Abstract
CD45 is a family of transmembrane protein tyrosine phosphatases exclusively expressed by hematopoietic cells and critically involved in the regulation of T cell activation signals. We now demonstrate that three 100-μg doses of anti-CD45RB mAb MB23G2 can induce long-term engraftment of islets into major histcompatibility complex-disparate chemically diabetic mice. Long-term graft survivors (>120 days) were tolerant to new islet allografts from the original donor strain. MB23G2 induced a temporary decrease in number circulating leukocytes but had no effect on leukocyte number in other lymphoid compartments. Histologic examination of allografts from treated and untreated recipients revealed a similar peri-islet infiltration on day 6. Eleven days after transplant, the peri-islet infiltrate in treated animals persisted, but in marked contrast to untreated control animals, there was no insulitis and islet integrity was preserved. The peri-islet infiltrate from treated animals showed a mild increase in CD4 cells, a decrease in CD8 cells, and decreased intensity of CD45RB expression. Treatment of naive animals with anti-CD45RB (MB23G2) resulted in a shift in CD45 isoform expression on T cells with a loss of higher molecular weight isoforms and increased expression of lower molecular weight (CD45R0) isoform. This shift in CD45 isoform expression from CD45RBHi to CD45RBLo was associated with an increase in the intragraft expression of transcripts for interleukin (IL) 4 and IL-10, consistent with the expected activity of this distinct immunoregulatory T cell subset. Antibody-mediated targeting of CD45 may induce tolerance through novel mechanisms and have direct applicability to clinical transplantation in humans.
Organ transplantation is now the treatment of choice for end-stage heart, kidney, and liver disease. Yet, current therapeutic regimens require life-long generalized immunosuppression with attendant risks of infection and malignancy. The quest for therapies able to induce long-term tolerance in humans after only a short treatment course remains unfulfilled. Transplantation of pancreatic islet cells has the potential to stabilize or even reverse diabetic neuropathy and retinopathy and should the avoid the morbidity associated with transplantation of the exocrine pancreas (1). However, because of inexorable rejection, human islet cell transplantation is not yet clinically feasible and new immunosuppressive approaches are required. Promising experimental strategies, specifically targeting T cell signaling molecules, appear to qualitatively alter T cell activation and induce peripheral tolerance in animal models (2–5).
The CD45 family of transmembrane protein tyrosine phosphatases plays a critical role in lymphocyte activation by regulating the phosphorylation and activity of src-family protein tyrosine kinases and their substrates (6). Multiple CD45 isoforms are generated by the alternative splicing of exons 4–6, commonly known as exons A, B, and C. The alternative exons give rise to isoforms that differ in the size of their extracellular domains but share identical cytoplasmic protein tyrosine phosphatase domains (6). The various isoforms range in size from 180 to 220 kDa, depending on which exons are used. Although some anti-CD45 mAbs recognize epitopes common to all isoforms (anti-CD45), others (anti-CD45R) recognize epitopes restricted to certain isoforms. For example, anti-CD45RB recognizes only those isoforms that include (epitopes encoded by) exon B.
Individual lymphocytes simultaneously express multiple CD45 isoforms (7, 8). However, the higher and lower molecular weight CD45 isoforms are differentially expressed by lymphocytes of distinct lineage (e.g., T versus B cells) as well as by subsets of T cells with distinct functions (9–12). For example, antibodies recognizing exon B-containing isoforms (anti-CD45RB) have been used to divide murine T cells into two largely reciprocal populations: T cells exhibiting high levels of CD45RB (CD45RBHi) express the higher molecular weight CD45 isoforms (which contain exon B alone or in combination with exons A or C), and T cells exhibiting low-level reactivity with anti-CD45RB (CD45RBLo) that primarily express the lower molecular weight CD45RO isoform (which lacks alternative exons) (13). In particular, freshly isolated CD45RBHi CD4 cells have been shown to preferentially secrete interleukin (IL) 2, whereas CD45RBLo CD4 cells preferentially secrete IL-4 (10, 12). Furthermore, these subsets have distinct immunoregulatory activities in vivo (14, 15). Although the exact function of each isoform is unknown, it is now clear that the individual isoforms preferentially regulate particular signal transduction pathways (16, 17) and this may contribute to the different cellular functions noted above.
Herein, we demonstrate that three 100-μg doses of anti-CD45RB (MB23G2) induces long-term survival of pancreatic islet cell allografts in 50% of murine recipients. This effect was directed at the recipient immune system and not at decreasing the immunogenicity of donor tissue. Despite MB23G2 treatment, a significant peri-islet infiltrate ensued. Eleven days after transplant, treated animals exhibited a persistent peri-islet infiltrate, but in marked contrast to untreated control animals, there was no invasion into the islets and islet integrity was preserved. Treatment with effective anti-CD45RB (MB23G2) but not with ineffective anti-CD45RB (MB4B4) mAb resulted in a shift in CD45 expression by T cells toward the lower molecular weight isoforms. This was associated with an increase in intragraft expression of transcripts for the T helper type 2 (Th2) cytokines IL-4 and IL-10, in keeping with the expected activity of CD45RBLo cells. Thus, CD45 is a potent immunotherapeutic target that may act through distinct mechanisms that include alteration of CD45 isoform expression and modulation of regulatory T cell subsets.
MATERIALS AND METHODS
Animals.
The 7- to 10-week-old male C57BL/6 (H-2b) recipient and BALB/c (H-2d) donor mice (Charles River Breeding Laboratories) were housed individually after transplantation with free access to food and water.
Antibodies and Immunofluorescence.
