Abstract
The conditional systems Tet-on and Geneswitch were compared and optimized for the tissue-specific expression of transgenes and manipulation of life span in adult Drosophila. Two versions of Tet-on system reverse-tetracycline-Trans-Activator (rtTA) were compared: the original rtTA, and rtTAM2-alt containing mutations designed to optimize regulation and expression. The rtTAM2-alt version gave less leaky expression of target constructs in the absence of doxycycline, however the absolute level of expression that could be achieved was less than that produced by rtTA, in contrast to a previous report. Existing UAS-rtTAM2-alt insertions were re-balanced, and combined with several tissue-general and tissue-specific GAL4 driver lines to yield tissue-specific, doxycycline-inducible transgene expression over three orders of magnitude. The Geneswitch (GS) system also had low background, but the absolute level of expression was low relative to Tet-on. Consequently, actin5C-GS multi-insert chromosomes were generated and higher-level expression was achieved without increased background. Moderate level over-expression of MnSOD has beneficial effects on life span. Here high-level over-expression of MnSOD was found to have toxic effects. In contrast, motor-neuron-specific over-expression of MnSOD had no detectable effect on life span. The results suggest that motor-neuron tissue is not the essential tissue for either MnSOD induced longevity or toxicity in adult males.
Keywords: aging, tet-on, Geneswitch, MnSOD, mitochondria, PTEN
1. Introduction
Conditional gene expression systems are a powerful tool for the study of Drosophila aging mechanisms (Aigaki et al., 2002; Giannakou et al., 2004; Hwangbo et al., 2004; Tower, 2000). The primary advantages of these systems are that experimental and control groups are genetically identical, produced from the same cross, and share developmental conditions, and that the experimenter can decide when in the life cycle to turn transgene expression on and off. The basic principle of all conditional systems is that an environmental stimulus is used to activate expression of a gene of interest. Here two conditional systems were compared and optimized for the tissue-specific expression of transgenes in Drosophila. The “Tet-on” system uses the tetracycline derivative doxycycline (DOX) as the stimulus, and the “Geneswitch” system uses the drug RU486/Mifepristone.
The reverse-tetracycline transactivator (rtTA) is an artificial transcription factor that was developed to allow doxycycline-regulated transgene expression in eukaryotes (Gossen and Bujard, 1995; Kistner et al., 1996). The rtTA protein consists of the DNA binding domain of the E. coli reverse-tet-repressor, fused to the transcriptional activation domain of the herpes simplex virus VP16 transcription factor. The result is an artificial transcription factor that will bind to its target site, the tet-operator sequence (TetO) and activate transcription of an adjacent promoter only in the presence of DOX or other tetracycline derivatives (the Tet-on system). The Tet-on system was introduced into Drosophila melanogaster by driving expression of rtTA with the tissue-general actin5C promoter (Figure 1A). A synthetic target promoter (the Tet-on promoter) was produced by placing 7 TetO sites adjacent to a truncated or “core” promoter derived from the hsp70 or other genes. This system yielded up to a 100-fold induction of reporter proteins such as beta-galactosidase in adult male flies without a detectable decrease in life span (Bieschke et al., 1998).
Fig. 1.
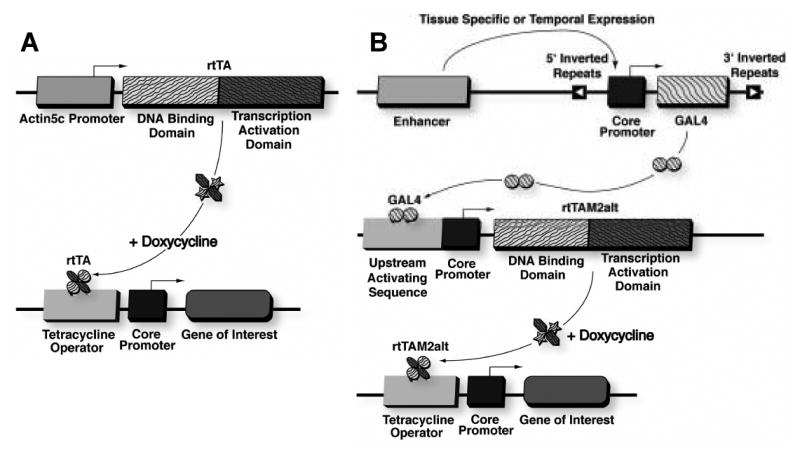
The Tet-on system. (A) The tissue-general actin5C promoter drives the expression of the artificial transcription factor rtTA (or in some cases, rtTAM2-alt). The rtTA transactivator protein will bind to a Tet-operator sequence only in the presence of doxycycline, and activate transcription of the downstream core promoter. (B) The tissue-specific Tet-on system. The “GAL4 driver” is usually a P element enhancer trap insertion that yields expression of the GAL4 transcription factor only in specific tissues. When GAL4 binds to the bridge construct, it drives expression of rtTAM2-alt. The rtTAM2-alt will in turn bind to a Tet-operator sequence only in the presence of doxycycline, and activate transcription of the downstream core promoter.
The rtTAM2-alt form of the rtTA transactivator was created with multiple mutations designed to yield greater expression and tighter regulation by DOX. The rtTAM2-alt transactivator was also introduced into Drosophila under the control of the actin5c promoter (Stebbins et al., 2001). While it was reported that rtTAM2-alt yielded higher levels of transgene expression than the original rtTA, a meaningful comparison was not possible, due to the lack of detectable rtTA activity in that study. In addition, the researchers developed a tissue-specific Tet-on system by combining rtTAM2-alt with GAL4/UAS. In the GAL4/UAS system, a tissue specific promoter or enhancer drives expression of the yeast transcription factor GAL4 in one transgenic construct. GAL4 then binds to an upstream activating sequence (UAS) site in the promoter of a second target construct and activates transcription (Brand and Perrimon, 1993; Seroude, 2002; Seroude et al., 2002). Stebbins and coworkers combined these two systems by creating a bridge construct where UAS promoter drives expression of rtTAM2-alt (Stebbins et al., 2001). In the combined system the “GAL4 driver” chromosome yields GAL4 expression only in specific tissues (Figure 1B). The GAL4 binds to the UAS promoter in the bridge construct, which drives rtTAM2-alt expression. The rtTAM2-alt will in turn bind to the TetO sites in a third target construct, but only in the presence of DOX. The result is transgene expression that is both tissue-specific and conditional.
A mobile Tet-on promoter has been developed in order to increase the number of genes that can be regulated by the Tet-on system. A Drosophila P type transposable element was constructed, called PdL, with a DOX-inducible promoter directed outward from its 3′ end (Landis et al., 2001). Transposable elements with outwardly directed promoters, like PdL, have been shown to be powerful tools for creating mutations by over-expression and/or mis-expression of genes near their insertion site (Rorth et al., 1998). When PdL transposes into a fly chromosome, it can cause DOX-dependent over-expression of a gene up to thousands of base-pairs downstream of the insertion site. It is estimated that up to a third of PdL insertions cause the over-expression of a downstream gene. Gene over-expression caused by PdL frequently, but not always, results in a conditional mutant phenotype.
The Geneswitch system also allows for conditional gene expression in Drosophila (Osterwalder et al., 2001; Roman and Davis, 2002; Roman et al., 2001). In this case the transactivator protein, Geneswitch, is a fusion of the DNA binding and transcriptional activation domains of yeast GAL4 with the regulatory domain of the human progesterone receptor. Geneswitch will bind to a UAS site and activate transcription only in the presence of progesterone, or analogs such as RU486. In this study the Tet-on and Geneswitch systems have been compared and optimized for use in Drosophila aging research.
2. Materials and Methods
Drosophila melanogaster strains are as previously described (Table 1) (Bieschke et al., 1998; Landis et al., 2001; Lindsley and Zimm, 1992). Northern blot procedures were as previously described (Landis et al., 2001). Briefly, 30 adult males of the indicated genotypes were cultured in the presence of drug, and 30 in the absence of drug, for one week with transfer to fresh food vials every-other day. Total RNA was extracted from the flies using RNAqueous kit (Ambion), fractionated on 1% agarose gels and transferred to GeneScreen membrane (Dupont/NEN). RNA loaded was 1X = 4μg and 2X = 8μg. The blot was hybridized in succession with P32-labelled DNA probe specific for the MnSOD gene and Rp49 gene as a loading control. Signals were visualized and quantified using a phosphoimager.
Table 1.
