Abstract
Interleukin 2 (IL-2) rapidly induces tyrosine phosphorylation of intracellular substrates, including the IL-2 receptor β chain (IL-2Rβ), Janus kinase 1 (Jak1), Jak3, signal transducer/activator of transcription proteins, and Shc, but the mechanism underlying dephosphorylation of these proteins is not known. The src homology 2 (SH2) containing tyrosine phosphatase 1 (SHP-1) is recruited by several hematopoietic surface receptors indicating that this phosphatase plays an important role as a regulator of signaling. We have found that IL-2 induces association of SHP-1 with the IL-2 receptor complex, and that once SHP-1 is recruited to the activated receptor it is able to decrease tyrosine phosphorylation of IL-2Rβ and the associated tyrosine kinases Jak1 and Jak3. This dephosphorylation is specific as expression of a catalytically inactive form of SHP-1, or expression of the related phosphatase SHP-2 did not result in dephosphorylation of the IL-2 receptor components. Furthermore, we have found that SHP-1 expression is greatly decreased or undetectable in a number of IL-2 independent HTLV-I transformed T cell lines that exhibit constitutive Jak/signal transducer/activator of transcription activation. In HTLV-I infected T cells, down-regulation of SHP-1 expression was also found to correlate with the acquisition of IL-2 independence. These observations suggest that SHP-1 normally functions to antagonize the IL-2 signal transduction pathway and that HTLV-I infection and oncogenic transformation can lead to loss of SHP-1 expression resulting in constitutive activation of IL-2 regulated T cell responses.
Binding of interleukin 2 (IL-2) to its receptor is a critical step in the regulation of T cell proliferation. High affinity IL-2 receptors are composed of IL-2 receptor α and β chains (IL-2Rα and IL-2Rβ) and the common cytokine receptor γ chain, γc, which is shared by the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15 (1). IL-2 rapidly induces tyrosine phosphorylation of a number of intracellular substrates, including IL-2 receptor components (2), presumably via the recruitment and activation of the Janus family of protein tyrosine kinases, Janus kinase 1 and 3 (Jak1 and Jak3, refs. 3 and 4). IL-2Rβ constitutively interacts with Jak1 (4–6), while Src family kinases(2), Syk kinase (2), and Shc (7–9) associate with IL-2Rβ following ligand binding; similarly, Jak3 is inducibly recruited to γc (4–6). Phosphorylation of tyrosine residues on IL-2Rβ has been shown to be essential for the coupling of signaling pathways that regulate T cell proliferation in response to IL-2 (8, 10).
The mechanism by which phosphorylation induced through the IL-2 receptor is controlled is not known. However, although tyrosine phosphorylation of IL-2Rβ and the signaling molecules associated with it is detected within seconds following IL-2 stimulation, it is transient as dephosphorylation also occurs. Therefore dephosphorylation of key tyrosine residues may be an important mechanism involved in the down modulation of signals activated through the IL-2 receptor.
To date no specific tyrosine phosphatase has been shown to be part of this process. SHP-1 and SHP-2 are cytoplasmic phosphatases that contain two src homology 2 (SH2) domains and are involved in the regulation of cellular proliferation and survival (11). SHP-1 expression is restricted to hematopoietic cells and has been shown to be an important negative regulator involved in signaling through receptors such as killer cell inhibitory receptor, erythropoietin-R, βc chain, colony-stimulating factor 1, c-Kit, CD22, and the T cell antigen receptor (reviewed in ref. 11). Association of SHP-1 to the majority of these receptors is mediated by phosphorylated tyrosine-based motifs known as immunoreceptor tyrosine-based inhibitory motifs (reviewed in ref. 12). The critical role of SHP-1 in regulating leukocyte responses is underscored by the phenotype of motheaten mice (me/me and mev/mev), which exhibit abnormalities in all hematopoietic cell lineages (reviewed in ref. 13). Interestingly thymocytes from motheaten mice show increased proliferative responses to IL-2 suggesting that SHP-1 may be required to modulate IL-2 signaling (14).
Deregulation of IL-2 receptor signaling appears to play an important role in the events that lead to immortalization and oncogenic transformation of CD4+ T cells by human T cell lymphotropic virus type I (HTLV-I) (reviewed in ref. 15). HTLV-I has been shown to be the etiological agent for adult T cell leukemia and tropical spastic paraparesis (15). Previous work has demonstrated that HTLV-I transformed T cell lines that proliferate independently of IL-2 exhibit constitutive tyrosine phosphorylation of the IL-2 receptor complex, including IL-2Rβ, Jak1, Jak3, signal transducer/activator of transcription 3 (STAT3), and STAT5 (16, 17). However, although in many HTLV-I-transformed T cell lines increased Jak/STAT activation has been observed, the mechanisms involved in this IL-2 independent growth remain unclear.
