Abstract
[Nielsen et al., 2001: Curr Biol 11:529–533], based on studies in Drosophila, have proposed that β tubulin in axonemal microtubules must contain a specific acidic seven amino acid sequence in its carboxyl terminus. In mammals, the two βIV isotypes (βIVa and βIVb) contain that sequence. In order to test the application of this hypothesis to mammals, we have examined the expression of β tubulin isotypes in four different ciliated tissues (trachea, ependyma, uterine tube, and testis) using isotype-specific antibodies and indirect immunofluorescence. We find that βIV tubulin is present in all ciliated cell types examined, but so is βI tubulin. Taken together with recent studies that show that βI and βIV tubulin are both present in the cilia of vestibular hair cells, olfactory neurons, and nasal respiratory epithelial cells, we propose that both βI tubulin and βIV tubulin may be required for axonemal structures in mammals.
Keywords: tubulin isotypes, cilia, trachea, ependyma, Fallopian tube, testis
INTRODUCTION
Tubulin is the ubiquitous structural protein in microtubules consisting of α and β monomers in 1:1 stoichiometry. β tubulin exists in seven different isotypes in mammals, each the product of a separate gene [Ludueña, 1998]. The seven different isotypes, named βI, βII, βIII, βIVa, βIVb, βV, and βVI, have unusually similar amino acid sequences. These sequences are considered among the most highly conserved in evolution. For example, the chicken and mouse βI isotypes differ in only two residues [Ludueña, 1998]. This sequence conservation in mammals and other vertebrates is consistent with the multitubulin hypothesis, the idea that the isotypes perform different functions [Fulton and Simpson, 1976]. However, functional correlations to specific isotypes have been hard to obtain.
Although it is now clear that cells selectively express β tubulin isotypes, it has been hard to ascribe a specific function to a particular isotype. In this context, Nielsen et al. [2001] have proposed that isotypes with a particular amino acid sequence in the carboxyl terminus (EGEFXXX, where X is any acidic amino acid) are required for normal axonemal function. The Nielsen et al. [2001] study was performed in Drosophila. In mammals, this sequence is present only in βIV tubulin, which is in fact two essentially identical isotypes, βIVa, and βIVb. Although four isotype-specific antibodies are now available [Roach et al., 1998], the distribution of βI, βII, βIII, and βIV tubulin isotypes has not been previously studied systematically in ciliated tissues (βIVa, and βIVb are indistinguishable). Previous reports have indicated that βIV tubulin is a common constituent of cilia, but that other isotypes are also present. In vestibular hair cells, βI and βIV tubulin occur in the single non-motile cilium [Perry et al., 2003]. In the sensory cilia of olfactory neurons, all four isotypes are found, while the cilia of cells of the adjacent nasal respiratory epithelium possess βI and βIV tubulin [Woo et al., 2002]. The latter cilia are motile. Thus, the isotypes common to cilia in these preparations are βI and βIV tubulin.
Therefore, we examined the distribution of β tubulin isotypes in adult gerbil ciliated tissues that have not previously been examined: brain ependyma, fallopian tube, testes, and trachea. Taken together with previous studies of vestibular hair cells and nasal sensory and respiratory epithelia, this encompasses nearly all ciliated tissues in the body. We find that both βI and βIV tubulin are common to all cilia. We suggest, therefore, that both βI and βIV tubulin may be required for axonemes in mammals.
MATERIALS AND METHODS
The distribution of β tubulin isotypes was examined in adult gerbil ciliated tissues using indirect immunofluorescence in frozen sections. Adult gerbils (20 days old or older) were anesthetized with Nembutal and cardiac-perfused with 10–15 ml of 4% paraformaldehyde in phosphate buffered saline (PBS). The tissues (trachea, brain, uterus, and testis) were dissected out and were processed to equilibration in 30% sucrose in PBS as a cryoprotectant and quickly frozen in O.C.T. (Miles Labs, Elkart, IN). Sections 8 to 10 µm thick were cut on a cryostat (Leica Microsystems, Bannockburn, IL).