Genotypes for transgeneic fly strains. The chromosome of insertion is given in parentheses.
| LacZ Lines | |
| 7T40 | w1118; P{7T40.B}E1(2) |
| 7TAdh | w1118; P{7TAdh.B}D1(3) |
| UAS-LacZ | w; P{UAS-LacZ.B}Bg4-1-2(2) |
| PdL Lines | |
| PdL 11A3 | yacw; P{PdL}ash1[11A3](3) |
| PdL 19B3 | yacw; P{PdL}alpha-Man-II[19B3](3) / TM3 |
| PdL 35B1 | yacw; P{PdL}35B1(3) / TM3 |
| PdL 3A | yacw; P{PdL}3A(3) / TM3 |
| PdL 3G | yacw; P{PdL}pum[3G] / TM3 |
| rtTA Driver Lines | |
| A1 | w1118; P{rtTA.B}A1(X) |
| C1 | w1118; P{rtTA.B}C1(2) |
| D1 | w1118; P{rtTA.B}D1(2) / CyO |
| E2 | w1118; P{rtTA.B}E2(3) / TM3 |
| F1 | w1118; P{rtTA.B}F1(2) |
| G1 | w1118; P{rtTA.B}G1(3) |
| I2 | w1118; P{rtTA.B}I2(3) / TM3 |
| K2 | w1118; P{rtTA.B}K2(3) / TM3 |
| M1 | w1118; P{rtTA.B}M1(3) / TM3 |
| rtTAM2-alt Driver Lines | |
| 901 | yacw; P{UAS-rtTA-M2-alt}901(2) / CyO |
| Act-962 | yacw; P{Act5C-rtTA-M2-alt}962(2) / CyO |
| Act-965 | yacw; P{Act5C-rtTA-M2-alt}965(2) |
| Act-966 | yacw; P{Act5C-rtTA-M2-alt}966(3) / TM3 |
| Tet-on Gal4/UAS Driver Lines | |
| TO-Actin (tissue-general) | w; P{UAS-rtTA-M2-alt}901(2) / CyO ; P{Act5C-GAL4}17bFO1(3) / TM6B |
| TO-D42 (motor neurons) | w; P{UAS-rtTA-M2-alt}901(2) ; P{GawB}D42(3) |
| TO-Elav (pan neuronal) | w; P{GawB}elavC155(X) ; P{UAS-rtTA-M2-alt}901(2) / CyO |
| TO-Tubulin (tissue-general) | w; P{UAS-rtTA-M2-alt}901(2) / CyO ; P{tubP-GAL4}LL7(3) / TM6B |
| Tet-on MnSOD Lines | |
| MnSOD12 | w1118; P{7T(MnSOD)12}(2) |
| MnSOD20 | w1118; P{7T(MnSOD)20}(2) |
| MnSOD22 | w1118; P{7T(MnSOD)22}(2) |
| MnSOD12,22 | w1118; P{7T(MnSOD)12}(2), P{7T(MnSod)22}(2) |
| Geneswitch Driver Lines | |
| GS-Elav | yw; P{elav-Switch.O}GSG301(3) |
| GS-Actin204 | w; P{Act5C(-FRT)GAL4.Switch.PR}204(2) |
| GS-Actin204-1 | w; P{Act5C(-FRT)GAL4.Switch.PR}204-1(2) |
| GS-Actin255 | w; P{Act5C(-FRT)GAL4.Switch.PR}255(2) |
| GS-Actin255-A | w; P{Act5C(-FRT)GAL4.Switch.PR}255A(2) |
| GS-Actin255-B | w; P{Act5C(-FRT)GAL4.Switch.PR}255B(2) |
| GS-Actin255-C | w; P{Act5C(-FRT)GAL4.Switch.PR}255C(2) |
| GS-Actin255-D | w; P{Act5C(-FRT)GAL4.Switch.PR}255D(2) |
| GS-Actin255-F | w; P{Act5C(-FRT)GAL4.Switch.PR}255F(2) |
| Geneswitch Expression Lines | |
| HS1 | w; P{UAS-Hsap\SOD1.P}(2) |
| PTEN(2) | w; P{UAS-Pten.Gb}(2) |
| PTEN(3) | w; P{UAS-Pten.Gb}(3) |
Tet-on MnSOD expression construct was created by cloning the MnSOD cDNA (Sun et al., 2002) into the USC1.0 vector (Allikian et al., 2002) and multiple independent transformant strains were generated as previously described. Strains containing UAS-rtTA-M2-alt constructs were provided by Jerry Yin (Stebbins et al., 2001). The GS-Act strains 204 and 255 were generously provided prior to publication by Hervé Tricoire, and contain a P element construct in which the Geneswitch cDNA is cloned downstream of the actin5C promoter (personal communication). Other GS strains were provided by T. Osterwalder and R. Davis (Osterwalder et al., 2001; Roman et al., 2001). Flies were cultured on standard agar/molasses/corn meal/yeast media (Ashburner, 1989). The Oregon-R control strain (provided by the Bloomington Drosophila stock center) and the experimental lines were crossed to the indicated driver strains and cultured at 25°C in urine specimen bottles. The bottles were subsequently cleared of the parent cross prior to the eclosion of progeny. Then, the male progeny were collected from the bottles 48 hours after the onset of eclosion, and designated one day old. These flies were maintained at 25°C at forty flies per vial of food, and transferred every other day into fresh vials. The number of deaths was recorded at each passage, and vials were periodically combined to maintain approximately forty flies per vial. After four days, the flies were split into control and experimental groups of 200 males each. For the Tet-on system, the experimental group was placed on vials supplemented with 2500 μg/ml of DOX and 640 μg/ml of ampicillin, while the control group received ampicillin only. The purpose of the ampicillin was to restrict bacterial growth on the surface of the food, and minimize any life span benefit due to the antibiotic activity of DOX. For the Geneswitch system, food vials were adjusted to 3200 μg/ml of RU486 (Mifepristone, Sigma). Each fly's life span was tabulated and the mean life spans for the experimental and control cohorts were calculated. Finally, the statistical significance between the mean life spans for each experimental group and the minus-drug control was determined using unpaired, two-sided t-tests (Table 2).
Table 2.
Summary of life span data.
| Driver Line | −RU486 | +RU486 | %change | (p) |
| P{elav-Switch.O}GSG301(3) | ||||
| Or-R | 41.74±0.91 | 42.57±1.03 | +1.98% | (0.5505) |
| Or-R | 45.45±0.99 | 41.93±0.99 | −7.73% | (0.0123) |
| PTENII | 41.56±0.83 | 34.97±1.10 | −15.87% | (2.33157E-06) |
| PTENII | 40.78±1.00 | 28.62±0.98 | −29.82% | (9.90101E-17) |
| PTENIII | 33.44±0.79 | 30.94±0.80 | −7.45% | (0.0274) |
| PTENIII | 32.86±0.76 | 26.14±0.93 | −20.44% | (4.23423E-08) |
| HS1 | 33.75±0.67 | 32.20±0.64 | −4.59% | (0.0934) |
| HS1 | 28.59±0.57 | 27.61±0.68 | −3.45% | (0.2566) |
| Driver Line | −DOX | +DOX | %change | (p) |
| P{rtTA.B}M1(3) | ||||
| Or-R | 56.30±1.37 | 55.43±1.41 | −1.55% | (0.6586) |
| Or-R | 62.47±0.63 | 65.82±0.63 | +5.37% | (0.0002) |
| MnSOD(2)22 | 47.95±1.31 | 37.65±0.96 | −21.48% | (1.0095E-09) |
| MnSOD(2)22 | 53.67±0.70 | 39.65±0.63 | −26.12% | (1.28385E-39) |
| MnSOD(2)20 | 50.54±1.34 | 37.08±1.12 | −26.63% | (5.83775E-13) |
| MnSOD(2)20 | 52.99±0.96 | 42.93±0.69 | −18.99% | (4.13052E-16) |
| MnSOD(2)12 | 53.30±1.69 | 39.07±0.98 | −26.69% | (9.82343E-13) |
| MnSOD(2)12 | 50.75±0.78 | 41.37±0.61 | −18.47% | (3.139E-19) |
| P{UAS-rtTA-M2-alt}901(2) ; P{GawB}D42(3) | ||||
| Or-R | 55.49±1.02 | 62.71±1.04 | +13.02% | (1.12687E-06) |
| MnSOD(2)22 | 54.28±1.20 | 57.20±1.18 | +5.38% | (0.0834) |
| MnSOD(2)20 | 57.93±1.21 | 54.97±1.52 | −5.11% | (0.1291) |
| MnSOD(2)12 | 55.93±1.09 | 56.90±1.33 | +1.75% | (0.5714) |
| P{UAS-rtTA-M2-alt}901(2) ; P{Act5C-GAL4}17bFO1(3) | ||||
| Or-R | 45.56±0.86 | 50.32±1.16 | +10.45% | (0.0010) |
| MnSOD(2)22 | 40.89±0.82 | 46.55±0.88 | +13.86% | (3.91948E-06) |
| MnSOD(2)12 | 46.29±0.74 | 48.35±1.04 | +4.44% | (0.1093) |
Mean life span +/− SEM, p value for unpaired, two-sided t-test.