We now report that SHP-1 is recruited to the IL-2 receptor upon IL-2 stimulation and that once present in the activated receptor complex it is able to down regulate phosphorylation of IL-2Rβ and of the associated Jak kinases, Jak1 and Jak3. We show that this dephosphorylation requires SHP-1 catalytic activity and cannot be achieved by expression of SHP-2. Moreover, we show that SHP-1 expression is not detected in a number of HTLV-I-transformed T cell lines that proliferate independent of IL-2, suggesting that loss of SHP-1 may participate in the events that lead to HTLV-I-mediated transformation.
METHODS
Plasmids.
Human IL-2Rβ, γc, and Jak3 cDNAs were cloned into pME18S, in which expression is driven by the SRα promoter (18); the murine Jak1 cDNA was cloned in pMLCMV; human SHP-1 and human SHP-1 mutants in pBabe have been described (19). The kinase inactive Jak3 K855A was prepared using an oligonucleotide designed to change Lys-855 in the JH1 domain to alanine.
Cell Culture and Transfections.
Peripheral blood lymphocytes were isolated from blood of normal donors by using lymphocyte separation medium (Organon Teknika–Cappel). Cells were rested overnight in RPMI 1640 medium/1% fetal bovine serum (FBS), prior to cytokine stimulation. Molt4β cells have been described (20) and were grown in RPMI 1640 medium/10% FBS. For IL-2 stimulation, cells were rested for 16–18 hr in RPMI 1640 medium/1% FBS, and then treated with 0 or 2 nM IL-2 for the indicated time. Alternatively, after 10 min of IL-2 stimulation, cells were washed twice in PBS and resuspended in RPMI 1640 medium containing 10% FBS for the indicated time. The IL-2 dependent T cell line Kit-225 was grown in RPMI 1640 medium/10% FBS containing 50 units IL-2/ml. 293 T+, and αβγ-3T3 (21) cells were maintained in DMEM with 10% FBS and transfected by the calcium phosphate method. Alternatively αβγ-3T3 cells were infected with supernatant from 293 T cells that had been cotransfected with the simian virus-ψ−-A-murine leukemia virus helper virus and either wild-type Jak3 or K855A Jak3 cloned in the retroviral vector pMX-puro (22, 23). Cells were starved in DMEM containing 1% FBS for 24 hr prior to IL-2 stimulation.
The HTLV-I-transformed T cell lines MT1, MT-2, MT-4, HUT102B2, and MJ, have been described (24, 25) and were grown in RPMI 1640 medium/10% FBS. The MB-B3 HTLV-I T cell line was established as described (26). Cells were thereafter maintained in media containing IL-2 (50 units/ml) and over a period of 12–16 months their requirement for IL-2 has decreased to 3 units IL-2/ml.
Immunoprecipitations and Western Blot Analysis.
Cells were washed in PBS, lysed in lysis buffer (10 mM Tris, pH 7.5/150 mM NaCl/2 mM EDTA/0.875% Brij 96/0.125% Nonidet P-40/1 mM Na3VO4/5 mM NaF/10 μg/ml each of leupeptin and aprotinin, and 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochlorine) and centrifuged at 18,000 × g at 4°C for 15 min. Lysates were immunoprecipitated by using either mAb 561 to IL-2Rβ, antisera to Jak1, SHP-1, SHP-2 (Santa Cruz Biotehnology), or to Jak3 (3) and protein A agarose beads for 2–4 hr at 4°C. Proteins were separated on SDS/8% polyacrylamide gels (NOVEX, San Diego), transferred onto polyvinylidene difluoride membranes (Millipore), and immunoblotted by using either antisera to Jak1, SHP-1, SHP-2, or IL-2Rβ (Santa Cruz Biotechnology), Jak3, or anti-phosphotyrosine mAb (4G10, Upstate Biotechnology, Lake Placid, NY).
Blots were visualized by enhanced chemiluminescence (Amersham).
RESULTS
Time Course of Tyrosine Phosphorylation of the IL-2 Receptor Complex.