Anti-CD45RB mAbs MB23G2 and MB4B4 (10) (American Type Culture Collection) were purified on protein G columns according to the manufacturer’s instructions (Pharmacia). CD45RB expression was detected by indirect immunofluorescence with fluorescein isothiocyanate-conjugated anti-rat Ig. Fluorochrome-conjugated mAbs reactive with CD3, CD4, CD8, CD25, CD44, CD45, and B220 (CD45RA) were from PharMingen. Anti-CD45 (TIB 122, ATCC) was a gift from Kim Bottomly (Yale University, New Haven, CT). Cell phenotype was analyzed by using a BD FACStar (5,000 cells per sample), as described (16). Negative fluorescence controls used rat IgG-fluorochrome conjugates or autofluorescence.
Islet Isolation and Transplantation.
Diabetes was induced in C57BL/6 mice with streptozotocin (200 mg/kg, i.p.) and confirmed by persistent hyperglycemia (blood glucose, >400 mg/dl). After in situ digestion with collagenase P (Sigma), islets were separated by density gradient centrifugation. Four hundred hand-picked islets per recipient were then transplanted under the left kidney capsule as described in ref. 18. Glycemia of <200 mg/dl by day 3 after transplantation and >250 mg/dl (after initial engraftment) defined primary graft function and graft loss, respectively.
Treatment Protocols.
On the basis of pilot studies, C57BL/6 recipients received 100 μg i.v. of anti-CD45RB (MB23G2) on days −1, 0, and 5. Control allograft recipients were untreated. Where indicated, naive C57BL/6 mice received three doses of anti-CD45RB (MB23G2), three doses of anti-CD45RB (MB4B4), or were left untreated (controls).
Data Analysis.
Actuarial curves of graft survival were compared by using the Wilcoxan test. Other statistical analyses used the unpaired Student’s t test.
Isolation of Lymphocytes/Mononuclear Cells.
Red blood cells were removed from single cell suspensions of lymph node, spleen, and peripheral blood leukocytes by hypotonic lysis. In some experiments, mononuclear leukocytes were obtained by density centrifugation. For immunoblotting studies, splenic mononuclear cells were separated into Ig+ (B cells) and Ig− cells with anti-IgG- and anti-IgM-coated immunomagnetic beads (PerSeptive Diagnostics, Cambridge, MA). T cells were enriched from the Ig− population by nonadherence to plastic plates.
Cell Lysis and Western Blotting.
Cell populations from treated and untreated animals were lysed in 1% Nonidet P-40 lysis buffer containing 25 mM Tris⋅HCl (pH 8.0), 150 mM NaCl, 1 mM aminoethyl-benzensulfonyl fluoride, aprotinin (10 μg/ml), and leupeptin (10 μg/ml), as described (16). Postnuclear supernatants (4 × 106 cell equivalents per lane) were boiled in Laemmli sample buffer (nonreducing), separated by SDS/PAGE on 6% gels, and transferred to nitrocellulose. Membranes were immunoblotted with pan anti-CD45 and developed with enhanced chemoluminescence (16, 17).
Histology.
Allografts excised 6 and 11 days after transplant were snap-frozen in liquid nitrogen, cryosectioned, fixed in paraformaldehyde, and stained with hematoxylin/eosin or incubated with primary mAb followed by peroxidase-conjugated secondary antibody. For quantitative analysis, all positive cells in peri-islet infiltrates were counted by using a grid (20 squares counted per tissue sample).
Quantitative Reverse Transcription-Coupled PCR (RT–PCR) for Cytokines.
RT–PCR was performed as detailed (19). RNA isolated from snap-frozen islets obtained 6 days after transplantation was reverse-transcribed into cDNA. For each cytokine, cDNA was coamplified with a specific competitive template (CT) and a single pair of specific primers in a same-tube reaction (19). Sample and CT PCR products were then separated by electrophoresis and the relative amount of each band was determined by opitical density. To control for the efficiency of each individual RT reaction, expression of the housekeeping gene GAPDH was determined by using the same PCR technique. The ratio of pg of target cDNA per pg of GAPDH cDNA was calculated for each sample.
RESULTS
Anti-CD45RB Can Induce Long-Term Survival of Islet Allografts.
Given its success in preventing renal allograft rejection (20), we examined the ability of the anti-CD45RB mAb MB23G2 to induce long-term survival of islet allografts. Because islet allografts are vigorously rejected in this model, the treatment regimen required optimization in pilot studies (18). Fully allogeneic BALB/c (H-2d) donor islets were transplanted under the left renal capsule of chemically diabetic C57BL/6 (H-2b) recipients. As shown in Fig. 1, untreated control animals rapidly rejected their grafts, with hyperglycemia ensuing within 12 days in all mice. As sole therapy, three doses of MB23G2, administered at 100 μg i.v. on days −1, 0, and 5, induced long-term allograft survival in 50% of the recipients. This is comparable to the survival of islet allografts induced by other biologics, including anti-B7 mAbs (21), anti-CD40L (22), and CTLA4/Fc (23), when used as single agents. To demonstrate that normoglycemia was indeed being maintained by the islet allografts, long-term survivors were subjected to left nephrectomy. This resulted in hyperglycemia in seven of eight animals, whereas the eighth animal died in the perioperative period and could not be assessed.
Figure 1.
Prolonged survival of islet allografts induced by anti-CD45RB mAb MB23G2. Kaplan–Meier plot of cumulative allograft survival vs. time. Treatment groups: ▴, untreated controls (n = 5); □, anti-CD45RB administered to recipient animals on days −1, 0, and 5 (n = 14, P = 0.002 vs. control); ○, anti-CD45RB administered to the donor animals on days −5, −1, and 0 (n = 4, P = NS vs. control).