Sections were blocked and permeabilized in PBS containing 1% bovine serum albumin (BSA), 0.25% Triton-X, and 5% normal goat serum. The presence of β tubulin isotypes was detected with monoclonal antibodies to the βI, βII, βIII or βIV tubulin isotypes, raised in mouse as previously described [Banerjee et al. 1988, 1990, 1992; Roach et al. 1998]. Each antibody was prepared to an epitope unique to the C-terminus of that isotype. The C-termini of βIVa and βIVb are almost identical, differing by only two residues, and therefore the anti-βIV antibody was unable to discriminate between them. The primary antibody was made visible by staining with goat anti-mouse IgG coupled to Alexa 488 (Molecular Probes, Eugene, OR). Sections and whole mounts were sealed under coverslips in 50% PBS: 50% glycerol containing 1% n-propylgallate as an anti-fade agent. Negative controls were performed by omitting the primary antibody.
Specimens were photographed using an Axioskop II microscope (Carl Zeiss Jena, Jena, Germany) equipped with 40X and 100X objectives. Images were obtained using a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI) and were analyzed and prepared for presentation using Photoshop (Adobe Systems, San Jose, CA). Confocal images were obtained on a Radiance 2000 confocal microscope (Bio-Rad, Hercules, CA) on a Nikon Eclipse 800 upright microscope equipped for epifluorescence (Nikon Instruments, Melville, NY). Some video images were obtained using a M.T.I. CCD72 video camera (Dage-M.T.I., Michigan City, IN) on a Nikon Eclipse 800 upright microscope equipped for epifluorescence (Nikon Instruments).
Animal care and handling was performed in conformance with approved protocols of the Creighton University School of Medicine Institutional Animal Care and Use Committee.
RESULTS
For Figure 1–Figure 4, we show on the left a transmitted light view (under Nomarksi differential interference contrast), and on the right the corresponding immunofluorescence view.
Fig. 1.

β tubulin isotypes in trachea. βI A: Transmitted light image of tissue lining the trachea. B: In the same section, the βI antibody labels cilia in trachea as well as non-ciliary microtubules in the pseudo-stratified epithelium. βII. C: Transmitted light image of tissue lining the trachea. D: The βII antibody does not label cilia in trachea. βIII. E: Transmitted light image of tissue lining the trachea. F: The βIII antibody does not label cilia in trachea. The arrow indicates possible labeling of innervation. βIV. G: Transmitted light image of tissue lining the trachea. H: The βIV antibody labels cilia in trachea. c, cilia; pe, psuedo-stratified epithelium; gc, goblet cell. The scale bars = 30 µm (A, B, G, H) and 50 µm (C–F).
Fig. 4.

(Overleaf) β tubulin isotypes in testis. βI. A: Transmitted light image of efferent ducts in testis. B: The βI antibody labels cilia in efferent ducts as well as non-ciliary microtubules in the connective tissue as well as non-ciliary microtubules in the connective tissue. βII. C: Transmitted light image of efferent ducts in testis. D: The βII antibody does not label cilia in efferent ducts. Note the presence of βII in smooth muscle. βIII. E: Transmitted light image of efferent ducts in testis. F: The βIII antibody does not label cilia in efferent ducts. βIV. G: Transmitted light image of efferent ducts in testis. H: The βIV antibody labels cilia in efferent ducts. C, cilia; sm, smooth muscle; ct, connective tissue. The scale bars = 15 µm for (A,B,G,H) and 40 µm (C–F).
Tracheal Epithelium
The lining of the trachea is a pseudo-stratified epithelium consisting of basal cells, mucous-producing (goblet) cells, brush cells, and ciliated cells [Geneser, 1986]. Cilia-bearing cells were observed in clumps lining the lumen of the trachea. In sections of trachea, cilia were labeled by the antibodies to βI tubulin (Fig. 1A,B) and βIV tubulin (Fig. 1G,H). Other cell types, many unidentified, were labeled by the antibody to βI tubulin. However, only ciliated cells were labeled by the βIV tubulin antibody. Cilia were not labeled by the antibodies to βII tubulin (Fig. 1C,D) and βIII tubulin (Fig. 1E,F). Some labeling for βIII tubulin was seen deep in the epithelium, possibly representing innervation (indicated by the arrow). In the Nomarski images, cilia are indicated by c, pseudostratified epithelium by pe, and goblet cells by gc.
Brain Ependyma
In brain, the ependyma is a simple cuboidal epithelium lining the ventricles [Geneser, 1986]. Ciliated ependymal cells were most often observed lining the third ventricle, but were also observed in the first and second ventricles. Ciliated cells were often seen lining the choroid epithelium. In ependyma, like trachea, cilia were labeled by the antibodies to βI tubulin (Fig. 2A,B) and βIV tubulin (Fig. 2G,H), but were not labeled by the antibodies to βII tubulin (Fig. 2C,D) and βIII tubulin (Fig. 2E,F). In the Nomarski images, the cilia are indicated by c and the ventricle is indicated by v.