The enzymatic activity of β-galactosidase was quantitated in whole fly extracts using previously described procedures (Wheeler et al., 1995). Young (4-7 day old) adult males of the indicated genotypes were fed the indicated drugs for one week and then assayed. For each assay, three flies were homogenized and total protein was extracted. The assays were performed under conditions where the reaction was linear with regard to the amount of extract and the duration of the reaction. The data are presented as the average activity, plus or minus the standard deviation of triplicate assays (nine flies total, three flies per extract), and the units are specific activity (ΔOD574/hr/fly). Unpaired, two-sided t-tests were used to determine the significance of the difference between the minus-drug control and experimental levels of average activity (Table 3).
Table 3.
Statistical analyses.
| Figure 3A | Figure 3B | ||
| w1118/+; P{7T40.B}E1(2)/+ | (p) | (p) | |
| +DOX | 0.8254 | +Atc | 0.2504 |
| +DOX 1:10 | 0.3521 | +Atc 1:10 | 0.0468 |
| +DOX 1:100 | 0.7727 | +Atc 1:100 | 0.1089 |
| +DOX 1:1000 | 0.6517 | +Atc 1:1000 | 0.2341 |
| +DOX 1:10000 | 0.5058 | +Atc 1:10000 | 0.0875 |
| w1118/w; P{7T40.B}E1(2) / P{UAS-rtTA-M2-alt}901(2); P{tubP-GAL4}LL7(3)/+ | |||
| +DOX | 3.1311E-04 | +Atc | 1.4981E-03 |
| +DOX 1:10 | 2.2759E-05 | +Atc 1:10 | 0.0110 |
| +DOX 1:100 | 4.2655E-03 | +Atc 1:100 | 0.4312 |
| +DOX 1:1000 | 9.8168E-04 | +Atc 1:1000 | 0.9043 |
| +DOX 1:10000 | 0.2279 | +Atc 1:10000 | 0.4310 |
| w1118/yacw; P{7T40.B}E1(2) / P{rtTA-M2-alt}Act-965(2) | |||
| +DOX | 1.4634E-05 | +Atc | 4.5216E-04 |
| +DOX 1:10 | 1.0041E-06 | +Atc 1:10 | 5.2606E-03 |
| +DOX 1:100 | 8.9457E-06 | +Atc 1:100 | 0.0730 |
| +DOX 1:1000 | 0.0775 | +Atc 1:1000 | 0.1581 |
| +DOX 1:10000 | 0.5535 | +Atc 1:10000 | 3.6787E-02 |
| w1118/yacw; P{7T40.B}E1(2) / P{rtTA-M2-alt}Act-966(2) | |||
| +DOX | 1.9900E-07 | +Atc | 2.3195E-03 |
| +DOX 1:10 | 2.0492E-05 | +Atc 1:10 | 0.1453 |
| +DOX 1:100 | 5.8211E-04 | +Atc 1:100 | 0.0582 |
| +DOX 1:1000 | 5.2494E-04 | +Atc 1:1000 | 0.9202 |
| +DOX 1:10000 | 0.1659 | +Atc 1:10000 | 0.5983 |
| w1118; P{7T40.B}E1(2)/+ ; P{rtTA.B}E2(3)/+ | |||
| +DOX | 3.9067E-04 | +Atc | 2.5480E-04 |
| +DOX 1:10 | 4.3683E-05 | +Atc 1:10 | 0.5705 |
| +DOX 1:100 | 1.9354E-04 | +Atc 1:100 | 0.3431 |
| +DOX 1:1000 | 0.7202 | +Atc 1:1000 | 0.7301 |
| +DOX 1:10000 | 0.2700 | +Atc 1:10000 | 0.8818 |
| w1118; P{7T40.B}E1(2)/+ ; P{rtTA.B}M1(3)/+ | |||
| +DOX | 3.2078E-06 | +Atc | 7.8894E-05 |
| +DOX 1:10 | 1.7527E-06 | +Atc 1:10 | 2.9795E-05 |
| +DOX 1:100 | 4.0452E-04 | +Atc 1:100 | 3.6414E-05 |
| +DOX 1:1000 | 3.7681E-03 | +Atc 1:1000 | 4.4618E-05 |
| +DOX 1:10000 | 0.8358 | +Atc 1:10000 | 3.7187E-05 |
| Figure 4A | (p) | ||
| w1118/+; P{7T40.B}E1(2) / + | 1.000 | ||
| w1118/w; P{GawB}elavC155(X);P{7T40.B}E1(2) / P{UAS-rtTA-M2-alt}901(2) | 5.4554E-03 | ||
| w1118/w; P{7T40.B}E1(2) / P{UAS-rtTA-M2-alt}901(2); P{GawB}D42(3)/+ | 7.9572E-04 | ||
| w1118/w; P{7T40.B}E1(2)/P{UAS-rtTA-M2-alt}901(2); P{Act5C-GAL4}17bFO1(3)/+ | 1.8618E-02 | ||
| w1118/w; P{7T40.B}E1(2) / P{UAS-rtTA-M2-alt}901(2); P{tubP-GAL4}LL7(3)/+ | 1.1997E-03 | ||
| w1118; P{7T40.B}E1(2)/+ / P{rtTA.B}E2(3)/+ | 3.0242E-03 | ||
| w1118; P{7T40.B}E1(2)/+ / P{rtTA.B}M1(3)/+ | 3.4995E-04 | ||
| Figure 4B | (p) | ||
| w1118/+; P{7Tadh.B}D1(3) / + | 0.6573 | ||
| w1118/+; P{GawB}elavC155(X); P{UAS-rtTA-M2-alt}901(2)/+ ; P{7Tadh.B}D1(3)/+ | 1.9906E-03 | ||
| w1118/w; P{UAS-rtTA-M2-alt}901(2)/+; P{7Tadh.B}D1(3) / P{GawB}D42(3) | 4.0657E-04 | ||
| w1118/w; P{UAS-rtTA-M2-alt}901(2); P{7Tadh.B}D1(3)/ P{Act5C-GAL4}17bFO1(3) | 1.2844E-04 | ||
| w1118/w; P{UAS-rtTA-M2-alt}901(2)/+; P{7Tadh.B}D1(3) /P{tubP-GAL4}LL7(3) | 6.8602E-07 | ||
| w1118; P{7Tadh.B}D1(3) / P{rtTA.B}E2(3) | 1.7529E-05 | ||
| w1118; P{7Tadh.B}D1(3) / P{rtTA.B}M1(3) | 1.4184E-05 | ||
| Figure 7A | (p) | ||
| w1118/+; P{7T40.B}E1(2) / + | 0.5297 | ||
| P{GawB}elavC155(X)/+; P{7T40.B}E1(2) / P{UAS-rtTA-M2-alt}901(2) | 6.7233E-05 | ||
| w/+; P{UAS-LacZ.B}Bg4-1-2(2) / + | 0.7664 | ||
| w/yw; P{UAS-LacZ.B}Bg4-1-2(2)/+ ; P{elav-Switch.O}GSG301(3)/+ | 1.5944E-02 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}3 204(2) | 2.6839E-02 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}3 255(2) | 1.0716E-03 | ||
| Figure 9B | (p) | ||
| w/+; P{UAS-LacZ.B}Bg4-1-2(2) / + | 0.1827 | ||
| w/yw; P{UAS-LacZ.B}Bg4-1-2(2)/+ ; P{elav-Switch.O}GSG301(3)/+ | 9.3055E-04 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}204(2) | 6.8552E-03 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}204-1(2) | 0.4746 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}255(2) | 2.4268E-04 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}255-H(2) | 0.1583 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}204-2(2) | 0.3414 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}255-A(2) | 2.8594E-06 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}255-B(2) | 2.8722E-03 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}255-C(2) | 2.5011E-06 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}255-D(2) | 3.0139E-04 | ||
| w; P{UAS-LacZ.B}Bg4-1-2(2) / P{Act5C(-FRT)GAL4.Switch.PR}255-F(2) | 4.9809E-06 | ||
3. Results
3.1 Comparison of the Tet-on actin5C-rtTA driver lines
The actin5C-rtTA constructs (rtTA) yield a tissue-general pattern of expression in the adult fly (Bieschke et al., 1998). However, it is important to characterize multiple transgenic lines, since the insertion site can affect both the absolute level and the tissue specificity of expression. Several rtTA lines have previously been compared using β-galactosidase assays to test their ability to activate a lacZ reporter construct in adult males, and a range of activities was observed (Bieschke et al., 1998) (Summarized in Figure 2). Here a separate group of experiments was performed to test the same drivers' relative ability to cause gene over-expression phenotypes during development. Two PdL mutations were used in this experiment: PdL3Gpum is an insertion in the pumilio gene and causes curly wings, while PdL3A results in larval lethality. The PdL lines were crossed to the indicated driver strains in the presence and absence of DOX and phenotype was scored. Weaker drivers, such as rtTA(2)F1 and rtTA(2)D1, failed to produce a phenotype. The rtTA(3)E2 driver gave complete DOX regulation, i.e. no phenotype in the absence of DOX, and complete penetrance of the phenotype in the presence of DOX. This is not unexpected since rtTA(3)E2 is the driver used to originally identify these two PdL mutations. In contrast, several of the strongest drivers, such as rtTA(3)M1, caused leaky expression, and resulted in complete penetrance of the phenotypes with and without DOX. Overall there was a general, but not perfect, correlation between the rtTA driver's propensity to cause phenotypic penetrance, and it's ability to drive LacZ expression (Figure 2).
