Abstract
B-cell accumulation and formation of ectopic germinal centers are characteristic changes in the diseased joints of patients with rheumatoid arthritis (RA). Earlier studies suggested that interactions between B lymphocytes and specialized synovial “nurse-like” cells peculiar to the RA synovium may be responsible for the homing and sustained survival of B cells in the synovium. However, in this study, we found that B cells spontaneously migrate beneath ordinary fibroblast-like synoviocytes (FLSs) and then experience prolonged survival. FLSs isolated from joints of patients with osteoarthritis also supported this activity, termed B-cell pseudoemperipolesis. We found that FLSs constitutively expressed the chemokine stromal cell–derived factor-1 (SDF-1), and that pertussis toxin or antibodies to the SDF-1 receptor (CXCR4) could inhibit B-cell pseudoemperipolesis. However, expression of SDF-1 is not sufficient, as dermal fibroblasts also expressed this chemokine but were unable to support B-cell pseudoemperipolesis unless previously stimulated with IL-4 to express CD106 (VCAM-1), a ligand for the α4β1 integrin, very-late-antigen-4 (VLA-4 or CD49d). Furthermore, mAb’s specific for CD49d and CD106, or the synthetic CS1 fibronectin peptide, could inhibit B-cell pseudoemperipolesis. We conclude that ordinary FLSs can support B-cell pseudoemperipolesis via a mechanism dependent upon fibroblast expression of SDF-1 and CD106.
Introduction
Rheumatoid arthritis (RA), the most common chronic inflammatory arthritis, is characterized by hyperplasia of the resident synoviocytes and synovial infiltration by a variety of hematopoietic cells, including T and B lymphocytes (1). Synovial infiltration with mononuclear cells presumably reflects an imbalance between factors that enhance cellularity (e.g., recruitment from the blood, retention, and local proliferation), and factors that decrease cellularity (e.g., cell death and emigration from the synovium) (2). Cytokine-mediated induction of adhesion molecules, in particular CD106 (VCAM-1) and CS1 fibronectin on vascular endothelium and fibroblast-like synoviocytes (FLSs), along with local production of chemoattractants, are the proposed mechanisms responsible for the recruitment and retention of leukocytes (1, 3, 4).
In vitro studies demonstrated that B lymphocytes could migrate beneath peculiar cells isolated from the RA synovium and thereby resist spontaneous apoptosis (5, 6). These supporting cells have been called RA synovial fibroblasts (7, 8), RA FLSs with properties of follicular dendritic cells (9), or simply RA synovial “nurse-like” cells (NLCs) (5, 6, 10). The latter term is derived from the NLCs found in marrow stroma that can protect B lymphocytes from undergoing apoptosis in vitro. The term “nurse-like” refers to “nurse cells” found within the thymus that form characteristic protective complexes with immature T lymphocytes (11). The active migration of thymocytes into the cytoplasm of thymic nurse cells is called “emperipolesis”. In contrast, T- or B-lineage cells migrate beneath marrow-derived NLCs (12, 13), but do not become internalized. As such, this process is called “pseudoemperipolesis.” Similar to marrow-derived NLCs, NLCs from RA synovium support B-cell pseudoemperipolesis (5, 7, 8). Some studies suggest that NLCs constitute a unique population of synovial cells peculiar to patients with RA (5, 6).
We examined whether conventional FLSs can also act as NLCs, and whether NLC activity is restricted only to FLSs isolated from the joints with active disease of patients with RA. In addition, we examined the factor(s) responsible for mediating pseudoemperipolesis of B cells in vitro.
Methods
Cytokines, antibodies, flow cytometry.
Synthetic human stromal cell–derived factor-1α (SDF-1α) (1–67) was purchased from Upstate Biotechnology Inc. (Lake Placid, New York, USA). Human IL-4 was purchased from R&D Systems Inc. (Minneapolis, Minnesota, USA). The following mAb’s specific for human surface antigens were used: anti-CXCR4 (12G5), anti-VCAM-1, anti-CD19, anti-CD20, anti-CD49d, and the appropriate isotype controls from PharMingen (San Diego, California, USA). For inhibition studies, V. Woods (University of California, San Diego) and E. Wayner (Seattle Biomedical Research Institute, Seattle, Washington, USA) kindly provided anti-VLA-4 mAb (8F2) and anti-VCAM-1 mAb (P3H12). Furthermore, anti-human VCAM-1 mAb’s (BBA6) were purchased from R&D Systems Inc. R. Houghten (Multiple Peptide Systems, La Jolla, California, USA) provided the cyclic peptide inhibitor containing the minimal CS1-VLA-4 binding motif “LDV” (H-CWLDVC-NH2) and a scrambled cyclic control peptide (H-CDLWC-OH) (14).
For flow cytometry, the cells were adjusted to a concentration of 5 × 106 cells/ml in FACS buffer (RPMI 1640 with 0.5% BSA). 5 × 105 cells were stained with saturating antibody concentrations for 30 minutes at 4°C, washed two times, and then analyzed on a FACSCalibur (Becton Dickinson Immunocytometry Systems, Mountain View, California, USA). Flow cytometry data were analyzed using the FlowJo 2.7.4 software (Tree Star Inc., San Carlos, California, USA).
Synoviocyte purification, culture and B-cell lines.
Synovial cells were isolated by enzymatic digestion of synovial tissue obtained from patients with RA or osteoarthritis (OA) who were undergoing joint replacement surgery, as previously described (3). Briefly, the tissues were minced and incubated with 2 mg/ml collagenase (Worthington, Freehold, New Jersey, USA) in serum-free DMEM (Life Technologies Inc., Grand Island, New York, USA) for 2 hours at 37°C, filtered through a nylon mesh, washed, and cultured in medium consisting of DMEM supplemented with 20% FCS and penicillin-streptomycin-glutamine (both from Life Technologies Inc.). Nonadherent cells were removed after overnight culture, and the adherent cells were cultured to confluence in 5% CO2 in air at 37°C. At confluence, cells were split at a 1:3 ratio, and recultured under identical conditions. Synoviocytes that had been passaged three to six times after the initiation of the culture were used in these experiments, during which time they were a homogenous population of FLSs, as described (3).
The human B-cell lines Ramos and BJAB were obtained from the American Type Culture Collection (ATCC; Rockville, Maryland, USA), and the pro–B cell line Reh and the pre–B cell line Nalm-6 were kindly provided by J. Scheele (University of California, San Diego). The murine stromal cell line M2-10B4 was purchased from ATCC. Cell lines were cultured at 37°C, 5% CO2 in RPMI 1640 supplemented with 10% FCS, and penicillin-streptomycin-glutamine (Life Technologies Inc.).
B-lymphocyte purification.
Human B cells were purified from the blood of healthy donors that was obtained from the San Diego Blood Bank (San Diego, California, USA). Mononuclear cells isolated via Ficoll gradient centrifugation (Ficoll Hypaque; Pharmacia Biotech AB, Uppsala, Sweden), were suspended in RPMI medium containing 1% FCS to a concentration of 5 × 106 cells/ml and incubated at 37°C, 5% CO2 for 2 hours in 125 cm2 tissue culture–treated plastic dishes (Falcon/Becton Dickinson Labware, Franklin Lakes, New Jersey, USA) to deplete monocytes. The cells were subsequently harvested and then incubated with CD19-DynalBeads (Dynal, Oslo, Norway) to isolate B cells. B cells were harvested and then detached from the magnetic spheres using DetachABead (Dynal), according to manufacturer’s instructions. The purity of the isolated B cells was ≥95%, as assessed by flow cytometric analysis after staining the cells with fluorochrome-labeled anti-CD20 mAb’s.
