Abstract
Concanavalin A (Con A) causes severe TNF-α–mediated and IFN-γ–mediated liver injury in mice. In addition to their other functions, TNF-α and IFN-γ both induce the inducible nitric oxide (NO) synthase (iNOS). Using different models of liver injury, NO was found to either mediate or prevent liver damage. To further elucidate the relevance of NO for liver damage we investigated the role of iNOS-derived NO in the Con A model. We report that iNOS mRNA was induced in livers of Con A–treated mice within 2 hours, with iNOS protein becoming detectable in hepatocytes as well as in Kupffer cells within 4 hours. iNOS–/– mice were protected from liver damage after Con A treatment, as well as in another TNF-α–mediated model that is inducible by LPS in D-galactosamine–sensitized (GalN-sensitized) mice. iNOS-deficient mice were not protected after direct administration of recombinant TNF-α to GalN-treated mice. Accordingly, pretreatment of wild-type mice with a potent and specific inhibitor of iNOS significantly reduced transaminase release after Con A or GalN/LPS, but not after GalN/TNF-α treatment. Furthermore, the amount of plasma TNF-α and of intrahepatic TNF-α mRNA and protein was significantly reduced in iNOS–/– mice. Our results demonstrate that iNOS-derived NO regulates proinflammatory genes in vivo, thereby contributing to inflammatory liver injury in mice by stimulation of TNF-α production.
Introduction
Activation of T lymphocytes and macrophages is an initial event during viral or autoimmune hepatitis (1, 2). Activated T cells exhibit direct cytotoxicity or release proinflammatory Th1 cytokines, which mediate liver damage in various experimental animal models (3). In two commonly used models of cytokine-mediated liver disease, mice are sensitized with the transcriptional inhibitor D-galactosamine (GalN) and severe TNF-α–dependent liver injury is induced either by administration of the macrophage activator LPS or by direct injection of recombinant TNF-α (3). These two models, however, are independent of T-cell activation.
A well-described mouse model of T-cell dependent liver injury is inducible by injection of the T-cell mitogenic plant lectin concanavalin A (Con A), which leads to fulminant hepatitis within 8 hours (4). The development of liver injury depends on TNF-α as well as on IFN-γ, since neutralizing Ab’s to either cytokine protect mice from liver failure (5–8). The central role of TNF-α in this model was further confirmed in various studies using either TNF-α or TNF-α–receptor knockout mice (9) or soluble TNF-α–receptor fusion proteins (10, 11). Although it was confirmed that both cytokines, i.e., TNF-α and IFN-γ, contribute to the development of liver injury after Con A treatment, the mechanism leading to liver damage is not fully understood. Since both cytokines induce inducible nitric oxide (NO) synthase (iNOS) in the liver (12), we investigated its function in acute cytokine-mediated liver injury models.
Many cell types are able to generate NO from L-arginine. As a free radical NO interacts in vivo with superoxide (O2–) to produce peroxynitrite (ONOO–), a potent oxidant (13). Peroxynitrite and other reactive nitrogen species react with free or protein-bound tyrosine residues to form 3-nitrotyrosine, a suitable marker for NO-mediated tissue damage (13, 14). At least three different isoforms of NOS are known. They differ in function, distribution, and regulation, but all catalyze the same redox reaction (15, 16). Two of them, NOS in neurons (nNOS, NOS1) (17) and NOS in endothelial cells of blood vessels (ecNOS, NOS3) (18), are constitutively expressed. They generate only small amounts of NO that are sufficient for cellular signaling under most circumstances. In contrast, the third type of NOS (iNOS, NOS2) is inducible by cytokines, e.g., TNF-α and IFN-γ (19, 12), and is able to generate large amounts of NO. While moderate levels of iNOS-derived NO are beneficial in principal (20), overexpression of iNOS was seen in many acute and chronic diseases, e.g., septic shock, hemorrhagic shock, and hepatitis (21–23). In vitro studies revealed that NO protects primary hepatocytes from ActD/TNF-α–induced apoptosis (24), but the role of NO in the liver is controversial. A protective function was observed after partial hepatectomy (25), in alcoholic hepatitis (26), and after GalN/TNF-α (27, 28) or CCl4 treatment (29). However, it was also reported that iNOS expression has hepatotoxic effects, e.g., in hemorrhagic shock (22, 30), during ischemia/reperfusion injury (31), in endotoxemia (32), or after GalN/LPS treatment (33).
Here we describe that iNOS is expressed in the livers of either Con A– or GalN/LPS–treated mice. Using either mice deficient in the inos gene (iNOS–/– mice) (34) or a specific pharmacological inhibitor of iNOS, i.e., L-N6-(1-iminoethyl)-lysine (L-NIL) (35), we found that reduced iNOS activity correlated with the prevention of liver injury in Con A– or GalN/LPS–treated, but not in GalN/TNF-α–treated, mice. The iNOS–/– mice treated with either Con A or GalN/LPS showed significantly reduced TNF-α production. Concordantly, hepatocyte damage was partially restorable in iNOS–/– mice treated with Con A plus recombinant TNF-α. In conclusion, TNF-α production in the liver, which is crucial for the development of inflammatory liver damage (9, 36), is regulated by NO.
Methods
Animals.
129/SvEv × C57BL/6, 129/Sv/Ev × C57BL/6-iNOS–/– mice (34), and BALB/c mice (age, 6–8 weeks; weight range, 18–22 g) were obtained from the animal facilities of the Institute of Experimental and Clinical Pharmacology and Toxicology and the Institute of Clinical Microbiology and Immunology of the University of Erlangen-Nuremberg (Erlangen, Germany). C57BL/6 × Sv129: IL-6+/+ and C57BL/6×Sv129: IL-6–/– mice were obtained from the Max Planck Institute for Immunobiology (Freiburg, Germany). All knockout mice are inbred into the C57BL/6 background for more than nine generations. All mice received human care according to the guidelines of the NIH, as well as to the legal requirements in Germany. They were maintained under controlled conditions (22°C, 55% humidity, and 12-hour day/night rhythm) and fed a standard laboratory chow.
