Abstract
The inhibitory effects of estrogen (17β-estradiol) on atherosclerosis have been well documented in numerous animal models, and epidemiological evidence supports this protective effect in humans. The detailed mechanisms for this protection are not understood, but most are thought to be mediated through estrogen receptors (ERs), of which two are known (ERα and ERβ). To investigate the role of ERα in the atheroprotective effect of 17β-estradiol (E2), we ovariectomized female mice that lack apoE (AAee) or lack both apoE and ERα (ααee), and treated half of them with E2 for three months. E2 treatment of ovariectomized AAee females dramatically reduced the size of the lesions as well as their histological complexity. Plasma cholesterol was significantly reduced in this group, although the observed extent of protection by E2 was greater than could be explained solely by the change in lipid levels. In contrast, E2 treatment of ovariectomized ααee females caused minimal reduction in lesion size and no reduction in total plasma cholesterol compared with ααee mice without E2, demonstrating that ERα is a major mediator of the atheroprotective effect of E2. Nevertheless, E2 treatment significantly reduced the complexity of plaques in the ααee females, although not to the same degree as in AAee females, suggesting the existence of ERα-independent atheroprotective effects of E2.
Introduction
Coronary heart disease (CHD) is the leading cause of death in the Western world (1). Epidemiological studies indicate that premenopausal women are protected, but that the risk of CHD rises sharply after menopause (2). This protection is thought to be in part through endogenous estrogen (17β-estradiol, or E2) as the risk is significantly reduced by estrogen replacement therapy (2), although a recently completed clinical trial using hormone replacement therapy did not show protection in women with preexisting CHD (3). Exogenous E2 clearly inhibits lesion progression in numerous animal models of atherosclerosis including the apoE-deficient mouse (Apoe–/–) (4–8). While E2 causes favorable alterations in lipoprotein metabolism, these changes appear to account for not more than 50% of the protection in humans (9). In addition, several studies in animals show full atheroprotection at E2 doses that do not alter the lipid profile (2). Thus attention has focused on the direct effects of E2 on the vessel wall and on the processes involved in the inflammatory and fibroproliferative components of atherosclerosis including: endothelial permeability to LDL (10), LDL oxidation (11, 12), cytokine and cell adhesion molecule expression (13–16), macrophage cholesterol homeostasis (17), vascular smooth muscle cell and neointimal proliferation and migration (18, 19), calcification (20, 21) and platelet adhesion and aggregation (22).
The long-term effects of E2 are generally ascribed to transcriptional modulation of target genes through estrogen receptors (ERs). These receptors are members of the steroid hormone receptor superfamily of ligand-activated transcription factors (23) that modulate gene expression directly through estrogen response elements in the promoter regions of target genes, or indirectly through interaction with transcription factors, coactivators, and transcription complexes, such as activating protein-1 (AP-1) (23–26). In addition, rapid, nongenomic, vasodilatory effects of E2 have been reported that may also involve ERs (27).
Two estrogen receptors, ERα and ERβ, have been characterized (28, 29). They are encoded in two separate genes (Esr1 and Esr2), are distinct structurally and functionally, and have tissue expression patterns that overlap but are not identical (29–31). Both receptors are expressed in cell types important in atherogenesis, including endothelial cells (32) and vascular smooth muscle cells (33, 34). Estrogen receptors have also been demonstrated in macrophages and T lymphocytes (35, 36). Determining the relative contribution of the two receptors to atheroprotection is of considerable interest in its own right, as well as in relation to the potential use of pharmaceutical agents specific to each receptor type.
Considerable success has been achieved in demonstrating the atheroprotective effect of E2 in Apoe–/– mice (7, 8) and in LDL-receptor deficient mice (37). For example, dose-response studies with E2 continuously administered to ovariectomized apoE-deficient mice have demonstrated that high physiological levels result in near complete inhibition of atherosclerotic lesion progression. We have therefore employed a similar study design to compare the effects of E2 treatment on the spontaneous development of atherosclerotic lesions in ovariectomized Apoe–/– mice (ee) that have or do not have ERα (AAee versus ααee). We find that the atheroprotection provided by E2 administration is substantially abrogated in the ααee mice, which lack ERα, but atheroprotection by non-ERα mechanisms is still clearly demonstrable.
Methods
Mouse experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Generation and use of AAee and ααee mice.
Mice heterozygous for an insertional mutation in ERα (Aα) and Apoe–/– mice (ee), each backcrossed over 99% to C57BL6/J, were intercrossed to yield males and females heterozygous for both mutations (AαEe). These double heterozygotes were further mated with ee mice to generate mice heterozygous for ERα and homozygous for the insertional mutation in ApoE (Aαee). Intercrossing the Aαee mice produced mice lacking ERα (ααee) and littermate controls having intact ERα (AAee). At day 30, females were weighed, ovariectomized, and randomly implanted subcutaneously with either pellets designed to release E2 at 6 μg/day for 60 days or a placebo control (C) (Innovative Research of America, Sarasota, Florida, USA). The four study groups were AAee with E2 (AAee/E2; n = 17), ααee with E2 (ααee/E2; n = 14), and their respective control groups (AAee/C; n = 17 and ααee/C; n = 11). Pellets were replaced 60 days after surgery and the mice were sacrificed 30 days later (day 120, or 4 months of age) after a 2- to 4-hour fast. Total body weight and uterine weight were recorded. Four of 17 AAee/E2 mice died during the third month of treatment and the data from another four mice were excluded for other reasons (see Results), leaving 9 AAee/E2 mice. Mice were maintained in specific-pathogen-free conditions on standard mouse chow (Prolab Isopro RMH 3000; PMI Nutrition International, Brentwood, Missouri, USA).
