Abstract
Specialized outer hair cells (OHCs) housed within the mammalian cochlea exhibit active, nonlinear, mechanical responses to auditory stimulation termed electromotility. The extraordinary frequency resolution capacity of the cochlea requires an exquisitely equilibrated mechanical system of sensory and supporting cells. OHC electromotile length change, stiffness, and force generation are responsible for a 100-fold increase in hearing sensitivity by augmenting vibrational input to non-motile sensory inner hair cells. Characterization of OHC mechanics is crucial for understanding and ultimately preventing permanent functional deficits due to overstimulation or as a consequence of various cochlear pathologies. The OHCs’ major structural assembly is a highly-specialized lateral wall. The lateral wall consists of three structures; a plasma membrane highly-enriched with the motorprotein prestin, an actin-spectrin cortical lattice, and one or more layers of subsurface cisternae. Technical difficulties in independently manipulating each lateral wall constituent have constrained previous attempts to analyze the determinants of OHCs’ mechanical properties. Temporal separations in the accumulation of each lateral wall constituent during postnatal development permit associations between lateral wall structure and OHC mechanics. We compared developing and adult gerbil OHC axial stiffness using calibrated glass fibers. Alterations in each lateral wall component and OHC stiffness were correlated as a function of age. Reduced F-actin labeling was correlated with reduced OHC stiffness before hearing onset. Prestin incorporation into the PM was correlated with increased OHC stiffness at hearing onset. Our data indicate lateral wall F-actin and prestin are the primary determinants of OHC mechanical properties before and after hearing onset, respectively.
Keywords: development, outer hair cell, specific stiffness, spectrin, actin, prestin
INTRODUCTION
The sensory apparatus of the mammalian cochlea, the organ of Corti, is composed of ~ 20,000 sensory hair cells interdigitated by several types of support cells (Fig. 1). There are two types of hair cells, the primarily afferent-innervated inner hair cell and the efferent-rich outer hair cell (OHC) [Slepecky, 1996]. Contraction and elongation of OHCs at acoustic frequencies in response to membrane potential changes, i.e., electromotility [Brownell, 1984 Brownell et al., 1985; Kachar et al., 1986], are fundamental for attaining high sensitivity and frequency selectivity in the mammalian cochlea [Ashmore, 1987; Ruggero and Rich, 1991; Evans and Dallos, 1993; Nuttall and Ren, 1995; Liberman et al., 2002; Fridberger and de Monvel, 2003]. Stiffness measurements from the second row of OHCs in intact organ of Corti are 4–10 times greater than those obtained from isolated OHCs, indicating that support cells in the organ of Corti exert a significant load on OHCs [Scherer and Gummer, 2004]. Therefore, to permit the generation high frequency resolution, OHCs must possess a significant amount of axial stiffness to contract and elongate at acoustic frequencies.
Fig. 1.
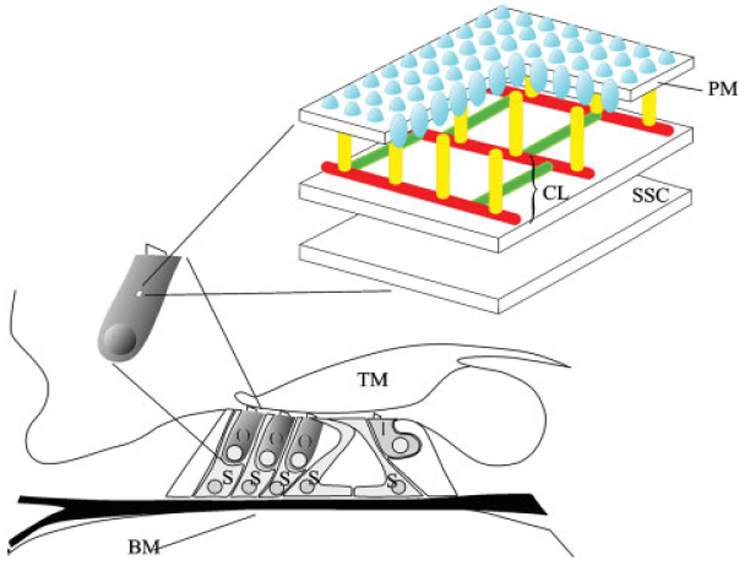