Chemotaxis assay.
The chemotaxis assay across bare polycarbonate was performed as described (15). B cells or B-cell lines were suspended in RPMI 1640 with 0.5% BSA. One hundred microliters of this cell suspension, containing 5 × 105 cells, was added to the top chamber of each 6.5-mm diameter Transwell culture insert (Corning-Costar Corp., Cambridge, Massachusetts, USA) with a pore size of 5 μm. Filters then were transferred to wells containing medium with or without SDF-1α. The chambers were incubated for 2 hours at 37°C in 5% CO2. After this incubation, the cells in the lower chamber were suspended and then counted using a FACSCalibur (Becton Dickinson Immunocytometry Systems) over 20 seconds at 60 μl/min in duplicates. A 1:20 dilution of input cells was counted under the same conditions to determine the relative proportion of migrated cells.
In vitro migration assay of B cells beneath FLSs (pseudoemperipolesis assay).
Spontaneous migration of B cells beneath FLSs from RA or OA patients, or normal dermal fibroblasts, was determined as previously described (16). Briefly, FLSs or dermal fibroblasts were seeded onto collagen-coated 24-well plates at a concentration of 1.5 × 105 cells per well in DMEM (supplemented with 10% FCS) and penicillin-streptomycin-glutamine. After overnight culture, B cells were suspended in RPMI 1640/10% FCS and added onto the confluent fibroblast cell layer to a final concentration of 5 × 106 cells/well, or the indicated cell number in the titration experiments. The plates were incubated at 37°C in 5% CO2. After incubation for 2 hours, the cells that had not migrated into the stromal cell layer were removed by vigorously washing the wells three times with RPMI medium. The complete removal of nonmigrated cells and the integrity of the stromal cell layer containing transmigrated cells was assessed by phase contrast microscopy and documented photographically. The fibroblast cell layer containing the migrated cells was then detached by incubation for 1 minute with trypsin/EDTA solution prewarmed to 37°C (ATV solution; Life Technologies Inc.). Thereafter, the cells were immediately suspended by adding 1 ml of RPMI/10% FCS, washed, and suspended in 0.5 ml medium for counting by flow cytometry. A lymphocyte gate was set using the different relative size and granularity (forward scatter and side scatter) characteristics to exclude fibroblasts. Duplicate samples were counted at high flow rates for 20 seconds to determine the relative number of migrated cells. Counts less than 400 events/20 seconds were not considered significant, because such low event counts were also obtained from wells without lymphocytes (background). For the inhibition studies, B cells were incubated with 30 μg/ml anti-CXCR4 mAb (12G5) or isotype control antibody for 30 minutes at 4°C, washed twice, and applied to the assay. Pertussis toxin pretreatment was performed as described (16).
Inhibition of B-cell apoptosis by FLSs.
B-cell viability was determined by staining the cells with 3,3′ dihexyloxacarbocyanine iodine (DiOC6), to assess mitochondrial transmembrane potential (Δψm), and propidium iodine (PI), to assess membrane permeability (17, 18). This allowed us to distinguish viable cells (Δψmbright, PI negative) from apoptotic cells (Δψmlow, PI negative) or dead cells (Δψmlow, PI positive). Synoviocytes (from RA or OA patients) or dermal fibroblasts were seeded into 24-well plates at a concentration of 1.5 × 105 cells/well. After overnight incubation, purified B cells were added to wells containing the different fibroblasts or control wells without fibroblasts at a concentration of 1 × 106 B cells per well. B cell viability was determined before initiation of the cocultures and subsequently at the time points indicated. Three hundred microliters of B-cell suspension were collected at the indicated time points from different wells by vigorous pipetting and transferred to FACS tubes containing 300 μl of 60 nM DiOC6 and 10 μg/ml PI (both from Molecular Probes Inc., Eugene, Oregon, USA) in FACS buffer. The cells were incubated at 37°C for 15 minutes, and analyzed by flow cytometry using a FACSCalibur (Becton Dickinson Immunocytometry Systems). Fluorescence was recorded at 525 nm (FL-1) for DiOC6 and at 600 nm (FL-3) for PI.
CXCR4 RT-PCR analysis.
RNA was isolated from 1 × 107 Nalm-6, Reh, Ramos, CD19-mAb purified blood B cells, or BJAB cells using the QIAGEN RNeasy kit (QIAGEN Inc., Valencia, California, USA). RNA was then used for first-strand cDNA synthesis with the SuperScript preamplification system (Life Technologies Inc.), according to the manufacturer’s instructions. The CXCR4-specific primers (5′ primer: GGA GAA TTC TTA CCA TGG AGG GGA TCA; 3′ primer: GGA GAA TTC AGC TGG AGT GAA AAC TTG) were used as described (16). The annealing temperature was 58°C and the reaction proceeded for 35 cycles. To control for the presence of intact RNA, RT-PCR for human GAPDH was performed under the same conditions, as described (19).
SDF-1 RT-PCR analysis.
For SDF-1 mRNA detection, RNA was extracted from 2–3 × 106 FLSs from RA patients (n = 3), OA patients (n = 3), or dermal fibroblasts, and used for cDNA synthesis as described above. The sequences of the human SDF-1β primers were: 5′ GAG AAT TCA TGA ACG CCA AGG TCG TGG 3′ (upper primer) and 5′ GAT CTA GAT CAC ATC TTG AAC CTC TTG 3′ (lower primer). The conditions of the PCR reaction were the same as described above. A sequenced plasmid containing the human SDF-1β cDNA was used as a positive control, and control RT-PCR for GAPDH to equalize for the amount of RNA was performed as described above.
SDF-1 protein detection in RA FLSs by immunofluorescence microscopy.
For SDF-1 detection by immunofluorescence, RA FLSs were cultured on Lab-Tek chambered cover glass (Nalge Nunc International, Naperville, Illinois, USA). GolgiStop (PharMingen), containing monensin, was added 6 hours prior to staining to accumulate cytoplasmic SDF-1. Then cells were fixed, made permeable by treatment with Cytofix/Cytoperm (PharMingen), and then stained with mouse anti-human vimentin (DAKO A/S, Glostrup, Denmark), and biotinylated goat anti-human SDF-1 (R&D Systems Inc.) in the presence of normal mouse and goat serum to mitigate nonspecific antibody binding. Mouse IgG1 and biotinylated goat IgG were used for negative controls, and fluorescein-labeled anti-mouse IgG1 or phycoerythrin-conjugated (PE-conjugated) streptavidin were to detect anti-vimentin or anti-SDF-1 staining, respectively. Hoechst 33258 (Sigma Chemical Co., St Louis, Missouri, USA) was used for nuclear staining, according to the manufacturer’s instructions. Optical sections of fluorochrome-labeled cells were captured with a Delta-Vision deconvolution microscope system (Applied Precision Inc., Issaquah, Washington, USA). In general, sections were spaced by 0.3 μm, and image stacks were deconvoluted using Applied Precision Inc. softWoRx software, and then rendered as volumes. Images were captured with a 60× Nikon oil lens using filters specific for blue, green, or red fluorescence.
Actin polymerization assay.
Actin polymerization was tested as described (16). B cells (1.25 × 106/ml) were suspended in RPMI 1640 medium containing 0.5% BSA and stimulated at 37°C with 100 ng/ml SDF-1α for varying amounts of time. At the indicated time points, 400 μl of the cell suspension was added to 100 μl of a solution containing 4 × 10–7 M FITC-labeled phalloidin, 0.5 mg/ml 1-α-lysophosphatidylcholine (both from Sigma Chemical Co.) and 18% formaldehyde (Sigma Chemical Co.) in PBS (pH 7.4). The fixed cells were analyzed by flow cytometry using a FACSCalibur (Becton Dickinson Immunocytometry Systems) and all time points were plotted relative to the mean relative fluorescence of the sample before addition of the chemokine.
