Summary
In previous studies in Drosophila, Nielsen et al. hypothesized that the β tubulin C-terminal axonemal motif ‘EGEFXXX’, where X is an acidic amino acid, is required for ciliary function and assembly (Nielsen et al., 2001, Curr. Biol. 11, 529–533). This motif is present in some but not all mammalian β tubulin isotypes. We therefore investigated whether this motif is important in ciliary function in mammals. In a preparation of isolated, ATP-reactivated bovine tracheal cilia, we found that monoclonal antibodies directed against the C-terminus of βI, βIV and βV tubulin blocked ciliary beating in a concentration dependent manner. Antibodies against other epitopes of β tubulin were ineffective, as were antibodies against α tubulin. Peptides consisting of the axonemal motif and motif-like sequences of these isotypes blocked ciliary beating. These results suggest that the axonemal motif sequences of βI, βIV and βV tubulin are essential for ciliary function. Peptides consisting of corresponding C-terminal sequences in α tubulin isotypes were also ineffective in blocking ciliary beating, which suggests that the C-terminus of α tubulin is not directly involved in cilia function in mammals.
Keywords: Axonemal function, β Tubulin isotype, Cilia, Ciliary beat frequency
Introduction
Microtubules are the main structures of cellular functions, such as the spatial distribution of organelles and cell motility. In addition, they constitute the scaffold within cilia and flagella. Microtubules are formed by protofilaments of αβ tubulin heterodimers stacked head to tail at 8 nm intervals (Meurer-Grob et al., 2001). The sequences of β tubulin isotypes are highly conserved in evolution and even isotypic differences are conserved (Ludueña, 1998). Their main differences lie in the C-terminal structure after helix H12 composed of 18 amino acid residues. Based on experiments with the testis-specific Drosophila β2 tubulin isotype, Nielsen et al. (Nielsen et al., 2001) suggested that a specific sequence in the C-terminus [termed here ‘the axonemal motif’ EGEFXXX, where X is either glutamic (E) or aspartic (D) acid] is required for correct axonemal function and assembly, assayed by fertility and electron microscopy. In mammals, this sequence is found in the C-terminus of βIV tubulin at positions 433–439, but axonemal motif-like sequences are also found in βI, βII and βV tubulin in the same positions (see Table 1). In recent studies, βI and βIV tubulin were found in all mammalian axonemal structures tested, including the cilia of vestibular hair cells, olfactory sensory neurons and ciliated epithelial cells of the nose, trachea, ependyma, fallopian tube and testis (Jensen-Smith et al., 2003; Perry et al., 2003; Renthal et al., 1993; Woo et al., 2002). In olfactory neurons, other isotypes (βII and βIII tubulin) were also present (Woo et al., 2002). Recently, βV tubulin has been found sporadically in axonemal structures, but is apparently not required for ciliary function and assembly (R. Hallworth and R. F. Ludueña, unpublished data). The only isotypes common to all cilia appear to be βI and βIV tubulin, and their C-termini likely play a key role in ciliary function.
Table 1.
Relationship between the β tubulin C-terminal sequence, the axonemal motif peptide, and isotype-specific β tubulin C-terminal tail peptides, by residue position
| AA | 432 | 433 | 434 | 435 | 436 | 437 | 438 | 439 | 440 | 441 | 442 | 443 | 444 | 445 | 446 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| βI | E | E | E | D | F | G | E | E | A | E | E | E | A | ||
| βII | E | Q | G | E | F | E | E | E | E | G | E | D | E | A | |
| βIII | E | G | E | M | Y | E | D | D | E | E | E | S | E | S | Q*: |
| βIV | E | E | G | E | F | E | E | E | A | E | E | E | V | A | |
| βV | G | E | E | A | F | E | D | E | D | E | E | E | I | N | E |
The shaded peptides indicate the axonemal motif (in βIV) or its nearest equivalent (in βI, βII, and βV). Notice that the axonemal motif and motif-like sequences pivot around F at position 436, indicated in red, whereas βIII contains the amino acid 436Y, shown in blue. The underlined peptides are those against which the isotype-specific antibodies used in this study were raised.
βIII tubulin is 449 amino acids long, and the C-terminal tail peptide is 443SESQGPK.
AA, amino acid. Data from Ludueña, 1998.
There are seven known isotypes of β tubulin in mammals. Their presence and composition are tissue-type specific, but no functional correlation has been made to date (Hallworth and Ludueña, 2000; Ludueña, 1993). To investigate the functional significance of the C-termini of β tubulin isotypes in mammals, we used a preparation of isolated, de-membranated ATP-activated beating bovine cilia (Hastie et al., 1986; Wyatt et al., 2005). We measured the effect on ciliary beat frequency (CBF) of isotype-specific monoclonal antibodies directed against the C-termini of tubulin and monoclonal antibodies directed against other epitopes of β tubulin, as well as monoclonal antibodies against various epitopes of α tubulin, using a new, rapid digital video motility analysis method (Sisson et al., 2003). In addition, we examined the effect on CBF of peptides containing (1) the C-terminal amino acid sequences of β tubulin isotypes against which the antibodies were raised (C-terminal tail peptides, CTT peptides) and (2) the peptides containing the amino acid sequences of the isotype-specific axonemal motif or the closest equivalent. This is the first study to investigate the isotype-specific differences of tubulin isotypes in mammalian cilia in a functional, motility-based assay.
Materials and Methods
Preparation of cilia
Isolated bovine tracheal cilia were prepared from fresh tracheas by the method of Hastie et al. (Hastie, 1995; Hastie et al., 1986). Tracheas from bovine lungs were obtained from a local abattoir, dissected, cleaned from debris and rinsed in cold PBS (pH 7.4). The orifices were clamped and the tracheas were then incubated with approximately 200 ml of a Triton X-100-based cilia extraction buffer under continuous shaking for 90 seconds [after Hastie, containing Tris HCl (20.0 mM), NaCl (50.0 mM), CaCl2 (10.0 mM), EDTA (1.0 mM), 2-mercaptoethanol (7.0 mM), Triton X-100 (100.0 mM) and dithiotreitol (DTT, 1.0 mM) pH 7.4]. The fluid containing cilia was filtered through micromesh (pore size 0.45 µm). After centrifugation at 10,000 g at 4°C for 7 minutes, the supernatant was discarded and the pelleted cilia were resuspended in resuspension buffer [Tris HCl (20.0 mM), KCl (50.0 mM), MgCl2-6H2O (4.0 mM), EDTA (0.5 mM), DTT (1.0 mM), Soybean Trypsin Inhibitor (10.0 mM), pH 8.0]. The protein concentration was determined using a Bradford-dye assay. The cilia preparation was frozen in a 25% (by weight) sucrose solution in a concentration of one mg/ml in 100 µl aliquots and stored at −80°C.