Diagram of the organ of Corti depicting OHC, IHC and support cell location. The OHC lateral wall consists of the plasma membrane (PM), cortical lattice (CL), and subsurface cisternae (SSC). The motor protein prestin (blue) is highly enriched in the PM. The cortical lattice, consisting of F-actin (red) and spectrin filaments (green), is connected to the plasma PM by pillar-like proteins of unknown identity (yellow). Two layers of SSC are depicted below the cortical lattice. O, outer hair cell; I, inner hair cell; S, support cell; BM, basilar membrane; TM, tectoral membrane. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The highly specialized lateral wall in mature OHCs has been hypothesized to generate this requisite stiffness and force, as well as maintain the OHC’s cylindrical shape [for review see Brownell et al., 2001]. This unique trilaminated structure is composed of the plasma membrane (PM), cortical lattice and subsurface cisternae (SSC) (Fig. 1). The mature OHC PM is highly enriched (7000/µm²) with 10–12 nm particles [Forge, 1991]. Although structural proteins and ion channels are clearly present, the motor-protein prestin is the primary PM component. The cortical lattice is composed of circumferential actin filaments cross-linked to longitudinal spectrin filaments [Holley and Ashmore, 1988b; Wada et al., 2003]. SSC located beneath the cortical lattice consist of varying numbers of complexed membrane bound organelles of unknown function. The absence of alternative structures capable of contributing to OHC axial stiffness [Pack and Slepecky, 1995; Vago et al., 1996; Hallworth et al., 2000; Jensen-Smith et al., 2003] solidifies the role of the lateral wall in establishing and maintaining the specialized mechanical properties of mature OHCs.
Unfortunately the relative contribution of each lateral wall component to OHC mechanics is currently unknown. Although membrane bound prestin can directly modulate OHC stiffness during electromotile length change [He and Dallos, 1999], other components (e.g., the actin-spectrin cortical lattice) may also regulate OHC stiffness and/or electromotilty [Dallos et al., 1997; Frolenkov et al., 2000; Kalinec et al., 2000; Zhang et al., 2003; Zelenskaya et al., 2005]. Independent manipulation of each lateral wall component is not possible at this time. However, during the first 3 weeks of life, the gerbil auditory epithelium undergoes a substantial morphological and functional reorganization. Although all cellular components of the gerbil organ of Corti are present at birth (P0), the gerbil does not hear until postnatal day 12 (P12) [Kuhn and Vater, 1996]. Furthermore, adult-like frequency selectivity and sensitivity are not attained until P14–P21 [Souter et al., 1997]. This maturational delay permits temporal correlations between anatomical development and function. Therefore, to dissect the relative contribution of each lateral wall component to OHC stiffness, we systematically investigated developmental correlations in OHC stiffness and lateral wall cytoarchitecture in isolated gerbil OHCs before and after hearing onset. We find that, of the several possible contributors to OHC mechanical properties during postnatal development described above, two stand out, F-actin and the motor-protein prestin.
MATERIALS AND METHODS
Measurement of Isolated OHC Specific Compliance
To generate isolated OHCs, temporal bones were removed from anesthetized gerbils. Cochleae were isolated and dissected from the temporal bone in a solution of Leibovitz’s L-15 (L-15, Sigma-Aldrich, St. Louis, MO). Individual strips of organ of Corti were dissected out and placed in an enzyme stock solution containing 0.5–1.0% papain (Calbiochem, La Jolla, CA) for 15–30 min. After rinsing gently with L-15 to remove excess enzyme, isolated cells were carefully transferred to the experimental chamber and allowed to equilibrate for 5 min. The experimental chamber was filled with L-15 (310 mOsm) containing cesium, tetraethyl ammonium chloride and cobalt to block active potassium and calcium currents [Hallworth, 1997b]. This chamber was sealed on the bottom with a glass cover slip and mounted on an inverted microscope.