Effects of VCAM-1 induction on the migration of B cells beneath dermal fibroblasts.
CD106 (VCAM-1) expression was induced on VCAM-1 negative dermal fibroblasts by incubating the cells with 10 ng/ml IL-4 for 12 hours at 37°C in 5%CO2. VCAM-1 expression was documented by flow cytometry, and migration experiments were performed with Nalm-6 and Ramos B cells on untreated and IL-4–treated dermal fibroblasts, as described above. For inhibition studies, anti-VCAM-1 mAb’s (BBA6, R&D Systems Inc.) were reconstituted in PBS/0.5% BSA and added to IL-4–pretreated dermal fibroblasts to a final concentration of 50 μg/ml. Mouse mAb’s of irrelevant specificity (MOPC21 mouse IgG1; Sigma Chemical Co.) were used as control. After incubation for 1 hour at room temperature, the wells were washed with RPMI 1640, and Nalm-6 or Ramos cells pretreated with heat aggregated human γ globulins (Sigma Chemical Co.) were added to triplicate wells of antibody-treated or control wells. Pseudoemperipolesis was then quantified by flow cytometry, as described above.
Data analysis, statistics.
Results are shown as mean ± SD, or SEM, of at least three experiments each. For statistical comparison between groups, the Student’s paired t test or Bonferroni t test was used. Analyses were performed using the Biostatistics software developed by Stanton A. Glantz (University of California San Francisco, San Francisco, California, USA). Flow cytometry data were analyzed using the FlowJo software (Becton Dickinson Immunocytometry Systems).
Results
FLSs can function as NLCs for human B cells.
We examined whether FLSs isolated from patients with RA or OA could function as NLCs for normal B cells and B-cell lines. RA-derived FLSs (RA FLSs) or OA-derived FLSs (OA FLSs) were cultured with a pro–B cell line (Nalm-6), a pre–B cell line (Reh), a B-cell line (Ramos), a B-lymphoblastoid cell line (BJAB), or normal adult blood B cells. Pseudoemperipolesis was monitored by phase contrast microscopy. We found that Nalm-6, Reh, Ramos, or blood B cells spontaneously migrated beneath RA FLSs or OA FLSs within hours of coculture (Figure 1). During this time, the lymphocytes migrated beneath or became trapped by cytoplasmatic projections of the adherent FLSs (13, 20). The B cells that migrated into the same focal plane as the FLSs had a dark appearance (Figure 1, a and b). In contrast, the lymphoblastoid B-cell line BJAB did not migrate under similar conditions and retained a light appearance (Figure 1c).
Figure 1.
Representative phase contrast photomicrographs demonstrating pseudoemperipolesis of B-cell lines cultured for 2 hours on FLSs from RA patients. Cells that had not migrated beneath the FLSs were washed off, and the FLS layer containing the migrated B cells was photographed (200× magnification). In contrast to Nalm-6 (a) and Ramos B cells (b), BJAB B cells did not display pseudoemperipolesis (c). (A few BJAB cells were added back to the well after washing in panel c to demonstrate the bright appearance of nonmigrated cells relative to that of cells that had migrated into the same focal plane as the FLSs, as seen in panels a and b).
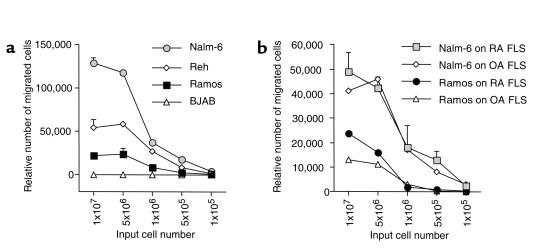
To measure relative B-cell migration beneath FLSs, we performed titration experiments in which 105 to 107 input B cells were added to wells containing a layer of confluent FLSs. BJAB cells did not migrate beneath RA FLSs or OA FLSs at any input cell number (data not shown). However, all other B-cell lines migrated beneath the RA FLSs (Figure 2a). No significant differences were noted between RA FLSs and OA FLSs in their ability to support pseudoemperipolesis of Nalm-6 or Ramos (Figure 2b). In either case, the numbers of migrated cells increased proportionately with the numbers of input cells provided that 5 × 106 or fewer input B cells were used. When greater numbers of input cells were added, we did not observe any further increase in the numbers of migrating cells over that seen with 5 × 106 input B cells (Figure 2). As such, 5 × 106 input cells were used for all subsequent experiments.
Figure 2.
(a) Measurement of B-cell pseudoemperipolesis on FLSs from RA patients. Nalm-6, Reh, Ramos, or BJAB B cells were added to separate wells of confluent RA FLSs from a representative RA patient at the input numbers indicated on the horizontal axis. After 2 hours, nonmigrated cells were removed and the adherent FLS layer was detached and made into single-cell suspensions. The numbers of B cells contained within the adherent FLS layer were assessed by flow cytometry. Displayed are the mean (± SEM) numbers of events acquired in 20 seconds in the lymphocyte scatter gate at high flow from duplicate wells. (b) Comparison between RA FLSs and OA FLSs in their capacity to support pseudoemperipolesis. Nalm-6 B cells or Ramos B cells were incubated on confluent layers of FLSs from a representative patient with RA or a patient with OA. After 2 hours, the B cells that had migrated beneath the FLSs were assessed, as outlined in a. Displayed are the mean relative numbers (± range) of migrated cells, collected from duplicate wells with the numbers of input cells indicated on the horizontal axis.
High numbers of cells from the immature B-cell lines Nalm-6 (111,870 ± 2,263 cells, mean ± range from duplicate wells) or Reh (58,943 ± 2,345 cells), and lower numbers from the Ramos cell line (24,161 ± 6,484 cells), migrated beneath RA FLSs. In contrast, dermal fibroblasts were relatively ineffective in supporting pseudoemperipolesis of the various B-cell lines. Spontaneous migration of Nalm-6 beneath dermal fibroblasts was only 13 ± 2.5% or 12.6 ± 1.7% of that noted with OA FLSs or RA FLSs. Moreover, the migration of Ramos cells beneath dermal fibroblasts was 50 ± 1.8% or 42 ± 5% of that noted with OA FLSs or RA FLSs.
Coculture of purified normal B cells with RA FLSs or OA FLSs protected the B cells from apoptosis. As shown in Figure 3a, only a small proportion of normal B cells retained characteristics of viable cells (high mitochondrial membrane potential and PI exclusion) when cultured in medium alone for a period of 5 days. In contrast, the majority of B cells remain viable when cultured with RA FLSs or OA FLSs. In contrast, dermal fibroblasts were significantly less effective in supporting B-cell survival than either RA FLSs or OA FLSs at all time points (Figure 3, a and b). The effect of RA FLSs or OA FLSs on B-cell survival was most prominent during the initial 5–7 days of culture (Figure 3b).
Figure 3.
(a) FLSs from RA and OA patients protect normal blood B lymphocytes from spontaneous apoptosis in vitro. The viability of purified B cells was determined by staining with DiOC6 and PI. Presented are contour maps of B cells from a representative donor defining the relative green (DiOC6) and red (PI) fluorescence intensities of the B cells on the horizontal and vertical axes, respectively. The vital cell population (DiOC6bright, PI-exclusion) was determined for B cells cultured in medium alone, or on a confluent layer of FLSs from a patient with RA (RA FLSs) or OA (OA FLSs), or dermal fibroblasts. The vital cells were gated as indicated by the lines. The relative proportions of vital cells are displayed above each of these gates. (b) The viability of B cells from three different donors was determined by DiOC6/PI staining at the time points indicated on the horizontal axis. Displayed is the mean (± SD) viability of B cells cultured with RA FLSs (diamonds), OA FLSs (circles), dermal fibroblasts (triangles), or medium alone (squares).