Activation of cilia and measuring CBF
A thawed aliquot of frozen demembranated cilia was mixed with resuspension buffer and cAMP (in resuspension buffer, final concentration 1.0 mM) in a 1:2:1 dilution (by volume). 20 µl of the solution containing 0.25 mg/ml protein were then plated into wells of a 48 well culture plate (Corning Inc., Corning, NY). The plate was centrifuged at 400 g for 2 minutes at room temperature to attach the cilia to the bottom of each well. A first measurement was taken (time t=0) and 20 µl of ATP in resuspension buffer (Sigma, MO) (final concentration 1.25 mM) were added to activate ciliary beating, typically at a frequency of 8–10 Hz. Test solutions containing antibodies or peptides in resuspension buffer were added at time t=3 minutes, after three readings of activated cilia. Measurements of CBF were made at intervals of 1 minute for 15–20 minutes. ATP depletion occurred at approximately 25 minutes after ATP addition. Reactivation of cilia was possible with further ATP addition, but was performed for control reasons only. For CBF measurements, antibodies and peptides were diluted in resuspension buffer.
CBF was measured using the rapid automated digital analysis system described earlier (Sisson et al., 2003). Cilia were visualized using phase contrast microscopy (Olympus IMT-2 inverted phase-contrast microscope, Olympus America Inc., Melville, NY) directly connected to a digital camera (Kodak Megaplus ES 310 analog/digital video camera; Eastman Kodak Motion Analysis System Division, San Diego, CA) and a PC workstation (Dell Inc., Round Rock, TX). CBF was determined using Fourier analysis of the entire field of view from the digitized video.
Generation of antibodies against β tubulin isotypes
Antibodies against the C-termini of β tubulin isotypes were raised in mouse hybridoma cells and purified as previously described (Banerjee et al., 1990; Banerjee et al., 1992; Banerjee et al., 1988; Roach et al., 1998). Each monoclonal antibody was prepared to an epitope unique to the C-terminus of that isotype. The C-termini of βIVa and βIVb are identical in amino acid sequence and therefore the antibody against βIV tubulin was unable to discriminate between them. All antibodies used in this study were purified monoclonal IgG class 1 antibodies except for anti βII and βIII tubulin, which were IgG2b (Table 2). The antibody against the βV tubulin C-terminus has not been previously reported (A.B. et al., unpublished). The monoclonal antibody SHM 12G11 specific for mouse βV tubulin was generated by immunizing mice with the C-terminal peptide EEEINE. The peptide, coupled to keyhole limpet hematocyanin (KLH), was used to immunize mice. The antibody was purified from the hybridoma supernatant using a protein-G-sepharose column. Initial immunization was performed with peptide-KLH while the subsequent immunizations were performed with peptide coupled to BSA, according to Banerjee et al. (Banerjee et al., 1988)
Table 2.
Epitopes of antibodies
| Antibody | Epitope | Ig isotype | Host species | Concentation used for immunoblots | Concentration used for immunohistochemistry | Order number |
|---|---|---|---|---|---|---|
| βI tubulin | EEAEEEA | IgG1 | Mouse | 1:50,000 | 1:200 | Sigma T7816 |
| βII tubulin | EGEDEA | IgG2b | Mouse | 1:500 | 1:500 | Sigma T8535 |
| βIII tubulin | SESQGPK | IgG2b | Mouse | 1:500 | 1:1000 | Sigma T8660 |
| βIV tubulin | EAEEEVA | IgG1 | Mouse | 1:5,000 | 1:400 | Sigma T7941 |
| βV tubulin | EEEINE | IgG1 | Mouse | 1:1,000 | 1:200 | Not commercially available |
| TUB 2.1 | Unspecified conserved epitope within AA 281–446 of rat brain β tubulin (Matthes et al., 1988) | IgG1 | Mouse | 1:10,000 | 1:200 | Sigma T4026 |
| DM1A | AA 426–450 of α tubulin (Breitling and Little, 1986) | IgG1 | Mouse | 1:30,000 | 1:200 | Sigma T9026 |
| α tubulin C-terminus | SEAREDMAALEKDYEEV (AA 419–435 of human α tubulin) | IgG1 | Mouse | 1:10,000 | 1:1000 | Synaptic Systems # 302 201 |
| α tubulin N-terminus | AA 65–97 of α tubulin | IgG1 | Mouse | 1:10,000 | 1:1000 | Abcam ab7750 |
Other antibodies were commercially obtained. The details are summarized in Table 2. Antibodies were used at a concentration of 20 µg/ml, which was within the range of concentrations used in several previous studies that employed antibodies to interfere with ciliary and flagellar beating (Bré et al., 1996; Audebert et al., 1999; Cosson et al., 1996; Gagnon et al., 1996). Controls to assure specific antibody binding to axonemal tubulin were performed by immunohistochemistry and immunoblotting.
Visualization of antibody-labeled bovine cilia
Isolated cilia were plated onto concanavalin A-coated glass microscope slides (Sigma), allowed to settle for 1 hour and fixed using a solution of 1% paraformaldehyde in PBS. No permeabilizing step was necessary since the cilia are already demembraneted. Bound antibodies were visualized using goat anti-mouse secondary antibodies conjugated to Alexa 488 (Molecular Probes, Eugene, OR). Specimens were viewed using an Axioskop II microscope (Carl Zeiss, Jena, Germany) equipped with 40X and 100X objectives and captured using a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI). Images were prepared for presentation using Photoshop (Adobe Systems, San Jose, CA). Negative controls were performed by omitting the primary antibody. Positive controls for the isotype-specific β tubulin antibodies were performed by staining organ of Corti tissue. The results seen were in concordance with previous observations (Hallworth and Ludueña, 2000; Hallworth et al., 2000; Vent et al., 2004) (see supplementary material Fig. S1).
Immunoblotting
Immunoblots were performed under standard conditions with all antibodies used in the above experiments. A uniform amount of homogenized axonemal proteins (15 µg) was loaded onto each lane of a 10% polyacrylamide gel (Biorad, Hercules, CA). The protein content had been determined in a Bradford dye assay prior to aliquoting the extracted cilia using a microplate reader and protein (BSA) standards (MPM III 1.080, microplate reader, Biorad). The lanes were run at 100 V and 250 mA for 1 hour in sodium dodecyl sulfate electrophoresis running buffer. Proteins were then transferred from the gels to nitrocellulose sheets at 100 V and 100 mA for 1 hour in a transfer buffer containing Trizma base and glycine (Sigma). The nitrocellulose sheets containing the transferred protein lanes were then cut into strips and were exposed to one of the primary antibodies overnight at 4°C. After thorough rinsing with 1% milk in PBS, the secondary antibody (anti-mouse IgG linked to biotin, Cell Signaling Technology, Beverly, MA), was added and the strips were incubated on a rocker for 1 hour at room temperature. The protein strips were then rinsed three times in 1% milk in PBS for 10 minutes and twice in PBS for 10 minutes. They were then treated with SuperSignal West Pico Chemiluminiscent Substrate (Pierce Biotechnology, Inc., Rockford, IL) for 3 minutes and exposed to a CL-XPosure blue X-ray film (Pierce Biotechnology, Inc., Rockford, IL) for three seconds.