Fig. 2.
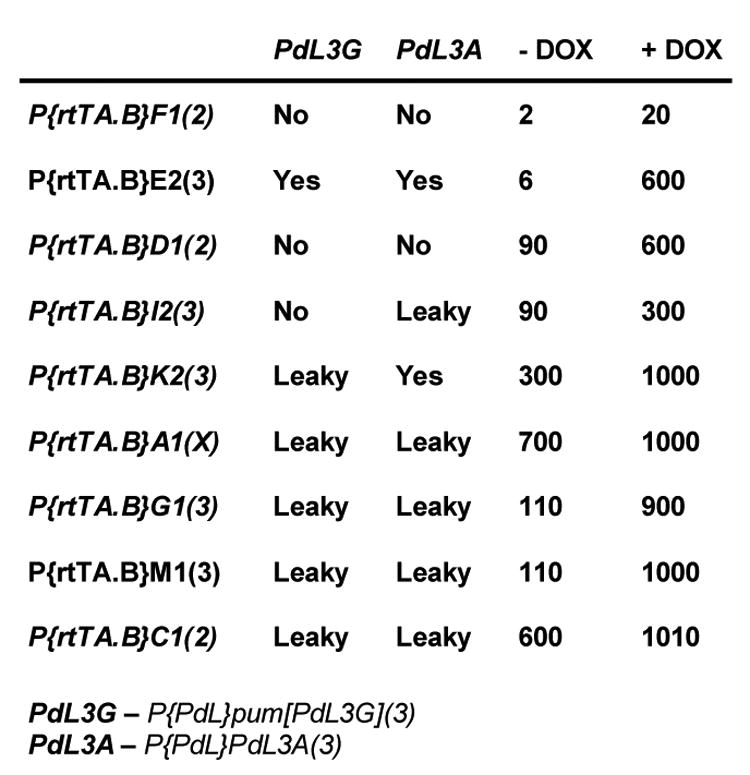
Effect of rtTA driver insertions on penetrance of two PdL mutant phenotypes. Each of the indicated rtTA driver strains has previously been assayed for ability to drive expression of a lacZ target construct in young adult males (Bieschke et al., 1998) and this data is summarized on the right (β-galactosidase units ΔOD/hr/fly × 10−3). In this study each driver was assayed for its ability to cause a DOX-dependent phenotype in combination with two different PdL insertions in multiple experiments. PdL3G is an insertion in the pumilio gene and the phenotype is curly wings. PdL3A is an insertion in an unknown gene that causes larval lethality. The rtTA(3)E2 driver was used to originally identify both mutations (Landis et al., 2001). “No” indicates complete lack of phenotype in presence and absence of DOX. “Yes” indicates phenotype observed only in presence of DOX, i.e, conditional regulation. “Leaky” indicates complete penetrance of phenotype observed in both presence and absence of DOX. The chromosome of insertion is indicated in parentheses.
3.2 Comparison of the rtTA and rtTAM2-alt driver lines
The rtTAM2-alt is a mutant form of rtTA that was designed to yield higher expression and lower background. Existing rtTAM2-alt insertions (Stebbins et al., 2001) were re-balanced and combined with other P element insertions to generate useful strains. In the Act-965 and Act-966 insertion strains, rtTAM2-alt is driven directly by the actin5C promoter. In the rtTA(3)E2 and rtTA(3)M1 strains the rtTA is also driven directly by the actin5C promoter. These drivers were compared for the relative ability to activate a LacZ reporter construct in young adult males across several DOX concentrations (Figure 3A, Table 4). Flies were cultured on control food or food supplemented with the indicated concentration of drug for one week, and then whole-fly extracts were prepared: three flies per extract and three extracts per data point. The rtTA(3)E2 line gave more than a 100 fold induction and good DOX regulation, while the rtTA(3)M1 line was both more active and more leaky (Figure 3A), consistent with previous characterization and the previous figure. In contrast, the two rtTAM2-alt lines produced an absolute level of expression that was 12-15 fold lower than that of rtTA. Because rtTAM2-alt also had a lower background signal it produced the same fold induction as rtTA (∼100 fold).
Fig. 3.
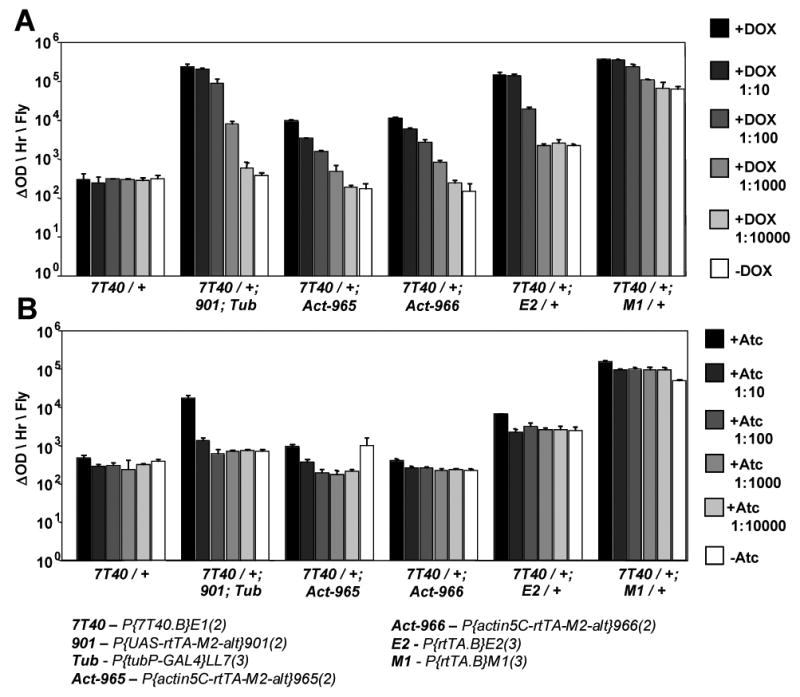
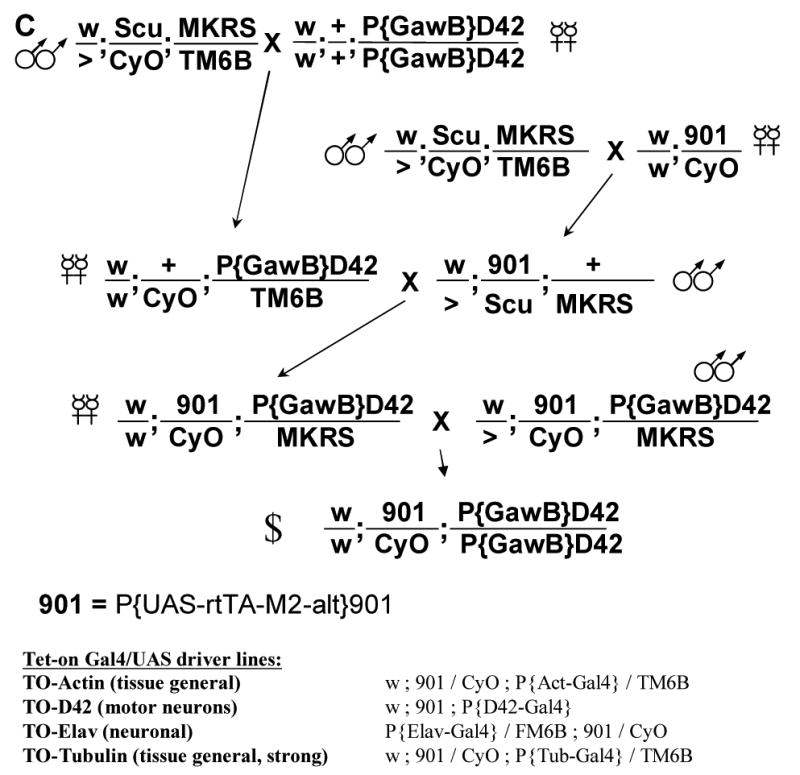
Tet-on system drivers were assayed for their ability to drive expression of a LacZ reporter construct in adult male flies. (A) Doxycycline was used to activate expression at the indicated dilutions. (B) Anhydrotetracycline was used to activate expression. (C) The cross to construct Drosophila strains with both the “901” UAS-rtTAM2-alt bridge construct and the desired GAL4 driver. D42 is used as an example. Note: 901 chromosome homozygotes are observed in some stocks and not others.