Dosage and application routes.
Con A was purchased from Sigma Chemical Co. (St. Louis, Missouri, USA) and 20 mg/kg were administered intravenously in 200-μl pyrogen-free saline. L-NIL (15 mg/kg) was purchased from Alexis Deutschland GmbH (Grünberg, Germany) and was administered intraperitoneally in 200 μl saline, 36, 24, and 12 hours before the induction of liver injury. GalN (Sigma Chemical Co.) was administered intraperitoneally at 700 mg/kg as saline solution. Recombinant murine TNF (rmuTNF) (kindly provided by G.R. Adolf, Bender & Co., Vienna, Austria), dissolved in saline/0.1% human serum albumin, was administered intravenously at 9 μg/kg or intraperitoneally at 1 μg/mouse. LPS from Salmonella abortus equi was purchased from Metalon (Ragow, Germany) and administered intraperitoneally at a concentration of 5 μg/kg, together with GalN.
Analysis of liver enzymes.
Hepatocyte damage was assessed 8 hours after Con A or GalN administration by measuring plasma enzyme activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (37) using an automated procedure.
Cytokine determination using ELISA.
Sandwich ELISAs for murine plasma TNF-α, IL-6, IL-2, and IFN-γ were performed using flat-bottom high-binding polystyrene microtiter plates (Greiner GmbH, Frickenhausen, Germany). Ab’s were purchased from BD Biosciences (Heidelberg, Germany). Streptavidin-peroxidase (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) and the peroxidase chromogen tetramethylbenzidine (Boehringer Mannheim, Mannheim, Germany) were used according to the manufacturers’ instructions. Plasma levels of TNF-α, IL-6, and IL-2 were measured at 2 hours and plasma levels of IFN-γ at 8 hours after the hepatotoxic challenge (5–7, 38). For determination of intrahepatic TNF-α concentrations livers were prepared as described previously (39). Liver lysates were adjusted to equal protein concentrations and analyzed for murine TNF-α by the Quantikine M Kit (Bio-Rad Laboratories GmbH, Munich, Germany).
Detection of mRNA by RT-PCR and real-time RT-PCR.
Isolation of total RNA from liver tissue was carried out using the Nucleo Spin RNA Purification Kit (CLONTECH, Palo Alto, California, USA). To analyze altered gene expression, mRNA was transcribed into cDNA using SuperScript II RNase H– reverse transcriptase (Life Technologies Inc., Grand Island, New York, USA). Oligonucleotides and Taq polymerase for subsequent PCR reactions were also obtained from Life Technologies Inc. The following oligonucleotide pairs were used: iNOS, 2588-2604 and 2914-2898 in GenBank M84373; CD25, 14-36 and 712-689 in GenBank NM008367; β-actin, 729-752 and 1076-1053 in GenBank X03765. Semiquantitative evaluation was done using the Gel Doc 2000 System (Bio-Rad Laboratories GmbH). For quantitative evaluation of TNF-α mRNA expression real-time RT-PCR primers and probes for murine TNF-α and β-actin were purchased from TIB Molbiol (Berlin, Germany) and performed as described elsewhere (39). Amplification and detection were done with an ABI PRISM 7700 Sequence Detection System (Perkin Elmer Applied Biosystems, Bad Wildbad, Germany).
Immunohistochemistry and confocal microscopy.
For immunohistochemistry on cryostat sections, liver samples were embedded with GSV 1 tissue-embedding medium (Slee Technik GmbH, Mainz, Germany), frozen in 2-methyl-butane (Carl Roth GmbH, Karlsruhe, Germany), and stored at –20°C until use. Cryostat sections of 10 μm were thawed on glass slides, air-dried, fixed for 10 minutes at 4°C in acetone/methanol (1+1), and used immediately or stored at –20°C. For immunostaining slides were rinsed with PBS and blocked for 1 hour at room temperature with PBS containing 3% BSA. Subsequently, slides were incubated at 4°C overnight with a primary Ab in PBS/3% BSA. After rinsing with PBS, binding sites were detected with a secondary Ab for 1 hour at room temperature. After prolonged rinsing with PBS, slides were coverslipped using PBS/glycerol, pH 8.6, and examined by MRC 1000 (Bio-Rad Laboratories Inc., Richmond, California, USA).
Analysis of DNA fragmentation.
DNA fragmentation was detected in liver sections using the in situ Cell Death Detection Kit, Fluorescein (terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling [TUNEL] test; Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s instructions and examined by confocal laser-scanning microscopy (MRC 1000, Bio-Rad Laboratories Inc.). Quantification of DNA fragmentation was performed using the cell death detection ELISA (Roche Diagnostics GmbH, Mannheim, Germany).
Western blot analysis.
Livers were homogenized in lysis buffer containing 0.5% Nonidet P-40 (NP40), 137 mM NaCl, 2 mM EDTA, 50 mM Tris/HCl, pH 8.0, and 10% glycerol. After centrifugation, supernatants were stored at –80°C. For Western blot analysis 10 μg of protein were fractionated by 10% SDS-polyacrylamide gel electrophoresis and blotted onto a nitrocellulose membrane. The Western blots were developed using an enhanced chemiluminescence system (Amersham Pharmacia Biotech Europe, Freiburg, Germany), according to the manufacturer’s instructions. Semiquantitative evaluation was done using the Gel Doc 2000 System (Bio-Rad Laboratories GmbH).
Antibodies.
For detection of iNOS we used a rabbit anti-mouse iNOS Ab (kindly provided by J. Pfeilschifter, University of Frankfurt, Frankfurt am Main, Germany) (40). Macrophages were detected with a rat mAb directed against a murine pan-macrophage marker (clone BM8; Dianova, Hamburg, Germany). Β-actin was detected using a goat anti-human polyclonal Ab (sc-1615; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA). Secondary Ab’s for immunofluorescence were goat anti-rabbit IgG tagged with Cy3 (Jackson ImmunoResearch Laboratories Inc.), swine anti-rabbit IgG tagged with FITC (DAKO Corp., Hamburg, Germany), or rabbit anti-rat IgG tagged with Texas red (DAKO Corp.). Secondary Ab’s for Western blots were goat anti-rabbit horseradish peroxidase (Dianova) and streptavidin peroxidase (Boehringer Mannheim).