Preliminary reports suggested that ex vivo perfusion of human renal allografts with pan anti-CD45 mAbs before transplantation decreased the incidence of rejection (24), possibly by removing passenger leukocytes and decreasing graft immunogencity. Whether or not anti-CD45 flowing out of the donor kidney after reanastamosis had any effect on the recipient immune system was not addressed. To determine whether anti-CD45RB in our model was primarily directed at the host immune system as opposed to decreasing allograft immunogenicity, we treated donor animals with three doses of anti-CD45RB (days −5, −1, and 0) before transplantation. This had no effect on allograft survival (Fig. 1). Thus, a short course of anti-CD45RB can induce long-term islet allograft survival through its effects on the recipient immune system.
To determine whether long-term allograft survivors (>120 days) were actually tolerant to their islet allografts, four such animals underwent left nephrectomy inducing diabetes. Animals were then retransplanted with fresh islets of original donor strain under the capsule of their contralateral kidney, without further treatment. Three animals remained normoglycemic >50 days, whereas the fourth became hyperglycemic on day 10 (data not shown). In contrast, third-party (CBA; H-2k) islets were promptly rejected. We previously demonstrated tolerance to donor strain skin allografts in 4 of 13 long-term renal allograft survivors without further MB23G2 treatment, whereas major histcompatibility complex-mismatched third-party skin grafts were rejected (20).
Anti-CD45RB Treatment Is Associated with a Peri-Islet Infiltrate.
As noted (20), anti-CD45RB (MB23G2) treatment caused a fall in peripheral WBCs to ∼33% of control values on day 6. However, white blood cells returned to baseline by day 14 (data not shown). Furthermore, MB23G2 had no effect on the number of leukocytes or lymphocytes recovered from lymph node or spleen (data not shown).
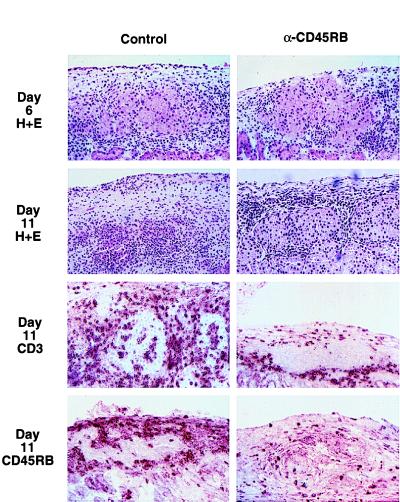
To determine the effect of MB23G2 on the immune response to the allograft, we compared the histology of allografts from treated and untreated animals 6 and 11 days after transplantation (Fig. 2). On day 6, histologic sections from MB23G2-treated recipients revealed a significant peri-islet mononuclear cell infiltrate. This was similar in character and magnitude to that observed on day 6 in untreated control recipients. This finding makes it unlikely that simple depletion of circulating lymphocytes by anti-CD45RB underlies the potent and long-lasting effects of anti-CD45RB therapy.
Figure 2.
Comparison of islet allograft histology from untreated control (Left) vs. anti-CD45RB (MB23G2)-treated animals (Right). Representative histology in the renal subcapsular area of the allograft. Shown are hematoxylin/eosin-stained sections on day 6 and day 11 after transplantation and immunoperoxidase staining for CD3 and CD45RB expression (day 11).
On day 11 after transplantation, tissue sections from treated animals revealed a persistent peri-islet infiltrate, superficially similar to that seen on day 6. There was little or no invasion into the islet capsule and islet architecture was well preserved (Fig. 2 Middle). Similarly, histology from long-term survivors demonstrates an ongoing lymphocytic peri-islet infiltrate surrounding intact islets (data not shown). In marked contrast, by day 11, allografts from untreated control recipients revealed frank insulitis and islet destruction.
Quantitative analysis of the peri-islet infiltrate on day 6 revealed a relative increase in CD4 and decrease in CD8 cells (Table 1). By day 11, islets from control animals exhibited significant invasion by CD3, CD4, and CD8 cells that was not seen in anti-CD45RB-treated animals. IL-2 receptor (CD25) expression in treated and untreated animals was similar on day 6. Although CD25 expression in untreated control animals was stable or increased by day 11, CD25 expression in treated animals decreased, suggesting down-regulation of the immune response. The number of cells expressing CD45RB was similar in treated and untreated animals (Table 1); however, treatment was associated with a significant decrease in the intensity of CD45RB staining (Fig. 2 Bottom). In one control animal at each time, rejection had already occurred leaving residual fibrosis, a scattered mononuclear infiltrate, and no discernible islets. Thus, lymphocytes from anti-CD45RB-treated animals are able to recognize and home to the graft. However, the quality of the infiltrate is altered and the capacity of the infiltrating cells to invade the graft and mediate its destruction is dramatically attenuated.
Table 1.
Quantitative analysis of allograft histology
| Animal | CD3 | CD4 | CD8 | CD45RB | CD25 |
|---|---|---|---|---|---|
| D6: Control | |||||
| 1 | 227 (2.2) | 112 (0.6) | 98 (0.5) | 126 | 12 |
| 2 | 390 (1.2) | 169 (0) | 154 (0.5) | 214 | 18 |
| 3‡ | |||||
| D6: Treated | |||||
| 1 | 238 (0.6)* | 150 (0.2) | 84 (0.4) | 106 | 14 |
| 2 | 310 (0.7) | 234 (0.5) | 84 (0.4) | 112 | 24 |
| 3 | 268 (0) | 146 (0.4) | 47 (0) | 63 | 44 |
| D11: Control | |||||
| 4 | 268 (14.3) | 106 (3.5) | 103 (4.9) | 78 | 19 |
| 5 | 526 (8.2) | 338 (2.7) | 300 (3.2) | 255 | 95 |
| 6‡ | |||||
| D11: Treated | |||||
| 4 | 274 (0)* | 216 (0)* | 78 (0)* | 105 | 4† |
| 5 | 214 (1.6) | 113 (0.3) | 91 (1.3) | 62 | 3 |
| 6 | 295 (1) | 196 (0.4) | 97 (0.4) | 122 | 14 |
Immunoperoxidase positive cells in peri-islet infiltrates from untreated and MB23G2-treated animals 6 and 11 days after transplantation were counted (in 20 squares of a grid). Cells actually invading the islets were counted separately and are presented within the parentheses as the mean number of cells/islet.