Table 4.
Phenotypic penetrance of PdL mutations PdL11A3ash (lethal), PdL19B3alpha-Man-II (rough eye), PdL35B1 (blister wing), and PdL3Gpum (curly wing) with various drivers and DOX concentrations.
| Driver Line | +DOX 1:1 | +DOX 1:10 | +DOX 1:102 | +DOX 1:103 | +DOX 1:104 | −DOX |
|---|---|---|---|---|---|---|
| P{rtTA.B}E2(3) | ||||||
| PdL(3)11A3 | 100 | 100 | 100 | 0 | 0 | 0 |
| PdL(3)19B3 | 100 | 100 | 100 | 0 | 0 | 0 |
| PdL(3)35B1 | 100 | 98 | 3 | 0 | 0 | 0 |
| PdL(3)3G | 100 | 47 | 3 | 0 | 0 | 0 |
| P{rtTA.B}M1(3) | ||||||
| PdL(3)11A3 | 100 | 100 | 100 | 99 | 56 | 33 |
| PdL(3)19B3 | 100 | 100 | 100 | 100 | 100 | 100 |
| PdL(3)35B1 | Lethal | Lethal | 100 | 100 | 100 | 100 |
| PdL(3)3G | 100 | 100 | 95 | 97 | 93 | 91 |
| P{Act5C-rtTA-M2-alt}966(2) | ||||||
| PdL(3)11A3 | 100 | 71 | 0 | 0 | 0 | 0 |
| PdL(3)19B3 | 0 | 0 | 0 | 0 | 0 | 0 |
| PdL(3)35B1 | 100 | 100 | 100 | 100 | 100 | 100 |
| PdL(3)3G | 100 | 92 | 34 | 0 | 0 | 0 |
| P{Act5C-rtTA-M2-alt}965(2) | ||||||
| PdL(3)19B3 | 27* | 49* | 0 | 0 | 0 | 0 |
| PdL(3)35B1 | 100 | 100 | 100 | 100 | 100 | 100 |
| P{Act5C-rtTA-M2-alt}962(2) | ||||||
| PdL(3)19B3 | 0 | 0 | 0 | 0 | 0 | 0 |
| PdL(3)35B1 | 100 | 100 | 100 | 100 | 100 | 100 |
Indicates female-lethal phenotype.
To optimize both low background and absolute level of expression with the Tet-on system, a configuration was generated that utilized the low background of rtTAM2-alt and the powerful tissue-general tubulin gene regulatory sequences. In the bridge construct, rtTAM2-alt is driven by the upstream activating sequence (UAS) promoter (Figure 1B). Two insertion strains of the UAS-rtTAM2-alt construct on the second chromosome (“901” and “902”) (Stebbins et al., 2001) were compared for relative ability to support tissue-general gene expression. The 901 insert was found to yield higher levels of expression of lacZ target constructs (data not shown), and so was used for further strain construction. A general strategy for creating tissue-specific Tet-on driver strains containing both a GAL4 driver of interest on the third chromosome and the 901 bridge construct insertion is presented (Figure 3C). This strategy was used to combine the 901 bridge construct insert and a commonly used GAL4 driver inserted in the tubulin gene. The resultant “TO-tub” combination-driver produces tissue-general expression and 1000-fold induction of LacZ reporter transgenes in adult males (Figure 3A).
The DOX analogue anhydrotetracycline (Atc) has a potential advantage for life span experiments in that it does not have the antibiotic properties of DOX (Gossen and Bujard, 1993). Anhydrotetracycline was found to have a reduced ability to activate both rtTA and rtTAM2-alt when used at the same concentrations as DOX (Figure 3B). However, Atc concentrations that were three times higher than that normally used for doxycycline yielded similar expression based on inspection of GFP reporter flies (unpublished observations).
The rtTA and rtTAM2-alt drivers were also compared for their ability to activate various PdL insertions that cause mutant phenotypes during development (Table1). The drivers were crossed to four different PdL mutations: PdL11A3ash (lethal), PdL19B3alpha-Man-II (rough eye), PdL35B1 (blister wing), and PdL3Gpum (curly wing), at a range of DOX concentrations. Phenotypic penetrance varied significantly for individual drivers and DOX concentrations, and generally correlated with the concentration of DOX used. However, there were several notable exceptions. For example, Act-966-rtTAM2-alt and Act-962-rtTAM2-alt failed to induce a rough eye phenotype when crossed to PdL19B3alpha-Man-II, while Act-965 gave a new phenotype of female lethality. It is unclear why these drivers caused an unexpected penetrance in the PdL phenotypes. However, it was most likely due to chromosomal position effects on the temporal and tissue-specificity of the expression of the otherwise tissue-general actin5C promoter.
3.3 Nervous system-specific tet-on drivers
The Elav-Gal4 driver is reported to yield pan-neuronal GAL4 expression, while the D42 driver yields expression preferentially in motorneurons (Parkes et al., 1998). These commonly-used GAL4 drivers were combined with the 901 bridge insert to generate TO-Elav and TO-D42 driver strains, analogous to the TO-tubulin strain described above. These combinations were compared for their ability to drive conditional expression of LacZ reporters, using quantitative β-gal assay of adult fly extracts. Each of the TO drivers yielded good DOX regulation with a low background (Figure 4). The absolute activity observed with Elav and D42 was lower than that for the ubiquitous drivers, consistent with their limited expression patterns. The appropriate tissue-general or nervous-system-specific expression pattern was confirmed for each new “TO” driver combination (Figure 3C) by staining dissected flies in situ for β-galactosidase activity (data not shown).
Fig. 4.
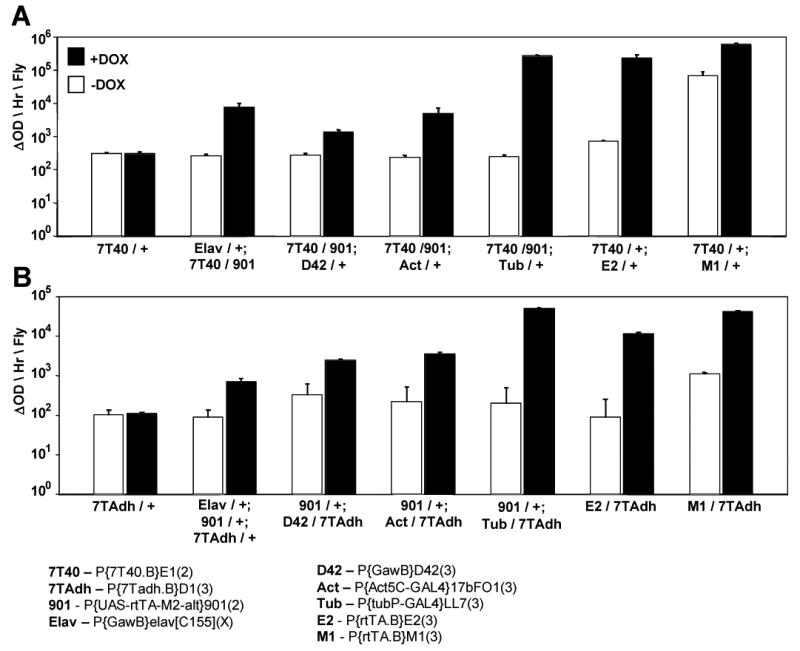
The tissue-specific and tissue-general Tet-on driver lines were assayed for activation of LacZ target constructs. Beta-galactosidase enzyme activity was assayed in extracts of whole adult male flies as above. Elav is a nervous-tissue driver, while D42 is expressed only in the motor-neurons. Both actin gene (Act) and tubulin gene (Tub) drivers are expressed in tissue-general pattern. Assays were performed using two different LacZ reporter constructs: (A) 7T40, containing a core promoter derived from the hsp70 gene. (B) 7TAdh, containing a core promoter derived from the alcohol dehydrogenase (Adh) gene.