Statistical analysis.
The results were analyzed using Student’s t test if two groups were compared or by ANOVA followed by the Dunnett test if more groups were tested against a control group. If variances were inhomogeneous in the Student’s t test, the results were analyzed using the Welsh test. All data in this study are expressed as a mean ± SEM. P values less than or equal to 0.05 were considered significant.
Results
iNOS expression is induced in the livers of Con A–treated mice.
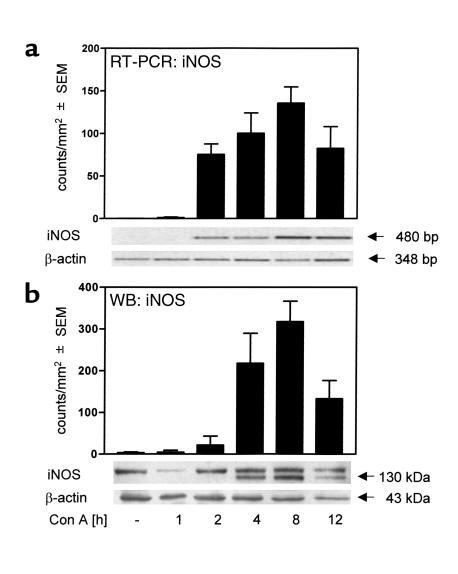
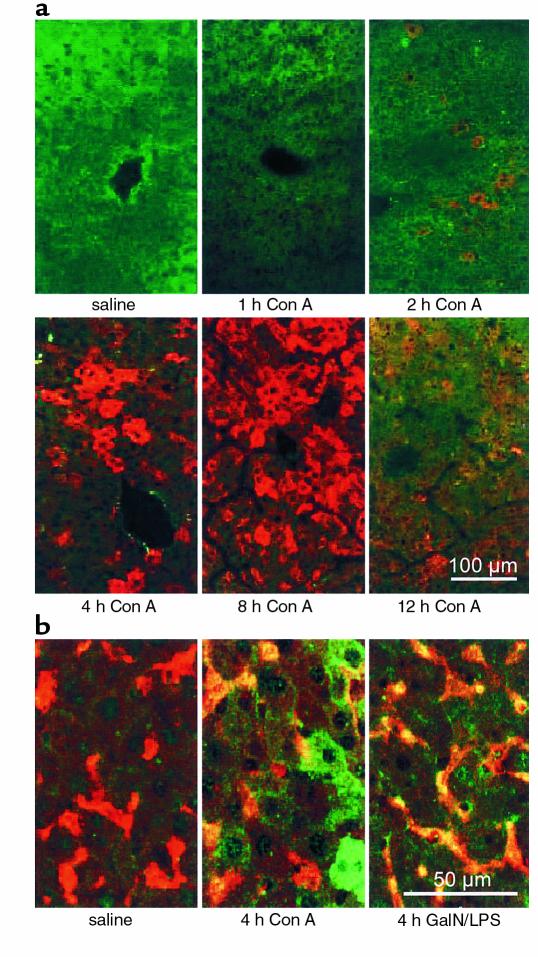
Liver injury in mice treated with Con A is characterized by an increase of plasma-transaminase levels becoming evident at 8 hours after intravenous injection (41). TNF-α and IFN-γ, two cytokines crucial for the development of liver injury in the Con A model (6, 7), reach their maximum plasma concentrations after 2 hours and 8 hours, respectively (7, 38). Since the expression of iNOS is inducible by TNF-α and IFN-γ (12, 19), we investigated the time course of iNOS induction in livers of Con A–treated mice. Figure 1a shows that intrahepatic iNOS mRNA is induced within 2 hours after Con A treatment. Protein expression was detectable at 2–4 hours after intervention, reaching maximum levels at 8 hours (Figure 1b and 2a). Double-immunofluorescent staining of iNOS and macrophages (Figure 2b) revealed that iNOS was expressed by macrophages as well as by hepatocytes. The gross expression of iNOS in livers of Con A–treated mice prompted us to investigate its functional role in Con A–induced liver damage.
Figure 1.
iNOS is expressed in livers of Con A–treated mice. Con A–induced iNOS expression was detected in livers of BALB/c mice by RT-PCR (a) or Western blot (WB) analysis (b). Semiquantitative evaluation of RT-PCR and Western blot was performed using the Gel Doc 2000 System (Bio-Rad Laboratories GmbH). Data are expressed as the mean ± SEM (n = 3). The gels shown are one example of three independent experiments.
Figure 2.
Detection of iNOS in livers of Con A–treated mice by immunofluorescence staining. (a) Con A–induced iNOS expression in livers of BALB/c mice was detected by immunofluorescence staining of liver cryostat sections (rabbit anti-mouse iNOS antiserum, secondary goat anti-rabbit IgG tagged with Cy3; red fluorescence). (b) Costaining of iNOS (rabbit anti-mouse iNOS antiserum, secondary swine anti-rabbit IgG tagged with FITC; green fluorescence) and Kupffer cells (BM8, secondary goat anti-rat IgG tagged with Texas red; red fluorescence) 4 hours after Con A or GalN/LPS treatment. All sections were examined by confocal laser-scanning microscopy. Costaining is represented by yellow fluorescence. Seen in a and b is one example of three independent experiments, respectively.
Mice deficient in iNOS are protected against Con A–induced liver injury.