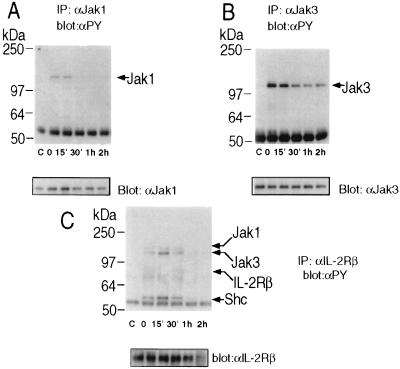
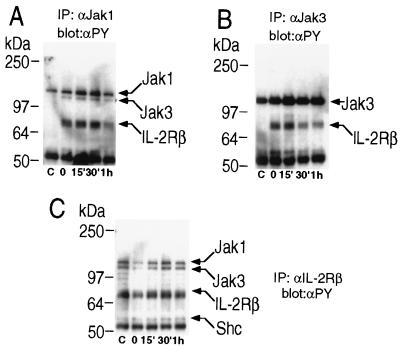
To examine the duration of tyrosine phosphorylation of proteins in the IL-2 receptor complex, we analyzed IL-2 responses in Molt4β cells, a human T cell line stably transfected with IL-2Rβ. Cells were treated with IL-2 for 10′, washed and left in culture in the absence of IL-2 for periods up to 2 hr. Markedly reduced Jak1 tyrosine phosphorylation was observed 30 min following removal of IL-2, while complete absence of detectable tyrosine phosphorylation of Jak1, IL-2Rβ, and signaling molecules associated with IL-2Rβ (Jak1, Jak3, and Shc, Fig. 1 A and C) occurred an hour following removal of IL-2. Tyrosine phosphorylation of Jak3 was also rapidly decreased, but was still detected at 2 hr following removal of IL-2 (Fig. 1B). This time course of tyrosine phosphorylation suggests that a phosphatase may be involved in the regulation of signals following IL-2 stimulation.
Figure 1.
Time course of tyrosine phosphorylation of IL-2 receptor components after IL-2 stimulation. Molt4β cells were stimulated for 10′ with 2nM IL-2 at 37°C. Cells were washed in PBS and resuspended in 10% FBS/RPMI 1640 medium and left in culture for the indicated time. In control lanes (marked c), cells were not exposed to IL-2. Cells were then washed, lysed and immunoprecipitations were performed with the indicated antibodies: (A) anti-Jak 1; (B) anti-Jak3; (C) anti-IL-2Rβ and were followed by Western blot analysis with mAb 4G10. Immunoblots were stripped and reprobed to control for equal protein loading.
IL-2 Induces Association of SHP-1 with IL-2 Receptor.
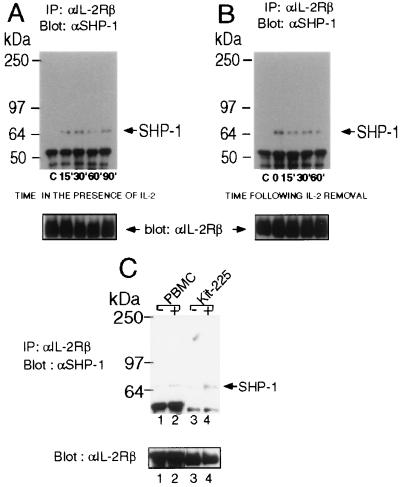
The protein tyrosine phosphatase SHP-1 is known to be recruited to a number of cytokine receptors including EpoR and the common β chain of the IL-3 receptor. We therefore examined whether SHP-1 might be recruited to the IL-2 receptor complex. As the association of SHP-1 to the IL-2 receptor might be inhibited in the presence of IL-2, to allow for continuous activation of proliferative signals, we either treated Molt4β cells with IL-2 for periods up to one hour (Fig. 2A) or stimulated them for 10 min with IL-2 and then cultured them in the absence of cytokine (Fig. 2B). To detect interactions of SHP-1 with the IL-2 receptor we immunoprecipitated IL-2Rβ and probed the immunocomplexes with an anti-SHP-1 antibody. Following continuous or pulse treatment with IL-2, SHP-1 became inducibly associated with IL-2Rβ. Interestingly SHP-1 remained in the IL-2 receptor complex up to 90 min following IL-2 treatment. Similar inducible association of SHP-1 with the IL-2 receptor complex, was seen in the IL-2-dependent T cell line Kit-225 and in freshly isolated peripheral blood mononuclear cells (Fig. 2C). These results indicate that SHP-1 is recruited to the IL-2 receptor following stimulation with IL-2.
Figure 2.
IL-2 induces association of SHP-1 with IL-2Rβ. (A) Molt4β cells were either stimulated with 2nM IL-2 for the indicated time or (B) stimulated for 10′ with 2nM IL-2, washed and resuspended in 10% FBS/RPMI 1640 medium in the absence of IL-2 for the indicated time. Cells were then washed, lysed and immunoprecipitations were performed with mAb 561 to hIL-2Rβ, followed by immunoblotting with anti-SHP-1 (Upstate Biotechnology). Immunoblots were then stripped and reprobed with an antiserum to human IL-2Rβ (Santa Cruz Biotechnology). (C) Freshly isolated peripheral blood mononuclear cells and Kit-225 cells were rested for 4 hr and then stimulated for 10′ with 2nM IL-2 at 37°C. Immunoprecipitations and Western blot analyses were performed as above.