Serum E2 concentration.
E2 levels were assayed on plasma from individual mice obtained at sacrifice using a radioimmunoassay kit for E2 and following the manufacturer’s instructions (Diagnostic Systems Laboratories Inc., Webster, Texas, USA).
Atherosclerotic lesion analysis.
Hearts were perfusion fixed with 4% paraformaldehyde under physiological pressure (pH 7.4). The proximal aorta and the part of the heart containing the aortic root were removed, embedded, sectioned, and stained with hematoxylin and eosin (H&E) and Sudan IV (Fisher Scientific, Fair Lawn, New Jersey, USA) as described (38). Lesion size was measured using NIH 1.59 Imaging Software from four sections chosen by strict anatomical criteria. The average of the four sections was taken as the lesion size of each animal and logarithmically transformed for statistical analyses. The plaques in the aortic sinus were morphologically graded in a blinded fashion by R.L. Reddick, an experienced cardiovascular pathologist, for lesion complexity using as parameters absence or presence of foam cells, fully formed or developing fibrous cap, calcifications, cholesterol clefts, acellular cores, inflammatory cells, and medial extensions.
Plasma lipid analysis.
Total cholesterol, HDL, and triglycerides were measured colorimetrically (Sigma-Aldrich, Milwaukee, Wisconsin, USA) on plasma collected at sacrifice. HDL cholesterol levels were determined after precipitating the apoB-containing particles. Plasma samples were stored at –20°C until assayed.
Statistics.
Statistical analyses were performed with the PC-based software package SYSTAT 5.0 (SPSS, Chicago, Illinois, USA), JMP (SAS Institute Inc., Cary, North Carolina, USA), and LogXact 2.0 (Cytel Corp., Cambridge, Massachusetts, USA). Data were analyzed with a factorial ANOVA, using genotype (AAee versus ααee) and E2 status (E2 versus C) as the two grouping variables. Further analyses to interpret significant main effects and/or interactions were conducted using the Tukey-Kramer honestly significant difference multiple comparison procedure. Nonparametric analyses of lesion histopathological characteristics were conducted with multiple logistic regression. Comparisons of females with E2 versus C across each genotype were with Fisher’s exact test using the Bonferoni procedure to correct for multiple comparisons. Correlations between different parameters were analyzed using simple linear regression. P values less than 0.05 were considered statistically significant for all comparisons.
Results
Experimental design.
The experimental animals used for investigating the role of ERα in atheroprotection by E2 were female apoE-deficient mice with or without ERα (AAee or ααee) on a C57BL6/J genetic background. However, intact ERα-deficient females have plasma E2 levels three times higher than wild-type mice as a consequence of their lack of an ERα-dependent functional negative feedback mechanism (39) and also have increased plasma levels of testosterone, which inhibits lesion progression in Apoe–/– mice (8). We therefore chose, as a means of removing these confounding variables, to ovariectomize all experimental animals and replace their endogenous E2 with subcutaneously implanted hormone-releasing pellets at 30 days of age. Pellets which nominally release 6 μg of E2 a day and attain plasma levels within the range of animals at peak estrus (7) were chosen, based on previously published work demonstrating that this dosage was the lowest able to reproducibly lower plasma lipids and plaque size in Apoe–/– mice (7, 8).
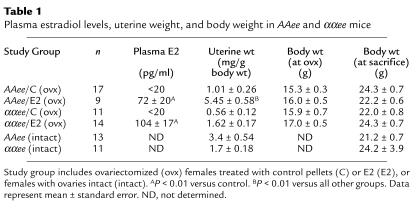
The data presented in Table 1 demonstrate that we were successful in achieving the desired hormonal status of the experimental animals. Ovariectomy reduced plasma E2 to below detection limits, and the E2 replacement attained plasma levels in the high physiological range. On gross inspection, the uteri were smaller and atrophic in AAee/C, ααee/C, and ααee/E2 females compared with AAee mice with intact ovaries, while uteri in AAee/E2 mice were enlarged. Uterine weight in the ovariectomized females was increased about fivefold by E2 treatment in AAee mice (P < 0.0005) and was 60% increased over intact AAee females (P < 0.005). The uteri in ααee/E2 females tended to be larger than in ααee/C (P = 0.075) but were similar to the uterine size of ααee females with intact ovaries. There were no differences in total body weight between groups at ovariectomy or at the end of the study. ERα-deficient mice have been reported to gain more body weight than wild-type controls, but the difference is not significant until after 4 months of age (39).
Table 1.
Plasma estradiol levels, uterine weight, and body weight in AAee and ααee mice
All of the ααee/E2 and control females completed the study. E2 treatment in some of the AAee females, however, was associated with decreased survival (four animals) and urinary and reproductive tract pathology (enlarged bladder, cystitis, hydronephrosis, and uterine hyperplasia and endometritis; four animals), most likely secondary to an increased susceptibility to infection. Data collected from these mice were excluded from the final analysis (final n = 9), though inclusion of the data did not alter the mean values for lesion size or lipid profiles.