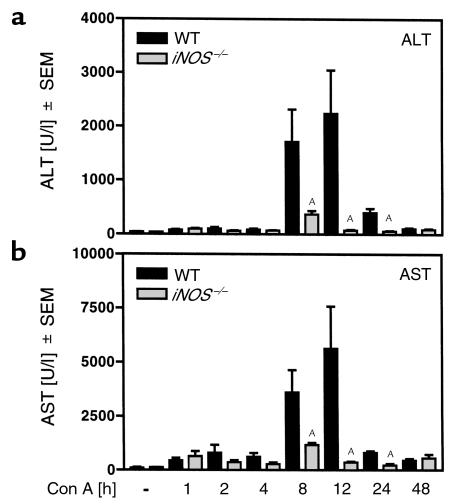
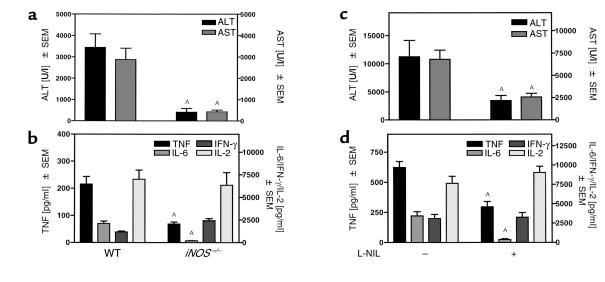
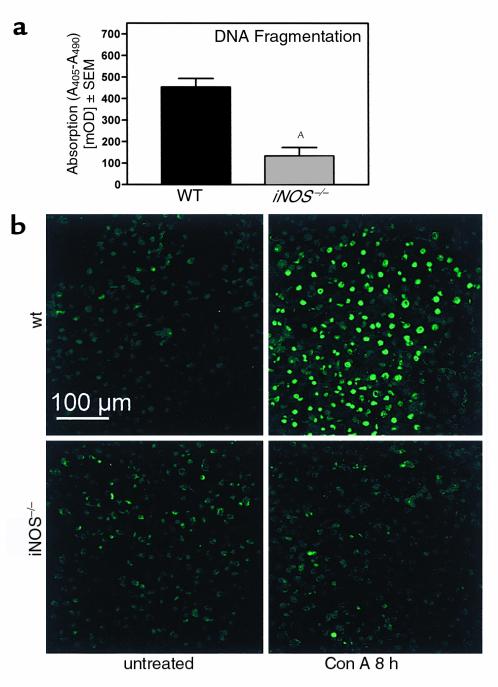
To study the role of iNOS in immune hepatitis we treated iNOS–/– mice (34) with Con A. Figure 3 (see also Figure 5a) shows that iNOS–/– mice failed to develop liver injury after Con A treatment. In a time-course experiment, enhanced plasma transaminase activities for ALT (Figure 3a) and AST (Figure 3b) could be detected in wild-type (WT) mice 8–24 hours after Con A treatment. In contrast, in iNOS–/– mice plasma transaminase levels were significantly reduced (Figure 3). Accordingly, intrahepatic DNA fragmentation was significantly reduced in Con A–treated iNOS–/– mice (Figure 4). RT-PCR analysis revealed that protection was not due to an inability of T cells to respond to Con A, since expression of IL-2 Rα-chain (CD25), a factor upregulated in activated T cells, did not differ in livers of Con A–treated iNOS–/– versus WT mice (WT: untreated 2 ± 3 counts/mm2; Con A: 109 ± 35 counts/mm2; iNOS–/–: untreated 2 ± 2 counts/mm2; Con A: 89 ± 16 counts/mm2).
Figure 3.
iNOS knockout mice are protected against Con A–induced liver injury. iNOS–/– mice and their corresponding WT were treated with Con A. Activities of plasma transaminases, i.e., ALT (a) and AST (b), were determined 1, 2, 4, 8, 12, 24, and 48 hours after Con A challenge. Data are expressed as the mean ± SEM (n = 4; AP ≤ 0.05 vs. Con A–treated WT mice).
Figure 5.
iNOS deficiency or pharmacological inhibition of iNOS protects mice against Con A–mediated liver injury. iNOS–/– mice (a and b) or BALB/c mice pretreated either with the specific iNOS inhibitor L-NIL or with saline at –36, –24, and –12 hours (c and d) were treated with Con A. Plasma transaminases, i.e., ALT and AST (a and c) and plasma concentrations of IFN-γ (b and d) were determined 8 hours after Con A treatment. Plasma concentrations of TNF-α, IL-6, and IL-2 (b and d) were determined 2 hours after Con A treatment. Data are expressed as the mean ± SEM (n = 6–8, AP ≤ 0.05 vs. Con A–treated WT or saline pretreated mice).
Figure 4.
DNA fragmentation is significantly reduced in the livers of Con A–treated iNOS–/– mice. The iNOS–/– mice and their corresponding WT were treated with Con A. DNA fragmentation was determined 8 hours after Con A administration by cell-death detection ELISA (a) and TUNEL staining of mouse liver cryostat sections (b) as described in Methods. Sections were examined by confocal laser-scanning microscopy. Evaluation of DNA fragmentation by ELISA (a) is expressed as the mean ± SEM (n = 5; AP ≤ 0.05 WT vs. Con A–treated iNOS–/– mice). DNA fragmentation in the corresponding untreated mice was subtracted as background. TUNEL staining (b) represents one example of three independent experiments. mOD, milli–optical density.
In Con A–treated iNOS–/– mice, maximum levels of plasma TNF-α and IL-6 (5–7) were significantly reduced compared with WT animals (Figure 5b). This was not an overall effect on cytokine synthesis, since IL-2 production was unaltered, and these mice released even more IFN-γ into the circulation than the WT mice in response to Con A in vivo (Figure 5b). Accordingly, pretreatment of WT mice with L-NIL, a potent and specific inhibitor of iNOS (35), had a comparable effect on the release of transaminases (Figure 5c vs. Figure 5a), as well as on plasma concentrations of TNF-α, IL-6, and IL-2 (Figure 5d vs. Figure 5b). Plasma levels of IFN-γ in L-NIL–pretreated mice did not differ from those observed in mice treated with Con A alone (Figure 5d). Hence, it seems that macrophage- but not T cell-derived cytokines are upregulated by NO. However, IL-6 is unlikely to mediate Con A–induced liver injury, since IL-6–/– mice were not protected from Con A–induced liver injury (WT: ALT: 1,393 ± 663 U/l; AST: 954 ± 523 U/l; IL-6–/–: ALT: 3,645 ± 879 U/l; AST: 2,524 ± 1,096 U/l).