Generation of peptides
Axonemal motif peptides were synthesized using a previously published solid phase method (Taylor et al., 2005). Briefly, N-α-butyloxycarbonyl (Boc)-amino acid derivatives (Bachem Biosciences Inc., King of Prussia, PA and ChemImpex, Wood Dale, IL) were coupled to methylbenzhydrylamine resin (Bachem Biosciences Inc., King of Prussia, PA) using the coupling reagent 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate in excess base. All coupling yields were >99% as determined by the quantitative ninhydrin method (Sarin et al., 1981). Boc groups were removed in trifluoroacetic acid (TFA, Acros Organics, Pittsburgh, PA). Peptides were cleaved from the resin using a mixture of trifluoromethanesulphonic acid/TFA/ethanedithiol/thioanisole (1/9/0.5/1, v/v) and immediately desalted on a BioGel P6 column (90X2.5 cm) (Bio Gel P6, Bio Rad, Hercules, CA) in 5% aqueous acetic acid. The desalted material was loaded on to a Vydac C18 HPLC column (25X2.5 cm) (Vydac C18 column, The Sep/a/ra/tions Group, Hesperia, CA) previously equilibrated with a mixture of water and acetonitrile (9/1, v/v) (Fisher Scientific, Pittsburgh, PA) containing 0.1% TFA. The concentration of acetonitrile in the eluent was increased from 10 to 40% over 50 minutes to elute the peptides from the column. Fractions containing the desired peptides were pooled and lyophilized. Peptides were judged to be >95% pure by analytical RP-HPLC and possessed satisfactory amino acid compositions.
The peptides containing the sequences EGEAEEE, EGEGEEE, EDEDEGE and GEMYEDD were custom made peptides purchased from Bachem Biosciences Inc. (King of Prussia, PA). The C-terminal tail peptides were the peptides against which the β tubulin isotype specific antibodies were raised (see Table 1 and Table 2 for sequences). Their synthesis is described elsewhere (Banerjee et al., 1990; Banerjee et al., 1992; Banerjee et al., 1988; Renthal et al., 1993) (A.B. et al., unpublished). Unless otherwise indicated, chemicals were purchased from Sigma-Aldrich Co. (St Louis, MO).
Results
Antibodies against βI, βIV and βV tubulin block ciliary beating
We hypothesized that the C-terminus of β tubulin has functional importance in ciliary beating. Thus, blocking this domain with antibodies should interfere with ciliary beating in isolated bovine cilia. On application of ATP solution (final concentration 1.25 mM) after the initial reading at time t=0, isolated cilia began to beat continuously at about 10 Hz. The CBF reached a typical steady state value by time t=3 minutes, at which time solutions containing the antibody were added at a final concentration of 20 µg/ml (equivalent to 0.33 µM). Axonemes maintained a steady CBF until ATP was depleted, typically after 25 minutes. Fig. 1A shows the effect of application of antibodies on CBF. Each data point is plotted as the mean and one standard error of the mean for at least six experiments. Antibodies against βI, βIV and βV tubulin reduced CBF to zero within 8–10 minutes. By contrast, antibodies against βII and βIII tubulin, as well as the buffer only controls (negative control), did not affect CBF. These results indicate that the isotype-specific antibodies against βI, βIV and βV tubulin interfere with the motility-determining structures of microtubules, and that only those isotype-specific antibodies raised against the C-terminal sequences of βI, βIV and βV tubulin are able to block ciliary beating.
Fig. 1.
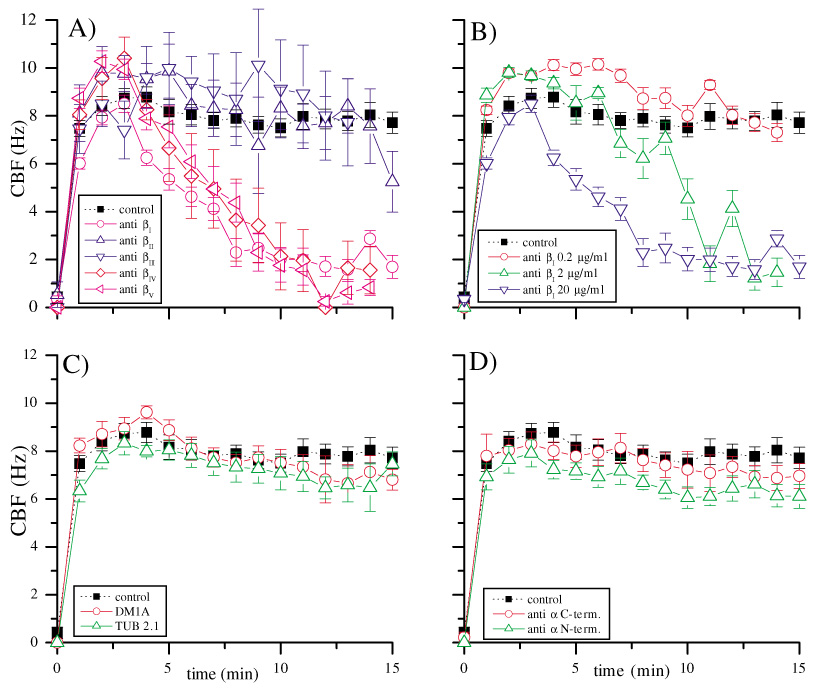
(A) Effect of isotype-specific antibodies against β tubulin on CBF. Final antibody concentrations 0.33 µM, error bars indicate 1 s.e.m. Filled black squares, control; open pink circle, anti βI tubulin; open upward blue triangle, anti βII tubulin; open downward navy triangle, anti βIII tubulin; open red diamond, anti βIV tubulin; open purple triangle, anti βV tubulin. The antibodies depicted in shades of red blocked ciliary beating, the antibodies in blue had no effect on CBF. (B) Concentration dependence of anti βI tubulin; error bars indicate 1 s.e.m. Antibody concentrations: blue triangle, 0.33 µM; green triangle, 0.033 µM; red circle, 3.3 nM. Similar concentration curves were obtained for anti βIV tubulin and anti βV tubulin. (C) Effect of non-isotype-specific, non-C-terminal antibodies against α and β tubulin (DM1A, red circle and TUB 2.1, green triangle), error bars indicate 1 s.e.m.; antibody concentrations 0.33 µM. (D) Effect of anti α tubulin C- (red circle) and N-terminal (green triangle), non-isotype-specific antibodies, error bars indicate 1 s.e.m.; antibody concentrations 0.33 µM.
The effect of the antibodies on CBF is concentration dependent
We hypothesized that the inhibition of ciliary beating by antibodies would be specific and thus concentration dependent. To test this hypothesis, we examined the effect of antibodies at different concentrations. When the concentration of antibodies against βI, βIV and βV tubulin was decreased in a logarithmic manner, the time taken for reduction of CBF to zero was increased. Fig. 1B shows the effect of the antibody against βI tubulin on CBF at varying concentrations (20 µg/ml, 2 µg/ml and 0.2 µg/ml final concentrations, equivalent to 0.33 µM, 0.033 µM and 3.3 nM, respectively). Comparable results were obtained with antibodies against βI and βV tubulin (data not shown). Reduction of antibody concentration by at least two orders of magnitude from the initial concentration was required to reduce the effect of the antibodies to control levels.