Fig. 2.
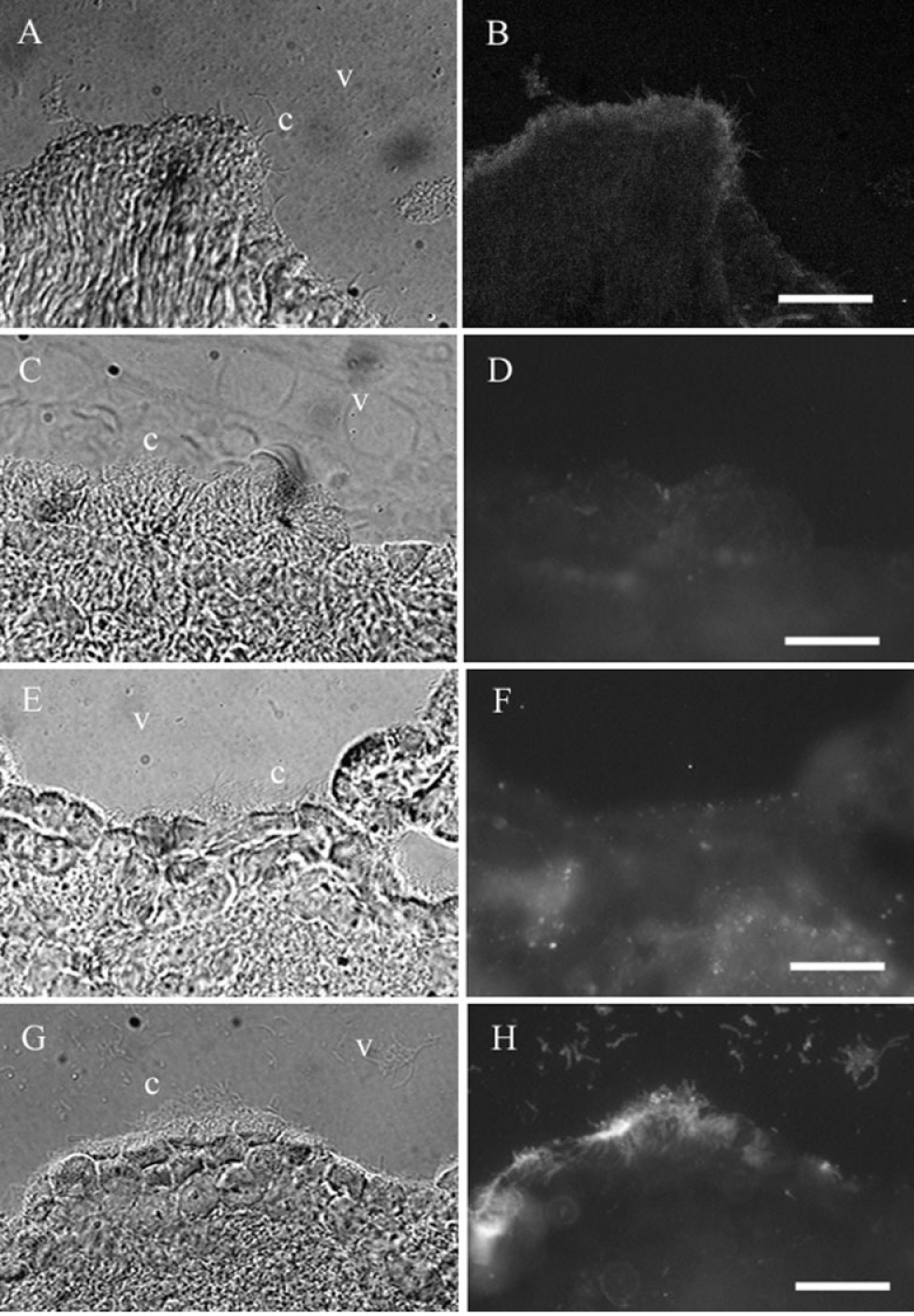
β tubulin isotypes in brain ependyma. βI. A: Transmitted light image of ependyma in ventricles of brain. B: The βII antibody labels cilia in ependyma. βII. C: Transmitted light image of ependyma in ventricles of the brain. D: The βII antibody does not label cilia in ependyma. βIII. E: Transmitted light image of ependyma in ventricles of the brain. F: The βIII antibody does not label cilia in ependyma. βIV. G: Transmitted light image of ependyma in ventricles of the brain. Note the presence of βIII in non-ciliary neuronal tissue. H: The βIV antibody labels cilia in ependyma. c, cilia; v, ventricle. The scale bars = 30 µm (A,B) and 50 µm (C–H).