TNF-α expression is significantly reduced in the livers of iNOS–/– mice.
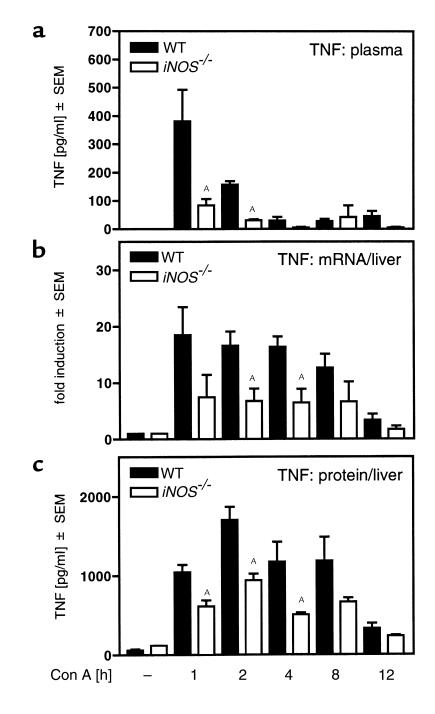
To quantify the intrahepatic expression of TNF-α in iNOS–/– versus WT mice, we performed time-course studies of intrahepatic TNF-α mRNA and protein appearance in WT and iNOS–/– animals. TNF-α protein in plasma (Figure 6a) and liver tissue (Figure 6c), as well as TNF-α mRNA in liver tissue (Figure 6b), were significantly increased within 1 hour after Con A injection in WT mice. Both TNF-α mRNA as well as the protein were significantly reduced in livers of Con A–treated iNOS–/– mice (Figure 6, b and c). These results indicate that iNOS-deficiency protects mice from Con A–induced liver injury by inhibiting the induction of its central mediator, TNF-α. To confirm this we treated iNOS–/– mice with Con A plus rmuTNF. This treatment restored the release of ALT in iNOS–/– mice to approximately 20% of the WT level (Con A-treated WT mice: 3507 ± 1254 U/l; Con A–treated iNOS–/– mice: 163 ±1 26 U/l; iNOS–/– mice treated with Con A plus rmuTNF: 768 ± 553 U/l; n = 5; P ≤ 0.05 versus Con A–treated iNOS–/– mice).
Figure 6.
Reduced TNF-α expression in plasma and livers of Con A–treated iNOS–/– mice. iNOS–/– mice and their corresponding WT were treated with Con A. The release of TNF-α into the plasma (a) and the expression of TNF-α mRNA (b) and protein (c) in the liver were determined 1, 2, 4, 8, and 12 hours after Con A challenge. TNF-α protein in plasma and liver tissue was measured by ELISA, and TNF-α mRNA in liver tissue was measured by real-time RT-PCR. Data are expressed as the mean ± SEM (n = 3–6, AP ≤ 0.05 vs. Con A–treated WT mice).
NO-induced TNF-α production is a key event in immune-mediated hepatitis.
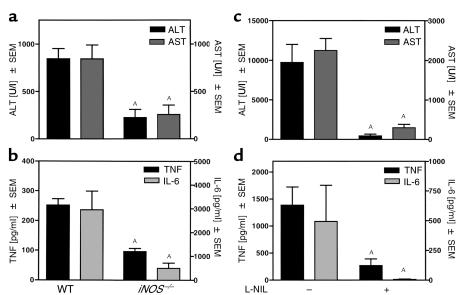
Since rmuTNF only partially restored liver damage in iNOS–/– mice challenged with Con A, we studied the relationship between iNOS deficiency and TNF-α induction in the GalN/LPS versus the GalN/TNF-α model of liver injury. LPS administration to GalN-sensitized mice results in TNF-α production and subsequent development of liver injury. In this model, Kupffer cells, but not hepatocytes, expressed iNOS (compare Figure 2b). However, as in the Con A model, iNOS–/– mice (Figure 7a), as well as L-NIL–pretreated mice (Figure 7c), were protected from GalN/LPS–induced liver damage. Again, protection was associated with decreased plasma levels of TNF-α and IL-6 (Figure 7, b and d). In contrast, in the GalN/TNF-α model liver injury is induced by direct administration of rmuTNF to GalN-sensitized mice and therefore is independent of endogenous TNF-α production. Here neither iNOS–/– mice (Figure 8a) nor L-NIL–pretreated mice (Figure 8b) were protected.
Figure 7.
The iNOS-deficient mice are protected against GalN/LPS–induced liver injury. iNOS–/– mice and their corresponding WT (a and b) or BALB/c mice pretreated either with the specific iNOS inhibitor L-NIL or with saline at –36, –24, and –12 hours (c and d) were treated with GalN/LPS. Eight hours after GalN/LPS challenge, activities of plasma ALT and AST (a and c) and plasma levels of IL-6 (b and d) were determined. TNF-α plasma concentrations were detected 2 hours after GalN/LPS treatment (b and d). Data are expressed as the mean ± SEM (n = 6; AP ≤ 0.05 vs. Con A–treated WT or saline-pretreated mice).
Figure 8.
The iNOS-deficient mice are sensitive toward GalN/TNF-α–induced liver injury. iNOS–/– mice and their corresponding WT (a) or BALB/c mice pretreated either with the specific iNOS inhibitor L-NIL or with saline at –36, –24, and –12 hours (b) were treated with GalN 30 minutes before intravenous administration of TNF-α (9 μg/kg). Eight hours after GalN treatment, activities of plasma ALT and AST were determined. Data are expressed as the mean ± SEM (n = 3; P ≤ 0.05 vs. Con A–treated WT or saline-pretreated mice).