P ≤ 0.05; treatment vs. control groups on same day.
P ≤ 0.05; treated animals on day 11 vs. day 6.
Not quantitated due to severe fibrosis and lack of discernable islets in the area of the allograft.
Anti-CD45RB MB23G2 but Not MB4B4 Induces a Decrease in CD45RB Expression.
Given the phenotypic differences observed in the peri-islet infiltrates and the differential binding of anti-CD45RB to distinct functional T cell subsets, we next determined whether or not MB23G2 had any direct effect on the expression of lymphocyte surface markers. To avoid any confounding effects induced by transplantation and immune system activation, otherwise naive animals were used. Leukocytes isolated from lymph node, spleen, and peripheral blood of MB23G2-treated animals on days 6 and 11 were compared with those from untreated control animals by using immunofluorescence. MB23G2 had no effect on CD3 expression and caused a 1.5- to 2-fold decrease in the density of CD4 and CD8 expression on day 6 that resolved by day 11 (data not shown).
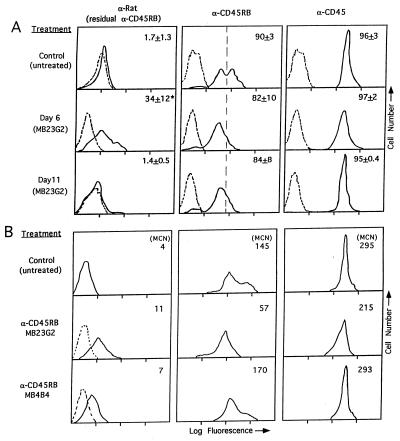
To determine the extent of residual anti-CD45RB mAb coating leukocytes after treatment, leukocytes were stained with anti-rat Ig secondary antibody only (Fig. 3A). By day 6, residual anti-CD45RB was only present on an average of 34% of the cells, and by day 11, no residual MB23G2 was detectable. Incubation of the cells with anti-CD45RB in vitro revealed that leukocytes from treated animals actually expressed much more CD45RB on their cell surface than that coated by residual anti-CD45RB mAb. In fact, the percentage of cells expressing CD45 or CD45RB was not statistically different from that in untreated animals. Although the percentage of cells expressing CD45RB was unchanged on day 6, cells from treated animals exhibited a 2- to 4-fold decrease in the level of CD45RB expression compared with untreated animals (as determined by mean channel fluorescence). By day 11, CD45RB expression was still decreased, at a time when no residual anti-CD45RB remained on the cells. Despite this decrease in CD45RB expression, total CD45 expression was only minimally affected (20–30% decrease in mean fluorescence). This suggests a relatively selective loss in exon B-containing isoforms. Similar results were obtained from peripheral blood and spleen (data not shown). Interestingly, the expression of B220 (CD45RA), which primarily recognizes the largest CD45 isoform (containing exons A, B, and C) expressed by murine B cells, was unchanged (data not shown). Because this isoform also contains exon B, the lack of change in B220 expression suggests that the decrease in exon B-containing isoforms might be limited to T cells.
Figure 3.
Representative expression of residual anti-CD45RB (anti-rat); CD45RB; and pan-CD45 by immunofluorescence. (A) Lymph node cells isolated on day 6 and 11 from animals treated with three doses of anti-CD45RB (MB23G2) vs. untreated animals. The number in each histogram is the percent of positive cells (mean ± SEM) in 3–5 experiments per marker. (* P < 0.5 vs. control). (B) Spleen cells isolated on day 6 from untreated controls vs. animals treated with three doses of either anti-CD45RB mAb MB23G2 or MB4B4. Mean channel number (MCN) is indicated in each histogram. Data are representative of three experiments. Negative controls are indicated as dotted histograms.
Such changes in CD45RB expression could provide a clue into the mechanism of MB23G2 action or serve as a convenient marker for effective versus ineffective anti-CD45RB mAbs. To examine this point, we compared the consequences of MB23G2 treatment with those of a second anti-CD45RB mAb, MB4B4, that we previously found to be completely ineffective in preventing the rejection of murine renal allografts (20). As seen in Fig. 3B, MB23G2 treatment resulted in a 2.5-fold decrease in the level of CD45RB expression, whereas MB4B4 induced little if any detectable change. Thus, treatment with the effective anti-CD45RB mAb MB23G2 but not the ineffective mAb MB4B4 induced a preferential decrease in the expression of exon B-containing CD45 isoforms.
MB23G2 mAb Induces a Shift in CD45 Isoform Expression in Vivo.
