and nontransfected cells (Figure 2, e and f). For quantitative estimations, we compared the numbers of vacuolated cells under each experimental condition. As depicted in Figure 3, the inhibitory effect of dominant-negative dynamin-1 on VacA-induced vacuolation was statistically significant. These data suggest that overexpression of dominant-negative dynamin-1 inhibits vacuolation induced by VacA.
Figure 2.
Inhibition of VacA-induced vacuolation in HeLa cells by transient transfection of dominant-negative dynamin-1. (a, c, e) Immunostaining of transiently transfected dominant-negative dynamin-1 (a and c) or wild-type (e) in VacA-treated HeLa cells using anti–dynamin-1 antibody. (b, d, f) Phase-contrast images of the same fields as in a, c, and e, respectively. VacA failed to induce cytoplasmic vacuolation in HeLa cells transfected with dominant-negative dynamin-1 (arrowheads in a–d). In contrast, VacA induced multiple cytoplasmic vacuoles in HeLa cells transfected with wild-type dynamin-1 (arrows in e and f). Bars, 10 μm. ×400.
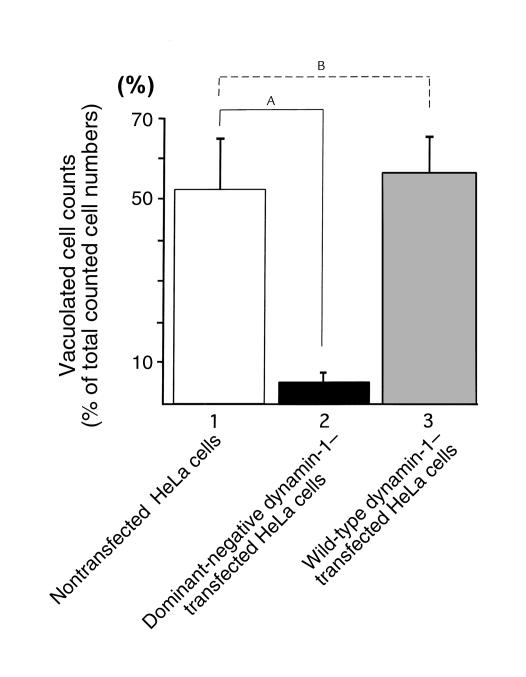
Figure 3.
Numbers of VacA-vacuolated cells in HeLa cells transiently transfected with dominant-negative dynamin-1 or wild-type, and in nontransfected HeLa cells. Results are expressed as percent of vacuolated cells. Values are means ± SE for six independent experiments with triplicate determinations in which more than 100 cells were counted. The numbers of VacA-vacuolated cells in dominant-negative dynamin-1–transfected HeLa cells (lane 2) were significantly lower than in nontransfected HeLa cells (lane 1). In contrast, no significant difference was observed between wild-type dynamin-1–transfected and nontransfected HeLa cells (lanes 3 and 1). AP < 0.01; Bnot significant.
Effect of stable transfection with dominant-negative or wild-type dynamin-1 on neutral red dye uptake in VacA-treated HeLa cells.
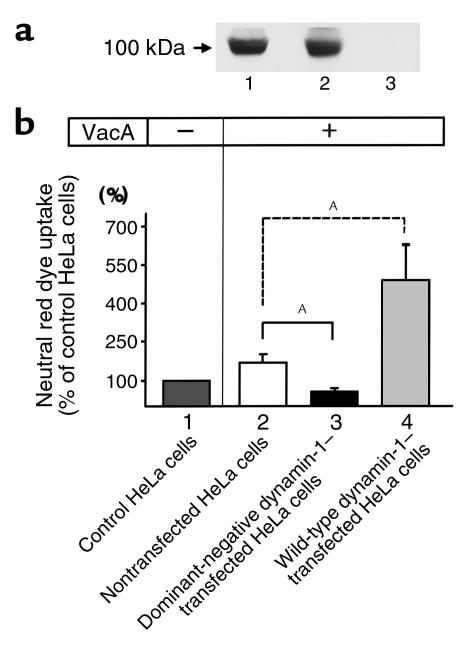
For a precise quantitation of the inhibitory effect of dominant-negative dynamin-1 on VacA-induced vacuolation, we constructed HeLa cell lines stably transfected with dominant-negative dynamin-1 or wild-type dynamin-1, and then examined their neutral red dye uptake under VacA-treated conditions. Because neutral red dye is predominantly taken up by VacA-induced vacuoles, it has been widely used as a marker thereof. Thus, higher uptake of neutral red dye by VacA-treated cells implies that more vacuoles are formed. During the preparation of stably transfected cell lines, we obtained multiple clones transfected with either dominant-negative or wild-type dynamin-1. We selected clones that expressed a sufficiently large amount of dominant-negative or wild-type dynamin-1 protein for further experiments (Figure 4a). As shown in Figure 4b, VacA treatment markedly increased neutral red dye uptake in control HeLa cells, compared with findings in VacA-nontreated control HeLa cells (lanes 1 and 2). However, VacA treatment did not enhance neutral red uptake in HeLa cells stably transfected with dominant-negative dynamin-1 (compare lane 3 with lanes 1 and 2), indicating that stable transfection of dominant-negative dynamin-1 inhibited the vacuolation induced by VacA. Furthermore, neutral red uptake in VacA-treated HeLa cells stably transfected with wild-type dynamin-1 was markedly enhanced compared with that in VacA-treated control HeLa cells (lanes 2 and 4). This suggests that overexpression of wild-type dynamin-1 enhances VacA-induced vacuolation. These data indicate that dynamin participates in the vacuolation induced by VacA.
