Corticosteroids are by far the most effective treatment available for the control of allergic diseases, including asthma, allergic rhinitis, and atopic dermatitis. Their beneficial effects are mainly mediated through multiple anti-inflammatory mechanisms (1). One of the most important actions of corticosteroids in suppressing allergic inflammation is the repression of genes encoding the multiple inflammatory cytokines and chemokines that are important in amplifying and perpetuating allergic inflammation. The molecular mechanisms of suppression of inflammatory genes involve an interaction of glucocorticoid receptors (GRs) activated by corticosteroids interacting with transcription factors that have been activated by inflammatory stimuli. This does not involve binding of GR to DNA recognition sequences, since anti-inflammatory effects of corticosteroids are preserved in mutant forms of GR that do not dimerize and that therefore fail to bind to glucocorticoid-response elements (GREs) in the upstream promoter regions of inflammatory genes (2). Inflammatory stimuli activate transcription factors, such as NF-κB and activator protein-1 (AP-1), that bind to and activate coactivator proteins at the start site of transcription, resulting in acetylation of core histones and increased transcription of inflammatory genes. Corticosteroids suppress the transcription of these inflammatory genes by reversing histone acetylation, in part by recruiting histone deacetylases to the transcription start site, thus repressing inflammatory genes (3). This mechanism accounts for many of the therapeutic effects of corticosteroids in the treatment of allergic diseases.
But although corticosteroids are highly effective in clinical management of allergic diseases, they have some cellular and molecular effects that are difficult to reconcile with this beneficial effect. In atopy there is a switch from the characteristic predominance of Th1 lymphocytes toward Th2 lymphocytes that characteristically secrete IL-4 and IL-5. IL-4, together with the related cytokine IL-13, is important for isotype switching of B lymphocytes to secrete IgE, the characteristic antibody that underlies atopy. IL-5 is critical for eosinophilic inflammation in allergic disease, as recently demonstrated by the profound fall in circulating eosinophils after administration of an anti–IL-5 antibody in atopic asthmatic patients (4). Corticosteroids inhibit the transcription of IL-4, IL-5, and IL-13 genes, and it is likely that switching off these key cytokines contributes importantly to their efficacy in controlling allergic diseases. Curiously, corticosteroids tip the balance toward Th2-cell predominance, through an effect that may be due to suppression of IFN-γ, which normally inhibits Th2 differentiation in response to IL-4 (5), and suppression of IL-12 receptors that result in increased differentiation of Th1 cells (6). Corticosteroids also suppress IL-12 production, while having little effect on the anti-inflammatory cytokine IL-10 (7). While this suggests that corticosteroids would be detrimental in the treatment by further polarizing the immune response toward a Th2 pattern, the inhibitory effects of corticosteroids on the secretion of IL-4, IL-5, and IL-13 override this detrimental effect. In addition, corticosteroids decrease the survival of T cells and eosinophils by increasing apoptosis, contributing to their suppression of chronic allergic inflammation.
Another effect of corticosteroids that appears to be detrimental to the allergic process is an increased production of IgE from B lymphocytes stimulated with IL-4 (8). This has also been demonstrated in vivo in asthmatic patients after 1 week of treatment with oral prednisolone, when there is a small, but significant, rise in polyclonal IgE in asthmatic patients (9). This explains why treatment with corticosteroids, even at high systemic doses, does not inhibit skin prick tests to common allergens. The molecular mechanisms underlying this paradoxical effect of corticosteroids are further elucidated by the study of Jabara et al. in this issue of the JCI (10). They have demonstrated that corticosteroid-induced IgE synthesis, in the presence of IL-4, is dependent on increased expression of the costimulatory molecule CD40 ligand (CD40L), a transmembrane glycoprotein that belongs to the TNF superfamily. CD40L is normally expressed on activated T lymphocytes and interacts with CD40, a surface glycoprotein related to TNF receptors that is expressed on all B lymphocytes (Figure 1). The interaction between CD40L and CD40 is critical to the induction of IgE synthesis by IL-4 and IL-13 (11). The gene for CD40L maps to the X chromosome, and patients with X-linked hyper-IgM syndrome have low levels of Ig’s, as well as dysfunctional mutations of the CD40L gene, with defective expression of CD40L (12). In patients with X-linked hyper-IgM syndrome, corticosteroids fail to induce any IgE synthesis, and a blocking CD40-Ig fusion protein inhibits the effects of hydrocortisone in vitro (10). The effect of corticosteroids on CD40L is mediated by GR, as it is blocked by the GR antagonist mifepristone (RU486). It is likely to involve DNA binding, resulting in increased gene transcription and expression of CD40L on the cell surface of T lymphocytes but also on B lymphocytes that do not normally express CD40L. These B cells may then interact with other B cells that express CD40.
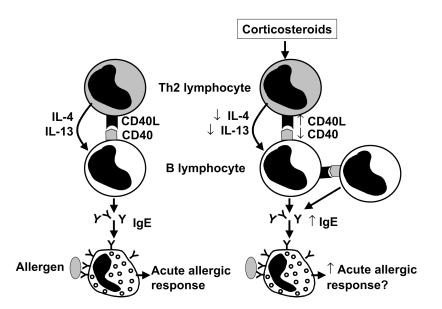
Figure 1.