Preincubation of axonemes with antibodies inhibited reactivation
As a further test of the specificity of the antibody effect, we pre-incubated cilia with a molar excess (10 µM) of antibody for 1 hour on ice. The high concentration was chosen to assure all epitopes would be bound by antibody. After equilibration to room temperature, ATP was added to activate ciliary beating. Normal activation to a CBF of 10 Hz was achieved in all samples except the ones containing antibodies against βI, βIV and βV tubulin, which did not activate (see supplementary material Fig. S2).
Antibodies against other epitopes of β tubulin, or against α tubulin, have no effect on CBF
We hypothesized that the effects of the antibodies against the C-termini of βI, βIV and βV tubulin on CBF were specific to the C-terminus of tubulin isotypes. To test this hypothesis, we applied monoclonal antibodies against other epitopes of β and α tubulin. Fig. 1C shows the effect on CBF of an antibody against a C-terminal, non-isotype-specific epitope of α tubulin (DM1A) and an antibody against a non-isotype-specific epitope (within position 281–446) of β tubulin (TUB 2.1). The antibodies had no effect on CBF at the same concentration as the maximum concentration used in the above described studies. Even higher concentrations (200 µg/ml or 0.66 µM) did not affect CBF (data not shown). Fig. 1D shows the effect of two monoclonal non-isotype specific antibodies raised against the C- and N-termini of α tubulin, respectively. They had no effect on CBF at comparable concentrations. These experiments further support the inference that the effects of the antibodies against βI, βIV and βV tubulin were specific to the binding sites of those antibodies, and that axonemal motility is dependent on the C-terminal epitope of β tubulin isotypes or its direct proximity against which the antibodies are directed. Furthermore, the results indicate that there is no steric hindrance by ineffective antibodies, even against the C-terminus of α tubulin, to the dynein binding site. They also exclude any non-specific inhibitory effect of the antibodies.
Specific peptides reduce CBF
Nielsen et al. (Nielsen et al., 2001) hypothesized that a certain amino acid sequence, the axonemal motif, is required for ciliary function and assembly. Because of our antibody findings, we tested the axonemal motif hypothesis using synthesized heptapeptides containing the axonemal motif or amino acid sequences in corresponding positions of mammalian βI, βII, βIII, βIV and βV tubulin, hypothesizing that the peptides would mimic the C-terminus of β tubulin and thus competitively inhibit beating. We compared their effects to the effects of the C-terminal tail peptides against which the antibodies were raised.
Fig. 2A–E and Table 3 show the results of these experiments. Each plot shows the effect on CBF of the axonemal motif peptide for each isotype (open red triangles), compared to the effect of the corresponding C-terminal tail peptide (open green circles), at the same molar concentrations of peptide (final concentration 0.33 µM) as was used for the antibodies. Each plot also shows the average of a series of negative control experiments (filled squares). The amino acid sequences of the C-terminal peptides are given in Table 1 and Table 3.
Fig. 2.
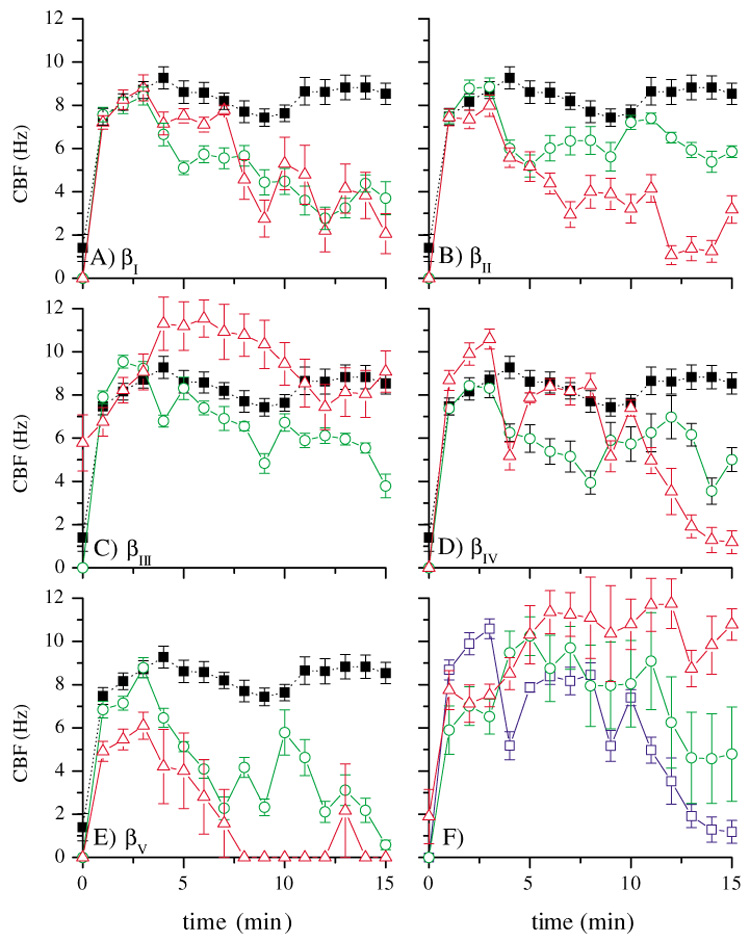
Comparison of the effect of axonemal motif (red triangles) and C-terminal tail peptides (green circles) on CBF and controls (dotted line, black solid squares); error bars indicate 1 s.e.m.; peptide concentrations: 0.33 µM. For sequences, see Table 1. (A) sequences derived from βI tubulin (433EEDFGEE and 438EEAEEEA). (B) sequences derived from βII tubulin (433QGEFEEE and 440EGEDEA). (C) sequences derived from βIII tubulin (433GEMYEDD and 443SESQGPK). (D) sequences derived from βIV tubulin (433EGEFEEE and 439EAEEEVA). (E) sequences derived from βV tubulin (433EEAFEDE and 441EEEINE). (F) Concentration-dependent effect of the axonemal motif peptide (EGEFEEE), concentrations: 0.33 µM (blue squares), 0.033 µM (green circles) and 3.3 nM (red triangles).
Table 3.
Effects of Peptides on CBF
| Axonemal motif peptide (in βIV) or heptapeptide in corresponding position | Effect | C-terminal tail peptide | Effect | Control peptides | Effect |
|---|---|---|---|---|---|
| βI(433EEDFGEE) | + | βI(438EEAEEEA) | + | EGEAEEE | − |
| βII(433QGEFEEE) | ++ | βII(440EGEDEA) | − | EGEGEEE | − |
| βIII(433GEMYEDD) | − | βIII(443SESQGPK) | − | EDEDEGE | − |
| βIV(433EGEFEEE) | +++ | βIV(439EAEEEVA) | ++ | ||
| βV(433EEAFEDE) | +++ | βV(441EEEINE) | ++ |
−, no effect; +, some inhibitory effect; ++, moderate inhibitory effect; +++, strong inhibitory effect leading to reduction of CBF to 0 Hz
The results of these experiments are shown in Fig. 2A–E and are summarized in Table 3. In general, the axonemal motif peptides were effective in reducing CBF while the C-terminal tail peptides were much less so. For example, the βIV and βV tubulin axonemal motif peptides completely abolished ciliary beating in 15 minutes or less, while the corresponding C-terminal tail peptides had only weak effects on CBF (Fig. 2D,E). Both the axonemal motif and C-terminal tail peptide of βIII tubulin were ineffective in reducing CBF (Fig. 2C), as expected since βIII tubulin is not present in the bovine cilia preparation. However, the axonemal motif peptide of βII tubulin, which is also not present in bovine tracheal cilia, was effective in abolishing ciliary beating, whereas the corresponding C-terminal tail peptide had only marginal effect (Fig. 2B). Further, both βI tubulin peptides were effective in reducing CBF (Fig. 2A).