Expression of SDF-1 and CXCR4.
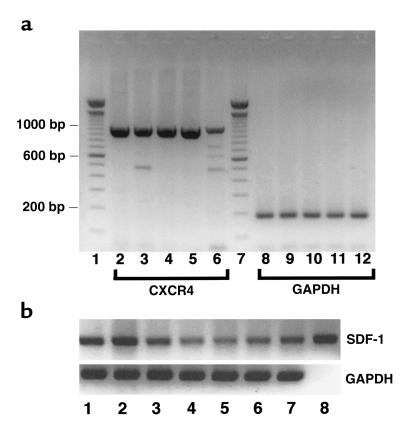
SDF-1 is a powerful chemoattractant cytokine (chemokine) that promotes the migration and activation of B lymphocytes and other hematopoietic cells (15, 21). To determine whether SDF-1 plays a role in the B-cell pseudoemperipolesis mediated by FLSs, we examined B cells, RA FLSs, OA FLSs, and dermal fibroblasts for expression of SDF-1 and its receptor CXCR4 by RT-PCR. CXCR4 mRNA was detected in Reh, Nalm-6, Ramos, and normal B cells (Figure 4a, lanes 2–5). However, RT-PCR of BJAB cDNA generated relatively small amounts of the CXCR4-specific 1,058 bp product (Figure 4a, lane 6). This did not appear to be due to differences in the amounts of input cDNA, as indicated by control RT-PCR for the GAPDH gene (Figure 4a, lanes 8–12). Using SDF-1β–specific primers we found expression levels of SDF-1 mRNA among RA FLSs isolated from three different patients with RA (Figure 4b, lanes 1–3) or OA (Figure 4b, lanes 4–6), respectively. SDF-1 mRNA was also detected in dermal fibroblasts by RT-PCR (Figure 4b, lane 7).
Figure 4.
(a) RT-PCR analysis for CXCR4 expressed by different B-cell lines or normal blood B cells. A CXCR4 PCR product of the expected size (1,058 bp) was generated using cDNA obtained from Reh, Nalm-6, Ramos, normal blood B cells, or BJAB cells in lanes 2–6, respectively. Similarly, a GAPDH PCR product was generated using the cDNA from Reh, Nalm-6, Ramos, normal blood B cells, or BJAB cells in lanes 8–12, respectively. Lanes 1 and 7 contain DNA fragments of known size, allowing for calibration of the migration distances, as indicated on the far left-hand side of the figure. (b) RT-PCR analysis for SDF-1 expression by FLSs from RA and OA patients, or dermal fibroblasts. SDF-1 PCR products (top row) or GAPDH PCR products (bottom row) were generated using cDNA obtained from the FLSs of three different RA patients (lanes 1–3), three different patients with OA (lanes 4–6), dermal fibroblasts (lane 7), or a DNA plasmid containing the human SDF-1β cDNA (lane 8). All tested samples displayed amplification of a PCR fragment of the expected size for SDF-1.
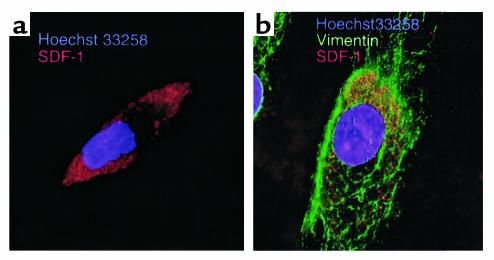
We examined FLSs for expression of SDF-1 protein by immunofluorescence microscopy. For this we stained RA FLSs with anti-SDF-1 antibodies by intracytoplasmic immunofluorescence staining. Figure 5 displays examples of representative FLSs from RA patients stained with anti-SDF-1 (Figure 5a) or stained with anti-SDF-1 and anti-vimentin Ab’s (Figure 5b). In both images, specific granular staining for cytoplasmatic SDF-1 can easily be seen as red fluorescence. These cells did not display nonspecific staining with either mouse IgG1 or biotinylated goat IgG (data not shown).
Figure 5.
SDF-1 protein detection in RA FLSs by immunofluorescence microscopy. This figure depicts fluorescence micrographs of representative RA FLSs stained with anti-SDF-1 Ab’s (red fluorescence) and Hoechst 33258 (blue; a), or anti-SDF-1 Ab’s (red fluorescence), anti-vimentin mAb’s (green fluorescence), and Hoechst 33258 (blue; b).
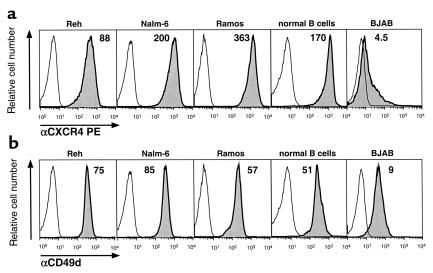
Flow cytometry revealed high-level expression of CXCR4 on Reh, Nalm-6, Ramos, and normal B cells, but relatively low levels of this receptor on BJAB (Figure 6a). In addition, we also examined for CD49d. This molecule was expressed at high levels on all B cells examined, with the exception of a lower display of CD49d on BJAB cells (Figure 6b).
Figure 6.
Expression of CXCR4 or CD49d (VLA-4) by different B-cell lines or normal blood B cells, as indicated. Displayed are fluorescence histograms depicting the relative red fluorescence intensity of the cells stained with anti-CXCR4 mAb’s (a, shaded histograms) or with anti-CD49d mAb’s (b, shaded histograms) compared with that of the same cells stained with a PE-conjugated isotype control mAb of irrelevant specificity (open histograms). The mean fluorescence intensity ratio of each specifically-stained cell population is displayed above the histograms.
Functional significance of CXCR4 on human B cells.
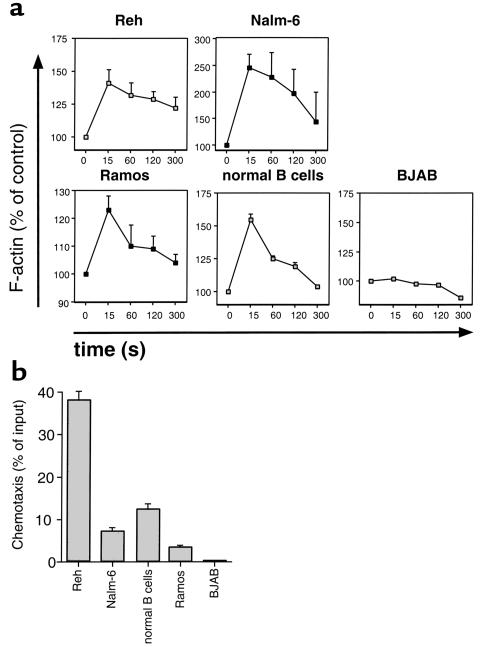
To determine whether differences in the capacity of B cells or B-cell lines to migrate beneath FLSs correlated with the function of CXCR4 on the respective cells, we examined the CXCR4 function by actin polymerization and chemotaxis in response to 100 ng/ml SDF-1α. In contrast to all other cell lines tested, BJAB cells did not show the characteristic transient increase in filamentous actin (F-actin) following SDF-1α stimulation (Figure 7a). Moreover, normal blood B cells and all B-cell lines except BJAB displayed chemotaxis with 100 ng/ml SDF-1 (Figure 7b). Background migration was always less than 1% of the input cells for all cell lines examined. As such, BJAB cells apparently lack a functional CXCR4 receptor.