Interaction of T and B lymphocytes. The left panel shows interaction of a CD4+ Th2 cell with a B lymphocyte. The release of cytokines IL-4 and IL-13 and interaction of CD40L and CD40 result in IgE synthesis and the sensitization of mast cells, which can then be triggered by allergen to activate an acute allergic response. The right panel shows the complex effects of corticosteroids, which increase the expression of CD40L in T and B cells, thereby increasing IgE formation and potentially acute allergic responses. On the other hand, corticosteroids also decrease expression of CD40L and the synthesis of IL-4 and IL-5, thus counteracting these effects.
The 5′-upstream promoter region of CD40L has several potential GRE sites. The molecular mechanism for increasing transcription involves interaction of GR with coactivators and subsequent acetylation of core histones, leading to increased gene transcription (3). By contrast, a previous study showed that corticosteroids inhibit CD40L expression in human peripheral blood CD4+ lymphocytes (13), so that the response to steroids may differ between cell types and in different conditions, such as the absence or presence of IL-4. Although corticosteroids increase CD40L in B lymphocytes, other studies show that in the same cells they suppress the expression of CD40, which acts as a receptor for CD40L, thus potentially diminishing any functional effect of corticosteroids on IgE production (14). Furthermore, as noted above, corticosteroids suppress the synthesis of IL-4 and IL-13, which are necessary for IgE production. The suppressive effects of corticosteroids on inflammatory genes, such as IL-4 and CD40, are seen at lower concentrations than the effects that involve increased transcription, such as the increase in CD40L.
The clinical implications of these findings are not yet clear. Corticosteroids may induce CD40L in T lymphocytes, which may also activate other CD40-expressing inflammatory cells, such as macrophages and eosinophils. This would paradoxically increase inflammation in the short-term, although presumably corticosteroids may suppress expression of CD40 in these other cells. Corticosteroids, while very effective at suppressing chronic allergic inflammation, are less effective against acute allergic events that are mediated via interaction of allergen with IgE bound to mast cells. However, there is no clinical evidence that IgE-mediated responses are worsened by corticosteroid treatment. In part, this may be because of counteracting beneficial effects of corticosteroids on these acute responses, as treatment with topical steroids reduces the number of mast cells in the mucosa, so that allergen is less able to activate these cells (15). With respect to therapy, this apparent adverse effect of corticosteroids on the acute allergic response suggests that therapies that deal with the increased IgE levels may be complementary to the effects of corticosteroids. A humanized monoclonal antibody to IgE, which profoundly reduces circulating IgE concentrations (16), has recently been found to provide surprising benefit in patients with severe steroid-dependent asthma, reducing the requirement for oral corticosteroids and allowing some patients to discontinue oral steroids completely (17). These findings can now be understood, as the high doses of systemic corticosteroid therapy may maintain high IgE levels, via the mechanisms described by Jabara and colleagues (10). This effect can be overcome by anti-IgE therapy, which appears to be of greater benefit in patients with more severe disease who are steroid-dependent.
References
- 1.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci. 1998; 94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 2.Reichardt HM, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998; 93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 3.Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits IL-1β-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000; 20:6891–6903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leckie MJ, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyperresponsiveness and the late asthmatic response. Lancet. 2000; 356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez F. Glucocorticoids induce a Th2 response in vitro. Dev Immunol. 1998; 6:233–243. doi: 10.1155/1998/73401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CY, Wang K, McDyer JF, Seder RA. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J Immunol. 1998; 161:2723–2730. [PubMed] [Google Scholar]
- 7.Visser J, et al. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood. 1998; 91:4255–4264. [PubMed] [Google Scholar]
- 8.Jabara HH, Ahern DJ, Vercelli D, Geha RS. Hydrocortisone and IL-4 induce IgE isotype switching in human B cells. J Immunol. 1991; 147:1557–1560. [PubMed] [Google Scholar]
- 9.Zieg G, Lack G, Harbeck RJ, Gelfand EW, Leung DY. In vivo effects of glucocorticoids on IgE production. J Allergy Clin Immunol. 1994; 94:222–230. doi: 10.1016/0091-6749(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 10.Jabara HH, Brodeur SR, Geha RS. Glucocorticoids upregulate CD40 ligand expression and induce CD40L-dependent immunoglobulin isotype switching. J Clin Invest. 2001; 107:371–378. doi: 10.1172/JCI10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spriggs MK, et al. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992; 176:1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aruffo A, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993; 72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- 13.Bischof F, Melms A. Glucocorticoids inhibit CD40 ligand expression of peripheral CD4+ lymphocytes. Cell Immunol. 1998; 187:38–44. doi: 10.1006/cimm.1998.1308. [DOI] [PubMed] [Google Scholar]
- 14.Jirapongsananuruk O, Leung DY. The modulation of B7.2 and B7.1 on B cells by immunosuppressive agents. Clin Exp Immunol. 1999; 118:1–8. doi: 10.1046/j.1365-2249.1999.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laitinen LA, Laitinen A, Haahtela T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and of a β2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma. J Allergy Clin Immunol. 1992; 90:32–42. doi: 10.1016/s0091-6749(06)80008-4. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ. Anti-IgE therapy in asthma: rationale and therapeutic potential. Int Arch Allergy Immunol. 2000; 123:196–204. doi: 10.1159/000024444. [DOI] [PubMed] [Google Scholar]
- 17.Milgrom H, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. N Engl J Med. 1999; 341:1966–1973. doi: 10.1056/NEJM199912233412603. [DOI] [PubMed] [Google Scholar]