It was observed that the content of acidic amino acids (E and D) accounts for much of the inhibitory effect on CBF. The axonemal motif-like peptides generally had five acidic amino acids (with the exception of the peptides of βII tubulin and βIII tubulin that have four), while the C-terminal tail peptides have four acidic amino acids (with the exception of the peptides of βI tubulin and βIII tubulin that have five and one, respectively). The C-terminal tail peptide of βI tubulin (438EEAEEEA) was noted to be equally effective in reducing CBF as its axonemal motif peptide (433EEDFGEE).
The effect of the axonemal motif peptide is concentration dependent
We hypothesized that the effect of the βIV tubulin axonemal motif peptide EGEFEEE is specific and therefore concentration dependent. To test this, the peptide was added to the activated cilia preparation at concentrations of 0.33 µM, 0.033 µM and 3.3 nM. The highest concentration of peptide reduced CBF the fastest, while the lowest concentration was essentially ineffective (Fig. 2F).
The central phenylalanine is important in the axonemal motif sequence
We observed that the axonemal motif peptides that reduced CBF contained, in addition to several acidic residues, a central F at position 436. Also, F436 is not present in the axonemal motif peptide of βIII tubulin, which did not reduce CBF. To test the hypothesis that F436 is important, we replaced F436 in the axonemal motif sequence (EGEFEEE) with alanine, resulting in the sequence EGEAEEE. As shown in Fig. 3A, this peptide had no effect on CBF, demonstrating the importance of F436.
Fig. 3.
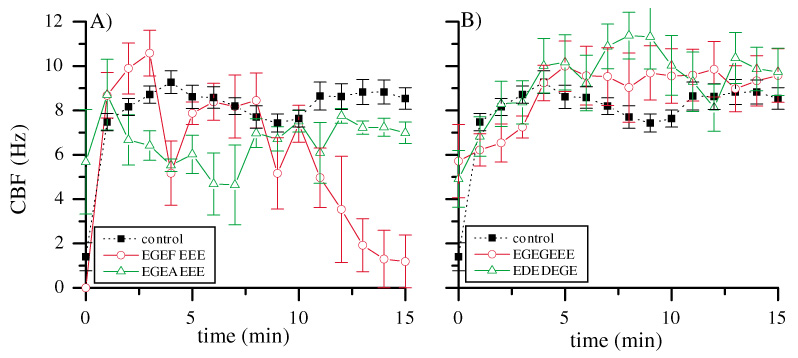
(A) Alanine scan of the axonemal motif peptide: when the central F is replaced by an A, there is no more effect of the peptide on CBF, red circles, 433EGEFEEE, green triangles, 433EGEAEEE. Peptide concentrations: 0.33 µM. Error bars indicate 1 s.e.m. (B) The effect of α tubulin axonemal motiflike peptides on CBF, red circles, 441EGEGEEE, green triangles, 441EDEDEGE. Peptide concentrations: 0.33 µM. Error bars indicate 1 s.e.m.
Axonemal motif-like sequences in the C-terminus of α tubulin are not involved in ciliary beating
An E-rich sequence similar to the axonemal motif is present in the C-termini of several α tubulin isotypes between positions 441 and 447, but these sequences lack the central F (Table 4). To test the hypothesis that the C-terminus of α tubulin is not involved in ciliary beating, we synthesized two peptides that represented the sequences of four α tubulin isotypes: EGEGEEE (αII, αIII) and EDEDEGE (αI, αIV) (Ludueña and Banerjee, 2005a; Ludueña and Banerjee, 2005b; Ludueña and Banerjee, 2005c; Ludueña and Woodward, 1975). Neither peptide affected ciliary beating at comparable concentrations to previous experiments, supporting the antibody observations that the C-terminus of α tubulin may not be directly involved in ciliary beating (Fig. 1C,D and Fig. 3B).
Table 4.
Comparison of α tubulin sequences
| AA | 438 | 439 | 440 | 441 | 442 | 443 | 444 | 445 | 446 | 447 | 448 | 449 | 450 | 451 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| αI | D | S | Y | E | D | E | D | E | G | E | E | |||
| αII | D | S | V | E | G | E | G | E | E | E | G | E | E | Y |
| αIII | D | S | V | E | G | E | G | E | E | E | G | E | E | Y |
| αIV | D | S | Y | E | D | E | D | E | G | E | E | |||
| αVII | D | S | V | E | A | E | A | E | E | G | E | E | Y | |
| αVIII | D | S | F | E | E | E | N | E | G | E | E | F |
Data from Ludueña and Banerjee, 2005a. Shaded peptides indicate axonemal motif-like sequences.
Antibodies are specific for axonemal tubulin
We hypothesized that the effects of antibodies against βI, βIV and βV tubulin on ciliary beating were specific for binding at the β tubulin isotype specific epitopes and are not due to unspecific inhibition of beating. To test this hypothesis, all antibodies were applied to fixed preparations of bovine cilia and processed for immunofluorescence as described in the Materials and Methods. Antibodies against βI, βIV and βV tubulin, which blocked ciliary beating, labeled cilia (Fig. 4). The same was observed for the antibodies against the C- and N-termini of α tubulin, as well as for antibodies against conserved, non-isotype-specific epitopes in the α and β tubulin protein (DM1A and TUB 2.1). However, they did not inhibit ciliary beating, making steric hindrance as a reason for CBF inhibition by anti βI, βIV and βV tubulin antibodies unlikely. As expected, antibodies against β tubulin isotypes not present in tracheal cilia (anti βII and anti βIII tubulin) did not label isolated cilia in the described preparation (Fig. 4A,B).
Fig. 4.
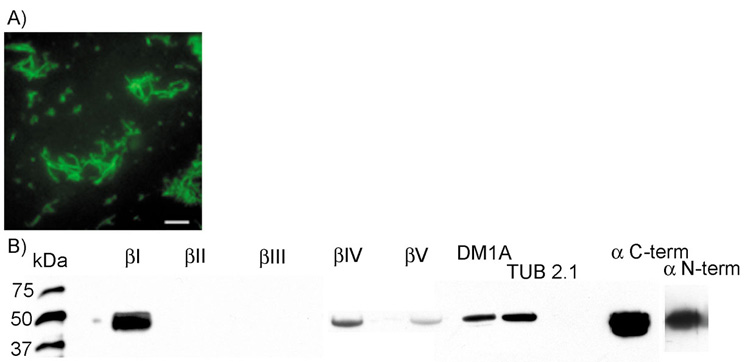
(A) Example of a labeling of isolated bovine tracheal cilia by the isotype-specific antibody against βIV tubulin. Scale bar 10 µm. (B) Immunoblotting of isolated bovine tracheal cilia by antibodies against α and β tubulin.