Uterine Tube
The lining of the uterine tube (also referred to as the oviduct and the Fallopian tube) is a columnar epithelium consisting of mucosal cells and ciliated cells [Geneser, 1986]. Ciliated cells were most often observed lining the uterine tube in the infundibulum and ampulla, close to the ovary. As in ependyma and trachea, ciliated cells were labeled by the antibodies to βI tubulin (Fig. 3A,B) and βIV tubulin (Fig. 3G,H), but were not labeled by the antibodies to βII tubulin (Fig. 3C,D) and βIII tubulin (Fig. 3E,F). In the Nomarski images, the cilia are indicated by c and the lumen is indicated by l.
Fig. 3.

(Overleaf) β tubulin isotypes in uterine tube. βI. A: Transmitted light image of ciliated tissue in uterine tube. B: The βI antibody labels cilia in uterine tube. βII. C: Transmitted light image of ciliated tissue in uterine tube. D: The βII antibody does not label cilia in uterine tube. βIII. E: Transmitted light image of ciliated tissue in uterine tube. F: The βIII antibody does not label cilia in uterine tube. βIV. G: Transmitted light image of ciliated tissue in uterine tube. H: The βIV antibody labels cilia in the lumen of the uterine tube. c, cilia; l, lumen. The scale bars = 30 µm (A,B,G,H) and 50 µm (C–F).
Testis
In the efferent duct of the testis, the lining is a columnar epithelium consisting of ciliated cells and absorptive cells [Geneser, 1986]. As in the uterine tube, ependyma, and trachea, cilia were labeled by the antibodies to βI tubulin (Fig. 4A,B) and βIV tubulin (Fig. 4G,H). They were not labeled by the antibodies to βII tubulin (Fig. 4C,D) and βIII tubulin (Fig. 4E,F). In the Nomarski images, the cilia are indicated by c, smooth muscle by sm, and connective tissue by ct.
DISCUSSION
Our results are in agreement with previous studies of β tubulin isotype expression in other ciliated tissues. Perry et al. [2003] showed that vestibular hair cells, each of which possesses a single cilium, display βI and βIV tubulin in the cell body and cilium. Woo et al. [2002] demonstrated that all four isotypes were present in olfactory neurons, including the sensory cilia. Nasal respiratory epithelial cells, which possess motile cilia, displayed βI and βIV tubulin. Thus both βI and βIV tubulin are common to cilia. In a previous study, Renthal et al. [1993] demonstrated the presence of βIV tubulin in bovine tracheal epithelial cells and in the cilium of photoreceptors. However, the βI tubulin was not available for that study. Roach et al. [1998] showed βIV tubulin in ciliated cells of the uterine wall but they did not find βI tubulin there.
The axoneme-specific sequence (EGEFXXX) proposed by Nielsen et al. [2001] is present only in the carboxyl terminus of the βIV tubulins [Lu et al., 1998]. However, the analogous sequence in the carboxyl terminus of βI tubulin (EEDFGEE) is perhaps the closest of the other β tubulins. This is not surprising since βI tubulin is closely related to βIV tubulin and represents perhaps a relatively recent evolutionary divergence [Ludueña, 1998].
Why are the other isotypes present in only some ciliated cells? We have seen so far that whatever isotypes are present in cilia are also present in microtubules in the remainder of the cell. It is possible that ciliated cells will use any available isotype to synthesize axonemes but must use βI and βIV tubulin. It is interesting in this regard to note the observations of Woo et al. [2002] in olfactory sensory epithelia. Expression of βIV tubulin was demonstrated in mature olfactory neurons but not in basal cells, the stem cells of olfactory neurons. Olfactory neurons undergo a regular cycle of replacement every 40 days or so, in which they are replaced by maturing basal cells. Basal cells express the other three isotypes but not βIV tubulin. It appears then that basal cells only express βIV tubulin when it is needed to make axonemes.