Figure 4.
Effect of stable transfection of wild-type or dominant-negative dynamin-1 on neutral red dye uptake by VacA-treated HeLa cells. (a) Stable transfection of dominant-negative or wild-type dynamin-1 in HeLa cell lines was confirmed by Western blotting. Crude lysates (30 μg protein) of HeLa cells stably transfected with dominant-negative (lane 1) or wild-type dynamin-1 (lane 2), or of nontransfected controls (lane 3), were applied to each lane for Western blotting, using an anti–dynamin-1 antibody. Wild-type or dominant-negative dynamin-1 protein was expressed in each stably transfected HeLa cell line. (b) For the neutral red dye uptake assay, cells were seeded into 96-well plates and cultured for 24 hours, then treated with VacA and incubated for a further 24 hours. After the incubation, neutral red uptake into intracellular acidic compartments was determined by measuring absorbance at 540 nm. Results are expressed as a percent of the neutral red dye uptake of nontransfected control HeLa cells without VacA treatment (lane 1). Values are means ± SE for three independent experiments with triplicate determinations. Dominant-negative dynamin-1 transfection (lane 3) significantly inhibited neutral red uptake compared with control cells (lane 2) under VacA-treated conditions. Wild-type dynamin-1 transfection (lane 4) markedly enhanced dye uptake compared with controls (lane 2) under VacA-treated conditions. AP < 0.05.
Dominant-negative dynamin-1 does not affect VacA internalization into HeLa cells.
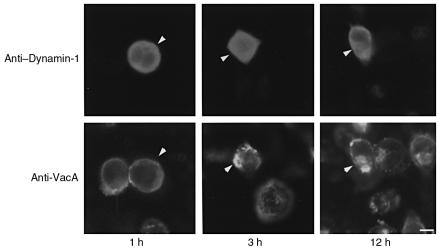
Although our observations with transfection approaches strongly suggest direct involvement of dynamin in VacA-induced vacuolation mechanisms, because dominant-negative dynamin is known to inhibit clathrin-dependent endocytosis, the possibility remained that dominant-negative dynamin might inhibit VacA-induced vacuolation by perturbing its internalization. To exclude this possibility, we investigated the effect of dominant-negative dynamin-1 overexpression on VacA internalization into HeLa cells. To this end, we incubated HeLa cells transiently transfected with dominant-negative dynamin-1 with VacA and then analyzed the time course of VacA internalization using immunocytochemistry with an anti-VacA antibody. As shown in Figure 5, signals of VacA toxin staining were observed at the plasma membrane in both dominant-negative dynamin-1–transfected and nontransfected cells, 1 hour after incubation with VacA. This indicates that dominant-negative dynamin-1 did not affect VacA binding to the HeLa cells. Furthermore, 3 hours after incubation with VacA, intracellular signals of VacA staining were detected both in cells transfected with dominant-negative dynamin-1 and in nontransfected controls. By 12 hours after the incubation, intracellular signals of VacA staining were localized to the perinuclear region in dominant-negative dynamin-1–transfected and nontransfected cells. These data indicate that dominant-negative dynamin-1 did not affect either binding or internalization of the VacA toxin to HeLa cells. Dynamin may be directly involved in molecular mechanisms of VacA-induced vacuole formation.
Figure 5.