Immunoblots of purified de-membranated cilia with all of the above mentioned primary antibodies indicated that labeling is restricted to a single band of molecular mass close to 55 kDa (Fig. 4B), the expected molecular mass of β tubulin (Ludueña, 1998). The amino acid sequence difference between β tubulin isotypes is so small that the isotypes are indistinguishable by molecular mass alone. Strong labeling appeared with anti βI, DM1A and Tub 2.1 (antibodies against conserved, non-isotype specific epitopes of α and β tubulin) as well as the C- and N-terminus specific antibodies against α tubulin. As expected, antibodies against βII and βIII tubulin did not label the axonemal proteins at all, because βII and βIII tubulins are not synthesized in bovine tracheal cilia. A weak band occurred at 55 kDa with labeling by antibodies against βIV and βV tubulin. Controls for the well-established antibodies against βII and βIII tubulin were performed by staining the organ of Corti (supplementary material Fig. S1).
Discussion
The axonemal motif is essential for ciliary function
Our findings suggest that the axonemal motif, EGEFEEE, is required for ciliary function and dynein binding. Generally, the results were consistent with the number of acidic amino acids (five E or Ds) determining its effectiveness, with the exception of the axonemal motif-like peptide of βII tubulin, which contains only four acidic amino acids but is equally effective as the axonemal motif. Mizuno et al. (Mizuno et al., 2004) inferred that dynein and kinesin share an overlapping binding site on the tubulin C-terminus. That paper indicated that dynein binds to either α or β tubulin, but not both and their results were suggestive of β tubulin being the target protein. Skiniotis et al. (Skiniotis et al., 2004) further support the importance of the tubulin C-terminus by its subtilisin digestion. However, we do not exclude the possibility that other regions of tubulin are also required, analogous to the weak and strong binding states of kinesin (Skiniotis et al., 2004). Nevertheless, findings from kinesin cannot definitively be applied to dynein. Kinesin contains a lysine (K)-rich sequence on its tubulin-binding site, termed the K-loop, which acts as a counterpart to the acidic C-terminus of tubulin (E-hook) (Okada and Hirokawa, 2000). No such sequence exists in dynein (Asai and Koonce, 2001; Gee et al., 1997). Inferences drawn from the interaction of kinesin with tubulin to dynein must be made carefully.
The axonemal motif sequence pivots around the central F
We found that, in general, C-terminal acidic residues (E or D) are required for ciliary beating. The higher the content of acidic amino acids in the peptide, the stronger was the inhibitory effect on beating. Further, we showed that this region must pivot around the central F436 found in some β tubulin isotypes. However, the study referred to earlier by Okada and Hirokawa did not evaluate dynein and did not focus on the isotype-specific differences in the C-termini of β tubulins (Okada and Hirokawa, 2000). In our study, the further requirement of a central F was established in addition to acidity as a key characteristic for axonemal function. In general, the C-terminal tail peptides, which did not contain F, were less effective than the axonemal motif peptides, with the exception of the peptides for βI and βV tubulin, which were equally effective. Our hypothesis is supported by the lack of effect of the two α tubulin peptides (Fig. 3) which, despite being highly acidic, lack the central F.
α tubulin is not directly involved in ciliary beating
Our evidence that α tubulin is not directly involved in ciliary beating is, on first inspection, surprising. Three different antibodies against α tubulin, two of which were directed against a conserved, non-isotype specific C-terminal amino acid sequence (419–435 and 426–450), had no effect on ciliary function. Furthermore, peptides of the α tubulin axonemal motif sequence did not affect CBF. Our results are consistent with recent literature that suggests no involvement of α tubulin in ciliary beating (Audebert et al., 1999; Cosson et al., 1996). Some earlier investigations favoured α tubulin being important for ciliary function (Gagnon et al., 1996; Hirose et al., 1999). Goldsmith et al. (Goldsmith et al., 1991; Goldsmith et al., 1995) suggest the involvement of both α and β tubulin for dynein binding (Goldsmith et al., 1991; Goldsmith et al., 1995). The role of α tubulin might lie in other, yet undetermined functions. For example, α tubulin may function in binding of microtubule-associated proteins (MAPs) (Rodionov et al., 1990) or interact with dynein for control of microtubule dynamics (Hunter and Wordeman, 2000). However, those studies were conducted on cytoplasmic dynein. Hunter and Wordeman showed that the C-terminus of tubulin is necessary for MAP binding and further suggested a tubulin-binding site of dynein outside the C-terminus. Hoenger et al. describe that each kinesin dimer occupies two microtubule-binding sites (Hoenger et al., 2000). That might be true for dynein also, but has not been investigated so far.
The periodicity of the αβ tubulin heterodimer in the microtubule is 8 nm. In cytoplasmic dynein, the step size has been proposed to be dependent on cargo load and to have a minimum of 8 nm (Mallik et al., 2004). If the step size of axonemal dynein is similar, dynein may skip α tubulin and interact only with the C-terminus of β tubulin.
The sequences of the peptides EDEDEGE447 (in αI and αIV tubulin) and EGEGEEE447 (in αII and αIII tubulin) were derived from Ludueña and Banerjee (Ludueña and Banerjee, 2005a). Due to the absence of isotype specific antibodies for α tubulins, previous information on the possible distribution of α tubulin isotypes was obtained by in situ hybridization. The presence of these α isotypes (I–IV) in the bovine tracheal cilia preparation is plausible but not established (Ludueña and Banerjee, 2005a), thus there is a small possibility that the peptides have no effect due to the absence of these α tubulin isotypes in bovine cilia. However, antibodies against various non-isotype-specific epitopes of α tubulin, including the C-terminus, also did not affect CBF.
Post-translational modifications may alter the secondary structure of the C-terminus
Post-translational modifications (PTM) in tubulins occur mainly at the highly flexible C-terminus (Ludueña, 1998). The most recently discovered PTM, polyglycylation, has been shown to occur in axonemal tubulin from Paramecium to sea urchin and mammalian spermatozoa (Bré et al., 1996). Bré et al. also suggested an involvement of polyglycylated tubulin in axoneme motility since AXO 49 and TAP 952, monoclonal antibodies against mono- and polyglyclyated C-terminal peptides from Paramecium axonemal tubulin, specifically inhibited the reactivated motility of sea urchin spermatozoa. Polyglycylation occurs at the C-terminus and is highly variable in its amount (up to 32 glycines on a side chain off the γ-carboxyl chain of glutamic acids E435, E437 and E438). Polyglycylation has not yet been shown to be functionally required, but may affect the secondary structure of the highly flexible C-terminus in tubulin. Gagnon et al. reported that a different PTM, polyglutamylation, in the lateral chain of α tubulin plays a dynamic role in spermatozoan motility (Gagnon et al., 1996). Possibly due to steric hindrance by the large antibody, Gagnon et al. could not determine the amino acid sequence required for ciliary beating.