ERα is required for the major atheroprotective effect of E2.
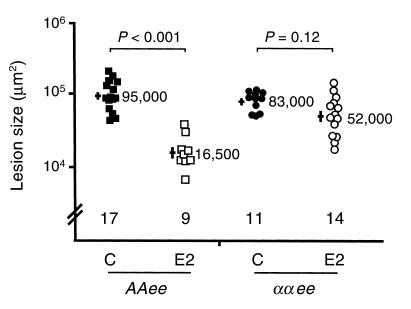
To determine the role of ERα in the inhibition of atherosclerotic progression by E2, lesion size in the proximal aorta was measured at 4 months of age in AAee and ααee females ovariectomized at 1 month of age and treated with or without E2 from the time of ovariectomy. As expected, exogenous E2 was highly effective in inhibiting lesion progression in AAee females. Lesions in AAee/E2 females were more than 80% smaller than those of AAee/C females (P < 0.001) (Figure 1). In contrast, E2 only minimally reduced lesion size in ααee mice. Mean lesion size in ααee/E2 females was 35% less than the mean lesion size in ααee/C females, but the effect did not reach significance (P = 0.12). Interaction between genotype and hormone treatment was highly significant by two-way ANOVA (P < 0.0001), demonstrating that ERα is important for the E2 effect. Furthermore, while all E2-deficient females and ααee/E2 females had at least one plaque in the descending aorta that was readily visible under a dissecting microscope, the AAee/E2 mice had none, correlating with the markedly reduced lesion sizes in the aortic sinus. Thus, ERα is required for a major part, if not all, of the E2-mediated reduction in lesion size.
Figure 1.
Atherosclerotic lesion size expressed on a log scale in ovariectomized 4-month-old AAee and ααee females after 3 months of treatment with control pellets (C) (filled squares and circles, respectively) or with E2 pellets (E2) (open squares and circles, respectively). Each point represents one mouse. Bars on the left represent the logarithmic mean ± standard error with mean lesion size (in μm2) shown on the right.
E2 reduces advanced-lesion characteristics in the absence of ERα.
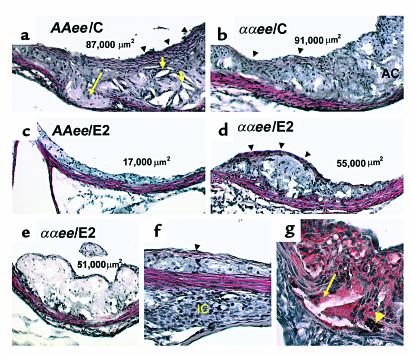
Figure 2 shows examples of atherosclerotic lesions chosen from females whose mean lesion size was near the mean for its respective group (actual lesion size for each example is shown in panels). The lesions from AAee/C (Figure 2a) and ααee/C females (Figure 2b), which are not distinguishable, are substantially more complex than lesions from AAee/E2 females (Figure 2c), which are essentially fatty streaks containing lipid-laden foam cells and have few advanced characteristics. Lesions in ααee/E2 females (Figure 2, d and e) are also reduced by the E2 treatment not only in size but also in complexity compared with the untreated ααee/C females (Figure 2b).
Figure 2.
H&E (a–f) and Sudan IV (g) staining of representative lesions from individual mice, 10× (a–e) and 20× (f and g) magnification. Numbers in lumen (top) indicate the lesion size for each example. Lesions from E2-deficient AAee/C (a) and ααee/C (b) females have multiple features of complex lesions including fibrous caps (black arrowheads), acellular cores (AC) with cholesterol clefts (short yellow arrows), and medial extension into the adventitia (long yellow arrow). Note that the lesion in the AAee/E2 female (c) is essentially a fatty streak composed of lipid-laden macrophages (foam cells). A lesion from an ααee/E2 female (d) has a fibrous cap (black arrowheads) in addition to many foam cells. A second example of a lesion from an ααee/E2 female (e) is composed primarily of foam cells. In (f), a focal collection of inflammatory cells (IC) is present adjacent to a small lesion that contains foam cells and a thin fibrous cap (black arrowhead) in an ααee/C female. Sudan IV staining of the base of a lesion found in an ααee/C female (g) illustrate examples of fragmentation of the media, calcifications (yellow arrowhead), and extension of the lesion into the adventitia (yellow arrow). Lipid-laden foam-cell macrophages are stained red.