SHP-1 Down-Regulates Phosphorylation of IL-2Rβ and Associated Jak1.
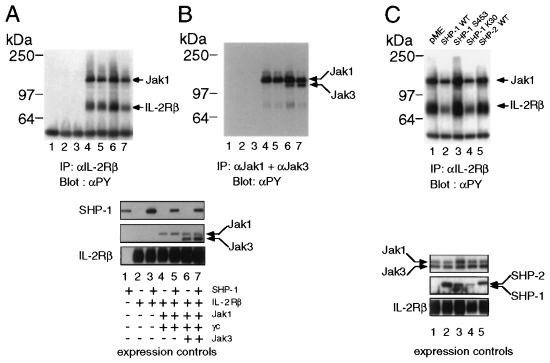
Clearly all the cells that are IL-2 responsive express SHP-1 and therefore are not suitable for studies aimed at examining the effect of SHP-1 on this signaling pathway. Therefore, to explore the role of SHP-1 once it is recruited to the IL-2 receptor complex, we reconstituted the IL-2 receptor by transient expression in 293 T+ cells. Due to overexpression, Jak1 and Jak3 are activated and are able to phosphorylate IL-2Rβ in the absence of ligand. Expression of SHP-1 caused a decrease in tyrosine phosphorylation of IL-2Rβ and of Jak1 that is constitutively associated with IL-2Rβ (Fig. 3A, lanes 4 and 5). A more marked effect was seen when Jak3 and γc were additionally expressed (Fig. 3A, lanes 6 and 7), suggesting that a fully assembled activated receptor is required to see optimal dephosphorylation by SHP-1. Similarly, decreased tyrosine phosphorylation of Jak1 was observed in immunoprecipitates with specific antisera. The small reduction of phosphotyrosine levels of Jak1 may result from overexpression, as most of the Jak kinases expressed in the cells will not be present in the receptor and therefore not accessible to SHP-1 (Fig. 3B).
Figure 3.
SHP- 1 expression down-regulates tyrosine phosphorylation of IL-2 receptor components. (A) 293T+ cells were transfected with the indicated combination of plasmids; 48 hr posttransfection cells were washed, lysed and proteins were immunoprecipitated with mAb 561 to IL-2Rβ or (B) with antibodies to Jak1 and Jak3, followed by Western blot analysis with antiphosphotyrosine mAb. Also shown are controls for equal expression of the transfected plasmids. (C) A catalytically inactive form of SHP-1 does not affect tyrosine phosphorylation of the IL-2 receptor complex. 293 T+ cells were transfected with cDNAs for IL-2Rβ, γc, Jak1, Jak3, and either empty vector or the indicated forms of SHP-1 or SHP-2. Immunoprecipitations and immunoblotting were performed as described in A. Also shown are controls for equal expression of the transfected plasmids.
A Catalytically Inactive Form of SHP-1 Does Not Affect Tyrosine Phosphorylation of the IL-2R Complex.
To assess whether the decrease in tyrosine phosphorylation seen in the presence of SHP-1 was specific, we analyzed two mutant forms of SHP-1 (19): the SHP-1 S453 lacks phosphatase activity, while in the SHP-1 K30 mutant the N-terminal SH2 domain is unable to bind phosphotyrosine residues. In addition we analyzed SHP-2 for its ability to down-modulate phosphorylation of the IL-2 receptor complex, as SHP-2 has been shown to be tyrosine phosphorylated following IL-2 stimulation, but its role in the regulation of IL-2 signals is not clear (27).
We therefore expressed IL-2Rβ, γc, Jak1, and Jak3 in 293 T+ cells and compared the effects of the various forms of SHP-1 and wild-type SHP-2 on the phosphorylation of the IL-2 receptor complex. Lysates from these cells were immunoprecipitated with mAb to IL-2Rβ and probed with antiphosphotyrosine. While expression of wild-type SHP-1 resulted in markedly reduced tyrosine phosphorylation, expression of the SHP-1 S453 mutant did not (Fig. 3D, lanes 2 and 3). In contrast the K30 mutant form of SHP-1, that carries a mutation in the N-terminal SH2 domain, showed an effect similar to the wild-type form, suggesting that this domain is not involved in binding to phosphotyrosine residues that are critical for modulation of signals through the IL-2 receptor. Finally, expression of SHP-2 did not result in a decrease in tyrosine phosphorylation of either IL-2Rβ or Jak1, perhaps indicating that SHP-2 is not involved in down-regulation of IL-2 signaling.
SHP-1 Down-Regulates IL-2-Induced Tyrosine Phosphorylation of Jak1 and Jak3.