Figure 7.
Responses of B-cell lines or normal blood B cells to SDF-1α. (a) Intracellular F-actin was measured using FITC-labeled phalloidin after the addition of 100 ng/ml SDF-1α at time 0. Results are displayed as percent of intracellular F-actin relative to that prior to the addition of SDF-1α, for each cell line, as indicated above the box for each graph. The lines connect the data points that are the mean ± the range of two independent experiments. (b) SDF-1α induces chemotaxis of B-cell lines or normal blood B cells, but not BJAB B cells. Blood B lymphocytes or B-cell lines, as indicated, were assayed in the bare filter chemotaxis assay for migration toward 100 ng/ml of SDF-1α. The bars represent the mean (± range) relative proportion of input B cells that had migrated in response to SDF-1. Migration to control wells containing medium alone was less than 1% in all cases.
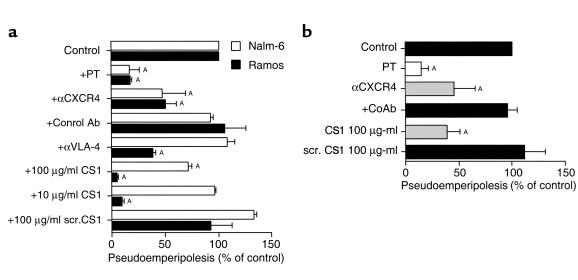
To determine whether B-cell activation through CXCR4 was required for pseudoemperipolesis, Nalm-6 or Ramos B cells were preincubated with either pertussis toxin, anti-CXCR4 mAb (12G5), or a control mAb of irrelevant specificity prior to culture with RA FLSs from different patients. Pertussis toxin, which inhibits signaling through G-protein coupled, seven transmembrane receptors, such as CXCR4, significantly reduced pseudoemperipolesis of Nalm-6 or Ramos cells to levels that were 16 ± 9.2% or 16.8 ± 0.9%, respectively, of those noted of untreated controls (n = 4, P < 0.005; Figure 8a). Anti-CXCR4 antibody pretreatment also significantly inhibited migration of Nalm-6 or Ramos cells beneath RA FLSs (Nalm-6: 46.9 ± 21.6%, n = 4, P < 0.005; Ramos: 49.5 ± 10.1% of the untreated controls, n = 4, P < 0.005; Figure 8a). Control antibody pretreatment had no effect (92.1 ± 1.8% or 105 ± 19.4%, n = 4). Similar results were obtained using purified blood B cells. For such cells, migration beneath RA FLSs was inhibited significantly by pretreatment with pertussis toxin (14 ± 7.1%, n = 5, P < 0.05), or anti-CXCR4 mAb (45.3 ± 19.8%, n = 5, P < 0.05), but not with a control mAb (95.1 ± 10%, n = 7; Figure 8b). Migration beneath OA FLSs was inhibited to a similar degree with either pertussis toxin or anti-CXCR4 mAb (data not shown).
Figure 8.
Inhibition of B-cell pseudoemperipolesis of RA FLSs by CXCR4 and VLA-4 antagonists. (a) Nalm-6 (open bars) or Ramos cells (filled bars) were preincubated with pertussis toxin (PT), αCXCR4 mAb’s, control mAb’s, αVLA-4 mAb’s, 100 μg/ml or 10 μg/ml of CS1 peptide, or a control peptide, as indicated on the left-hand side. Cells then were incubated on RA FLSs and allowed to migrate beneath the FLSs for 2 hours. Then, the FLS layer containing the migrated cells was harvested, and the relative numbers of migrated cells were determined by flow cytometry. The bars represent the mean (± SD) B-cell migration relative to that of untreated samples. AThe difference between the percent migration under a given condition is significantly less than that noted for same cell population for FLSs in the absence of inhibitors (e.g., P values < 0.05, Bonferroni’s t test). (b) Pseudoemperipolesis of normal blood B cells beneath RA FLSs was also inhibited by PT, αCXCR4 mAb’s, or CS1 peptide. The bars represent the mean (± SD) relative B-cell migration of B cells from five different donors. ASignificant inhibition of migration with P values < 0.05 using Bonferroni’s t test.
Pseudoemperipolesis is dependent on interaction of CD106 with VLA-4.
Interactions between CD49d/CD29 (VLA-4) on B cells and its respective ligands (CD106 and the CS1 portion of fibronectin) play an important role in the adhesion between B cells and FLSs (3) or NLCs derived from RA synovium (5, 10). We examined the influence of a VLA-4 mAb or a synthetic CS1 peptide on B-cell migration beneath RA FLSs. Both anti-VLA-4 and CS1 peptide were active inhibitors of pseudoemperipolesis for Ramos and normal B cells (Figure 8, a and b), but had little or no inhibitory effect on the migration of immature Nalm-6 cells (Figure 8a). For instance, Nalm-6 migration was not inhibited by anti-VLA-4 mAb (108 ± 7% of untreated controls, n = 4), but the antibody reduced migration of Ramos cells to 38 ± 2% of respective controls (n = 4, P < 0.05; Figure 9a). Moreover, CS1 peptide inhibited Ramos cell migration to levels that were 4 ± 0.6% (100 μg/ml CS1) and 8.7 ± 1.3% (10 μg/ml CS1) of untreated controls (n = 4, P < 0.05; Figure 9a), while 100 μg/ml of CS1 was required for significant inhibition of pseudoemperipolesis with Nalm-6 (71.2 ± 2.6%, n = 4, P < 0.05; Figure 8a). One hundred micrograms per milliliter of CS1 peptide also inhibited the migration of normal B cells to 37.9 ± 12% of cultures without added peptide (mean ± SD, n = 4, P < 0.05). In contrast, the scrambled CS1 control peptide did not inhibit migration of Nalm-6 or Ramos even at the highest peptide concentration of 100 μg/ml (Nalm-6: 132.2 ± 2.3%, n = 4; Ramos: 91.8 ± 20%, mean ± SD, n = 4). Moreover, normal B-cell pseudoemperipolesis in the presence of 100 μg/ml of scrambled CS1 peptide was 111 ± 20% of control cultures without added peptide (mean ± SD, n = 4; Figure 8b).
Figure 9.