VacA internalization by HeLa cells is not affected by dominant-negative dynamin-1. HeLa cells transiently transfected with dominant-negative dynamin-1 were double-stained with anti–dynamin-1 antibody (upper panels) and anti-VacA antibody (lower panels) 1, 3, and 12 hours after VacA intoxication. VacA attached to (1 hour) and internalized into (3 hours and 12 hours) dominant-negative dynamin-1–transfected cells (arrowheads) as well as nontransfected cells. Bar, 10 μm. ×400.
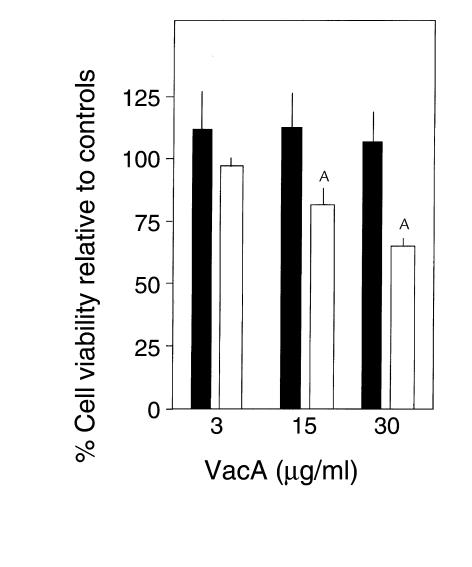
Dominant-negative dynamin-1 inhibits VacA cytopathic effects on the viability of HeLa cells.
We next examined whether dominant-negative dynamin-1 prevents VacA cytotoxity. To this end, we investigated the effect of VacA on the viability of HeLa cells stably transfected with dominant-negative dynamin-1, using the MTT assay. As shown in Figure 6, 3 μg/ml VacA, the concentration at which vacuolation can be strongly induced in naive HeLa cells, did not affect the viability of either the dominant-negative dynamin-1–transfected cells or the nontransfected controls. Higher concentrations of VacA were required before a cytopathic effect on HeLa cell viability was observed. These findings are consistent with a previous report by Peek et al. that concentrations of VacA greater than those inducing vacuolation were required for significant increases in cell death (18). Therefore, we treated HeLa cells with 15 μg/ml or 30 μg/ml VacA. We first confirmed that, when incubated with 15 μg/ml or 30 μg/ml VacA for 24 hours, marked vacuolation was induced in nontransfected HeLa cells, whereas intracellular vacuolation in cells stably transfected with dominant-negative dynamin-1 was inhibited (data not shown). Concomitantly, the viability of nontransfected HeLa cells was decreased by about 20–30% compared with controls, whereas it was not affected under these conditions in cells stably transfected with dominant-negative dynamin-1 (Figure 6). These data indicate that dominant-negative dynamin-1 prevented VacA cytotoxicity to HeLa cells.
Figure 6.
Prevention of VacA cytopathic effects on cell viability by stable transfection of dominant-negative dynamin-1 into HeLa cells. HeLa cells stably transfected with dominant-negative dynamin-1 (filled bars) or nontransfected HeLa cells (open bars) were seeded into 96-well plates and cultured for 24 hours, then treated with the indicated amounts of VacA and incubated for a further 24 hours. After the incubation, cell viability was determined by MTT assay measuring absorbance at 595 nm. Results are expressed as a percent of the viability of controls incubated without VacA. Values are means ± SE for three independent experiments with triplicate determinations. Stable transfection of dominant-negative dynamin-1 prevented the cytopathic effect of 15 and 30 μg/ml VacA on cell viability. AP < 0.05 by Student’s t test.
VacA does not affect intracellular distribution of clathrin.
Dynamin is involved in multiple processes of clathrin-dependent endocytosis and vesicle formation. Knowing that dynamin is also involved in VacA-induced vacuolation, we next examined whether such vacuolation is clathrin-dependent. To this end, we investigated the effect of VacA intoxication on the intracellular localization of clathrin. Immunocytochemistry using an anti-clathrin antibody revealed that clathrin was localized to the perinuclear region in untreated HeLa cells (Figure 7, a and b). In VacA-treated cells, clathrin was also localized to the perinuclear region as in controls. No discernible clathrin signals were detected on the vacuoles induced by VacA. These data indicate that VacA did not affect the intracellular distribution of clathrin and that clathrin was not present on VacA-induced vacuoles, thereby implying that VacA-induced intracellular vacuolation is independent of clathrin. We also examined the effect of perturbation of clathrin function on VacA-induced vacuolation, using hypertonic medium supplemented with sucrose. This method was previously reported to inhibit clathrin function by preventing its interaction with its adapter (19). When HeLa cells were incubated in hypertonic medium with 100 mM sucrose, clathrin-dependent endocytosis analyzed with Texas red–conjugated transferrin was indeed blocked. However, VacA still successfully induced intracellular vacuolation in HeLa cells (data not shown). These data therefore reinforce the notion that VacA-induced vacuolation is independent of clathrin.