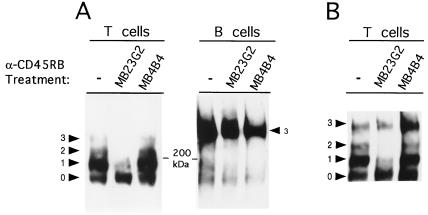
As mentioned, the relative expression of CD45RB has been used to delineate subsets of murine T cells expressing different CD45 isoforms and having distinct functions. The decrease in high-density CD45RB expression on lymphocytes after treatment with MB23G2 might be explained by selective down-regulation of the CD45(RB) molecules to which it binds. However, the CD45 isoforms expressed by CD45RBHi cells largely contain exon B alone or in combination with exons A and C. Thus, significant loss of CD45RB expression by these cells should result in almost equivalent loss in overall CD45 expression. Yet CD45 expression was only modestly reduced after MB23G2 and no CD45− or CD45-low subpopulation was observed. These findings suggest that loss of exon-B containing isoforms might be accompanied by up-regulation of non-exon-B-containing isoforms. Such isoforms might be of low (CD45R0) or higher molecular weight (for example, containing exons A and/or C). Unfortunately, murine mAbs specifically recognizing CD45RO do not exist and other anti-CD45R mAbs recognize several isoforms. Therefore, to more precisely determine the effects of MB23G2, CD45 isoforms were resolved by SDS/PAGE and analyzed by Western blotting. Naive animals were untreated or received three doses of either anti-CD45RB MB23G2 or anti-CD45RB MB4B4. On day 6, lysates from splenic T and B cells were subjected to immunoblotting with anti-CD45. As seen in Fig. 4A, T cells from untreated (control) animals express large amounts of CD45 containing a single alternative exon and lesser amounts of the CD45RO isoform that lacks alternative exons. Small amounts of larger isoforms containing two or three alternative exons were also detected. Probing similar immunoblots with anti-CD45RB confirmed that the single isoform band contains large amounts of exon B, whereas none was detected in the CD45R0 band, as expected (13) (data not shown).
Figure 4.
Effective anti-CD45RB MB23G2 induces a shift towards expression of the low molecular weight CD45 isoforms in T cells. Anti-CD45 immunoblot on day 6 using cell lysates from animals that were untreated or treated wtih MB23G2 (effective) or MB4B4 (ineffective) anti-CD45RB mAb. (A) Splenic T cells (Left) and B cells (Right) from naive (untransplanted) animals. (B) Splenic T cells from islet allograft recipients. The numbered arrowheads indicate the number of alternative exons used to generate CD45 bands of each size, as defined (7, 25) and confirmed using lysates from transfectants expressing single CD45 isoforms (from K. Bottomly, Yale University).
Treatment with the effective mAb MB23G2 induces loss of the higher molecular weight CD45 isoforms and up-regulation of the CD45RO isoform (Fig. 4A). Thus, relative expression of the CD45RB and CD45RO bands is dramatically altered by MB23G2 compared with untreated controls. In contrast, the ineffective mAb MB4B4 did not significantly alter the relative expression of the various CD45 bands. On B cells, CD45 isoform expression was unaffected by either mAb. Thus, effective mAb induces a phenotypic conversion of CD45RBHi T cells (primarily expressing the exon B-only isoform) to CD45RBLo cells (primarily expressing the CD45RO isoform). This shift was not the result of cellular activation induced by MB23G2, because neither CD25 expression nor CD44 expression, as markers of cellular activation, were up-regulated (data not shown).
Although the above findings were obtained in naive animals, the decreased intensity of peri-islet CD45RB staining noted in MB23G2-treated allograft recipients (Fig. 2) suggests that MB23G2 induces a similar shift in CD45 isoform expression after transplantation. To confirm this point, experiments similar to that in Fig. 4A were performed, only this time in animals having received islet allografts. After transplantation, MB23G2-treatment once again induces a significant shift toward CD45RO isoform expression compared with untreated controls (Fig. 4B). As before, treatment with MB4B4 induces little relative change in CD45 isoform expression.
Cytokine Expression in Allografts of MB23G2-Treated Recipients: CD45RBLo.
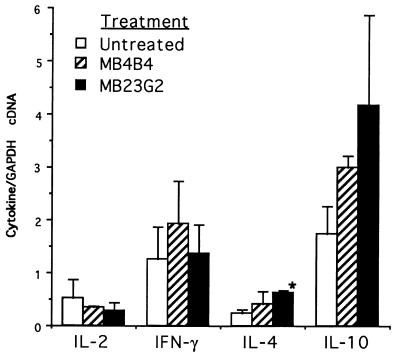
CD4 cells preferentially secrete IL-4 in vitro (10). Thus, if the shift in CD45 isoforms induced by MB23G2 were physiologically significant, one might expect augmented secretion of IL-4 and possibly IL-10 in the allografts of MB23G2-treated animals. By using quantitative RT–PCR, we examined cytokine mRNA in transplanted tissue from untreated animals and those treated with MB23G2 or MB4B4. As seen in Fig. 5, compared with control animals, MB23G2 had little effect on secretion of IL-2 or interferon γ. However, there was a 3-fold increase in mRNA levels for IL-4 and IL-10. Interestingly, MB4B4 showed a suggestive trend toward an increase in these cytokines but at a level not statistically different from control animals.
Figure 5.
Cytokine transcript expression by quantitative RT–PCR in allografts from untreated control animals, anti-CD45RB (MB23G2)-treated animals, and anti-CD45RB (MB4B4)-treated animals. Peri-islet tissue obtained 6 days after transplantation was analyzed by RT–PCR for each cytokine mRNA using competitive templates and normalized to the amount of GAPDH in each sample. Two animals were analyzed for each treatment group. Data are the ratio of pg of cytokine cDNA per pg of GAPDH mRNA. ∗, P ≤ 0.025 compared with control (for comparisons between other groups, P > 0.05).
DISCUSSION
Antibodies against CD45 have been shown to affect a wide-range of in vitro biological assays (9). It has now been established in vivo that a short course of anti-CD45RB can induce long-term tolerance to both vascularized renal (20) and neovascularized islet allografts. The efficacy of MB23G2 is not limited to a single strain, because we also have used the reverse donor/recipient strain combination (18, 20).