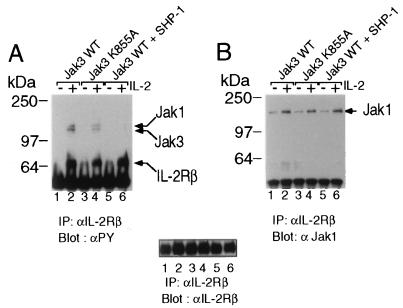
To analyze the effect of SHP-1 on cells in which Jak activation and subsequent tyrosine phosphorylation of the IL-2 receptor can be stimulated by IL-2, we used NIH 3T3 fibroblasts that stably express IL-2Rα, IL-2Rβ, and γc (21). To render these cells IL-2 responsive we infected them with retroviral constructs driving expression of wild-type Jak3 (Fig. 4, lanes 1 and 2), a kinase inactive form of Jak3 denoted K855A (Fig. 4, lanes 3 and 4), or wild-type Jak3 in combination with wild-type SHP-1. Following 30 min of IL-2 stimulation, IL-2Rβ immunoprecipitates were probed with antiphosphotyrosine: when compared with wild-type Jak3, cells expressing Jak3 K855A showed a reduction in IL-2 inducible tyrosine phosphorylation of IL-2Rβ and the associated Jak1 and Jak3, consistent with the ability of this mutant to act as a dominant negative. When SHP-1 was expressed in the presence of WTJak3 tyrosine phosphorylation of Jak1 and Jak3 associated with IL-2Rβ was not detected, consistent with our observation in 293 T+ cells. Although SHP-1 expression reduced the tyrosine phosphorylation of Jak1 and Jak3, it did not affect their ability to associate with IL-2Rβ, because when the same immunocomplexes were then probed with anti Jak1 (Fig. 4B), or antiJak3 antibody (data not shown), no differences in the amounts of Jak kinases present in the IL-2 receptor complex were detected. This finding suggests that even in the presence of SHP-1 Jak kinases are recruited to the IL-2 receptor, but that expression of the phosphatase results in a more rapid reduction of Jak1 and Jak3 tyrosine phosphorylation.
Figure 4.
SHP-1 expression down regulates IL-2 induced phosphorylation of Jak1 and Jak3 in αβγ-3T3 cells, but does not affect their ability to associate with IL-2Rβ. (A) αβγ-3T3 cells were infected with virus encoding wild-type Jak3 (lanes 1 and 2), kinase inactive Jak3 (lanes 3 and 4), and wild-type Jak3 and SHP-1 (lanes 5 and 6). Cells were stimulated for 30 min with 2nM IL-2 at 37°C. Immunoprecipitations and Western blot analysis were performed as above. (B) The membrane was stripped and reprobed with anti Jak1 and anti-Jak3 antibody to analyze the effect of SHP-1 on IL-2Rβ-Jak1 interactions. Additionally, the same membrane was reprobed with anti-IL-2Rβ antibody as a control for equal protein loading.
SHP-1 Expression in HTLV-I-Transformed T Cells.
Previous work has established that HTLV-I-transformed T cell lines, that grow independent of IL-2, exhibit constitutive activation of Jak kinases and STAT proteins normally regulated by IL-2 binding to its receptor (15, 16). Transition from IL-2-dependent to IL-2-independent growth in HTLV-I-infected peripheral blood lymphocytes in vitro occurs, by an unknown mechanism over an extended period; this process was also shown to correlate to acquisition of constitutive activation of the Jak-STAT pathway.