Figure 7.
Intracellular localization of clathrin in VacA-treated and nontreated HeLa cells. Naive HeLa cells (a) and VacA-treated HeLa cells (c) were fixed, stained with an anti-clathrin antibody, and visualized with FITC-conjugated anti-mouse IgG. (b and d) Phase-contrast images of a and c, respectively. VacA-treatment did not affect intracellular distribution of clathrin in HeLa cells. Bars, 10 μm. ×400.
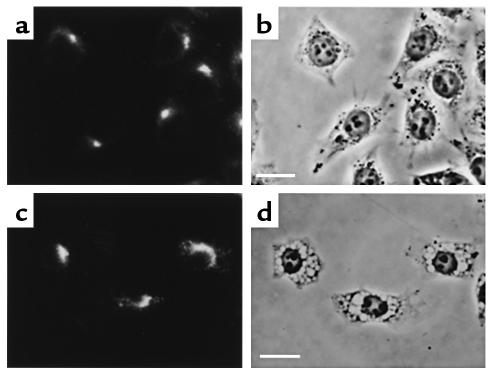
Dominant-negative dynamin-1 inhibits VacA-induced vacuolation in MKN28 human gastric mucosal cells.
Finally, we examined the inhibitory effect of dominant-negative dynamin-1 on VacA-induced vacuolation in target cells of greater physiological relevance for H. pylori infection. To this end, we transfected dominant-negative dynamin-1 into MKN28 human gastric mucosal cells and examined its effect on VacA-induced vacuolation. Because it is hard to transfect recombinant genes into MKN28 cells using the LipofectAMINE reagent, we used an adenovirus-mediated technique for transfecting dominant-negative dynamin-1 into these cells. Although MKN28 cells are derived from human gastric tubular adenocarcinoma (9), they retain the characteristics of gastric epithelial cells and have been used for studies on gastric epithelial cell functions (20, 21). As shown in Figure 8, VacA induced vacuolation in nontransfected MKN28 cells (arrows). In contrast, VacA failed to induce vacuolation in MKN28 cells transfected with dominant-negative dynamin-1 (arrowheads). Quantitating the numbers of vacuolated cells under each experimental condition, in the same way as for Figure 3, revealed that 62% of nontransfected MKN28 cells, but only 6% of the dominant-negative dynamin-1–transfected MKN28 cells, were vacuolated. These data indicate that dominant-negative dynamin-1 can inhibit VacA-induced vacuolation in gastric mucosal cells as well as HeLa cells.
Figure 8.
Inhibition of VacA-induced vacuolation in MKN28 human gastric mucosal cells by adenovirus-mediated transfection of dominant-negative dynamin-1. (a and c) Immunostaining of transiently transfected dominant-negative dynamin-1 in VacA-treated MKN28 cells using anti–dynamin-1 antibody. (b and d) Phase-contrast images of the same fields as in a and c, respectively. VacA induced cytoplasmic vacuolation in nontransfected MKN28 cells (arrows), whereas it failed to induce vacuolation in MKN cells transfected with dominant-negative dynamin-1 (arrowheads). Bars, 10 μm. ×400.
Discussion
We obtained evidence for the involvement of dynamin in the cytoplasmic vacuolation induced by VacA. Immunocytochemistry revealed the localization of endogenous dynamin-2 to the VacA-induced vacuoles. Dominant-negative dynamin-1 inhibited the vacuolation induced by VacA in both transiently and stably transfected HeLa cells. Moreover, overexpression of wild-type dynamin-1 markedly augmented VacA-induced vacuolation in stably transfected cells. Sequential immunocytochemical analysis confirmed that dominant-negative dynamin-1 did not affect VacA internalization by HeLa cells. Additionally, we demonstrated that dominant-negative dynamin-1 prevented deleterious VacA cytopathic effects on cell viability. Finally, we also observed that dominant-negative dynamin-1 inhibited VacA-induced vacuolation in MKN28 gastric mucosal cells. Accordingly, it is suggested that dynamin participates directly in VacA-induced vacuolation.