How anti-CD45RB exerts this potent immunomodulatory effect is not yet clear. Although a transitory drop in circulating leukocytes occurs, the lack of effect on leukocyte number in the spleen or lymph nodes and the presence of an early peri-islet infiltrate make this mechanism unlikely to be primary. We have now shown that treatment with effective anti-CD45RB MB23G2 results in down-regulation of high molecular weight isoforms and up-regulation of the low molecular weight CD45RO isoform. T cells expressing the high and low molecular weight CD45 isoforms have been shown to exhibit distinct in vitro immunoregulatory functions in mouse, rat, and human (9–12). Specifically, in mice, the CD45RBHi subset has been shown to primarily secrete T helper type 1 (Th1) cytokines, whereas the CD45RBLo subset preferentially secretes the Th2 cytokine IL-4 (11, 12). Although the function of these T cell subsets in vivo is less clear, cytokine secretion patterns seen after subset reconstitution appear to corroborate the in vitro data (15). In concordance, the shift in CD45 isoform expression induced by MB23G2 was associated with increased expression of IL-4 and more variably of IL-10 transcripts within the allografts.
The precise function of individual CD45 isoforms is as yet poorly understood. However, we and others (16, 17, 25) have shown that distinct CD45 isoforms differentially regulate tyrosine phosphorylation of particular signaling intermediates including Vav and SLP-76 and the secretion of IL-2. Thus, the expression of distinct CD45 isoforms by T cell subsets alters the activation signals resulting from TCR ligation and is likely to directly contribute to the functional differences observed. We hypothesize that the change in CD45 isoforms induced by MB23G2 causes a shift in the functional repertoire of responding T cells that skews the immune response toward tolerance.
In this regard, the development of tolerance in a number of transplant models has been associated with an increase in Th2 cytokines (5, 26–28). This has led to the hypothesis that Th1 cytokines induce rejection, whereas Th2 cytokines are required for the induction of tolerance (28). One difference between our findings and those referenced above is that although intragraft Th2 cytokine mRNAs were increased after MB23G2 treatment, Th1 cytokine mRNAs persisted. However, Larsen et al. (3, 29) have shown that long-term graft survival induced by anti-CD40L, CTLA4-Ig, or their combination was associated, respectively, with no change in Th1 or Th2 cytokines, decreased expression of IL-4, or lack of both Th1 and Th2 cytokines. Indeed, gene-ablation studies have failed to establish a causal role between Th1/Th2 cytokine expression and rejection/tolerance. For example, tolerance can occur in the absence of IL-4 and associated Th2 immune deviation (30). Conversely, ablation of the IL-2 or interferon γ gene have no effect on acute rejection (31, 32) and, in fact, lack of IL-2 prevents the induction of allograft tolerance with CTLA4-Ig (33). Although such results indicate that this Th1/Th2 paradigm in transplantation may be an oversimplification, they do not abrogate an important role for Th2 cytokines in the prevention of rejection and promotion of tolerance. In fact, IL-4 appears necessary for the induction of tolerance in naive animals after adoptive transfer of regulatory CD4 cells from tolerant hosts (34). In addition, viral-encoded IL-10 can prolong cardiac allograft survival in gene transfer experiments (35). Thus, in the microenvironment of the allograft, the combination of cytokines secreted after treatment with MB23G2 may well contribute to the development of tolerance. Regardless of their ultimate role, this deviation in cytokine production clearly indicates that treatment with effective anti-CD45RB mAb alters activation signals and/or the functional repertoire of responding T cells.
Although altered cytokine secretion may not be solely responsible for tolerance induced by anti-CD45RB, other functions ascribed to CD45RBLo T cells might contribute to the generation of tolerance. Several lines of evidence indicate that T cells expressing the lower molecular weight CD45 isoforms exhibit down-regulatory activity. Reconstitution of SCID mice with congenic CD45RBHi CD4 cells results in severe inflammatory bowel disease not seen when animals are reconstituted with CD45RBLo CD4 cells (14, 15). Importantly, cotransfer of CD45RBLo with CD45RBHi CD4 cells prevents colitis altogether. Thus, the CD45RBLo subset down-regulates autoimmune attack by cells expressing the higher molecular weight CD45 isoforms. Furthermore, adoptive transfer studies in nude rats reveal that T cells expressing high, but not those expressing low, molecular weight CD45 isoforms mediate graft-versus-host disease and cardiac allograft rejection (36). On the basis of these studies, we hypothesize that cells in the CD45RBLo subset are capable of down-regulating allo responses after transplantation. Thus, treatment with anti-CD45RB may skew the T cell repertoire toward cells that promote the development of long-lasting tolerance. Precedence for induction and maintenance of transplantation tolerance by immunoregulatory T cells has been amply demonstrated and in some cases may depend upon IL-4 secretion (4, 34). Indeed, tolerance can be adoptively transferred with T cells from MB23G2-tolerized renal allograft recipients to naive syngeneic recipients (A.L., unpublished data). Although anti-CD45RB might also act on B cells and recipient antigen-presenting cells, the ability of treated animals to maintain tolerance after reexposure to antigen and to adoptively transfer tolerance is likely to reside within the T cell repertoire.
CD45 isoform expression is highly regulated by individual T cells (8–11). For example, in mice and humans, recent thymic émigrés largely express the high molecular weight isoforms (6, 11, 36). T cell activation results in down-regulation of the high molecular weight isoforms and concomitant up-regulation of low molecular weight isoforms. Such findings led to the popular view that CD45RBHi cells are naive and convert to CD45RBLo memory cells after antigen exposure (10, 11, 37). However, subsequent in vitro and in vivo studies in both humans and rats have demonstrated that cells expressing high and low molecular weight isoforms can interconvert and that long-lived memory cells actually express high molecular weight isoforms (8, 38, 39). In any case, the effect of MB23G2 on CD45 isoform expression does not appear to be secondary to cellular activation because the activation markers CD25 and CD44 are not increased.
