Abstract
Compartmentalization of β-tubulin isotypes within cells according to function was examined in gerbil olfactory and respiratory epithelia by using specific antibodies to four β-tubulin isotypes (βI, βII, βIII, and βIV). Isotype synthesis was cell-type-specific, but the localization of the isotypes was not compartmentalized. All four isotypes were found in the cilia, dendrites, somata, and axons of olfactory neurons. Only two isotypes (βI and βIV) were present in the cilia of nasal respiratory epithelial cells. The βIV isotype, thought to be an essential component of cilia, was present in olfactory neurons and respiratory epithelial cells, which are ciliated, but was not found in basal cells (the stem cells of olfactory sensory neurons, which have no cilia). Olfactory neurons therefore do not synthesize βIV-tubulin until they mature, when functioning cilia are also elaborated. The failure to observe compartmentalization of β-tubulin isotypes in olfactory neurons sheds new light on potential functions of the β-tubulin isotypes.
Keywords: Tubulin isotypes, β-Tubulin, Cilia, Olfactory neuron, Respiratory epithelium, Gerbil
Introduction
Microtubules perform a variety of functions in the eukaryotic cell, including providing cell form and rigidity and facilitating the transport of organelles. Microtubules consist of heterodimers of α- and β-tubulin. The seven β-tubulin isotypes in mammals (βI, βII, βIII, βIVa, βIVb, βV, and βVI) are among the most highly conserved proteins known (Ludueña 1998). Nevertheless, the isotype-specific sequence differences are also highly conserved, which suggests that there are important functional differences between the isotypes.
The multi-tubulin hypothesis (Fulton and Simpson 1976) proposes that the isotypes have specific functional roles. Consistent with this idea, different cell types have been found to synthesize different isotypes even within the same tissue (Roach et al. 1998; Hallworth and Ludueña 2000). However, little is known about the functions of the seven isotypes, except that the βIV isotype appears to be associated with axonemal microtubules and with actin stress fibers (Renthal et al. 1993; Lu et al. 1998; Roach et al. 1998; Walss-Bass et al. 2001). If β-tubulin isotypes have specific functional roles, they may be sequestered to different compartments of the same cell according to function.
In olfactory epithelia, microtubules exist in discrete populations (Burton 1992). For example, olfactory neurons have four identifiable microtubule compartments (cilium, dendrite, soma, and axon; Graziadei 1973), each of which may have microtubules with different functions. Olfactory cilia contain the 9+2 arrangement of axonemal microtubules but are not motile. Within the dendrite, microtubules are longitudinally arranged. The dendrite, soma, and axon of the olfactory neuron each represent potentially distinct compartments for microtubules. Bordering the olfactory epithelium is the respiratory epithelium, whose cells bear motile cilia with an entirely different function from that of the olfactory cilia. The varied microtubule populations in olfactory and respiratory epithelia therefore offer an excellent opportunity to test this aspect of the multi-tubulin hypothesis.
Materials and methods
The synthesis of β-tubulin isotypes was examined by using indirect immunofluorescence microscopy in frozen sections of gerbil olfactory and respiratory epithelium. Adult gerbils (22 days old or older) were anesthetized with Nembutal and cardiac-perfused with formaldehyde that had been freshly prepared by dissolving 4% paraformaldehyde in phosphate-buffered saline (PBS). After decapitation, the dorso-posterior nasal epithelium of the gerbil was dissected away from the septum, rinsed in PBS, cut into strips, equilibrated in 30% sucrose in PBS as a cryoprotectant, and quickly frozen in O.C.T. (Tissue-Tek, Miles Laboratories, Elkart, Ind., USA). Transverse sections (10 µm thick) were prepared on a cryostat (Leica Microsystems, Bannockburn, Ill., USA). Sections were blocked and permeabilized in PBS containing 1% bovine serum albumin (BSA), 0.25% Triton-X100, and 5% normal goat serum and then rinsed in PBS plus 0.1% BSA. Sections were labeled with primary mouse monoclonal antibodies specific for each β-tubulin isotype, rinsed, and labeled with secondary antibody, viz., goat anti-mouse IgG coupled to fluorescein isothiocyanate (Sigma, St. Louis, Mo., USA) or to Alexa 488 (Molecular Probes, Eugene, Ore., USA). Sections were sealed under coverslips on glass slides in 50% PBS:50% glycerol containing 1% n-propylgallate. All sections were examined on an Axioskop II epifluorescence microscope (Carl Zeiss Jena, Jena, Germany). Images were acquired digitally by using a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, Mich., USA). Confocal images were obtained on a Radiance 2000 confocal microscope (Bio-Rad, Hercules, Calif., USA) or an Eclipse 800 epifluorescence microscope (Nikon Instruments, Kawasaki, Japan).