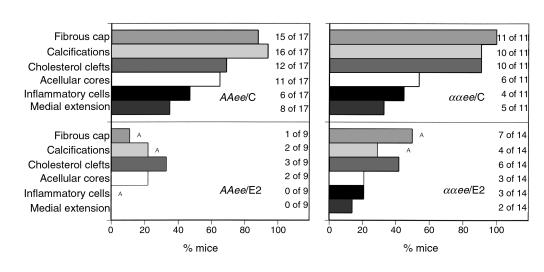
To analyze the effects of E2 and ERα on the development of lesion complexity, tabulations of lesion characteristics from the aortic sinus were conducted in a blinded fashion. Figure 3 presents the percentage of mice in each group that had evidence of advanced lesions (absence or presence), including developing or well-formed fibrous caps (vascular smooth muscle cells and extracellular matrix deposition), calcifications, cholesterol clefts (extracellular crystallized cholesterol), acellular cores, inflammatory cells in the adventitia and plaque shoulders, and medial extension (vessel wall involvement by lesion) (see Figure 2 for specific examples). While the lesions in all four groups of mice contained lipid-laden foam cells (by Sudan IV and H&E staining, see Figure 2f for example), the frequencies of advanced lesion characteristics varied across the different groups. Thus when AAee/E2 females are compared with AAee/C females, the frequencies of all advanced characteristics were reduced, although only reductions in fibrous cap formation, inflammatory cells, and calcifications reached significance (P < 0.05). Similarly E2 treatment reduced the frequencies of all the advanced lesion characteristics in ααee/E2 females compared with their ααee/C controls (P < 0.05 for fibrous cap and calcifications only). The effect of E2 treatment was statistically significant by multiple logistic regression for all characteristics (fibrous caps, P < 0.00005; calcifications, P < 0.00005; cholesterol clefts, P < 0.02; acellular cores, P < 0.02; inflammatory cells, P < 0.02) except medial extension (P = 0.095). However, interaction between genotype and E2 treatment was not statistically significant, nor was there a statistically demonstrable effect of genotype. These results show that E2 reduces lesion complexity, but that the effect is probably independent of ERα. However, we note that lesion size and complexity are not entirely independent measures of atherosclerotic progression, and factors that affect the growth of the lesion at any stage are likely to have an impact on the development of complex features.
Figure 3.
Histopathological lesion characterization of AAee (left) and ααee (right) females treated with control pellet (C) or E2 pellets (E2) shown as percentage of mice with the presence of the characteristic. ASignificant differences (P < 0.05). The number of animals scoring positively for each characteristic are indicated.
E2 alters plasma lipids in AAee but not in ααee females.
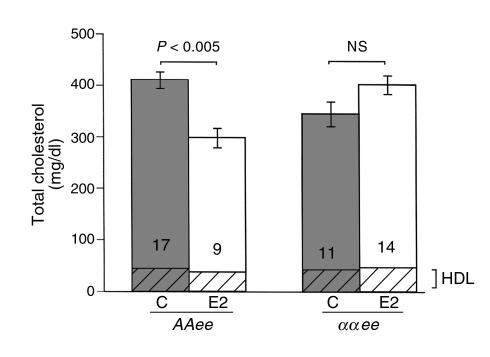
To determine the role of ERα in lipid metabolism, fasting plasma was obtained at sacrifice from E2-treated and control ovariectomized AAee and ααee animals on normal chow and assayed for total cholesterol and HDL (Figure 4). Fasting plasma total cholesterol was significantly reduced by exogenous E2 in the AAee females (P < 0.005), but not in the ααee females (P = 0.17). Interaction between genotype and E2 treatment was highly significant by two-way ANOVA (P < 0.001), indicating that significant cholesterol reduction by E2 requires ERα. Fast performance liquid chromatography demonstrated that the reduction of total cholesterol by E2 was a result of decreases in VLDL, IDL, and LDL range fractions (not shown). Plasma HDL–cholesterol (the cross-hatched bases of the bars in Figure 4) was unchanged across all groups.
Figure 4.
Total cholesterol in plasma of AAee and ααee mice after 3 months of treatment with control pellets (C) or E2 pellets (E2). Vertical bars represent ± SEM. Filled bars, C; open bars, E2; cross-hatched bases of bars, HDL.
In the AAee group (/C and /E2 combined), but not the ααee group, lesion area was positively correlated with plasma total cholesterol (r2 = 0.51, P < 0.0001 and r2 = 0.06, P = 0.25, respectively). This was expected since E2 was able to reduce both lesion size and total cholesterol only in AAee/E2 females. Subgroup analysis revealed a positive correlation between lesion size and plasma total cholesterol only in AAee/E2 females (r2 = 0.47, P = 0.041). These results should be interpreted cautiously because E2 release in individual mice may vary, although it could suggest a small reduction in size consequent to small reductions in total cholesterol. Comparison of plasma total cholesterol between individual females of the AAee/C and AAee/E2 groups demonstrated an overlap in about half of each group (AAee/C = 9 and AAee/E2 = 5) within the 300–400 mg/dl range. Total cholesterols above 400 mg/dl included only AAee/C females and cholesterols below 300 mg/dl included only AAee/E2 females. In this midrange group of total cholesterol values in control and E2-treated AAee females, there was no overlap in lesion size. This demonstrates that a substantial reduction in lesion size occurs with E2 treatment in ERα-intact females, and that this effect cannot entirely be explained by the reduction in plasma levels of total cholesterol.
ERα and E2 are required for xanthoma formation in Apoe–/– mice.