Discussion
Liver protection as well as liver toxicity have been attributed to NO (42). Administration of NO donors provides cytoprotection from inflammatory liver damage and hepatocellular apoptosis. However, pharmacological blockade of iNOS either aggravates or attenuates liver injury or is ineffective (42). Here we describe in two mouse models of immune-mediated liver injury, i.e., Con A–induced hepatitis (3, 4) and LPS-induced liver injury in GalN-sensitized mice (3, 43) that endogenously produced iNOS-derived NO mediates liver injury. In both models, liver injury is mediated by TNF-α and IFN-γ (3, 5–8, 44), i.e., strong inducers of iNOS in macrophages (45) and hepatocytes (46). In fact, we could show that after Con A administration, iNOS was expressed by hepatocytes and Kupffer cells of WT animals, whereas LPS-induced iNOS expression was detected only in Kupffer cells of GalN-sensitized mice, most likely because GalN selectively blocks transcription within hepatocytes. Although it is conceivable that iNOS-derived NO produced by hepatocytes and/or Kupffer cells is directly toxic for liver cells, we could not detect 3-nitrotyrosine as a marker of NO-mediated tissue damage within livers of Con A–treated mice, as has been described for hepatic allograft rejection (47). Moreover, there was no iNOS immunoreactivity in TUNEL-positive liver cells in the Con A model (data not shown), indicating that the iNOS-expressing hepatocyte was not directly damaged. This could even demonstrate a partially cytoprotective effect of iNOS expression in hepatocytes. Accordingly, a hepato-cytoprotective effect of iNOS has been demonstrated in vitro against TNF-α–induced apoptosis (48) and in vivo in the case of liver regeneration (25). Furthermore, NO donors have been described to prevent inflammatory liver injury. The protective effects of NO have been discussed in terms of caspase-3 inhibition, induction of cytoprotective proteins, or beneficial vascular effects (42). Despite potentially cytoprotective effects of NO on hepatocytes, liver damage induced by either Con A or GalN/LPS treatment was prevented in iNOS–/– mice or in mice pretreated with the specific iNOS-inhibitor L-NIL given in a dose that has been described to inhibit NO formation in vivo (49). Hence, direct hepatotoxicity of NO cannot explain the iNOS dependence of Con A– or GalN/LPS–induced liver injury.
Most strikingly, in both models we observed a significant inhibition of TNF-α as well as of IL-6 release in iNOS–/– or L-NIL–pretreated mice (compare Figure 5 and 7). This was not an overall effect on cytokine synthesis, since IL-2 plasma levels were unaltered and IFN-γ production was even increased in iNOS–/– mice (compare Figure 5). Hence, it seems that macrophage-derived cytokines, i.e., TNF-α and IL-6, but not T cell–derived cytokines, i.e., IL-2 and IFN-γ, are upregulated by NO in vivo. The increase of IFN-γ might have been due to the fact that NO limits the processing of IFN-γ–inducing factor, i.e., IL-18, in macrophages by inhibition of caspase-1 (50).
Taken together, the mechanism leading to protection from liver injury in NO-deficient mice most likely depends on downregulation of either IL-6 or TNF-α. However, IL-6 seems not to mediate Con A–induced liver injury, since IL-6–/– mice were not protected from Con A–induced liver injury. Accordingly, pretreatment of mice with recombinant IL-6 was shown to be hepatoprotective in the Con A (51, 52) as well as in the GalN/LPS model (53).
In contrast, TNF-α was clearly shown to mediate liver damage induced by Con A or GalN/LPS (3, 5, 6, 9, 10). In Con A–induced liver injury IFN-γ cooperates with TNF-α (7), i.e., neutralizing one of both cytokines in the presence of high concentrations of the other is sufficient to block Con A hepatitis (7, 9). Thus, a reduction of plasma TNF-α levels without reduction of IFN-γ production, as observed here, is sufficient to block liver injury. The time courses of intrahepatic TNF-α and iNOS expression nearly matched, suggesting that iNOS-derived NO might potentiate and prolong intrahepatic TNF-α production. Indeed, in iNOS–/– mice, the intrahepatic expression of TNF-α was significantly reduced (compare Figure 6). This result was further confirmed by showing that the lack of iNOS activity (a) protected mice against liver injury induced by GalN/LPS administration, i.e., another model depending on TNF-α induction, and (b) failed to prevent hepatic damage induced by direct administration of rmuTNF to GalN-sensitized mice. Furthermore, administration of rmuTNF to Con A–treated iNOS–/– mice partially restored liver injury. In contrast to the induction of liver injury after endotoxin treatment, which depends on soluble TNF (sTNF) and TNF receptor 1 (TNFR1) only (54), the development of liver injury after Con A treatment depends on the activation of both TNF receptors, i.e., TNFR-1 and TNFR-2, as well as of membrane-bound TNF (mTNF) (9, 10), which primarily activates TNFR-2. Therefore, full restoration of liver injury by administration of soluble TNF-α, as it was seen in iNOS–/– mice treated with GalN/LPS versus GalN/TNF-α, could not be expected in the Con A model.
In vitro, either activation or inhibition of gene expression by NO have been reported for a variety of inflammation-related genes, including TNF-α and IL-6 (55–59). To determine physiologically relevant effects, NO-mediated gene expression must be investigated in in vivo models of inflammatory diseases. To date, only limited data on a possible role of NO acting as a transcriptional regulator in vivo are available. For example, iNOS-dependent expression of IL-6 and G-CSF in lungs and livers of mice during hemorrhagic shock has been reported (22). In this model, inflammatory organ damage depends on an ischemia/reperfusion insult and accumulation of neutrophils in a variety of tissues (60). However, it is not known whether liver failure in this model depends on TNF-α and whether TNF-α is affected by an alteration of NO production.
Our results demonstrate in two models of immune-mediated liver injury that endogenously produced NO causes hepatocellular damage by stimulating the expression of TNF-α. They also provide further knowledge regarding the modulation of gene expression by NO in vivo and underline the necessity to critically evaluate the therapeutic use of NO donors and iNOS inhibitors.