The isotype-specific antibodies had previously been prepared against an epitope unique to the C-terminus of that isotype (Banerjee et al. 1988, 1990, 1992; Roach et al. 1998). Since the C-termini of βIVa and βIVb are virtually identical, the anti-βIV antibody is unable to discriminate between them. Positive controls were performed by using the same procedures as above but with a non-isotype-specific antibody against β-tubulin (Sigma). Negative controls were performed by omitting the primary antibody.
For immunoblotting, strips of nasal epithelium were removed from anesthetized and decapitated gerbils and homogenized in SDS sample buffer. Approximately 18 µg of protein was loaded onto each lane of a 8% poly-acrylamide TRIS-glycine gel (Gradipore, French’s Forest, N.S.W., Australia), and this was run at 150 V for 90 min in SDS electrophoresis buffer. The proteins were then transferred to nitrocellulose sheets soaked in chilled TRIS-glycine/methanol transfer buffer. Lanes were cut into strips for labeling with the β-tubulin isotype antibodies or with a non-isotype-specific antibody against β-tubulin (Sigma). The strips were rinsed in TRIS-buffered saline (TBS) with 0.1% Tween, blocked in 2% powdered milk in TBS, and exposed to one of the five primary antibodies overnight at 4°C. After being rinsed, the strips were incubated in anti-mouse IgG conjugated with biotin-horseradish peroxidase (Cell Signaling Technology, Beverly, Mass., USA) in blocking buffer for 1 h at room temperature. Strips were then rinsed and treated with Luminol reagent (Santa Cruz Biotechnology, Santa Cruz, Calif., USA). After again being rinsed, the protein strips were wrapped in plastic and exposed to XAR5 scientific imaging film (Kodak, Rochester, N.Y., USA).
Preservation of olfactory cilia in frozen sections was achieved only with great difficulty, whereas the more robust cilia of the respiratory epithelium were usually well preserved. Most often, olfactory cilia were seen only in truncated form. As a supplementary approach to identifying the isotypes expressed in sensory cilia, we obtained confocal microscopic images of labeled sections at high magnification. The images obtained demonstrated clear, albeit shortened, processes emanating from dendritic knobs, consistent with olfactory cilia (see below).
Animal care and handling was performed according to approved protocols of the University of Texas Health Science Center at San Antonio and Creighton University School of Medicine Institutional Animal Care and Use Committees.
Results
Western blots of nasal epithelia indicated that labeling for β-tubulin isotypes was restricted to a single band of molecular weight of approximately 50 kDa (Fig. 1), close to the molecular weight of β-tubulin (Ludueña 1998).
Fig. 1.

Western blots showing immunolabeling of gerbil nasal epithelial tissue with an antibody to β-tubulin (right) and the isotype-specific β-tubulin antibodies used in this study (lanes 2–4). Marker Sample of protein standards with molecular weights indicated left
The olfactory epithelium consists of a pseudo-stratified layer of round cell bodies. The lowest layer of cell bodies consists of basal cells, which are the stem cells for olfactory neurons. The middle layer consists of the somata of mature olfactory neurons. Each neuron sends a dendritic process to the epithelial surface and an axonal process to the olfactory bulb. Long sensory cilia arise from a small swelling at the apical surface of the dendrite. Above the neuronal somata are the somas of supporting cells, whose processes span the epithelium and intercalate between the neurons. The adjacent respiratory epithelium consists of a thin uniform layer of epithelial cells bearing multiple short cilia.