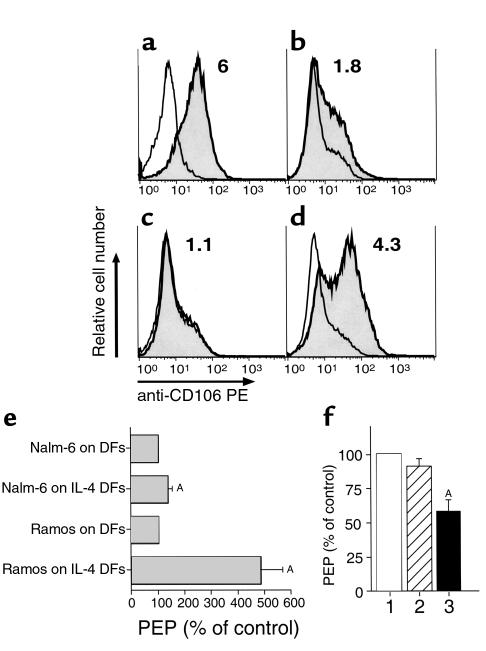
(a–d) CD106 (VCAM-1) expression by RA FLSs (a), OA FLSs (b), dermal fibroblasts (c), or IL-4–stimulated dermal fibroblasts (d). a–d depict histogram graphs that display the relative red fluorescence intensity of cells stained with anti-CD106 mAb’s (shaded histograms) or a nonspecific isotype control antibody (open histograms). The mean fluorescence intensity ratios of cells stained for CD106 relative to that of control antibody–stained cells are displayed in the upper right-hand corner of each histogram. (e) B-cell migration beneath dermal fibroblasts (DFs) is significantly enhanced by IL-4 treatment of dermal fibroblasts. The bars represent the mean (± SD) Nalm-6 or Ramos B-cell migration beneath IL-4–treated dermal fibroblasts relative to the migration beneath untreated dermal fibroblasts, corresponding to 100%. ASignificant inhibition of migration with P values < 0.05 using Bonferroni’s t test. (f) Migration of Ramos B cells and Nalm-6 B cells beneath IL-4–treated dermal fibroblasts is significantly inhibited by anti-CD106 mAb’s. Compared with the migration beneath IL-4–treated dermal fibroblasts without antibody treatment (column 1), control antibody treatment did not significantly affect pseudoemperipolesis (PEP) of Ramos and Nalm-6 cells (column 2). In contrast, treatment with anti-CD106 mAb significantly decreased the migration of Ramos cells (column 3). ASignificant inhibition of migration compared with that of control cultures (P < 0.05, Bonferroni’s t test). The bars represent the mean (± SEM) PEP of Ramos B cells in test conditions relative to that of Ramos B cells in control conditions without antibody (n = 6).
Because CD49d appeared necessary for B-cell pseudoemperipolesis, we examined FLSs and dermal fibroblasts for expression of the ligand for this β1 integrin, namely CD106 (VCAM-1). We found that RA FLSs and OA FLSs express high levels of cell-surface CD106 (Figure 9, a and b). However, nonstimulated dermal fibroblasts did not express detectable levels of this adhesion molecule (Figure 9c). We hypothesized that this could account for the inability of dermal fibroblasts to support B-cell pseudoemperipolesis.
To test this hypothesis, we stimulated dermal fibroblasts with exogenous IL-4, a cytokine that we noted could induce fibroblasts to express CD106 (Figure 9d). We next tested whether IL-4–stimulated dermal fibroblasts could support B-cell pseudoemperipolesis. Migration of Nalm-6 or Ramos cells beneath IL-4–stimulated dermal fibroblasts was comparable to that observed for RA FLSs or OA FLSs. Whereas only 5,691 ± 1,076 (mean ± SD, n = 4; Figure 9e) Ramos cells migrated beneath untreated dermal fibroblasts, 29,000 ± 5,733 cells migrated beneath IL-4–stimulated dermal fibroblasts (P < 0.05, Student’s t test). The effect on Nalm-6 migration was more modest in that 31,312 ± 4,307 Nalm-6 cells migrated beneath untreated dermal fibroblasts, but 38,786 ± 2,255 cells migrated beneath IL-4–treated dermal fibroblasts. Nevertheless, the difference between Nalm-6 migration beneath IL-4–stimulated dermal fibroblasts versus that noted for IL-4–treated dermal fibroblasts was statistically significant (P < 0.05, Student’s t test).
We examined whether pseudoemperipolesis mediated by IL-4–treated dermal fibroblasts was dependent upon CD106. Pseudoemperipolesis of Ramos or Nalm-6 beneath IL-4–treated dermal fibroblasts was significantly reduced by anti-CD106 mAb’s to levels that were 57.9 ± 8.5% (mean ± SEM, n = 6) or 85.4 ± 3.1% (n = 6), respectively, of that observed with each cell line beneath IL-4–treated dermal fibroblasts without mAb’s (100%) (P < 0.05). In contrast, addition of control mAb’s (MOPC21) to the cultures did not significantly inhibit pseudoemperipolesis beneath IL-4–treated dermal fibroblasts (90.8 ± 5.5%, n = 6; Figure 9f). Pseudoemperipolesis of Nalm-6 cells was also significantly inhibited by anti-CD106 mAb’s (85.4 ± 3.1% of untreated controls, P < 0.05, n = 6), whereas control mAb’s also did not display a significant effect on Nalm-6 pseudoemperipolesis (106.1 ± 4, n = 6; Figure 9f).
Discussion
In the RA synovium there is B-lymphocyte accumulation and clonal expansion (22), formation of ectopic germinal centers, plasma cell accumulation (23), and deposits of immune complexes (24), suggesting that B cells and their products participate in disease progression. Moreover, a recently described animal model that simulates RA demonstrates a critical need for B cells in the transition of T-cell autoreactivity to immunoglobulin-mediated joint destruction (25, 26).
Prior in vitro studies of requirements for B-cell survival in the synovial membrane and local differentiation into plasma cells concluded that cell contact between synovial fibroblasts and B cells is essential (7, 27). Subsequently, synovial NLCs were characterized that inhibited B-cell apoptosis through the engagement of CD49d/CD29 with CD106 (5), resulting in an upregulation of Bcl-xL expression in the B cells (10). Similarly, Reparon-Schuijt and colleagues recently reported that FLSs also could protect synovial fluid B cells from undergoing spontaneous apoptosis through a contact-dependent mechanism requiring VLA-4 interactions with CD106 expressed on FLSs (28).
The present study demonstrates that the CXC chemokine SDF-1 and its receptor CXCR4 are involved in the nurse-like properties of FLSs for B cells. B cells that express functional CXCR4 receptors migrate beneath FLSs. In contrast, BJAB B cells, which express CXCR4 mRNA but do not display functional CXCR4 surface receptors (Figures 4, 6, and 7), do not migrate beneath FLSs. Moreover, both pertussis toxin and a specific anti-CXCR4 mAb (12G5) significantly inhibited B-cell migration beneath FLSs, demonstrating an important role for B-cell activation through the CXCR4 chemokine receptor. The failure to achieve higher levels of inhibition with 12G5 αCXCR4 mAb’s has been noted earlier (21, 29), and may reflect the competition of secreted and/or surface-bound SDF-1 with anti-CXCR4 mAb’s during a 2 hour assay. Internalization and recycling of CXCR4 receptors after mAb binding (30), or partial dissociation of this mAb at the physiologic temperatures of the pseudoemperipolesis assay may also play a role in this context.
In the hematopoietic microenvironment, SDF-1 secreted by marrow stromal cells retains developing B cells in close contact with stromal cells. This may be the most important of the many properties of SDF-1, as demonstrated in SDF-1– or CXCR4-deficient mice (31, 32). The role of SDF-1 in pseudoemperipolesis could be interpreted in a similar fashion, namely, that SDF-1 made by FLSs attracts and retains B cells in the synovium.
We noticed that immature B cells (Nalm-6, Reh) migrated better beneath FLSs from either RA or OA patients than did mature B cells (Ramos, normal blood B cells), a result that correlates with recently recognized differences in SDF-1 responsiveness between immature and mature B cells. Although B cells at all stages of maturation express functional CXCR4 receptors (33), pre- and pro-B cells, and in particular Nalm-6 and Reh B cells (34), have a greater migratory and signaling response to SDF-1 than mature B cells (35, 36). Our functional CXCR4 characterization supports this interpretation (Figure 7). Reh cells had a significantly stronger chemotactic response than mature B cells to 100 ng/ml SDF-1, suggesting that the degree to which B cells migrate beneath FLSs correlates with the CXCR4 responsiveness of the respective B cells.