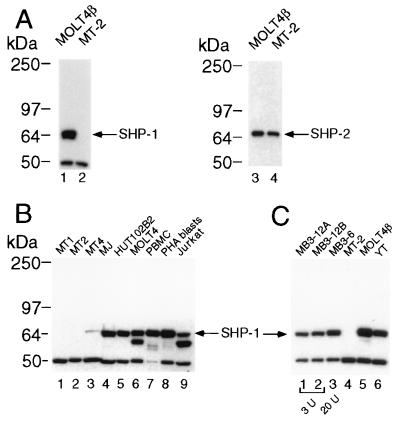
We therefore analyzed the HTLV-I-transformed T cell line MT-2 as this line shows constitutive activation of Jak1 and Jak3 (15) that can be further induced by IL-2: upon stimulation with IL-2 we observed an enhanced and sustained phosphorylation of Jak1 and Jak3 (Fig. 5 A and B). In contrast to Molt4β cells (Fig. 1), levels of Jak and receptor tyrosine phosphorylation were not significantly decreased when IL-2 was removed. These cells also exhibit a constitutively assembled and phosphorylated receptor complex (Fig. 5C). As SHP-1 might be an important regulator of IL-2 signaling, we investigated whether a defect in either function or expression of SHP-1 could explain the constitutive activation of the Jak-STAT pathway observed in these cells. We therefore compared the levels of expression of SHP-1 and SHP-2 in Molt4β cells and MT-2 and found that while similar levels of SHP-2 were observed in the two cell lines, SHP-1 expression was not detected in MT-2 cells (Fig. 6A). We then analyzed a number of HTLV-I-transformed T cells and found a marked decrease in SHP-1 expression in two additional cell lines, MT1 and MT4. However, two other lines, HUT102B2 and MJ, showed levels of SHP-1 similar to non-HTLV-I-transformed T cells (Fig. 6B). Again, similar levels of SHP-2 expression were observed in all the cell lines examined (data not shown). Finally we analyzed two clones of HTLV-I-infected peripheral blood lymphocytes that are becoming IL-2-independent MB3–12A and MB3–12B, and compared them to the parental cells, MB3–6 that require a higher amount of IL-2 for cell growth. Both clones in which dependency on IL-2 was diminished, showed decreased SHP-1 expression when compared with the parental cell line (Fig. 6C). These findings indicate that the loss of SHP-1 expression may contribute to the constitutive Jak-STAT activation and IL-2-independence acquired by these HTLV-I-transformed T cell lines.
Figure 5.
IL-2 receptor phosphorylation in HTLV-I-transformed T cells. The HTLV-I-transformed T cell line MT-2 was stimulated as described in Fig. 1. Immunoprecipitations and Western blot analysis were performed as described in Fig. 1.
Figure 6.
SHP-1 expression is not detected in some HTLV-I-transformed T cell lines. (A) Lysates (40 μg) from MOLT4β or MT-2 cells probed with antisera to either SHP-1 or SHP-2. (B) Lysates (40 μg) from the HTLV-I-transformed T cell lines MT1, MT2, MT4, MJ, Hut102B, or from MOLT4β, freshly isolated peripheral blood mononuclear cells, phytohemagglutinin-activated peripheral blood lymphocytes, and Jurkat, were immunoblotted with anti-SHP-1. (C) 40 μg of lysates from the HTLV-I-infected T cells MB3–12A, MB3–12B (grown in medium containing 3 units IL-2/ml), MB3–6 (grown in medium containing 20 units IL-2/ml) were compared with lysates from MT-2, MOLT4β, and YT cells by immunoblotting with anti-SHP-1.
DISCUSSION
Previous reports have established an important role for SHP-1 as a negative regulator in hematopoietic cells. In this study we have found that SHP-1 is inducibly recruited to the IL-2 receptor where it is able to dephosphorylate components of the IL-2 receptor complex, therefore regulating the duration of signals transduced through this receptor system. Expression of a phosphatase dead form of SHP-1 or wild-type SHP-2 did not result in the dephosphorylation of the IL-2 receptor, indicating that the effect seen with wild-type SHP-1 was specific. This finding suggests that SHP-1 is a negative regulator of the IL-2 signaling pathway.
The mechanism by which SHP-1 is recruited to the IL-2 receptor remains unclear as no known signaling molecule that is part of the activated complex contains any immunoreceptor tyrosine-based inhibitory motif. These tyrosine-based motifs have been found on a number of receptors that are known to associate with SHP-1, via interaction with an SH2 domain on SHP-1 (12). Yet interaction with and phosphorylation of Jak2 in an overexpression system (19) have been reported to occur independently of SH2 domains on SHP-1, suggesting that direct substrate binding, not mediated by immunoreceptor tyrosine-based inhibitory motifs, is an additional mechanism through which SHP-1 might function. Our findings show that the association of SHP-1 to the IL-2 receptor was seen only following IL-2 stimulation (Fig. 2), suggesting that SHP-1 may be recruited to an activated Jak kinase. Dephosphorylation of Jak1 and IL-2Rβ was seen when SHP-1 was expressed in the absence of γc and Jak3 (Fig. 3A, lanes 4 and 5), indicating that direct binding of SHP-1 to Jak3 is not required, yet SHP-1-mediated dephosphorylation appeared to be more efficient in the presence of a fully assembled receptor complex (when Jak3 and γc were additionally expressed).