Isotype specificity of β tubulin in axonemes
This leads us back to the initial question of the existence of functional correlations of β tubulin isotypes: why do some cells synthesize β tubulin isotypes in one pattern and others synthesize different isotypes? Why, if the isotypes are so similar in amino acid sequence, is there still a requirement for the different isotypes? The answer may lie in the highly variable C-terminus.
It was previously found that βI and βIV tubulin are synthesized by all ciliated cells types tested in the gerbil (Jensen-Smith et al., 2003). It was inferred that both isotypes are required for axonemal assembly and/or function. However, those observations were based on immunohistochemistry alone. βV tubulin was recently found to be in some but not all motile cilia (R. Hallworth and R. F. Ludueña, unpublished observations). Therefore, βV tubulin may be capable of supporting ciliary beating, but may not be absolutely required for ciliary function. βII tubulin has so far not been detected in cilia. However, its axonemal motif-like sequence differs only in the first amino acid from the axonemal motif sequence of βIV tubulin (E433 to Q433). Further, the axonemal motif peptide of βII tubulin was nearly as potent as that of βIV tubulin in blocking ciliary beating. Is βII tubulin incompatible with the correct axonemal assembly or function? A change in only position 433 from glutamate to glutamine is observed, resulting in a removal of one charge in βII tubulin. This may indicate that the secondary structure of the synthetic peptide resembles the axonemal motif peptide more closely than does the corresponding sequence in βII tubulin. It is worth noting that the unusual β tubulin isotypes βII and βIII are in some circumstances capable of supporting axonal assembly, if not motility. In the globose basal cells of the nose (the olfactory stem cells) βI, βII and βIII tubulin are synthesized and incorporated into microtubules. As soon as the cell matures and develops long, immotile sensory cilia, βIV tubulin is also synthesized (Woo et al., 2002). The isotypes do not compartmentalize, that is, all synthesized isotypes are incorporated into all microtubule structures in the cell, including the cilia. Thus βII tubulin and βIII tubulin are at least capable of assembling into immotile cilia. It remains to been seen if βII tubulin and βIII tubulin are capable of supporting ciliary motility and the assembly into a 9+2 structure, or if they disrupt these.
Acknowledgments
We thank Elisabeth Raff for critically reading the manuscript, Jonathan Howard for constructive input and Jacqueline Pavlik for outstanding technical assistance. Supported by N.I.H. grant DC02053 to R.H., N.I.H. grant CA26376, Department of Defense Breast Cancer Research Program grant DAMD17-01-1-0411 and Welch Foundation grant AQ-0726 to R.F.L., N.I.H. grant AA-08769 to J.H.S. and a Veterans Affairs Merit Review Grant to T.A.W., who is an American Lung Association Career Investigator. The mass spectrometry was supported by N.I.H. grant 1P20RR16469 from the INBRE Program of the National Center for Research Resource. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program grant 1 C06 RR17417-01 from the National Center for Research Resources, N.I.H.
References
- Asai DJ, Koonce MP. The dynein heavy chain: structure, mechanics and evolution. Trends Cell. Biol. 2001;11:196–202. doi: 10.1016/s0962-8924(01)01970-5. [DOI] [PubMed] [Google Scholar]
- Audebert S, White D, Cosson J, Huitorel P, Edde B, Gagnon C. The carboxy-terminal sequence Asp427-Glu432 of beta-tubulin plays an important function in axonemal motility. Eur. J. Biochem. 1999;261:48–56. doi: 10.1046/j.1432-1327.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Wall KA, Lopata MA, Cleveland DW, Ludueña RF. A monoclonal antibody against the type II isotype of beta-tubulin. Preparation of isotypically altered tubulin. J. Biol. Chem. 1988;263:3029–3034. [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Trcka P, Ludueña RF. Increased microtubule assembly in bovine brain tubulin lacking the type III isotype of beta-tubulin. J. Biol. Chem. 1990;265:1794–1799. [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Trcka P, Ludueña RF. Preparation of a monoclonal antibody specific for the class IV isotype of beta-tubulin. Purification and assembly of alpha beta II, alpha beta III and alpha beta IV tubulin dimers from bovine brain. J. Biol. Chem. 1992;267:5625–5630. [PubMed] [Google Scholar]
- Bré MH, Redeker V, Quibell M, Darmanaden-Delorme J, Bressac C, Cosson J, Huitorel P, Schmitter JM, Rossler J, Johnson T, et al. Axonemal tubulin polyglycylation probed with two monoclonal antibodies: widespread evolutionary distribution, appearance during spermatozoan maturation and possible function in motility. J. Cell Sci. 1996;109:727–738. doi: 10.1242/jcs.109.4.727. [DOI] [PubMed] [Google Scholar]
- Breitling F, Little M. Carboxy-terminal regions on the surface of tubulin and microtubules. Epitope locations of YOL1/34, DM1A and DM1B. J. Mol. Biol. 1986;189:367–370. doi: 10.1016/0022-2836(86)90517-6. [DOI] [PubMed] [Google Scholar]
- Cosson J, White D, Huitorel P, Edde B, Cibert C, Audebert S, Gagnon C. Inhibition of flagellar beat frequency by a new anti-beta-tubulin antibody. Cell Motil. Cytoskeleton. 1996;35:100–112. doi: 10.1002/(SICI)1097-0169(1996)35:2<100::AID-CM3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Gagnon C, White D, Cosson J, Huitorel P, Edde B, Desbruyeres E, Paturle-Lafanechere L, Multigner L, Job D, Cibert C. The polyglutamylated lateral chain of alpha-tubulin plays a key role in flagellar motility. J. Cell Sci. 1996;109:1545–1553. doi: 10.1242/jcs.109.6.1545. [DOI] [PubMed] [Google Scholar]
- Gee MA, Heuser JE, Vallee RB. An extended microtubule-binding structure within the dynein motor domain. Nature. 1997;390:636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- Goldsmith M, Connolly JA, Kumar N, Wu J, Yarbrough LR, van der Kooy D. Conserved beta-tubulin binding domain for the microtubule-associated motors underlying sperm motility and axonal transport. Cell Motil. Cytoskeleton. 1991;20:249–262. doi: 10.1002/cm.970200308. [DOI] [PubMed] [Google Scholar]
- Goldsmith M, Yarbrough L, van der Kooy D. Mechanics of motility: distinct dynein binding domains on alpha- and beta-tubulin. Biochem. Cell Biol. 1995;73:665–671. doi: 10.1139/o95-074. [DOI] [PubMed] [Google Scholar]
- Hallworth R, Ludueña RF. Differential expression of beta tubulin isotypes in the adult gerbil cochlea. Hear. Res. 2000;148:161–172. doi: 10.1016/s0378-5955(00)00149-0. [DOI] [PubMed] [Google Scholar]