3.4 The effect of high-level MnSOD over-expression on life span
Previously, over-expression of manganese superoxide dismutase (MnSOD) was found to be sufficient to extend Drosophila life span using the FLP-recombinase system (FLP-out) to induce transgene expression. MnSOD enzyme levels in whole-fly extracts were increased in proportion to the increased expression of a MnSOD cDNA. Life span extension was in proportion to the amount of MnSOD enzyme over-expression, however there was an apparent ceiling of MnSOD over-expression and life span extension that could be achieved with FLP-out in those studies (Sun et al., 2004). Here the Tet-on system was used to determine if greater increases in life span could be achieved by higher level MnSOD over-expression. Moderate level over-expression of MnSOD using the rtTA(3)E2 driver yielded 15-20% increases in life span, and those flies will be described in detail elsewhere (Landis et al, in preparation). As expected from the characterization above, the more active rtTA(3)M1 driver yielded very high levels of MnSOD transgene expression, as detected by Northern blot of total RNA from young adult males (Figure 5). Strikingly, this high-level MnSOD over-expression had a negative effect on life span, as observed with MnSOD transgenic lines 22, 20, and 12 (Figure 6A). The levels of MnSOD transgene expression were also increased by using two Tet-on MnSOD target transgenes simultaneously with the rtTA(3)E2 driver, and again negative effects on life span were observed (Figure 6B). Not surprisingly, use of the more powerful rtTA(3)M1 driver in combination with two MnSOD inserts also dramatically decreased life span (Figure 7C). Strains with quadruple MnSOD inserts were constructed; however, even in the absence of DOX these resulted in pupal lethality when combined with rtTA(3)M1, presumably due to leaky expression of toxic levels of MnSOD during development. Taken together the data indicate that there is a limit to the amount of life span extension that can be achieved by ubiquitous over-expression of MnSOD, and that the highest levels of over-expression are increasingly toxic. Notably, over-expression of MnSOD with the motor-neuron-specific TO-D42 driver had no detectable effect on life span using either a single or double insert of MnSOD to vary expression (Figure 6D). It is also important to note that DOX dilutions resulted in a corresponding decrease in MnSOD over-expression, consistent with the results presented above for DOX dilutions with LacZ expression and phenotypic penetrance (Figure 5B).
Fig. 5.
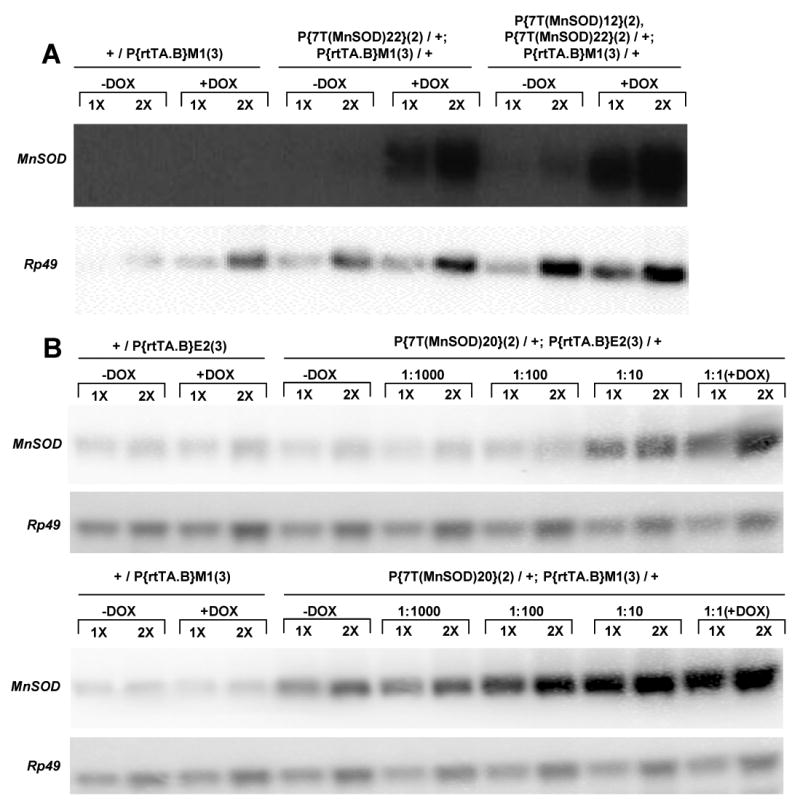
Northern blots of MnSOD over-expression. Total RNA was isolated from adult male flies containing no MnSOD transgene, and single, and double MnSOD insertions, and loaded at 1X and 2X concentrations as indicated. Rp49 is used as a loading control. (A) Northern blot of MnSOD over-expression using the powerful P{rtTA}M1(3) driver. Endogenous MnSOD transcript is readily detected with longer exposures (data not shown). (B) Doxycycline was used to activate expression of MnSOD at the indicated dilutions using the P{rtTA}E2(3) and P{rtTA}M1(3) drivers.
Fig. 6.
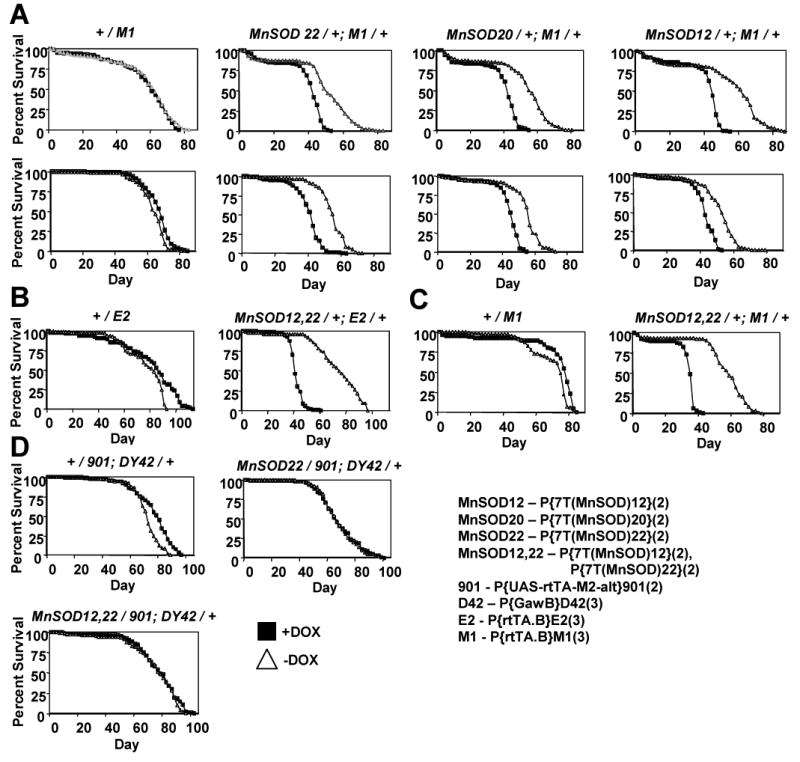
Survival curves. (A) Over-expression of a single MnSOD insert with the rtTA(3)M1 driver. Results are presented for three independent MnSOD target gene insertions on the second chromosome: MnSOD22, 20 and 12. Results of duplicate experiments are presented. (B) rtTA(3)E2 combined with two MnSOD inserts. The MnSOD12 and 22 insertions assayed in (A) were combined onto the same second chromosome for this experiment. (C) Over-expression of two MnSOD inserts with the rtTA(3)M1 driver. (D) Motor-neuron specific over-expression of MnSOD single and double inserts.
Fig. 7.
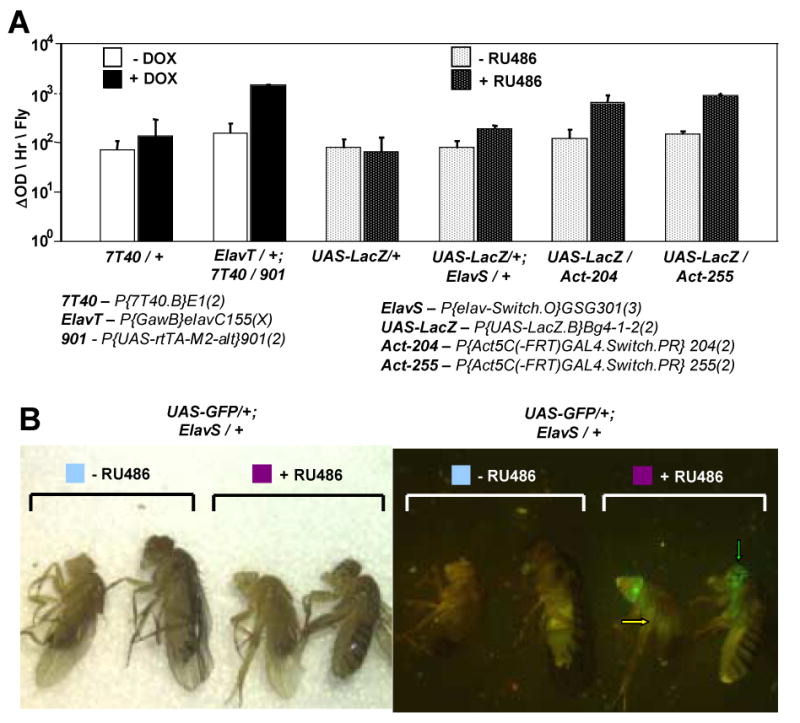
Relative ability of Tet-on and Geneswitch drivers to drive expression of their corresponding LacZ reporter constructs. (A) Beta-galactosidase assay of extracts of whole adult males. (B) The Leica MZFLIII fluorescence-dissecting microscope was used to visualize GFP expression in live flies anesthetized with CO2 gas (image on right) and the corresponding visible light image is presented on the left. Autofluorescence in the gut is indicated with a yellow arrow, GFP fluorescence specific to the CNS is indicated with a green arrow. Genotypes and drug treatments are as indicated. Males are to the left and females are to the right in each sample. Drug treatment was for one week.