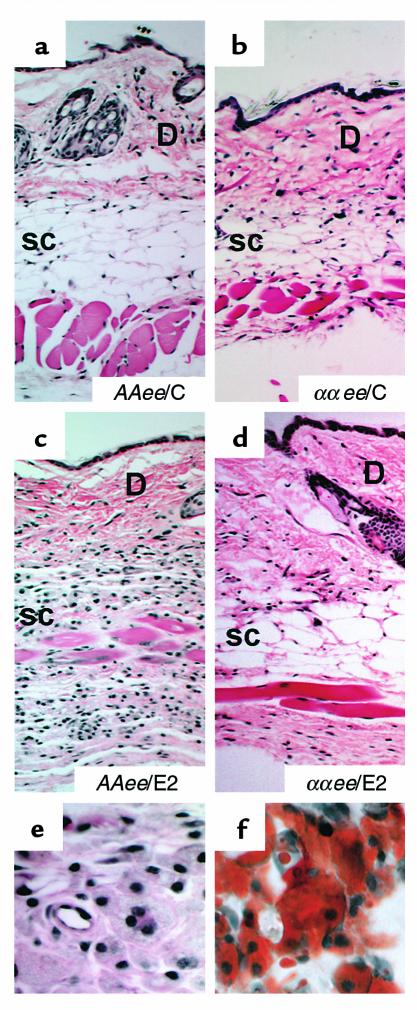
At sacrifice, we observed that the dorsal skin of all the E2-treated Apoe–/– females with wild-type ERα (AAee/E2) was difficult to remove and frequently required sharp dissection. Histological sections of the skin from the AAee females given E2 (Figure 5, c and e), when compared with AAee/C (Figure 5a) and ααee/C (Figure 5b) females not treated with E2, demonstrated subcutaneous infiltration of lipid-laden macrophages. The foam cells stained intensely with Sudan IV (Figure 5f) and had obliterated most of the subcutaneous fat. In general, the ααee/E2 females (Figure 5d) did not show this phenotype, although two of the 14 showed minor collections of foam cells in the flank areas (not shown). These observations demonstrate that the absence of ERα prevents the development of xanthomas in ovariectomized Apoe–/– females treated with E2.
Figure 5.
Histological sections of dorsal skin at sacrifice. H&E stain; 10×. (a) AAee/C; (b) ααee/C; (c) AAee/E2; (d) ααee/E2; (e) 40× magnification of AAee/E2 skin; (f) Sudan IV stain of AAee/E2 skin, 40× magnification. Note the infiltration of lipid-laden macrophages or foam cells (xanthoma formation) in the subcutaneous fat (SC) of AAee/E2 skin. The dermis (D) is positioned at the top.
Discussion
The genetic and treatment studies described here demonstrate that continuous administration of E2 to ovariectomized ααee mice, which lack ERα, largely fails to reduce the size of their atherosclerotic lesions compared with the effects of E2 in ovariectomized AAee mice, which have the receptor. This establishes ERα as a major mediator of the atheroprotective effects of exogenous E2 in atherosclerosis-prone mice. Our histological analysis reveals that the complexity of the lesions in the ααee mice is nevertheless reduced by E2 treatment, demonstrating that some protective effects of E2 are still present in the absence of ERα. Finally, we demonstrate that the cholesterol-lowering effects of E2 in ovariectomized Apoe–/– mice is entirely dependent on the presence of ERα.
Our experiments provide some clues to the stage at which the ERα protection occurs. Thus our observation that the lesions in the AAee/E2 mice at 4 months of age have rarely progressed beyond small and uncomplicated fatty streaks indicates that E2 must be affecting an early stage of atherogenesis. A possible factor in the protection is nitric oxide, since multiple studies have shown that estrogen increases the synthesis of nitric oxide through increased expression and activity of endothelial nitric oxide synthase (27, 40, 41). Previous work by Rubanyi et al. (42) has also shown that the basal release of nitric oxide is compromised in ERα-deficient male mice, implicating the importance of ERα for this effect. It is likely that nitric oxide can scavenge harmful oxidants in the subendothelial space and thereby protect vascular cells from injuries that activate the cascade of atherosclerotic processes (43, 44). Protection from injurious oxidants would be expected to delay the formation of fatty streaks and so reduce their sizes at a defined time.
The role of ERα on the effects of E2 in fatty-streak formation has not been directly investigated, but studies using estrogen receptor antagonists have suggested receptor-dependent effects of E2 on mechanisms that contribute to macrophage foam-cell accumulation. Thus E2 decreases mRNA levels of vascular adhesion molecules (13) and monocyte chemoattractant protein 1 (15), both of which effects would likely reduce the recruitment of macrophages to the intimal area and their activation. Furthermore, Tomita et al. (45) have demonstrated that E2 stimulates neutral cholesterol esterase activity, thereby inhibiting the accumulation of cholesterol esters in macrophages, a key process of foam-cell formation. Finally, the ERα-dependent reduction of plasma cholesterol may still be contributing to a delay in atherosclerosis development in AAee/E2 mice, although the extent of protection is clearly greater than can be accounted for by the lipid changes, as suggested by our data and previously shown by others (2, 7, 8, 37).
The effect of E2 on the complexity of lesions requires comment. Previous investigators have shown that E2 inhibits smooth muscle cell proliferation, a component of fibrous cap formation, both in vitro (46, 47) and in various animal models of vascular injury in vivo (18, 48–50). E2 also reduces connective tissue deposition (51, 52), and possibly inhibits calcium phosphate deposition in the atherosclerotic plaque (21). Furthermore, Tse et al. (53) have demonstrated that E2 prevents calcified cartilaginous metaplasia in the aorta of streptozotocin-treated Apoe–/– mice. How much of each of these effects of E2 is mediated by the estrogen receptors remains to be determined. Though ERα is clearly a major mediator of atheroprotection by E2, there remains a demonstrable ERα-independent protective effect of E2 seen mainly as a decrease in plaque complexity in ααee females. This protection may be mediated by ERβ or by a yet-unknown ER, or it may be through a receptor-independent action of E2 (27, 40, 41). One possibility is that E2 may be inhibiting early steps of lesion formation even in the absence of ERα. Since lesion complexity is generally correlated with lesion size, a delay in lesion formation would also delay the development of advanced lesion characteristics. Receptor-independent antioxidant properties of E2 are possible factors (40), but recent evidence suggests that direct antioxidant actions of physiological concentrations of E2 are unlikely (54). Changes in plasma cholesterol levels can be excluded as a non-ERα mechanism, since we find that E2 does not lower total cholesterol in the ααee females.