SDF-1 mRNA and protein was detected in RA FLSs by RT-PCR and immunofluorescence, respectively, but SDF-1 mRNA was also found in all FLSs from OA patients and in dermal fibroblasts, indicating that SDF-1 expression is not restricted to RA FLSs. SDF-1 is needed to engage and activate the CXCR4 chemokine receptor on B cells, but in addition, adhesion to FLSs through α4 integrins (VLA-4; CD49d/CD29) is necessary for B-cell migration beneath FLSs or dermal fibroblasts. This is seen by the significant inhibition of Ramos or normal blood B-cell migration beneath RA FLSs by anti-VLA-4 mAb or synthetic CS1 peptide, but not by control mAb or control peptide (Figure 8). Moreover, when CD106 expression is induced on dermal fibroblasts by IL-4, it can support pseudoemperipolesis, in particular of mature (Ramos) B cells (Figure 9). This increased B-cell migration beneath IL-4–treated dermal fibroblasts was significantly inhibited by anti-CD106 mAb’s, but not by isotype-matched control mAb’s (Figure 9f), demonstrating that this increase in pseudoemperipolesis was dependent in part on the induction of CD106.
The anti-CD106 mAb’s were more effective in inhibiting the migration of mature Ramos cells than the migration of immature Nalm-6 cells. Furthermore, the migration of Nalm-6 cells beneath IL-4–treated dermal fibroblasts was more modest that that of Ramos cells. These observations suggest that Ramos cells are more dependent than Nalm-6 cells on the engagement of CD49d with CD106 for migration beneath fibroblasts. Nevertheless, the pseudoemperipolesis of Nalm-6 beneath IL-4–treated dermal fibroblasts was significantly greater than that beneath nontreated fibroblasts, and this increase was significantly reduced by CD106 mAb’s, suggesting that pseudoemperipolesis of even immature B cells can be facilitated through a CD106-dependent mechanism. As such, findings presented in this study suggest a multi-step process for immature or mature B-cell migration beneath FLSs, similar to the multi-step paradigm of leukocyte transendothelial migration (37, 38), which requires the sequential engagement of VLA-4 adhesion molecules and activation through CXCR4 chemokine receptors.
Based on the knowledge that synovial cells are not homogeneous, but contain one or more minority populations of mesenchymal cells (39, 40), Shimaoka and coworkers asked “whether the capacity to facilitate B-cell activation is a general property of synoviocytes, or alternatively reflects the activity of a small number of contaminating nurse-like cells (NLC)” (5). They and others (6, 41) emphasized the distinction between specialized NLCs from synovium or skin and the fibroblasts isolated from the synovium or dermis. Synovial NLCs are approximately twofold larger than FLSs or dermal fibroblasts. In addition, they have an adherent, polygonal shape with long slender cytoplasmic processes and are easily distinguished from the characteristic slender, elongated appearance of FLSs (see figure 1 in ref.39). Furthermore, NLCs could support pseudoemperipolesis of selected T- and B-cell lines. Moreover, RA NLCs, in particular, were argued to support the survival and function of normal B cells, a property found to be lacking in NLCs from OA or normal synovium or dermal fibroblasts (5). However, we found that conventional RA FLSs or OA FLSs could support B-cell pseudoemperipolesis and survival. These results agree with those obtained from other groups (7, 27, 42), demonstrating that support for B-cell survival is not a property restricted to synoviocytes from RA patients (42). Here, we found that FLSs from patients with OA or IL-4–stimulated dermal fibroblasts could also support B-cell pseudoemperipolesis (Figure 9). Moreover, we found that the ability of fibroblast cell lines to support B-cell pseudoemperipolesis was dependent upon their expression of SDF-1 and CD106, rather than their cell of origin.
An important difference between the current study and those conducted on synovial NLCs (5, 6, 41) is that the studies on NLCs used “conditioned medium” prepared by coculture of allogeneic blood mononuclear cells from several normal subjects for 48 hours. The allogeneic mixed lymphocyte reaction (MLR) generated a cytokine-rich conditioned medium that was added to primary synovial cultures twice a week prior to subcloning (5, 6, 10, 41). Moreover, 20–30% of the RA synovial cells used to initiate these cultures generally are comprised of monocytes and macrophages (43). In contrast, we generated suspensions of fibroblasts via enzymatic digestion of synovial tissues that subsequently were cultured and passaged in serum-containing medium. Conceivably, the allogeneic MLR-conditioned medium induces cells that are distinct from the FLSs used in this study. Although Shimaoka and colleagues hypothesized that synovial NLCs could be derived from stromal cells of mesenchymal origin, they noted that they had functional and phenotypic features similar to those of follicular dendritic cells (5). Induction and/or expansion of rare synovial cells possibly related to (follicular) dendritic cells on the one hand, as opposed to conventional FLSs in our study, may account for the noted differences between these studies.
Whichever interpretation of the NLC phenomenon is correct has direct bearing on ideas about the pathogenesis of RA. One theory proposes that RA joint tissues have a minority population of NLCs that facilitates the local accumulation, proliferation, and activation of B cells for immunoglobulin production. This minor population is necessary for the initiation of the process and becomes responsible for the perpetuation of the joint inflammation that is the hallmark of RA. Lacking synovial nurse cells, the OA joint does not become inflamed. An alternative view, supported by the findings reported in this paper, is that under the proper conditions all fibroblasts are capable of supporting B-cell pseudoemperipolesis and that all joints are susceptible to chronic inflammation. Thus, the difference between chronic inflammatory and noninflammatory forms of arthritis may be secondary to the enhanced microvascular permeability caused by the byproducts of inflammation, which allows immune competent cells from the circulation to enter the joint and become established in the nurturing environment provided by FLSs. In noninflammatory arthritis, like OA, insufficient numbers of circulating cells enter the synovium. Furthermore, serum and/or proinflammatory cytokines can induce normal fibroblasts and endothelial cells to produce higher amounts of SDF-1 (44, 45). This, coupled with the observation that certain cytokines, such as IL-4, can induce phenotypic changes in fibroblasts that allow for B-cell pseudoemperipolesis, suggests that inflammatory cytokines can act to enhance the nurse-like function of normal fibroblasts.
In any case, the observations reported in this paper support the notion that conventional FLSs can have a nurse-like function for mature B cells. This function is dependent upon expression of SDF-1 and CD106-CD49d integrin interactions. As such, B-cell pseudoemperipolesis is not an RA-restricted phenomenon, but rather a general response-mechanism of fibroblast-like cells to inflammatory activation. SDF-1 secretion by FLSs, in concert with the induction of adhesion molecules on the synovial microvasculature (3, 46, 47), may allow for the immigration and retention of B cells in the synovium during arthritis. Additional studies are needed to define the efficacy of CXCR4, VCAM-1, or CS1-directed approaches for inhibiting the accumulation of B cells and possibly other inflammatory cells in the RA synovium in vivo. Such approaches may lead to new therapeutic avenues for patients with RA.
Acknowledgments
The authors are grateful to David L. Boyle and Li Yang for providing the FLS samples. Furthermore, the authors are grateful to James R. Feramisco and Brian A. Smith for their excellent assistance with immunofluorescence detection of SDF-1. This work was supported in part by grants from the NIH, and AR44850 (D.A. Carlson), AR45347, and the Deutsche Krebshilfe, Bonn, Germany (D-96-17136).
References
- 1.Firestein, G.S. 1996. Etiology and pathogenesis of rheumatoid arthritis. In Textbook of rheumatology. Volume 1. W.N. Kelley, E.D. Harris, S. Ruddy, and C.B. Sledge, editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 851–897.