In the olfactory epithelium, label for β1-tubulin was found in the olfactory neurons and basal cells (Fig. 2A). The label consisted of a bright irregular strip along the apical surface of the epithelium, apparently representing olfactory cilia. Supporting cell perikarya, the most superficial layer of somata, were unlabeled. Fine strands of label were seen perpendicular to the cilia layer, probably representing the dendrites of olfactory neurons. Deeper in the epithelium, labeling was evident in the perikarya of olfactory neurons and basal cells. Irregularly shaped bundles of bright label, probably representing the axons of olfactory neurons, were present below the epithelium.
Fig. 2.
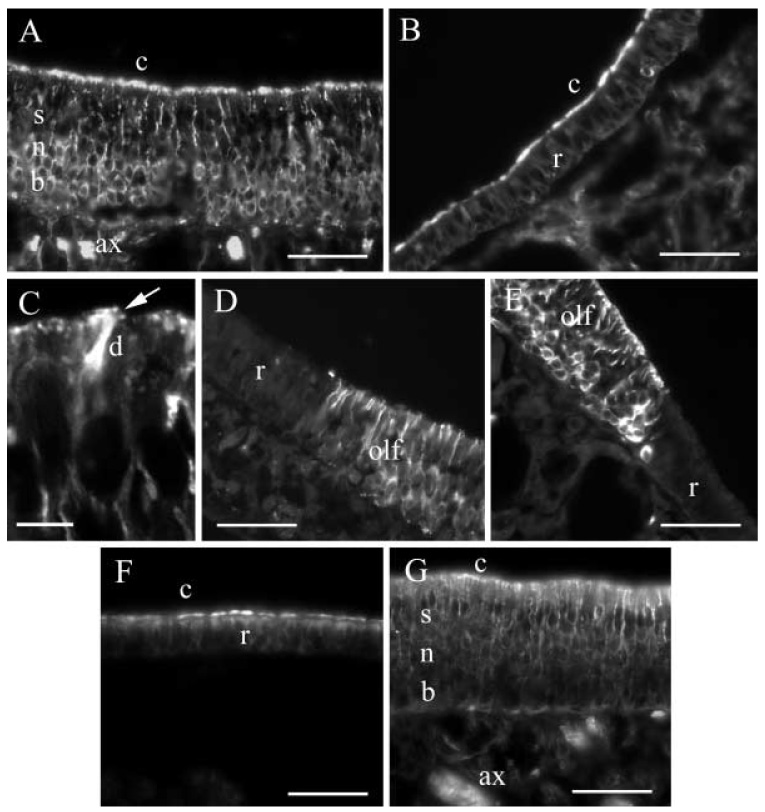
A–G Distribution of β-tubulin isotypes in gerbil nasal epithelia. A βI-Tubulin in a section of olfactory epithelium, showing label in olfactory neurons (n), in basal cells (b), in the axons of sensory neurons (ax), and in cilia (c), but not in supporting cells (s). B βI-Tubulin in respiratory epithelium (r) in which the label is present in the cilia (c) only. C Confocal view of a section of the olfactory epithelium with βI-tubulin in cilia (arrow) and a dendrite (d). D βII-Tubulin in a transition region between olfactory (olf) and respiratory epithelium (r); note the absence of label in the respiratory epithelium. E βIII-Tubulin in a transition region between olfactory (olf) and respiratory epithelium (r); again, note the absence of label in the respiratory epithelium. F βIV-Tubulin in respiratory epithelium (r), showing label in the cilia (c). G Section of olfactory epithelium stained for βIV-tubulin, with label in olfactory neurons (n), in cilia (c), and in the axons of sensory neurons (ax), but not in basal cells (b) or in supporting cells (s). Bars 50 µm in A, B. D–G, 6 µm in C
In the respiratory epithelium (Fig. 2B), labeling for βI-tubulin was strongest in the cilia layer. Only background levels of label were seen in the rest of the respiratory epithelium.