A second possibility is that, by an ERα-independent mechanism, E2 may play a role in a later stage of atherosclerosis lesion progression. Our finding of a significant reduction of fibrous caps and calcification in the plaques developing in ααee mice treated with E2 compared with the ααee control mice could be secondary to a general delay in lesion formation, or it could be due to a contribution of ERα-independent mechanisms to these later processes. In this context, it is notable that E2 reduces the proliferative response of smooth muscle cells to carotid injury in mice even in the absence of either ERα or ERβ (19, 55). Further study is needed to determine whether this effect of E2 is receptor-independent or is due to overlapping contributions of ERα and ERβ.
E2 alters the lipid profile in both animals and humans in part by regulating lipoprotein binding and clearance in the liver (40) where ERα is the predominant receptor (30, 39). In C57BL/6J mice, estrogen increases apoB, decreases apoAI (56), and increases apoE expression through an ERα-dependent mechanism (57). Our data demonstrate that E2 does not lower total plasma cholesterol in Apoe–/– mice when ERα is absent.
Although E2 treatment was highly effective in reducing atherosclerosis in AAee mice, it had some marked adverse effects at the dose we employed. Thus, in addition to an overt enlargement of uteri, we found evidence of urinary obstruction in 24%, and death in another 24% of AAee mice treated with E2. Marsh et al. (37) have reported renal tract obstruction in LDL receptor–deficient mice treated with a similar dose of E2. Our present experiments show that ERα mediates the adverse effects of E2 in Apoe–/– mice, because none of the ααee mice treated with E2 exhibited urinary or reproductive tract abnormalities. We also observed a striking infiltration of lipid-laden macrophages into the dorsal subcutaneous fat layer in all AAee mice treated with E2, even though E2 inhibits foam-cell accumulation in the aorta of AAee mice. Only two of 14 ααee/E2 mice demonstrated any infiltration of subcutaneous macrophages, and the extent was considerably less than in the AAee mice. We conclude that the development of xanthomas in our animals requires E2 and that ERα is the primary mediator of this E2-induced collection of macrophages in the fat of the skin. It is therefore conceivable that E2 induces alterations in the expression of cytokines from subcutaneous tissues that induce extravasation of monocytes. Hyperlipidemia is associated with cutaneous foam-cell formation (xanthoma) in humans, and Maeda and colleagues have previously shown that cholesterol feeding exacerbates planary xanthoma in the skin of Apoe–/– mice (58). Whether E2-induced xanthomatosis is related to the cholesterol-induced xanthoma remains to be determined.
In conclusion, our data show a dramatic loss of the atheroprotection induced by E2 in Apoe–/– mice lacking functional ERα, and demonstrate that ERα is the major mediator in this protection. The role played by the second receptor, ERβ, in the remaining protective effects of E2 remains to be established; mice lacking ERβ (59) should facilitate this investigation. Understanding the relative roles of the two known estrogen receptors and of other factors should further the development of improved therapies for preventing CHD in postmenopausal women.
Acknowledgments
The authors would like to thank John Couse, Dominic Ciavatta, Josh Knowles, Maggie Hicken, and Myron Hinsdale for helpful comments; Virginia Godfrey, Scott Trasti, Shin Ja Kim, and Jeffrey Moyer for technical assistance; Cindy Lawler and Thomas J. Prihoda for statistical assistance; and Chet Hodgin, Steve Kizer, and William Blythe for their encouragement and support. This work was supported by NIH grants HL42630 (N. Maeda), GM-20069 (O. Smithies), and HL-03470 (J.H. Krege).