The SHP-1 K30 mutant, that carries a point mutation in the N-terminal SH2 domain, was able to dephosphorylate IL-2Rβ and Jak1 to an extent similar to the wild-type form of SHP-1. This suggests that binding of phosphotyrosines to that SH2 domain is not required for SHP-1-IL-2 receptor complex interactions. Clearly the catalytically inactive SHP-1 (SHP-1 S453) was not capable of reducing phosphotyrosine levels of the components of the IL-2 receptor complex, indicating that the dephosphorylation observed is a specific effect of SHP-1 and does not result from the recruitment of other proteins by SHP-1, or the disruption of the IL-2 receptor complex by SHP-1. More recently the structurally related protein tyrosine phosphatase SHP-2 has been implicated in IL-2 signaling (27). SHP-2 has been found to enhance responses to growth factors such as erythropoietin and epidermal growth factor, perhaps via its association with Grb2 and the subsequent coupling to the Ras pathway. A role for SHP-2 in the IL-2 response has not been established, but the apparent lack of effect on tyrosine phosphorylation of proteins associated with the IL-2 receptor complex are inconsistent with an inhibitory role for SHP-2.
If SHP-1 functions as a negative regulator of IL-2 signaling, it might be expected that the lack of SHP-1 in me/me thymocytes would increase responsiveness to IL-2. Consistent with this, me/me thymocytes exhibit a more than two fold increase in proliferation in response to IL-2, suggesting that SHP-1 expression may be required to curtail IL-2 proliferative signals (14). Moreover, heightened T cell responses and perhaps IL-2 proliferation, may contribute to the autoimmunity observed in these mice.
The importance of SHP-1 in regulating IL-2 signaling was also suggested by our findings in HTLV-I-transformed T cells. These cells grow independently of IL-2 and exhibit constitutive activation of Jak kinases and STAT proteins that are normally regulated by IL-2. Acquisition of IL-2 independence in HTLV-I-infected T cells is a critical step in the events that lead to transformation. Therefore it was striking that three HTLV-I-transformed T cell lines that we examined showed no detectable expression of SHP-1. Furthermore, two HTLV-I-infected T cell lines, that are becoming IL-2 independent, showed decreased SHP-1 expression when compared with the parental line that requires higher amounts of IL-2 for cell growth. MT-1, MT-2, and MT-4 cells were derived by cocultivation of normal cord blood lymphocytes with leukemic T cells from adult T cell leukemia patients. In contrast the two other HTLV-I-transformed cell lines examined, MJ and HUT102B2, that still express SHP-1 were directly derived from peripheral blood of patients who suffered from cutaneous T cell lymphomas associated with Sezary syndrome. MJ and HUT102B2 are unusual in that they constitutively express IL-15 (28), a cytokine that employs IL-2Rβ and γc for its action, while other HTLV-I-transformed T cell lines do not produce detectable levels of either IL-2 or IL-15. Therefore in the cell lines MJ and HUT102B2, IL-15 may mediate the constitutive activation of the Jak-STAT pathway and hence overcome the requirement for down-regulation of SHP-1 expression.
Interestingly, an oncogenic mutation in the c-Kit receptor (D814Y) has been shown to induce rapid degradation of SHP-1, coupled with constitutive and increased tyrosine phosphorylation of the mature and immature forms of the c-kit receptor (29). Recently down-regulated expression of SHP-1 in Burkitt lymphomas and germinal center B lymphocytes has also been reported (30). This together with our findings in HTLV-I-transformed T-cells suggests that down-regulation of expression of SHP-1 may cause changes in the regulation of signaling pathways that result in factor independent growth and oncogenic transformation.
In summary, our results suggest that the recruitment of SHP-1 to the IL-2 receptor complex represents an important mechanism for the regulation of IL-2-induced responses, and that the loss of SHP-1 may contribute to the oncogenic transformation observed in adult T cell leukemia.
Acknowledgments
We wish to thank Dr. P. Sondel, University of Wisconsin, for the mAb 561 to IL-2Rβ, Dr. K. Sugamura for Molt4β cells, Dr. T. Taniguchi for αβγ-3T3 cells, and Drs. J. Bolen and R. Herbst for critical comments.