On examination under the confocal microscope, labeling for βI-tubulin could be seen in fine processes emanating from the dendrites of sensory neurons (Fig. 2C, arrow). These appeared to be truncated olfactory cilia, which confirmed the impression obtained from light-microscope observation that cilia were labeled.
In the olfactory epithelium, the labeling pattern for βII- and βIII-tubulin was essentially identical to that for βI-tubulin. Label was seen in olfactory neurons and in basal cells and not in supporting cells. In olfactory neurons, label was found in axons, perikarya, dendrites, and (by confocal inspection) in cilia. However, in the respiratory epithelium, no labeling for βII- or βIII-tubulin was detected. Thus, for both βII- and βIII-tubulin, there was an abrupt transition from label in the thicker olfactory epithelium to the absence of label in the thinner respiratory epithelium (Fig. 2D, E).
In the respiratory epithelium, label for βIV-tubulin was found strongly in the cilia but not in the perikarya (Fig. 2F) in essentially the same pattern as that for βI-tubulin. In the olfactory epithelium, however, label for βIV-tubulin was observed in the cilia, perikarya and dendrites of olfactory neurons (Fig. 2G). In the deeper layers of the sensory epithelium, label was strikingly absent from the perikarya of cells in the basal cell layer but was present in the axons. Thus, βIV-tubulin was the only isotype not observed in basal cells.
Discussion
In its original formulation, the multi-tubulin hypothesis (Fulton and Simpson 1976) envisaged specific functions for each isotype. The complexity of the isotype synthesis patterns that have so far been observed, in which even cells of similar function in the same organ express different isotypes (Roach et al. 1998; Hallworth and Ludueña 2000; B. Perry, H. Jensen-Smith, R. F. Ludueña, R. Hallworth, in preparation), seemingly refutes this form of the hypothesis. Here, we have examined whether cells might sequester different isotypes to different pools in the same cell, the olfactory neuron, for functional reasons. However, olfactory neurons were found to express all four studied isotypes (βI, βII, βIII, and βIV-tubulin) in all compartments. In contrast, respiratory epithelial cells selectively synthesize only two of the four β-tubulin isotypes examined, viz., βI and βIV.
We have not seen label for any β-tubulin isotypes in supporting cells. Undoubtedly, these cells have at least some microtubules, although they may be few in number. The isotypes found in basal cells do not include one found in olfactory neuron somas, viz., βIV-tubulin. Because basal cells develop into olfactory neurons, βIV gene expression in olfactory sensory neurons appears to coincide with the appearance of cilia. βIV-Tubulin is apparently a common feature of cilia. The presence of βIV-tubulin in axonemes is also consistent with the prediction of Raff et al. (1997) who have postulated that, for a β-tubulin isotype to be in an axonemal microtubule, it must have the sequence EGEFEEE near its C-terminus. βIV-Tubulin (both βIVa and βIVb) is the only vertebrate isotype that contains this sequence (Ludueña 1998). In the axoneme of rod outer segments, βIV-tubulin has been found but not βII- or βIII-tubulin (βI-tubulin was not tested; Renthal et al. 1993). In vestibular hair cells, both βI- and βIV-tubulin are found in cilia (B. Perry, H. Jensen-Smith, R. F. Ludueña, R. Hallworth, in preparation). βIV-Tubulin has been localized by immuno-electron microscopy to the axonemal microtubules of bovine retinal rod cells and bovine tracheal cilia (Renthal et al. 1993). It is also the only isotype that has been found in oviduct epithelial cilia (Roach et al. 1998) and appears to be the major isotype in mouse sperm flagella (Lu et al. 1998). This isotype, which seems to be essential for cilia and flagella, is apparently synthesized in the olfactory epithelium only when the basal cell matures into a sensory neuron and synthesizes cilia. It is likely that, during the developmental program of the olfactory neuron, βIV synthesis may be initiated only when it is needed for the synthesis of cilia. The presence of the βIV isotype could therefore function as a useful marker for the transition from basal cell to sensory neuron.
Acknowledgements
We thank Tom Adrian, Xinquan Li, and Xianzhong Ding for assistance with the Western blots.