References
- 1.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995; 57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 2.Chae CU, Ridker PM, Manson JE. Postmenopausal hormone replacement therapy and cardiovascular disease. Thromb Haemost. 1997; 78:770–780. [PubMed] [Google Scholar]
- 3.Hulley S, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998; 280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 4.Adams MR, et al. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990; 10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 5.Haarbo J, Leth-Espensen P, Stender S, Christiansen C. Estrogen monotherapy and combined estrogen-progestogen replacement therapy attenuate aortic accumulation of cholesterol in ovariectomized cholesterol-fed rabbits. J Clin Invest. 1991; 87:1274–1279. doi: 10.1172/JCI115129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holm P, Stender S, Andersen HO, Hansen BF, Nordestgaard BG. Antiatherogenic effect of estrogen abolished by balloon catheter injury in cholesterol-clamped rabbits. Arterioscler Thromb Vasc Biol. 1997; 17:1504–1511. doi: 10.1161/01.atv.17.8.1504. [DOI] [PubMed] [Google Scholar]
- 7.Bourassa PA, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 1996; 93:10022–10027. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhage R, et al. 17 beta-estradiol prevents fatty streak formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1997; 17:2679–2684. doi: 10.1161/01.atv.17.11.2679. [DOI] [PubMed] [Google Scholar]
- 9.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991; 265:1861–1867. [PubMed] [Google Scholar]
- 10.Wagner JD, et al. Estrogen and progesterone replacement therapy reduces low density lipoprotein accumulation in the coronary arteries of surgically postmenopausal cynomolgus monkeys. J Clin Invest. 1991; 88:1995–2002. doi: 10.1172/JCI115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sack MN, Rader DJ, Cannon RO., III Oestrogen and inhibition of oxidation of low-density lipoproteins in postmenopausal women. Lancet. 1994; 343:269–270. doi: 10.1016/s0140-6736(94)91117-7. [DOI] [PubMed] [Google Scholar]
- 12.Keaney JF, Jr, et al. 17 beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation. 1994; 89:2251–2259. doi: 10.1161/01.cir.89.5.2251. [DOI] [PubMed] [Google Scholar]
- 13.Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest. 1996; 98:36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakai K, et al. Estradiol-17 beta regulates the induction of VCAM-1 mRNA expression by interleukin-1 beta in human umbilical vein endothelial cells. Life Sci. 1994; 54:L221–L227. doi: 10.1016/0024-3205(94)00630-x. [DOI] [PubMed] [Google Scholar]
- 15.Frazier-Jessen MR, Kovacs EJ. Estrogen modulation of JE/monocyte chemoattractant protein-1 mRNA expression in murine macrophages. J Immunol. 1995; 154:1838–1845. [PubMed] [Google Scholar]
- 16.Kovacs EJ, et al. Estrogen regulation of JE/MCP-1 mRNA expression in fibroblasts. J Leukoc Biol. 1996; 59:562–568. doi: 10.1002/jlb.59.4.562. [DOI] [PubMed] [Google Scholar]
- 17.St. Clair RW. Effects of estrogens on macrophage foam cells: a potential target for the protective effects of estrogens on atherosclerosis. Curr Opin Lipidol. 1997; 8:281–286. doi: 10.1097/00041433-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Foegh ML, Asotra S, Howell MH, Ramwell PW. Estradiol inhibition of arterial neointimal hyperplasia after balloon injury. J Vasc Surg. 1994; 19:722–726. doi: 10.1016/s0741-5214(94)70047-8. [DOI] [PubMed] [Google Scholar]
- 19.Iafrati MD, et al. Estrogen inhibits the vascular injury response in estrogen receptor alpha-deficient mice. Nat Med. 1997; 3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 20.Demer LL. A skeleton in the atherosclerosis closet. Circulation. 1995; 92:2029–2032. doi: 10.1161/01.cir.92.8.2029. [DOI] [PubMed] [Google Scholar]
- 21.Balica M, Bostrom K, Shin V, Tillisch K, Demer LL. Calcifying subpopulation of bovine aortic smooth muscle cells is responsive to 17 beta-estradiol. Circulation. 1997; 95:1954–1960. doi: 10.1161/01.cir.95.7.1954. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblum WI, et al. Effects of estradiol on platelet aggregation in mouse mesenteric arterioles and ex vivo. Thromb Res. 1985; 39:253–262. doi: 10.1016/0049-3848(85)90220-8. [DOI] [PubMed] [Google Scholar]
- 23.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995; 83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umayahara Y, et al. Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. J Biol Chem. 1994; 269:16433–16442. [PubMed] [Google Scholar]
- 25.Katzenellenbogen JA, O’Malley BW, Katzenellenbogen BS. Tripartite steroid hormone receptor pharmacology: interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol Endocrinol. 1996; 10:119–131. doi: 10.1210/mend.10.2.8825552. [DOI] [PubMed] [Google Scholar]
- 26.Klein-Hitpass L, Schwerk C, Kahmann S, Vassen L. Targets of activated steroid hormone receptors: basal transcription factors and receptor interacting proteins. J Mol Med. 1998; 76:490–496. doi: 10.1007/s001090050243. [DOI] [PubMed] [Google Scholar]
- 27.Mendelsohn ME, Karas RH. Estrogen and the blood vessel wall. Curr Opin Cardiol. 1994; 9:619–626. doi: 10.1097/00001573-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Walter P, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA. 1985; 82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996; 93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuiper GG, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997; 138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 31.Paech K, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997; 277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 32.Lindner V, et al. Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ Res. 1998; 83:224–229. doi: 10.1161/01.res.83.2.224. [DOI] [PubMed] [Google Scholar]