3.5 The Geneswitch system
Geneswitch is a conditional system that is similar to Tet-on; however, in this case RU486 is used to induce expression instead of DOX. The Geneswitch system Elav driver (GS-Elav) was compared to the analogous driver of the Tet-on system, and found to yield low background and good induction. However, the absolute expression level for the GS-Elav driver was lower than that of TO-Elav (Figure 7A). The nervous-tissue specificity of the GS-ELAV drivers was confirmed by crossing them to a UAS-GFP reporter construct (Figure 7B). Notice that there is significant autofluorescence present in the flies, particularly in the gut (arrow), and it is typically more yellow in hue than the true GFP signal, present only the brain and ventral nerve cord (central nervous system, CNS) of flies fed RU486 (green arrow).
The GS-Elav driver was crossed to Or-R flies, and the progeny were assayed for life span with and without RU486 (Figure 8A). RU486 had no significant effect on life span under these conditions. This indicates that RU486 alone does not have either a significant beneficial or negative effect on life span, consistent with recent reports (Giannakou et al., 2004; Hwangbo et al., 2004). GS-Elav was crossed to a insert line (called HS1) where human Cu/ZnSOD expression is driven by a UAS promoter (Parkes et al., 1998). This was similar to a previous experiments where HS1 was non-conditionally expressed with the motor-neuron specific driver D42 (Parkes et al., 1998; Spencer et al., 2003). In the present case no effect on life span was observed (Figure 8B). However it is important to note this negative result might be due to a variety of reasons, such as amount of over-expression, the exact neurons of over-expression, the lack of expression at earlier stages, or a failure of the HS1 insertion to be efficiently expressed in males. The phosphatase and tensin homologue (PTEN) gene had a negative effect on life span when it was over-expressed using the same GS-Elav driver (Figure 9C, D), demonstrating that the over-expression system is indeed working.
Fig. 8.
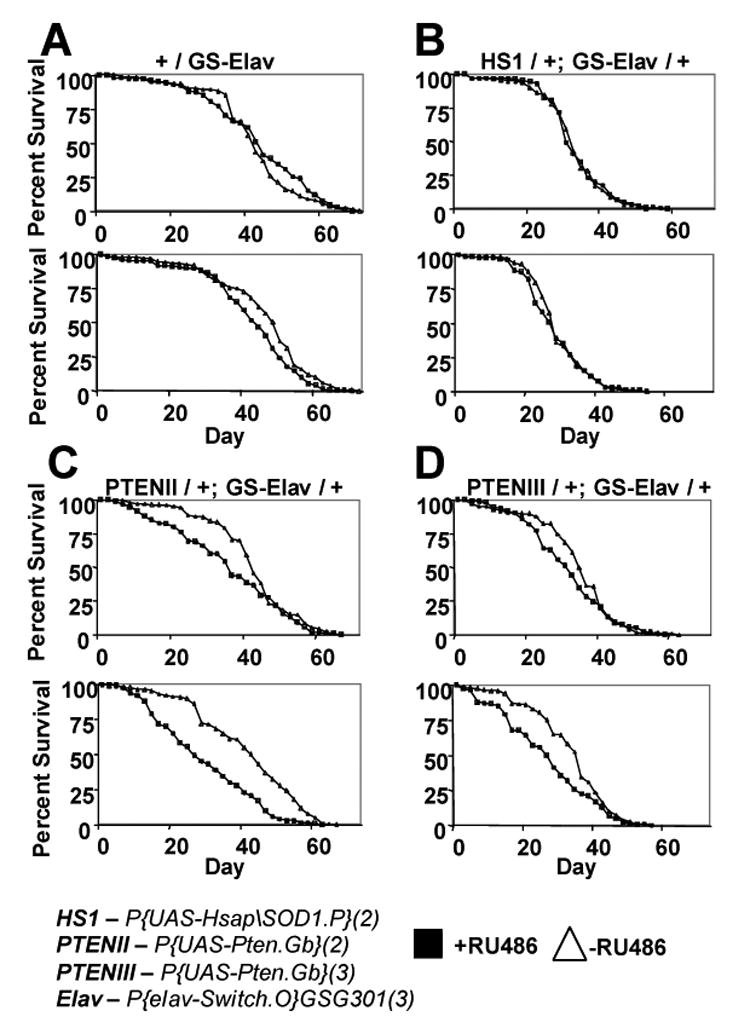
Life span assays performed with neuronal-specific Geneswitch drivers. Results of duplicate assays are presented. (A) Effect of RU486 on the life span of progeny of Or-R flies crossed to GS-Elav. (B) Neuron-specific over-expression of human Cu/ZnSOD. (C, D) GS-Elav driver was used to over-express PTEN in adult male neurons, using a UAS-PTEN construct insertion on the second chromosome (C) and a UAS-PTEN construct insertion on the third chromosome (D).
Fig. 9.
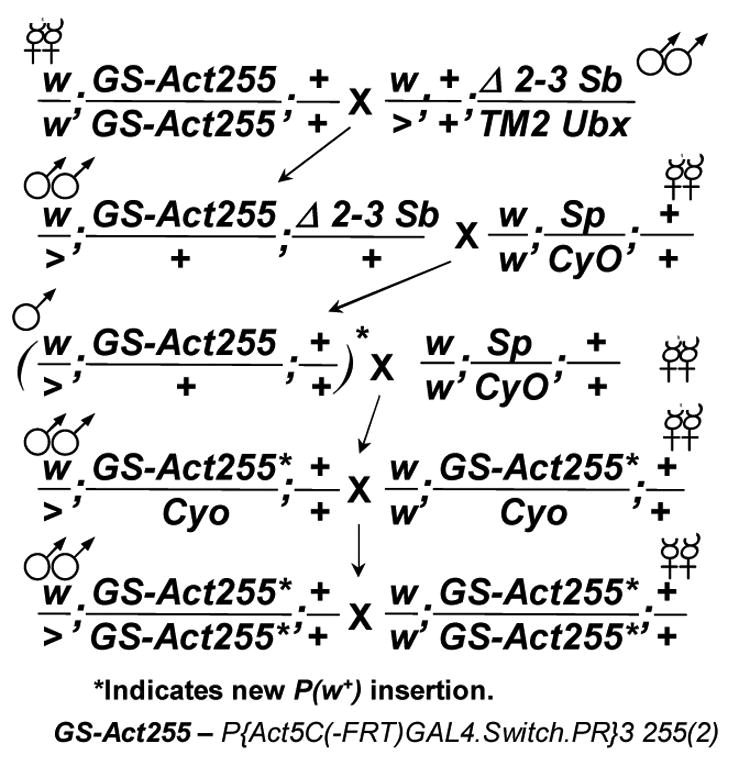
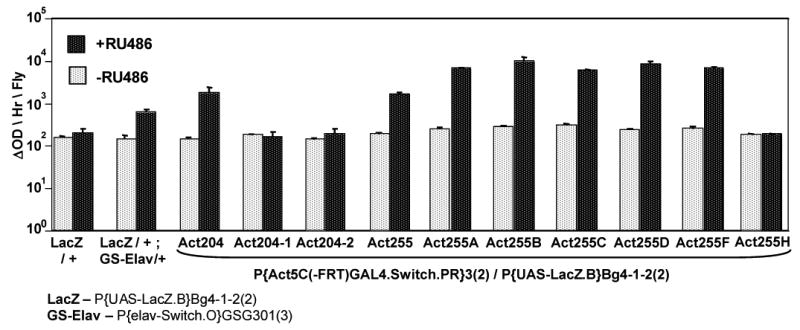
Geneswitch construct insertions were mobilized to cause duplications and increase expression levels. (A) The crossing scheme for the P element mobilization. (B) Assay of the starting Act204 and Act255 insertions and the new multi-insert derivative chromosomes for activation of a LacZ target construct in adult males.