- 2.Firestein GS, Yeo M, Zvaifler NJ. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995; 96:1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morales-Ducret J, et al. Alpha 4/beta 1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992; 149:1424–1431. [PubMed] [Google Scholar]
- 4.Oppenheimer-Marks N, Lipsky PE. Adhesion molecules in rheumatoid arthritis. Springer Semin Immunopathol. 1998; 20:95–114. doi: 10.1007/BF00832001. [DOI] [PubMed] [Google Scholar]
- 5.Shimaoka Y, et al. Nurse-like cells from bone marrow and synovium of patients with rheumatoid arthritis promote survival and enhance function of human B cells. J Clin Invest. 1998; 102:606–618. doi: 10.1172/JCI3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi E, et al. Establishment and characterization of nurse cell-like stromal cell lines from synovial tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1999; 42:221–228. doi: 10.1002/1529-0131(199902)42:2<221::AID-ANR3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Dechanet J, Merville P, Durand I, Banchereau J, Miossec P. The ability of synoviocytes to support terminal differentiation of activated B cells may explain plasma cell accumulation in rheumatoid synovium. J Clin Invest. 1995; 95:456–463. doi: 10.1172/JCI117685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merville P, et al. Bcl-2+ tonsillar plasma cells are rescued from apoptosis by bone marrow fibroblasts. J Exp Med. 1996; 183:227–236. doi: 10.1084/jem.183.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindhout E, et al. Fibroblast-like synoviocytes from rheumatoid arthritis patients have intrinsic properties of follicular dendritic cells. J Immunol. 1999; 162:5949–5956. [PubMed] [Google Scholar]
- 10.Hayashida K, Shimaoka Y, Ochi T, Lipsky PE. Rheumatoid arthritis synovial stromal cells inhibit apoptosis and up-regulate Bcl-xL expression by B cells in a CD49/CD29-CD106-dependent mechanism. J Immunol. 2000; 164:1110–1116. doi: 10.4049/jimmunol.164.2.1110. [DOI] [PubMed] [Google Scholar]
- 11.Wekerle H, Ketelsen UP, Ernst M. Thymic nurse cells. Lymphoepithelial cell complexes in murine thymuses: morphological and serological characterization. J Exp Med. 1980; 151:925–944. doi: 10.1084/jem.151.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyake K, Hasunuma Y, Yagita H, Kimoto M. Requirement for VLA-4 and VLA-5 integrins in lymphoma cells binding to and migration beneath stromal cells in culture. J Cell Biol. 1992; 119:653–662. doi: 10.1083/jcb.119.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiai H, et al. Symbiotic culture of mouse leukaemias: regulation of cell interaction by an activity of serum. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980; 32:261–279. doi: 10.1007/BF02889031. [DOI] [PubMed] [Google Scholar]
- 14.Boyle DL, Shi Y, Gay S, Firestein GS. Regulation of CS1 fibronectin expression and function by IL-1 in endothelial cells. Cell Immunol. 2000; 200:1–7. doi: 10.1006/cimm.2000.1610. [DOI] [PubMed] [Google Scholar]
- 15.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996; 184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999; 94:3658–3667. [PubMed] [Google Scholar]
- 17.Zamzami N, et al. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995; 181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000; 6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 19.Hanauer A, Mandel JL. The glyceraldehyde 3 phosphate dehydrogenase gene family: structure of a human cDNA and of an X chromosome linked pseudogene; amazing complexity of the gene family in mouse. EMBO J. 1984; 3:2627–2633. doi: 10.1002/j.1460-2075.1984.tb02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewson J, Bianchi A, Bradstock K, Makrynikola V, Gottlieb D. Ultrastructural changes during adhesion and migration of pre-B lymphoid leukaemia cells within bone marrow stroma. Br J Haematol. 1996; 92:77–87. doi: 10.1046/j.1365-2141.1996.00291.x. [DOI] [PubMed] [Google Scholar]
- 21.Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med. 1998; 187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1996; 93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Krenn V, Steinhauser G, Berek C. Plasma cell development in synovial germinal centers in patients with rheumatoid and reactive arthritis. J Immunol. 1999; 162:3053–3062. [PubMed] [Google Scholar]
- 24.Zvaifler NJ. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973; 16:265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]
- 25.Korganow AS, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999; 10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 26.Kouskoff V, et al. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996; 87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 27.Edwards JC, Leigh RD, Cambridge G. Expression of molecules involved in B lymphocyte survival and differentiation by synovial fibroblasts. Clin Exp Immunol. 1997; 108:407–414. doi: 10.1046/j.1365-2249.1997.4061306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reparon-Schuijt CC, et al. Regulation of synovial B cell survival in rheumatoid arthritis by vascular cell adhesion molecule 1 (CD106) expressed on fibroblast-like synoviocytes. Arthritis Rheum. 2000; 43:1115–1121. doi: 10.1002/1529-0131(200005)43:5<1115::AID-ANR22>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997; 94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forster R, et al. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998; 160:1522–1531. [PubMed] [Google Scholar]
- 31.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999; 10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 32.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996; 382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 33.Bowman EP, et al. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J Exp Med. 2000; 191:1303–1318. doi: 10.1084/jem.191.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Apuzzo M, et al. The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4. Eur J Immunol. 1997; 27:1788–1793. doi: 10.1002/eji.1830270729. [DOI] [PubMed] [Google Scholar]
- 35.Honczarenko M, et al. SDF-1 responsiveness does not correlate with CXCR4 expression levels of developing human bone marrow B cells. Blood. 1999; 94:2990–2998. [PubMed] [Google Scholar]
- 36.Fedyk ER, Ryyan DH, Ritterman I, Springer TA. Maturation decreases responsiveness of human bone marrow B lineage cells to stromal-derived factor 1 (SDF-1) J Leukoc Biol. 1999; 66:667–673. doi: 10.1002/jlb.66.4.667. [DOI] [PubMed] [Google Scholar]
- 37.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994; 76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 38.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996; 272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 39.Zvaifler NJ, et al. Pannocytes: distinctive cells found in rheumatoid arthritis articular cartilage erosions. Am J Pathol. 1997; 150:1125–1138. [PMC free article] [PubMed] [Google Scholar]
- 40.Marinova-Mutafchieve L, Taylor P, Funa K, Maini RN, Zvaifler NJ. Mesenchymal cells expressing bone morphogenetic protein receptors are present in the rheumatoid arthritis joint. Arthritis Rheum. 2000; 43:2046–2055. doi: 10.1002/1529-0131(200009)43:9<2046::AID-ANR16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Iwagami S, et al. Establishment and characterization of nurse cell-like clones from human skin. Nurse cell-like clones can stimulate autologous mixed lymphocyte reaction. J Immunol. 1994; 153:2927–2938. [PubMed] [Google Scholar]
- 42.Reparon-Schuijt CC, et al. Regulation of synovial B cell survival in rheumatoid arthritis by vascular cell adhesion molecule 1 (CD106) expressed on fibroblast-like synoviocytes. Arthritis Rheum. 2000; 43:1115–1121. doi: 10.1002/1529-0131(200005)43:5<1115::AID-ANR22>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 43.Burmester GR, Dimitriu-Bona A, Waters SJ, Winchester RJ. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983; 17:69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 44.Iyer VR, et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999; 283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 45.Pablos JL, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999; 155:1577–1586. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Postigo AA, et al. Increased binding of synovial T lymphocytes from rheumatoid arthritis to endothelial-leukocyte adhesion molecule-1 (ELAM-1) and vascular cell adhesion molecule-1 (VCAM-1) J Clin Invest. 1992; 89:1445–1452. doi: 10.1172/JCI115734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elices MJ, et al. Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J Clin Invest. 1994; 93:405–416. doi: 10.1172/JCI116975. [DOI] [PMC free article] [PubMed] [Google Scholar]