- Hallworth R, McCoy M, Polan-Curtain J. Tubulin expression in the developing and adult gerbil organ of Corti. Hear. Res. 2000;139:31–41. doi: 10.1016/s0378-5955(99)00165-3. [DOI] [PubMed] [Google Scholar]
- Hastie AT. Isolation of respiratory cilia. Methods Cell Biol. 1995;47:93–98. doi: 10.1016/s0091-679x(08)60795-5. [DOI] [PubMed] [Google Scholar]
- Hastie AT, Dicker DT, Hingley ST, Kueppers F, Higgins ML, Weinbaum G. Isolation of cilia from porcine tracheal epithelium and extraction of dynein arms. Cell Motil. Cytoskeleton. 1986;6:25–34. doi: 10.1002/cm.970060105. [DOI] [PubMed] [Google Scholar]
- Hirose K, Lowe J, Alonso M, Cross RA, Amos LA. 3D electron microscopy of the interaction of kinesin with tubulin. Cell Struct. Funct. 1999;24:277–284. doi: 10.1247/csf.24.277. [DOI] [PubMed] [Google Scholar]
- Hoenger A, Thormahlen M, Diaz-Avalos R, Doerhoefer M, Goldie KN, Muller J, Mandelkow E. A new look at the microtubule binding patterns of dimeric kinesins. J. Mol. Biol. 2000;297:1087–1103. doi: 10.1006/jmbi.2000.3627. [DOI] [PubMed] [Google Scholar]
- Hunter AW, Wordeman L. How motor proteins influence microtubule polymerization dynamics. J. Cell Sci. 2000;113:4379–4389. doi: 10.1242/jcs.113.24.4379. [DOI] [PubMed] [Google Scholar]
- Jensen-Smith HC, Ludueña RF, Hallworth R. Requirement for the betaI and betaIV tubulin isotypes in mammalian cilia. Cell Motil. Cytoskeleton. 2003;55:213–220. doi: 10.1002/cm.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludueña R, Banerjee A. The isotypes of tubulin: distribution and functional significance. In: Fojo T, editor. Microtubules. Totowa, NJ: Humana Press; 2005a. (in press) [Google Scholar]
- Ludueña R, Banerjee A. The post-translational modifications of tubulin. In: Fojo T, editor. Microtubules. Totowa, NJ: Humana Press; 2005b. (in press) [Google Scholar]
- Ludueña R, Banerjee A. The tubulin superfamily. In: Fojo T, editor. Microtubules. Totowa, NJ: Humana Press; 2005c. (in press) [Google Scholar]
- Ludueña RF. Are tubulin isotypes functionally significant. Mol. Biol. Cell. 1993;4:445–457. doi: 10.1091/mbc.4.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludueña RF. Multiple forms of tubulin: different gene products and covalent modifications. Int. Rev. Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- Ludueña RF, Woodward DO. Alpha- and beta-tubulin: separation and partial sequence analysis. Ann. New York Acad. Sci. 1975;253:272–283. doi: 10.1111/j.1749-6632.1975.tb19206.x. [DOI] [PubMed] [Google Scholar]
- Mallik R, Carter BC, Lex SA, King SJ, Gross SP. Cytoplasmic dynein functions as a gear in response to load. Nature. 2004;427:649–652. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- Matthes T, Wolff A, Soubiran P, Gros F, Dighiero G. Antitubulin antibodies. II. Natural autoantibodies and induced antibodies recognize different epitopes on the tubulin molecule. J. Immunol. 1988;141:3135–3141. [PubMed] [Google Scholar]
- Meurer-Grob P, Kasparian J, Wade RH. Microtubule structure at improved resolution. Biochemistry. 2001;40:8000–8008. doi: 10.1021/bi010343p. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Toba S, Edamatsu M, Watai-Nishii J, Hirokawa N, Toyoshima YY, Kikkawa M. Dynein and kinesin share an overlapping microtubule-binding site. EMBO J. 2004;23:2459–2467. doi: 10.1038/sj.emboj.7600240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MG, Turner FR, Hutchens JA, Raff EC. Axoneme-specific beta-tubulin specialization: a conserved C-terminal motif specifies the central pair. Curr. Biol. 2001;11:529–533. doi: 10.1016/s0960-9822(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Okada Y, Hirokawa N. Mechanism of the single-headed processivity: diffusional anchoring between the K-loop of kinesin and the C terminus of tubulin. Proc. Natl. Acad. Sci. USA. 2000;97:640–645. doi: 10.1073/pnas.97.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry B, Jensen-Smith HC, Ludueña RF, Hallworth R. Selective expression of beta tubulin isotypes in gerbil vestibular sensory epithelia and neurons. J. Assoc. Res. Otolaryngol. 2003;4:329–338. doi: 10.1007/s10162-002-2048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal R, Schneider BG, Miller MM, Ludueña RF. Beta IV is the major beta-tubulin isotype in bovine cilia. Cell Motil. Cytoskeleton. 1993;25:19–29. doi: 10.1002/cm.970250104. [DOI] [PubMed] [Google Scholar]
- Roach MC, Boucher VL, Walss C, Ravdin PM, Ludueña RF. Preparation of a monoclonal antibody specific for the class I isotype of beta-tubulin: the beta isotypes of tubulin differ in their cellular distributions within human tissues. Cell Motil. Cytoskeleton. 1998;39:273–285. doi: 10.1002/(SICI)1097-0169(1998)39:4<273::AID-CM3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rodionov VI, Gyoeva FK, Kashina AS, Kuznetsov SA, Gelfand VI. Microtubule-associated proteins and microtubule-based translocators have different binding sites on tubulin molecule. J. Biol. Chem. 1990;265:5702–5707. [PubMed] [Google Scholar]
- Sarin VK, Kent SB, Tam JP, Merrifield RB. Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. Anal. Biochem. 1981;117:147–157. doi: 10.1016/0003-2697(81)90704-1. [DOI] [PubMed] [Google Scholar]
- Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J. Microsc. 2003;211:103–111. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- Skiniotis G, Cochran JC, Muller J, Mandelkow E, Gilbert SP, Hoenger A. Modulation of kinesin binding by the C-termini of tubulin. EMBO J. 2004;23:989–999. doi: 10.1038/sj.emboj.7600118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CK, Abel PW, Hulce M, Smith DD. Solvent effects on coupling yields during rapid solid-phase synthesis of CGRP(8–37) employing in situ neutralization. J. Pept. Res. 2005;65:84–89. doi: 10.1111/j.1399-3011.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- Vent J, Ludueña R, Hallworth R. Distribution of beta tubulin isotypes in the developing rat organ of Corti in vivo and in vitro. Annual Meeting of the Association for Research in Otolaryngology (ARO) Abstract 417.2003. [Google Scholar]
- Woo K, Jensen-Smith HC, Ludueña RF, Hallworth R. Differential synthesis of beta-tubulin isotypes in gerbil nasal epithelia. Cell Tissue Res. 2002;309:331–335. doi: 10.1007/s00441-002-0591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Adams JM, Sisson JH. Both cAMP and cGMP are required for maximal ciliary beat stimulation in a cell-free model of bovine ciliary axonemes. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288:L546–L551. doi: 10.1152/ajplung.00107.2004. [DOI] [PubMed] [Google Scholar]