This work was supported by NIH grant CA26376, US Army grant DAMD17-98-1-8246, and Welch Foundation grant AQ-0726 to R.F.L., and NIH grant DC02053 to R.H. Purchase of the confocal microscope used in this study was made possible by grants from the Taub Foundation and the Nebraska Health Futures Foundation
Contributor Information
Karen Woo, The Medical School, University of Texas Health Science Center at San Antonio, San Antonio, TX 78229-3900, USA.
Heather C. Jensen-Smith, Department of Biomedical Sciences, Creighton University, Omaha, NE 68178, USA
Richard F. Ludueña, Department of Biochemistry, University of Texas Health Science Center at San Antonio, San Antonio, TX 78229-3900, USA
Richard Hallworth, Department of Biomedical Sciences, Creighton University, Omaha, NE 68178, USA, e-mail: hallw@creighton.edu Tel.: +1-402-2803057, Fax: +1-402-2802690.
References
- Banerjee A, Roach MC, Wall KA, Lopata MA, Cleveland DW, Ludueña RF. A monoclonal antibody against the type II isotype of β-tubulin. Preparation of isotypically altered tubulin. J Biol Chem. 1988;263:3029–3034. [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Trcka P, Ludueña RF. Increased microtubule assembly in bovine brain tubulin lacking the type III isotype of β-tubulin. J Biol Chem. 1990;265:1794–1799. [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Trcka P, Ludueña RF. Preparation of a monoclonal antibody specific for the class IV isotype of β-tubulin. Purification and assembly of αβII, αβIII, and αβIV tubulin dimers from bovine brain. J Biol Chem. 1992;267:5625–5630. [PubMed] [Google Scholar]
- Burton PR. Ultrastructural studies of microtubules and microtubule organizing centers of the vertebrate olfactory neuron. Microsc Res Tech. 1992;23:142–156. doi: 10.1002/jemt.1070230205. [DOI] [PubMed] [Google Scholar]
- Fulton C, Simpson PA. Selective synthesis and utilization of flagellar tubulin. The multi-tubulin hypothesis. In: Goldman R, Pollard T, Rosenbaum J, editors. Cell motility. vol 3. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 1976. pp. 987–1005. [Google Scholar]
- Graziadei PPC. The ultrastructure of vertebrates olfactory mucosa. In: Friedmann I, editor. The ultrastructure of sensory organs. Amsterdam: North Holland; 1973. pp. 267–305. [Google Scholar]
- Hallworth R, Ludueña RF. Differential expression of β tubulin isotypes in the adult gerbil organ of Corti. Hear Res. 2000;148:161–172. doi: 10.1016/s0378-5955(00)00149-0. [DOI] [PubMed] [Google Scholar]
- Lu Q, Moore GD, Walss C, Ludueña RF. Structural and functional properties of tubulin isotypes. Adv Struct Biol. 1998;5:203–227. [Google Scholar]
- Ludueña RF. The multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- Raff EC, Fackenthal JD, Hutchens JA, Hoyle HD, Turner FR. Microtubule architecture specified by a β-tubulin isoform. Science. 1997;275:70–73. doi: 10.1126/science.275.5296.70. [DOI] [PubMed] [Google Scholar]
- Renthal R, Schneider BG, Miller MA, Ludueña RF. βIV is the major β-tubulin isotype in bovine cilia. Cell Motil Cytoskel. 1993;25:19–29. doi: 10.1002/cm.970250104. [DOI] [PubMed] [Google Scholar]
- Roach MC, Boucher VL, Walss C, Ravdin PM, Ludueña RF. Preparation of a monoclonal antibody specific for the class I isotype of β-tubulin: the β isotypes of tubulin differ in their cellular distributions within human tissues. Cell Motil Cytoskel. 1998;39:273–285. doi: 10.1002/(SICI)1097-0169(1998)39:4<273::AID-CM3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Walss-Bass C, Prasad V, Kreisberg JI, Ludueña RF. Interaction of the βIV-tubulin isotype with actin stress fibers in cultured rat kidney mesangial cells. Cell Motil Cytoskel. 2001;49:200–207. doi: 10.1002/cm.1033. [DOI] [PubMed] [Google Scholar]