Acknowledgments
We thank J. Pfeilschifter, University of Frankfurt, Frankfurt am Main, Germany, for kindly providing rabbit anti-mouse iNOS antiserum and G.R. Adolffor for providing rmuTNF. The perfect technical assistance of S. Heinlein and A. Agli is gratefully acknowledged. We are indebted to C. Labahn and T. Mittmann for their professional help in the animal facilities. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) grants TI 169/4-2 and TI 169/4-3.
References
- 1.Koziel MJ. Cytokines in viral hepatitis. Semin Liver Dis. 1999; 19:157–169. doi: 10.1055/s-2007-1007107. [DOI] [PubMed] [Google Scholar]
- 2.McFarlane IG. Pathogenesis of autoimmune hepatitis. Biomed Pharmacother. 1999; 53:255–263. doi: 10.1016/S0753-3322(99)80096-1. [DOI] [PubMed] [Google Scholar]
- 3.Schümann J, Tiegs G. Pathophysiological mechanisms of TNF during intoxication with natural or man-made toxins. Toxicology. 1999; 138:103–126. doi: 10.1016/s0300-483x(99)00087-6. [DOI] [PubMed] [Google Scholar]
- 4.Tiegs G, Hentschel J, Wendel A. A T-cell dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992; 90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuhara H, et al. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994; 179:1529–1537. doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gantner F, Leist M, Lohse AW, Germann P, Tiegs G. Concanavalin A-induced T cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995; 21:190–198. doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- 7.Küsters S, Gantner F, Künstle G, Tiegs G. Interferon-γ plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996; 111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 8.Mizuhara H, et al. Critical involvement of interferon γ in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology. 1996; 23:1608–1615. doi: 10.1053/jhep.1996.v23.pm0008675184. [DOI] [PubMed] [Google Scholar]
- 9.Küsters S, et al. In vivo evidence for a functional role of both TNF receptors and transmembrane TNF in experimental hepatitis. Eur J Immunol. 1997; 27:2870–2875. doi: 10.1002/eji.1830271119. [DOI] [PubMed] [Google Scholar]
- 10.Solorzano CC, et al. Involvement of 26-kDa cell-associated TNFα in experimental hepatitis and exacerbation of liver injury with a matrix metalloproteinase inhibitor. J Immunol. 1997; 158:414–419. [PubMed] [Google Scholar]
- 11.Ksontini R, et al. Disparate roles for TNF-α and Fas ligand in concanavalin A-induced hepatitis. J Immunol. 1998; 160:4082–4089. [PubMed] [Google Scholar]
- 12.Taylor BS, Alarcon LH, Billiar TR. Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry. 1998; 63:766–781. [PubMed] [Google Scholar]
- 13.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996; 271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997; 411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- 15.Michel T, Feron O. Nitric oxide synthases: which, where, how and why? J Clin Invest. 1997; 100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997; 100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christopherson KS, Bredt DS. Nitric oxide in excitable tissues: physiological roles and diseases. J Clin Invest. 1997; 100:2424–2429. doi: 10.1172/JCI119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997; 100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997; 15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 20.Fang FC. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997; 99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993; 54:171–178. [PubMed] [Google Scholar]
- 22.Hierholzer C, et al. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998; 187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monzon-Garcia C, et al. Intrahepatic accumulation of nitrotyrosine in chronic viral hepatitis is associated with histological severity of liver disease. J Hepatol. 2000; 32:331–338. doi: 10.1016/s0168-8278(00)80080-x. [DOI] [PubMed] [Google Scholar]
- 24.Kim YM, de Vera ME, Watkins SC, Billiar TR. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-alpha-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997; 272:1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- 25.Rai RM, et al. Impaired liver regeneration in inducible nitric oxide synthase-deficient mice. Proc Natl Acad Sci USA. 1998; 95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanji AA, et al. Nitric oxide production in experimental alcoholic liver disease in the rat: role in protection from injury. Gastroenterology. 1995; 109:899–907. doi: 10.1016/0016-5085(95)90400-x. [DOI] [PubMed] [Google Scholar]
- 27.Bohlinger I, et al. Interleukin-1 and nitric oxide protect against tumor necrosis factor α-induced liver injury through distinct pathways. Hepatology. 1995; 22:1829–1837. [PubMed] [Google Scholar]
- 28.Saavedra JE, et al. Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-alpha-induced apoptosis and toxicity in the liver. J Med Chem. 1997; 40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]
- 29.Muriel P. Nitric oxide protection of rat liver from lipid peroxidation, collagen accumulation, and liver damage induced by carbon tetrachloride. Biochem Pharmacol. 1998; 56:773–779. doi: 10.1016/s0006-2952(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 30.Menezes J, et al. A novel nitric oxide scavenger decreases liver injury and improves survival after hemorrhagic shock. Am J Physiol. 1999; 277:G144–G151. doi: 10.1152/ajpgi.1999.277.1.G144. [DOI] [PubMed] [Google Scholar]
- 31.Isobe M, et al. Beneficial effects of inducible nitric oxide synthase inhibitor on reperfusion injury in the pig liver. Transplantation. 1999; 68:803–813. doi: 10.1097/00007890-199909270-00013. [DOI] [PubMed] [Google Scholar]
- 32.Thiermermann C, Ruetten H, Wu CC, Vane JR. The multiple organ dysfunction syndrome caused by endotoxin in the rat: attenuation of liver dysfunction by inhibitors of nitric oxide synthase. Br J Pharmacol. 1995; 116:2845–2851. doi: 10.1111/j.1476-5381.1995.tb15935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morikawa A, et al. Role of nitric oxide in lipopolysaccharide-induced hepatic injury in D-galactosamine-sensitized mice as an experimental endotoxic shock model. Infect Immun. 1999; 67:1018–1024. doi: 10.1128/iai.67.3.1018-1024.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacMicking JD, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995; 81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 35.Moore WM, et al. L-N6-(1-iminoethyl)-lysine: a selective inhibitor of inducible nitric oxide synthase. J Med Chem. 1994; 37:3886–3888. doi: 10.1021/jm00049a007. [DOI] [PubMed] [Google Scholar]
- 36.Schümann J, Angermüller S, Bang R, Lohoff M, Tiegs G. Acute hepatotoxicity of Pseudomonas aeruginosa exotoxin A in mice depends on T cells and tumor necrosis factor. J Immunol. 1998; 161:5745–5754. [PubMed] [Google Scholar]
- 37.Bergmeyer, H.U. 1984. Methods of enzymatic analysis. 3rd edition. Volume 82. Verlag Chemie. Weinheim, Germany. 416–456.