ABBREVIATIONS
- Jak
Janus kinase
- STAT
signal transducer/activator of transcription
- HTLV-I
human T-lymphotropic virus type I
- IL-2
interleukin 2
- SH2
src homology
- SHP
SH2 containing phosphatase
- IL-2Rα and IL-2Rβ
IL-2 receptor α and β chains
- FBS
fetal bovine serum
References
- 1.Leonard W J. Annu Rev Med. 1996;47:229–236. doi: 10.1146/annurev.med.47.1.229. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi T. Science. 1995;268:251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 3.Johnston J A, Kawamura M, Kirken R A, Chen Y Q, Blake T B, Shibuya K, Ortaldo J R, McVicar D W, O’Shea J J. Nature (London) 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 4.Russell S M, Johnston J A, Noguchi M, Kawamura M, Bacon C M, Friedmann M, Berg M, McVicar D W, Witthun B A, Silvennoinen O A S, et al. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 5.Boussiotis V A, Barber D L, Nakarai T, Freeman G J, Gribben J G, Bernstein G M, D’Andrea A D, Ritz J, Nadler L M. Science. 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki T, Kawahara A, Fujii H, Nkagawa Y, Minami Y, Liu Z J, Oishi I, Silvennoinen O, Witthun B A, Ihle J N, Taniguchi T. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 7.Evans G A, Goldsmith M A, Johnston J A, Xu W, Weiler S R, Erwin R, Howard O M, Abraham R T, O’Shea J J, Greene W C, Farrar W L. J Biol Chem. 1995;270:28858–28863. doi: 10.1074/jbc.270.48.28858. [DOI] [PubMed] [Google Scholar]
- 8.Friedmann M C, Migone T-S, Russell S M, Leonard W J. Proc Natl Acad Sci USA. 1996;93:2077–2082. doi: 10.1073/pnas.93.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravichandran K S, Burakoff S. J Biol Chem. 1994;269:1599–1602. [PubMed] [Google Scholar]
- 10.Goldsmith M A, Lai S A, Xu W, Amaral C, Kuczek E S, Parent L J, Mills G B, Tarr K L, Longmore G D, Greene W C. J Biol Chem. 1995;270:21729–21737. doi: 10.1074/jbc.270.37.21729. [DOI] [PubMed] [Google Scholar]
- 11.Neel B G. Curr Opin Immunol. 1997;3:405–420. doi: 10.1016/s0952-7915(97)80088-x. [DOI] [PubMed] [Google Scholar]
- 12.Unkeless J C, Jin J. Curr Opin Immunol. 1997;3:385–390. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 13.Schultz L D, Rajan T V, Greiner D L. Trends in Biotechnology. 1997;15:302–307. doi: 10.1016/S0167-7799(97)01060-3. [DOI] [PubMed] [Google Scholar]
- 14.Lorenz U, Ravichandran K S, Burakoff S J, Neel B G. Proc Natl Acad Sci USA. 1996;93:9624–9629. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yodoi J, Uchiyama T. Immunol Today. 1992;13:405–411. doi: 10.1016/0167-5699(92)90091-K. [DOI] [PubMed] [Google Scholar]
- 16.Migone T-S, Lin J-X, Cereseto A, Mulloy J C, O’Shea J J, Franchini G, Leonard W J. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Kang S H, Heidenreich O, Brown D A, Nerenberg M I. J Clin Invest. 1995;96:1548–1555. doi: 10.1172/JCI118193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao H, Berrada K, Yang W, Tabrizi M, Platanias L C, Yi T. Mol Cell Biol. 1996;16:6985–6992. doi: 10.1128/mcb.16.12.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshita T, Ohtani K, Asao H, Kumaki S, Nakamura M, Sugamura K. J Immunol. 1992;148:2154–2158. [PubMed] [Google Scholar]
- 21.Chen M, Cheng A, Chen Y-Q, Hymel A, Hanson E P, Kimmel L, Minami Y, Taniguchi T, Changelian P S, O’Shea J J. Proc Natl Acad Sci USA. 1997;94:6910–6915. doi: 10.1073/pnas.94.13.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landau N R, Littman D R. J Virol. 1992;66:5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onishi M, Kinoshita S, Morikawa Y, Shibuya A, Philips J, Lanier L L, Gorman D M, Nolan G P, Miyajima A, Kitamura T. J Exp Hematol. 1996;24:324–329. [PubMed] [Google Scholar]
- 24.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Nature (London) 1981;296:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 25.Gazdar A F, Carney D N, Bunn P A, Russell E K, Jaffe E S, Schecter G P, Guccion J G. Blood. 1980;55:409–417. [PubMed] [Google Scholar]
- 26.Kohn D B, Mitsuya H, Ballow M, Selegue J E, Barankiewicz J, Cohen A, Gelfand E, Anderson W F, Blaese R M. J Immunol. 1989;142:3971–3975. [PubMed] [Google Scholar]
- 27.Adachi M, Ishino M, Torigoe T, Minami Y, Matozaki T, Miyazaki T, Taniguchi T, Hinoda Y, Imai K. Oncogene. 1997;14:1629–1635. doi: 10.1038/sj.onc.1200981. [DOI] [PubMed] [Google Scholar]
- 28.Bamford R N, Grant A J, Burton J D, Peters C, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. Proc Natl Acad Sci USA. 1994;91:3005–3009. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piao D, Paulson R, Geer P V D, Pawson T, Bernstein A. Proc Natl Acad Sci USA. 1996;93:14665–14669. doi: 10.1073/pnas.93.25.14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delibrias C C, Floettmann J E, Rowe M, Fearon D T. J Exp Med. 1997;186:1575–1583. doi: 10.1084/jem.186.9.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]