Regulation of CD45 isoform expression is controlled at the level of alternative mRNA splicing (6). Rothstein et al. (40) have shown that generation of the smaller isoforms is dependent on expression of a negative regulatory alternative splicing factor (required to splice out alternative exons). Ultimately, MB23G2 binding must signal the cellular splicing machinery, although it is currently unknown how cells “sense” their overall level of CD45 expression and what signals are involved in regulating splicing factor(s).
An important unresolved question is whether MB23G2 acts solely through its ability to alter CD45 isoform expression on T cells or whether antibody binding has other effects on signal transduction that contribute toward peripheral T cell tolerance. In this regard, we have shown that effective (MB23G2), but not ineffective (MB4B4), anti-CD45RB augments anti-CD3-induced tyrosine phosphorylation of phospholipase Cγ1 in a murine hybridoma (20). A similar finding was observed upon induction of anergy in a Th1 clone (41). MB23G2 binding might conceivably alter protein tyrosine phosphatase activity or disrupt lateral interactions between CD45 and other cell surface molecules (42), thereby altering proximal signal transduction upon antigen recognition. Altered activation signals mediated through the T cell receptor (TCR) by partial agonists (altered peptide ligand) can induce a variety of functional responses ranging from anergy to lack of proliferation despite cytokine production and IL-2 receptor expression (43, 44). That transplantation up-regulates cytokine mRNAs (including that for IL-2) despite MB23G2 treatment and, in some reports, despite CTLA4-Ig or anti-CD40L (3, 29) suggests that classical anergy is not present. However, to the degree that T cells home to the graft yet are unable to invade and destroy the islets, some sort of “functional anergy”, at least of certain clones, may exist. Whether or not MB23G2 actually induces anergy or alters TCR-proximal signaling in vivo has not yet been examined. Regardless of its mechanism(s) of action, T cell activation appears necessary for induction of tolerance by anti-CD45RB. As has been shown for CTLA4-Ig and anti-CD40L (29), concomitant treatment with cyclosporin A inhibits MB23G2-induced allograft survival (A.L., unpublished data).
Peripheral tolerance is likely to depend upon a combination of mechanisms (2) and additional work will be required to define which of these are key to the activity of anti-CD45RB. In the meantime, the shift in CD45 isoforms induced by certain anti-CD45RB mAbs may serve as a convenient marker for efficacy. Because different mAbs reactive with the same isoforms differ widely in their ability to induce tolerance (20), identification of markers for efficacy will be of considerable practical importance when considering trials in nonhuman primates or humans. A number of mAbs recognizing the variably spliced exons of human CD45 are currently available. Thus, antibody-mediated targeting of CD45 isoforms offers a new immunotherapeutic strategy with important potential in human transplantation.
Acknowledgments
We thank Dr. David Leitenberg and Dr. Kim Bottomly for murine anti-CD45 mAbs and for lysates from unique single-isoform-expressing transfectants, and Dr. Charles B. Carpenter for critical reading of this manuscript. This work was supported in part by grants from Research Corporation Technologies, The American Heart Association (CT Affiliate), and The Juvenile Diabetes Association.
ABBREVIATIONS
- IL
interleukin
- Th1 and Th2
T helper types 1 and 2, respectively
- RT–PCR
reverse transcription-coupled PCR
References
- 1.Sutherland D, Greussner R, Gores P. In: Transplantation Immunology. Bach F, Auchincloss H J, editors. New York: Wiley-Liss; 1995. pp. 147–160. [Google Scholar]
- 2.Nickerson P, Streurer W, Steiger J, Strom T B. Kidney Int. 1994;45:S40–S49. [PubMed] [Google Scholar]
- 3.Larsen C, Alexander D, Hollenbaugh D, Elwood E, Ritchie S, Arruffo A, Hendrix R, Pearson T. Transplantation. 1996;61:4–9. doi: 10.1097/00007890-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bushell A, Morris P, Wood K. Eur J Immunol. 1995;25:2643–2649. doi: 10.1002/eji.1830250936. [DOI] [PubMed] [Google Scholar]
- 5.Sayegh M, Akalin E, Hancock W, Russell M, Carpenter C, Linsley P S, Turka L A. J Exp Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trowbridge I, Thomas M L. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 7.Rudd C E, Morimoto C, Wong L L, Schlossman S F. J Exp Med. 1987;166:1758–1773. doi: 10.1084/jem.166.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothstein D M, Yamada A, Schlossman S F, Morimoto C. J Immunol. 1991;146:1175–1183. [PubMed] [Google Scholar]
- 9.Thomas M L. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- 10.Birkeland M L, Metlay J, Sanders V M, Ferandez-Botran R, Vitetta E, Steinman R, Pure E. J Mol Cell Immunol. 1988;4:71–85. [PubMed] [Google Scholar]