- 33.Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation. 1994; 89:1943–1950. doi: 10.1161/01.cir.89.5.1943. [DOI] [PubMed] [Google Scholar]
- 34.Register TC, Adams MR. Coronary artery and cultured aortic smooth muscle cells express mRNA for both the classical estrogen receptor and the newly described estrogen receptor beta. J Steroid Biochem Mol Biol. 1998; 64:187–191. doi: 10.1016/s0960-0760(97)00155-6. [DOI] [PubMed] [Google Scholar]
- 35.Gulshan S, McCruden AB, Stimson WH. Oestrogen receptors in macrophages. Scand J Immunol. 1990; 31:691–697. doi: 10.1111/j.1365-3083.1990.tb02820.x. [DOI] [PubMed] [Google Scholar]
- 36.Suenaga R, Evans MJ, Mitamura K, Rider V, Abdou NI. Peripheral blood T cells and monocytes and B cell lines derived from patients with lupus express estrogen receptor transcripts similar to those of normal cells. J Rheumatol. 1998; 25:1305–1312. [PubMed] [Google Scholar]
- 37.Marsh MM, Walker VR, Curtiss LK, Banka CL. Protection against atherosclerosis by estrogen is independent of plasma cholesterol levels in LDL receptor-deficient mice. J Lipid Res. 1999; 40:893–900. [PubMed] [Google Scholar]
- 38.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992; 258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 39.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999; 20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 40.Nathan L, Chaudhuri G. Estrogens and atherosclerosis. Annu Rev Pharmacol Toxicol. 1997; 37:477–515. doi: 10.1146/annurev.pharmtox.37.1.477. [DOI] [PubMed] [Google Scholar]
- 41.Joswig M, Hach-Wunderle V, Ziegler R, Nawroth PP. Postmenopausal hormone replacement therapy and the vascular wall: mechanisms of 17 beta-estradiol’s effects on vascular biology. Exp Clin Endocrinol Diabetes. 1999; 107:477–487. doi: 10.1055/s-0029-1232556. [DOI] [PubMed] [Google Scholar]
- 42.Rubanyi GM, et al. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest. 1997; 99:2429–2437. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996; 20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 44.Heinecke JW. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis. 1998; 141:1–15. doi: 10.1016/s0021-9150(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 45.Tomita T, et al. Inhibition of cholesterylester accumulation by 17 beta-estradiol in macrophages through activation of neutral cholesterol esterase. . Biochim Biophys Acta . 1996; 1300:210–218. doi: 10.1016/0005-2760(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 46.Bhalla RC, Toth KF, Bhatty RA, Thompson LP, Sharma RV. Estrogen reduces proliferation and agonist-induced calcium increase in coronary artery smooth muscle cells. Am J Physiol. 1997; 272:H1996–H2003. doi: 10.1152/ajpheart.1997.272.4.H1996. [DOI] [PubMed] [Google Scholar]
- 47.Dai-Do D, et al. 17 beta-estradiol inhibits proliferation and migration of human vascular smooth muscle cells: similar effects in cells from postmenopausal females and in males. Cardiovasc Res. 1996; 32:980–985. [PubMed] [Google Scholar]
- 48.Chen SJ, Li H, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996; 93:577–584. doi: 10.1161/01.cir.93.3.577. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan TR, Jr, et al. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest. 1995; 96:2482–2488. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White CR, et al. Estrogen restores endothelial cell function in an experimental model of vascular injury. Circulation. 1997; 96:1624–1630. doi: 10.1161/01.cir.96.5.1624. [DOI] [PubMed] [Google Scholar]
- 51.Register TC, Adams MR, Golden DL, Clarkson TB. Conjugated equine estrogens alone, but not in combination with medroxyprogesterone acetate, inhibit aortic connective tissue remodeling after plasma lipid lowering in female monkeys. Arterioscler Thromb Vasc Biol. 1998; 18:1164–1171. doi: 10.1161/01.atv.18.7.1164. [DOI] [PubMed] [Google Scholar]
- 52.Frazier-Jessen MR, Mott FJ, Witte PL, Kovacs EJ. Estrogen suppression of connective tissue deposition in a murine model of peritoneal adhesion formation. J Immunol. 1996; 156:3036–3042. [PubMed] [Google Scholar]
- 53.Tse J, et al. Accelerated atherosclerosis and premature calcified cartilaginous metaplasia in the aorta of diabetic male Apo E knockout mice can be prevented by chronic treatment with 17 beta-estradiol. Atherosclerosis. 1999; 144:303–313. doi: 10.1016/s0021-9150(98)00325-6. [DOI] [PubMed] [Google Scholar]
- 54.Santanam N, et al. Estradiol as an antioxidant: incompatible with its physiological concentrations and function. J Lipid Res. 1998; 39:2111–2118. [PubMed] [Google Scholar]
- 55.Karas RH, et al. Estrogen inhibits the vascular injury response in female estrogen receptor beta-deficient mice. Proc Natl Acad Sci USA. 1999; 96:15133–15136. doi: 10.1073/pnas.96.26.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srivastava RA, Krul ES, Lin RC, Schonfeld G. Regulation of lipoprotein metabolism by estrogen in inbred strains of mice occurs primarily by posttranscriptional mechanisms. Mol Cell Biochem. 1997; 173:161–168. doi: 10.1023/a:1006896131186. [DOI] [PubMed] [Google Scholar]
- 57.Srivastava RA, et al. Estrogen up-regulates apolipoprotein E (ApoE) gene expression by increasing ApoE mRNA in the translating pool via the estrogen receptor alpha-mediated pathway. J Biol Chem. 1997; 272:33360–33366. doi: 10.1074/jbc.272.52.33360. [DOI] [PubMed] [Google Scholar]
- 58.Feingold KR, et al. Apolipoprotein E deficiency leads to cutaneous foam cell formation in mice. J Invest Dermatol. 1995; 104:246–250. doi: 10.1111/1523-1747.ep12612790. [DOI] [PubMed] [Google Scholar]
- 59.Krege JH, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998; 95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]