3.6 The generation of actin5C-Geneswitch driver lines with higher expression
The GS-Actin-204 and 255 driver contain a single P element insertion where the actin5C promoter drives expression of the Geneswitch coding region. The number of P element insertions on the chromosome was increased in order to yield higher expression levels. Specifically, the chromosomes were crossed to a Δ2-3 transposase line to mobilize the P element insertions (Figure 9A). The white+ marker gene in the GS-Actin P element yields only a partial rescue (orange eyes); therefore, multiple P inserts on the same chromosome could be scored as they resulted in a more complete rescue (red eyes). Several such chromosomes were generated and tested for their ability to transactivate a LacZ reporter construct in adult males. Derivatives of the Act255 chromosome yielded five-fold increased expression with no detectable increase in background (Figure 9B). One concern is that the GS protein itself might become toxic at high levels, however, both Act255A and Act255B are currently being used in various life span experiments and no significant effects on life span have been observed in the absence or presence of drug (unpublished observations).
4. Discussion
Both the Tet-on system and Geneswitch systems were found to have specific advantages for manipulating adult life span. The Tet-on system has a generally higher level of expression than the Geneswitch system, and a greater potential range of tissue specificity via combination with existing GAL4/UAS lines. A potential advantage of the Geneswitch system is that the drug RU486 has no significant effect on life span. DOX can sometimes have a small positive effect (∼0-4%) depending on the particular conditions and study. We attribute this to the antibiotic effect of DOX reducing the occasional growth of sticky bacteria on the surface of the food. This effect can be reduced, although not always eliminated, by adding ampicillin to both the −DOX and +DOX vials (Landis et al., 2003).
The three important criteria for any conditional expression system are a) the absence of expression in the control group, b) reproducible expression when induced, and c) the regulation of the level of over-expression. Using the original rtTA construct the Tet-on system yields high-level expression, good DOX regulation, and a 100-fold induction with LacZ reporters (Bieschke et al., 1998). Other investigators have reported that the rtTAM2-alt version of the transactivator yields lower background and stronger induction than rtTA (Stebbins et al., 2001). However, in that study no activity was detected for rtTA, making the comparison uninformative. Here rtTAM2-alt was indeed found to have a lower background than rtTA, and using a specific combination of constructs a 1000 fold induction of lacZ transgenes was routinely achieved.
Over-expression of human Cu/ZnSOD using the (non-conditional) GAL4/UAS system and the D42 driver has previously been reported to increase adult Drosophila life span, in experiments using two human Cu/ZnSOD transgenic inserts called HS1 and HS2 (Parkes et al, 1998). Here the Geneswitch Elav (GS-Elav) driver was used to conditionally over-express the HS1 transgene pan-neuronally in adult males. This did not result in a detectable change in life span, potentially because of the difference in expression patterns between the Elav and D42 drivers, or the fact that here over-expression was limited to adults.
A reduction in insulin-like signaling can extend life span in a number of different model organisms, such as C. elegans, flies, and rodents (Kenyon, 2005). PTEN is an evolutionarily conserved negative regulator of the pathway. Previous researchers used the Geneswitch system to over-express the PTEN gene in the fat body of the head, and observed a life span extension of 20% (Hwangbo et al., 2004). However, life span decreased when PTEN was over-expressed in the fat bodies of the head, thorax, and abdomen. Here, over-expression of the PTEN gene in the adult nervous system with the GS-Elav driver was found to have a negative effect on life span. This data supports the previous research, and suggests that PTEN has a positive effect when expressed in the head fat body, but a negative effect when expressed in other tissues. It is also consistent with other research showing nervous-system-specific over-expression of dFOXO, a target of the insulin-like pathway, does not significantly increase life span (Hwangbo et al., 2004).
Over-expression of the mitochondrial enzyme MnSOD in a tissue-general pattern over a moderate range increases adult life span in proportion to the increase in enzyme activity, as observed with both the FLP-out system (Sun et al., 2002) and Tet-on system (Landis et al, in preparation). Here very high levels of MnSOD over-expression were achieved using Tet-on drivers with stronger expression and by using two or more MnSOD target constructs. In these cases MnSOD over-expression had a progressively negative effect on survival suggesting that it is toxic at very high levels. The data suggest that there is an optimum level of MnSOD over-expression, and that above this level the activity of MnSOD enzyme, or some other aspect of the expression system, becomes toxic to the fly. It is also possible that MnSOD over-expression may be having a beneficial effect in certain tissues, and a toxic effect in other tissues. For example, the motor-neuron-specific driver TO-D42 did not alter life span when it was used to drive expression of either a single or double MnSOD insert. These results suggest that motor-neuron tissue is not the essential tissue for MnSOD induced longevity or toxicity. Therefore, future work in this area will involve the use of several tissue-specific drivers to attempt to determine critical tissues.
Acknowledgments
We thank Cheryl Teoh and Jie Shen for assisting with the lacZ expression assays. This work was supported by a grant from the Department of Health and Human Services to JT (AG11833). AB was supported in part by NIA training grant (AG00093).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigaki T, Seong KH, Matsuo T. Longevity determination genes in Drosophila melanogaster. Mech Ageing Dev. 2002;123:1531–41. doi: 10.1016/s0047-6374(02)00089-1. [DOI] [PubMed] [Google Scholar]
- Allikian MJ, Deckert-Cruz D, Rose MR, Landis GN, Tower J. Doxycycline-induced expression of sense and inverted-repeat constructs modulates phosphogluconate mutase (Pgm) gene expression in adult Drosophila melanogaster. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-5-research0021. research0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M. Drosophila: A Laboratory Handbook. Cold Spring Harbor Lab. Press; Plainview, NY: 1989. [Google Scholar]
- Bieschke ET, Wheeler JC, Tower J. Doxycycline-induced transgene expression during Drosophila development and aging. Mol Gen Genet. 1998;258:571–9. doi: 10.1007/s004380050770. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with Overexpressed dFOXO in Adult Fat Body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Anhydrotetracycline, a novel effector for tetracycline controlled gene expression systems in eukaryotic cells. Nucleic Acids Res. 1993;21:4411–2. doi: 10.1093/nar/21.18.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Efficacy of tetracycline-controlled gene expression is influenced by cell type: commentary. Biotechniques. 1995;19:213–6. discussion 216-7. [PubMed] [Google Scholar]
- Hwangbo DS, Gersham B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–6. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lubbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:10933–8. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis G, Bhole D, Lu L, Tower J. High-frequency generation of conditional mutations affecting Drosophila melanogaster development and life span. Genetics. 2001;158:1167–76. doi: 10.1093/genetics/158.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis GN, Bhole D, Tower J. A search for doxycycline-dependent mutations that increase Drosophila melanogaster life span identifies the VhaSFD, Sugar baby, filamin, fwd and Cctl genes. Genome Biol. 2003;4:R8. doi: 10.1186/gb-2003-4-2-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The Genome of Drosophila melanogaster. Academic Press; San Diego: 1992. [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes TL, Elia AJ, Dickson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nature Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- Roman G, Davis RL. Conditional expression of UAS-transgenes in the adult eye with a new gene-switch vector system. Genesis. 2002;34:127–31. doi: 10.1002/gene.10133. [DOI] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:12602–7. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth P, Szabo K, Bailey A, Laverty T, Rehm J, Rubin GM, Weigmann K, Milan M, Benes V, Ansorge W, Cohen SM. Systematic gain-of-function genetics in Drosophila. Development. 1998;125:1049–1057. doi: 10.1242/dev.125.6.1049. [DOI] [PubMed] [Google Scholar]
- Seroude L. GAL4 drivers expression in the whole adult fly. Genesis. 2002;34:34–8. doi: 10.1002/gene.10128. [DOI] [PubMed] [Google Scholar]
- Seroude L, Brummel T, Kapahi P, Benzer S. Spatio-temporal analysis of gene expression during aging in Drosophila melanogaster. Aging Cell. 2002;1:47–56. doi: 10.1046/j.1474-9728.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- Spencer CC, Howell CE, Wright AR, Promislow DE. Testing an ‘aging gene’ in long-lived Drosophila strains: increased longevity depends on sex and genetic background. Aging Cell. 2003;2:123–30. doi: 10.1046/j.1474-9728.2003.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins MJ, Urlinger S, Byrne G, Bello B, Hillen W, Yin JC. Tetracycline-inducible systems for Drosophila. Proc Natl Acad Sci U S A. 2001;98:10775–80. doi: 10.1073/pnas.121186498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–72. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Molitor J, Tower J. Effects of simultaneous over-expression of Cu/ZnSOD and MnSOD on Drosophila melanogaster life span. Mech Ageing Dev. 2004;125:341–9. doi: 10.1016/j.mad.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Tower J. Transgenic methods for increasing Drosophila life span. Mech Ageing Dev. 2000;118:1–14. doi: 10.1016/s0047-6374(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Wheeler JC, Bieschke ET, Tower J. Muscle-specific expression of Drosophila hsp70 in response to aging and oxidative stress. Proc Natl Acad Sci U S A. 1995;92:10408–12. doi: 10.1073/pnas.92.22.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]