- 38.Gantner F, et al. Protection from T cell-mediated murine liver failure by phosphodiesterase inhibitors. J Pharmacol Exp Ther. 1997; 280:53–60. [PubMed] [Google Scholar]
- 39.Schümann J, et al. Importance of Kupffer cells for T cell-dependent liver injury in mice. Am J Pathol. 2000; 157:1671–1683. doi: 10.1016/S0002-9440(10)64804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunz P, Walker G, Pfeilschifter J. Dexamethasone differentially affects interleukin 1β- and cyclic AMP-induced nitric oxide synthase mRNA expression in renal mesangial cells. Biochem J. 1994; 304:337–340. doi: 10.1042/bj3040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiegs, G., Küsters, S., and Künstle, G. 1997. T cell-mediated experimental liver injury. In Autoimmune liver disease. P. Berg, A.W. Lohse, G. Tiegs, and A. Wendel, editors. Kluwer Academic Publishers. Dordrecht, The Netherlands. 32–42.
- 42.Li J, Billiar TR. Nitric oxide. IV. Determinants of nitric oxide protection and toxicity in the liver. Am J Physiol. 1999; 276:G1069–G1073. doi: 10.1152/ajpgi.1999.276.5.G1069. [DOI] [PubMed] [Google Scholar]
- 43.Freudenberg MA, Keppler D, Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986; 51:891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Car BD, et al. Interferon γ receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994; 179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng W, Thiel B, Tannenbaum CS, Hamilton TA, Stuehr DJ. Synergistic cooperation between T cell lymphokines for induction of the nitric oxide synthase gene in murine peritoneal macrophages. J Immunol. 1993; 151:322–329. [PubMed] [Google Scholar]
- 46.Curran RD, et al. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990; 212:462–469. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaguchi Y, et al. Peroxinitrite formation during rat hepatic allograft rejection. Hepatology. 1999; 29:777–784. doi: 10.1002/hep.510290354. [DOI] [PubMed] [Google Scholar]
- 48.Tzeng E, et al. Adenovirus-mediated inducible nitric oxide synthase gene transfer inhibits hepatocyte apoptosis. Surgery. 1998; 124:278–283. [PubMed] [Google Scholar]
- 49.Salvemini D, et al. Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. J Clin Invest. 1995; 96:301–308. doi: 10.1172/JCI118035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim YM, Talanian RV, Li J, Billiar TR. Nitric oxide prevents IL-1β and IFNγ-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1β-converting enzyme) J Immunol. 1998; 161:4122–4128. [PubMed] [Google Scholar]
- 51.Mizuhara H, et al. Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology. 1996; 23:1608–1615. doi: 10.1053/jhep.1996.v23.pm0008675184. [DOI] [PubMed] [Google Scholar]
- 52.Nishikage T, et al. Inhibition of concanavalin A-induced hepatic injury of mice by bacterial lipopolysaccharide via the induction of IL-6 and the subsequent reduction of IL-4: the cytokine milieu of concanavalin A hepatitis. J Hepatol. 1999; 31:18–26. doi: 10.1016/s0168-8278(99)80159-7. [DOI] [PubMed] [Google Scholar]
- 53.Barton BE, Jackson JV. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993; 61:1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schümann J, Bluethmann H, Tiegs G. Synergism of Pseudomonas aeruginosa exotoxin A with endotoxin, superantigen, or TNF results in TNFR1- and TNFR2-dependent liver toxicity in mice. Immunol Lett. 2000; 74:165–172. doi: 10.1016/s0165-2478(00)00240-6. [DOI] [PubMed] [Google Scholar]
- 55.Beck KF, et al. Inducible NO synthase: role in cellular signaling. J Exp Biol. 1999; 202:645–653. doi: 10.1242/jeb.202.6.645. [DOI] [PubMed] [Google Scholar]
- 56.Eigler A, Sinha B, Endres S. Nitric oxide-releasing agents enhance cytokine-induced tumor necrosis factor synthesis in human mononuclear cells. Biochem Biophys Res Commun. 1993; 196:494–501. doi: 10.1006/bbrc.1993.2277. [DOI] [PubMed] [Google Scholar]
- 57.Harbrecht BG, Wang SC, Simmons RL, Billiar TR. Cyclic GMP and guanylate cyclase mediate lipopolysaccharide-induced Kupffer cell tumor necrosis factor-α synthesis. J Leukoc Biol. 1995; 57:297–302. doi: 10.1002/jlb.57.2.297. [DOI] [PubMed] [Google Scholar]
- 58.Won SJ, Huang WT, Lai YS, Lin MT. Staphylococcal enterotoxin A acts through nitric oxide synthase mechanisms in human peripheral blood mononuclear cells to stimulate synthesis of pyrogenic cytokines. Infect Immun. 2000; 68:2003–2008. doi: 10.1128/iai.68.4.2003-2008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eigler A, Moeller J, Endres S. Exogenous and endogenous nitric oxide attenuates tumor necrosis factor synthesis in the murine macrophage cell line RAW 264.7. J Immunol. 1995; 154:4048–4054. [PubMed] [Google Scholar]
- 60.Hierholzer C, et al. Interleukin-6 production in hemorrhagic shock is accompanied by neutrophil recruitment and lung injury. Am J Physiol Lung Cell Mol Physiol. 1998; 275:L611–L621. doi: 10.1152/ajplung.1998.275.3.L611. [DOI] [PubMed] [Google Scholar]