Cytoplasmic vacuolation induced by cell injury is a widely observed morphological phenomenon (22). It has been assumed to be a pathophysiological cellular response leading to cell death. Thus, understanding vacuolation-related mechanisms should contribute to knowledge of cellular injury and death, and the use of VacA may assist in the elucidation of such mechanisms. On the basis of recent findings that VacA-induced vacuoles contain both the late endosome marker Rab7 and the lysosome marker Lgp110 (2, 3), these vacuoles are assumed to be a hybrid product of late endosomes and lysosomes. Because vesicular fusion of intracellular compartments is required to form the hybrid, vesicle traffic machinery has been assumed to participate in VacA-induced vacuolation. Rab7 localized to VacA-induced vacuoles is a low–molecular weight G-protein functioning in vesicle traffic. Papini et al. reported that Rab7 plays an important role in VacA-induced vacuolation (4). Although rab proteins are indeed important in intracellular vesicle traffic in that they function as regulators of such traffic, they do not directly form or move intracellular vesicles. In intracellular vesicle traffic, multiple mechanoenzymes play essential roles. Kinesin and dynein are the ATPases that move intracellular vesicles along on microtubules toward their plus and minus ends, respectively (23). Dynamin is a high–molecular weight G-protein assumed to function in forming intracellular vesicles (24). Because endocytotic pathways are involved in VacA-induced vacuolation and dynamin is a mechanoenzyme functioning at an early step during vesicle formation, including endocytosis, we focused our study on examining the molecular mechanism of VacA-induced vacuolation by dynamin. Our observations that VacA failed to induce vacuolation in HeLa cells transfected with dominant-negative dynamin, and that stable transfection of wild-type dynamin augmented VacA-induced vacuolation, represent, to our knowledge, the first direct evidence that dynamin plays a crucial role in VacA-induced vacuolation.
Our immunocytochemistry data that VacA cytotoxin is internalized even into cells transfected with dominant-negative dynamin-1 are important. The dominant-negative dynamin-1 transfection inhibited VacA-induced vacuolation by blocking endogenous dynamin function, but not by perturbing VacA-internalization into HeLa cells. The VacA cytotoxin is internalized into cells through mechanisms independent of dynamin. We also observed that transfection of a GTPase-deficient dynamin-2 mutant tagged with green fluorescence protein (kindly provided by Mark A. McNiven, Mayo Clinic, Rochester, Minnesota, USA), which is known to inhibit endogenous dynamin-2 function (25), inhibited VacA-induced vacuolation in HeLa cells, but it did not perturb VacA internalization by these cells (H. Ohnishi and J. Suzuki, unpublished data). Various cytotoxins enter target cells through either clathrin-dependent or -independent endocytotic pathways, or both. To date, however, the mechanism of VacA internalization into target cells has been unclear (26). Because dominant-negative dynamin inhibits the endocytosis of clathrin-coated vesicles (17), it is reasonable to conclude that VacA is internalized, at least in part, by a mechanism distinct from the clathrin-dependent endocytotic machinery.
Although a number of studies demonstrated that VacA is the major virulence factor of H. pylori infection (27), the molecular mechanism by which VacA exerts its cytopathic effect is still unclear. Based on the knowledge that VacA-induced vacuoles are hybrids of late endosomes and lysosomes, Montecucco et al. suggested that a marked decrease of proteolytic activity essential for cell life, and an extensive alteration of protein traffic from the trans-Golgi network to late endosomes — both of which are functional defects caused by vacuolation — might account for VacA cytopathic effects (1). Our observation that dominant-negative dynamin-1 prevented the VacA cytopathic effect on cellular viability concurrently with the inhibition of VacA-induced vacuolation is consistent with their suggestion.
In summary, we have shown that dynamin plays a crucial role in VacA-induced vacuolation. These observations provide new insights related to the molecular pathogenesis of gastroduodenal diseases caused by the H. pylori bacterium.
Acknowledgments
We thank Richard B. Vallee, Mark McNieven, and Toshiyuki Takeuchi for providing the dynamin-1 cDNA, the GFP-tagged mutant dynamin-2 cDNA, and MKN28 cells, respectively.