- 11.Lee W, Yin X-M, Vitetta E. J Immunol. 1990;144:3288–3295. [PubMed] [Google Scholar]
- 12.Bottomly K, Luqman M, Greenbaum L, Carding S, West J, Pasqualini T, Murphy D. Eur J Immunol. 1989;19:617–623. doi: 10.1002/eji.1830190407. [DOI] [PubMed] [Google Scholar]
- 13.Hathcock K, Laslo G, Dickler H, Sharrow S, Laszlo Z, Pickler H, Sharrow S, Johnson P, Trowbridge J S, Hodes R. J Immunol. 1992;148:19–28. [PubMed] [Google Scholar]
- 14.Powrie F, Leach M, Mauze S, Caddle L, Coffman R. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 15.Powrie F, Correa-Oliveira R, Mauze S, Coffman R. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenney D W, Onodera H, Gorman L, Mimura T, Rothstein D M. J Biol Chem. 1995;270:24949–24954. doi: 10.1074/jbc.270.42.24949. [DOI] [PubMed] [Google Scholar]
- 17.Onodera H, Motto D G, Koretzky G A, Rothstein D M. J Biol Chem. 1996;271:2225–2230. doi: 10.1074/jbc.271.36.22225. [DOI] [PubMed] [Google Scholar]
- 18.Auersvald L, Rothstein D, Oliveira S, Khuong C, Onodera H, Basadonna G. Transplantation. 1997;63:1355–1358. doi: 10.1097/00007890-199705150-00026. [DOI] [PubMed] [Google Scholar]
- 19.Zheng X X, Strom T B, Steele A. Transplantation. 1994;58:87–92. [PubMed] [Google Scholar]
- 20.Lazarovits A, Poppema S, Zhang Z, Khandaker K, LeFeuvre C, Singhal S, Garcia B, Jevnikar A, White M, et al. Nature (London) 1996;380:717–720. doi: 10.1038/380717a0. [DOI] [PubMed] [Google Scholar]
- 21.Lenschow D, Zeng Y, Hathcock K, Zuckerman L, Freeman G, Thistlethwaite J R, Gary G, Hodes R, Bluestone J. Transplantation. 1995;60:1171–1178. doi: 10.1097/00007890-199511270-00019. [DOI] [PubMed] [Google Scholar]
- 22.Parker D, Greiner D, Phillips N, Appel M, Steele A, Durie F, Noelle R, Mordes J, Rossini A. Proc Natl Acad Sci USA. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streurer W, Nickerson P, Steele A, Steiger J, Zheng X, Strom T B. J Immunol. 1995;155:1165–1174. [PubMed] [Google Scholar]
- 24.Brewer Y, Taube D, Bewick M, Hale G, Dische F, Palmer A, Welsh K, Waldmann H, Parsons V, Snowden S. Lancet. 1989;II:935–937. doi: 10.1016/s0140-6736(89)90951-3. [DOI] [PubMed] [Google Scholar]
- 25.Novak T, Farber D L, Leitenberg D, Hong S-C, Johnson P, Bottomly K. Immunity. 1994;1:109–119. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 26.Salom R, Maguire J, Hancock W. Transplantation. 1993;56:1309–1314. doi: 10.1097/00007890-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Mottram P, Han W-R, Purcell L, McKenzie I, Hancock W. Transplantation. 1995;59:559–565. [PubMed] [Google Scholar]
- 28.Nickerson P, Steiger J, Zheng X, Steele A, Streurer W, Strom T B. Transplantation. 1997;63:489–494. doi: 10.1097/00007890-199702270-00001. [DOI] [PubMed] [Google Scholar]
- 29.Larsen C, Elwood E, Alexander D, Ritchie S, Hendrix R, Tuckerburden C, Cho H, Arruffo A, Hollenbaugh D, Linsley P, et al. Nature (London) 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 30.Nickerson P, Zheng X, Steiger J, Steele A, Streurer W, Roy-Chaudry P, Muller W, Strom T B. Transplant Immunol. 1996;4:81–85. doi: 10.1016/s0966-3274(96)80043-8. [DOI] [PubMed] [Google Scholar]
- 31.Steiger J, Nickerson P, Streurer W, Moscovitch-Lopatin M, Strom T B. J Immunol. 1995;155:489–498. [PubMed] [Google Scholar]
- 32.Saleem S, Konieczny B, Lowry R, Baddoura F, Lakkis F. Transplantation. 1996;62:1908–1911. doi: 10.1097/00007890-199612270-00039. [DOI] [PubMed] [Google Scholar]
- 33.Dai Z, Lahkis F G. J Am Soc Nephrol. 1997;8:674. (abstr.). [Google Scholar]
- 34.Davies J, Martin G, Phillips J, Marshall S, Cobbold S, Waldmann H. J Immunol. 1996;157:529–533. [PubMed] [Google Scholar]
- 35.Qin L, Chavin K, Ding Y, Tahara H, Favaro J, Woodward J, Suzuki T, Robbins P, Lotze M, Bromberg J. J Immunol. 1996;156:2316–2323. [PubMed] [Google Scholar]
- 36.Yang C, McDonagh M, Bell E. Transplantation. 1995;60:192–199. [PubMed] [Google Scholar]
- 37.Tedder T F, Clement L T, Cooper M D. J Immunol. 1985;134:2983–2988. [PubMed] [Google Scholar]
- 38.Michie C A, McLean A, Alcock C, Beverley P C L. Nature (London) 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 39.Bunce C, Bell E. J Exp Med. 1997;185:767–776. doi: 10.1084/jem.185.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothstein D M, Saito H, Streuli M, Schlossman S F, Morimoto C. J Biol Chem. 1992;267:7139–7147. [PubMed] [Google Scholar]
- 41.Gajewski T F, Qian D, Fields P, Fitch F W. Proc Natl Acad Sci USA. 1994;91:38–42. doi: 10.1073/pnas.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leitenberg D, Novak T, Farber D L, Smith B R, Bottomly K. J Exp Med. 1996;183:249–259. doi: 10.1084/jem.183.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.La Face D, Couture C, Anderson K, Shih G, Alexander J, Sette A, Mustelin T, Altman A, Grey H. J Immunol. 1997;158:2057–2064. [PubMed] [Google Scholar]
- 44.Jameson S, Bevan M. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]