It is perhaps not surprising that two highly conserved tubulin isotypes occur in many types of cilia. The ciliary axoneme, whose structure is extraordinarily conserved in evolution, is a highly complex structure in which three structurally and functionally distinct populations of microtubules occur. First, there are the two microtubules of the central pair, whose role may be to organize the beating motion of the axoneme. These are each singlet microtubules. Then there are the unusual outer doublet microtubules. Each of these nine outer doublets consists of two tubules designated A and B. The A-tubule is a complete microtubule with 13 protofilaments; the B-tubule is an incomplete microtubule of 10 protofilaments attached to the A-tubule. Among other proteins, the A-tubule binds to one end of the dynein complex. The B-tubule binds to the other end. By binding to the A-tubule of one outer doublet microtubule and “walking” on the B-tubule of the adjacent outer doublet, dynein causes sliding of the doublets and hence generates the bending motion of the axoneme. It is also possible that one of the protofilaments of the B-tubule is the unrelated protein tektin. In short, the central pair microtubules, the A-tubule and the B-tubule, differ in the proteins to which they bind as well as in their apparent functions. The A- and B-tubules are also structurally different. This is all consistent with axonemes containing more than one tubulin isotype. Future experiments with electron microscopy will address the specific distributions of the βI and βIV tubulin isotypes among the axonemal microtubules.
ACKNOWLEDGMENTS
Supported by N.I.H. grant CA26376, U.S. Army grant DAMD17-98-1-8246, and Welch Foundation grant AQ-0726 to R.F.L., and N.I.H. grant DC02053 to R.H. H.C.J-S. is a Clare Booth Luce fellow. We thank David Nichols for help in the dissections and Levi Ward, Becky Van Winkle, and Dayton Young for technical assistance.
Contract grant sponsor: NIH; Contract grant numbers: CA26376, DC02053; Contract grant sponsor: U.S. Army; Contract grant number: DAMD17-98-1-8246; Contract grant sponsor: Welch Foundation; Contract grant number: AQ-0726.
REFERENCES
- Banerjee A, Roach MC, Wall KA, Lopata MA, Cleveland DW, Ludeña RF. A monoclonal antibody against the type II isotype of β-tubulin. Preparation of isotypically altered tubulin. J Biol Chem. 1988;263:3029–3034. [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Trcka P, Ludueña RF. Increased microtubule assembly in bovine brain tubulin lacking the type III isotype of β-tubulin. J Biol Chem. 1990;265:1794–1799. [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Trcka P, Ludueña RF. Preparation of a monoclonal antibody specific for the class IV isotype of β-tubulin. Purification and assembly of βII, βIII, and βIV tubulin dimers from bovine brain. J Biol Chem. 1992;267:5625–5630. [PubMed] [Google Scholar]
- Fulton C, Simpson PA. Selective synthesis and utilization of flagellar tubulin. The multi-tubulin hypothesis. In: Goldman R, Pollard T, Rosenbaum J, editors. Cell motility. vol. 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1976. pp. 987–1005. [Google Scholar]
- Geneser F. Textbook of histology. Philadelphia: Lea and Fibiger; 1986. 831 pp. [Google Scholar]
- Lu Q, Moore GD, Walss C, Ludueña RF. Structural and functional properties of tubulin isotypes. Adv Struct Biol. 1998;5:203–227. [Google Scholar]
- Ludueña RF. The multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- Nielsen MG, Turner FR, Hutchens JA, Raff EC. Axoneme-specific β-tubulin specializations: a conserved C-terminal motif specifies the central pair. Curr Biol. 2001;11:529–533. doi: 10.1016/s0960-9822(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Perry B, Jensen-Smith H, Ludueña RF, Hallworth R. Differential expression of β tubulin isotypes in gerbil vestibular end organs. Journal of the Association for Research in Otolaryngology. 2003 doi: 10.1007/s10162-002-2048-4. (DOI: 10.1007/S10162-002-2048-4) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal R, Schneider BG, Miller MA, Ludueña RF. βIV is the major β-tubulin isotype in bovine cilia. Cell Motil Cytoskeleton. 1993;25:19–29. doi: 10.1002/cm.970250104. [DOI] [PubMed] [Google Scholar]
- Roach MC, Boucher VL, Walss C, Ravdin PM, Ludueña RF. Preparation of a monoclonal antibody specific for the class I isotype of β-tubulin: The β isotypes of tubulin differ in their cellular distributions within human tissues. Cell Motil Cytoskeleton. 1998;39:273–285. doi: 10.1002/(SICI)1097-0169(1998)39:4<273::AID-CM3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Woo K, Jensen-Smith HC, Ludueña RF, Hallworth R. Differential expression of β tubulin isotypes in gerbil nasal epithelia. Cell Tissue Res. 2002;309:331–335. doi: 10.1007/s00441-002-0591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