References
- 1.Montecucco C, Papini E, de Bernard M, Zoratti M. Molecular and cellular activities of Helicobacter pylori pathogenic factors. FEBS Lett. 1999; 452:16–21. doi: 10.1016/s0014-5793(99)00652-3. [DOI] [PubMed] [Google Scholar]
- 2.Molinari M, et al. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J Biol Chem. 1997; 272:25339–25344. doi: 10.1074/jbc.272.40.25339. [DOI] [PubMed] [Google Scholar]
- 3.Papini E, et al. Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc Natl Acad Sci USA. 1994; 91:9720–9724. doi: 10.1073/pnas.91.21.9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papini E, et al. The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J. 1997; 16:15–24. doi: 10.1093/emboj/16.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid SL, McNiven MA, De Camilli P. Dynamin and its partners: a progress report. Curr Opin Cell Biol. 1998; 10:504–512. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- 6.Jones SM, Howell KE, Henley JR, Cao H, McNiven MA. Role of dynamin in the formation of transport vesicles from the Trans-Golgi Network. Science. 1998; 279:573–577. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- 7.Kreitzer G, Marmorstein A, Okamoto P, Vallee R, Rodriguez-Boulan E. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nat Cell Biol. 2000; 2:125–127. doi: 10.1038/35000081. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhausen T. Vesicle formation: dynamic dynamin lives up to its name. Curr Biol. 1998; 8:R792–R794. doi: 10.1016/s0960-9822(07)00501-5. [DOI] [PubMed] [Google Scholar]
- 9.Hojo H. Establishment of cultured lines of human stomach cancer. Origin and their morphological characteristics. Niigata Igakukai Zasshi. 1977; 91:737–752. [Google Scholar]
- 10.Yahiro K, et al. Helicobacter pylori vacuolating cytotoxin binds to the 140-kDa protein in human gastric cancer cell lines, AZ-521 and AGS. Biochem Biophys Res Commun. 1997; 238:629–632. doi: 10.1006/bbrc.1997.7345. [DOI] [PubMed] [Google Scholar]
- 11.Yahiro K, et al. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase β. J Biol Chem. 1999; 274:36693–36699. doi: 10.1074/jbc.274.51.36693. [DOI] [PubMed] [Google Scholar]
- 12.Omata W, et al. Subcellular distribution of GLUT4 in Chinese hamster ovary cells overexpressing mutant dynamin: evidence that dynamin is a regulatory GTPase in GLUT4 endocytosis. Biochem Biophys Res Commun. 1997; 241:401–406. doi: 10.1006/bbrc.1997.7810. [DOI] [PubMed] [Google Scholar]
- 13.Forsayeth JR, Garcia PD. Adenovirus-mediated transfection of cultured cells. Biotechniques. 1994; 17:354–359. [PubMed] [Google Scholar]
- 14.Ohnishi H, Samuelson LC, Yule DI, Ernst SA, Williams JA. Overexpression of rab3D enhances regulated amylase secretion from pancreatic acini of transgenic mice. J Clin Invest. 1997; 100:3044–3052. doi: 10.1172/JCI119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 1987; 47:943–946. [PubMed] [Google Scholar]
- 16.Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998; 9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994; 127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peek RM, et al. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 1999; 59:6124–6131. [PubMed] [Google Scholar]
- 19.Hansen SH, Sandvig K, van Deurs B. Clathrin and HA2 adapters: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993; 121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romano M, Razandi M, Sekhon S, Krause WJ, Ivey KJ. Human cell line for study of damage to gastric epithelial cells in vitro. J Lab Clin Med. 1988; 111:430–440. [PubMed] [Google Scholar]
- 21.Sharma SA, Tummuru MK, Miller GG, Blaser MJ. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995; 63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henics T, Wheatley DN. Cytoplasmic vacuolation, adaptation and cell death: a view on new perspectives and features. Biol Cell. 1999; 91:485–498. doi: 10.1016/s0248-4900(00)88205-2. [DOI] [PubMed] [Google Scholar]
- 23.McNiven MA, Marlowe KJ. Contributions of molecular motor enzymes to vesicle-based protein transport in gastrointestinal epithelial cells. Gastroenterology. 1999; 116:438–451. doi: 10.1016/s0016-5085(99)70142-3. [DOI] [PubMed] [Google Scholar]
- 24.McNiven MA. Dynamin: a molecular motor with pinchase action. Cell. 1998; 94:151–154. doi: 10.1016/s0092-8674(00)81414-2. [DOI] [PubMed] [Google Scholar]
- 25.Gold ES, et al. Dynamin 2 is required for phagocytosis in macrophages. J Exp Med. 1999; 190:1849–1856. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garner JA, Cover TL. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect Immun. 1996; 64:4197–4203. doi: 10.1128/iai.64.10.4197-4203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricci V, et al. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect Immun. 1996; 64:2829–2833. doi: 10.1128/iai.64.7.2829-2833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]