To reduce variability due to developmental differences in cell length, specific stiffness (i.e., stiffness per unit length) was calculated. The method for determining specific stiffness was based on the work of Hallworth [Hallworth, 1995, 1997a]. Briefly, OHCs were held in a suction pipette while a fiber of known stiffness (0.5–2.0 mN/m) attached to a piezoelectric actuator and triaxial manipulator was placed against the apex of the cell. Specific stiffness (S) was calculated from the amplitude of the fiber tip motion during displacement of the fiber base by a 300 nm compressive rectangular pulse, using the relationship:
where k is the fiber stiffness, X and x are the motion amplitudes of the fiber base and tip, respectively, and L is the cell length, measured from a video microscopic image using a calibrated grid, with an accuracy of 1 µm.
Specific stiffness measurements were obtained from developing (P3–P18, at 3 day intervals) and mature OHCs (P21+). OHCs were harvested and pooled from all three cochlear turns. Care was taken to curtail OHC damage during isolation. Four specific criteria were used to ensure cell viability. First, since damaged OHCs tend to swell in the radial direction, the diameter of experimental cells was required to be that of an intact, healthy cell (8–10 µm). Second, the lateral walls of damaged OHCs bow out and become curved. Therefore, cells with a curvature deviating more than 25% from the cells’ diameter were excluded from analysis. Third, cells with nuclei shifted from the basal pole were not analyzed. Finally, cells with granular material moving in the cytoplasm were also excluded.
Immunohistochemistry
Incorporation of prestin, spectrin, F-actin, and cholesterol into the lateral wall of isolated OHCs from developing (P0–P18) and mature (P21+) gerbil organ of Corti was examined. Isolated OHCs were prepared in glass-bottomed culture dishes. After isolation, cells were allowed to settle onto poly-l-lysine coated glass culture dishes for 5 min (MatTek Corporation, Ashland, MA). To help prevent cell loss during subsequent solution changes, cells were covered with warm 2.5% agarose (Sigma-Aldrich, St. Louis, MO) dissolved in phosphate buffered saline (PBS). After agarose solidification, isolated cells were exposed to 4% paraformaldehyde in PBS for 30 min. Excess paraformaldehyde was removed with PBS.
OHCs were prepared for immunolabeling by blocking and permeablizing in a solution consisting of PBS containing 0.25% Triton-X 100, 1% bovine serum albumin (BSA), and 5% normal goat serum. OHCs were labeled with a rabbit anti-gerbil (C-terminus) prestin antibody (1:1000) generously donated by Dr. J. Zheng (Northwestern University, Evanston, IL). An affinity purified goat anti-rabbit Cy3 antibody (1:200) was used to visualize prestin-labeled OHCs (Jackson Immunolaboratories, West Grove, PA). The presence of F-actin was detected with phalloidin (1:500), a mushroom-derived toxin, directly coupled to Alexa 568 (Molecular Probes, Eugene, OR). The presence of spectrin was detected with a mouse anti-bovine monoclonal fodrin (spectrin) antibody (1:200, Chemicon, Temecula, CA). Spectrin antibodies were counterstained with anti-mouse IgG coupled to Alexa 488 (1:200, Molecular Probes, Eugene, OR). Negative controls were established by omitting the primary antibody.
Dishes containing isolated OHCs were exposed for 24 h to each antibody during the labeling process to ensure optimum antibody penetration through the thin covering of agarose. To remove excess antibody, dishes were rinsed with PBS containing 0.1% BSA (10 min, 2X) between each antibody step. Fluorescence was preserved by sealing specimens with a solution of equal parts PBS and glycerol containing 1% n-propylgallate and 1.5 µg/ml 4′,6-diamidino-2-phenylindole, dilactate or ClariØn® permanent mounting medium (Biomeda, Foster City, CA).
Filipin (Sigma-Aldrich, St. Louis, MO), a cholesterol-binding polyene fluorescent antibiotic was used to examine developmental alterations in lateral wall unesterfied cholesterol distribution. Isolated OHCs were stained with 0.75 mg/ml filipin in PBS for 1 h at RT. Isolated OHCs were imaged immediately after rinsing with PBS (5 min X 3). Images were obtained within 1 h of the final PBS rinse. Two-photon excitation was used to image isolated OHCs stained with filipin (740 nm excitation).
Quantitative Fluorescence
Temporal changes in lateral wall protein and cholesterol content were determined using quantitative fluorescence. All OHCs were imaged on a Zeiss LSM 510 META NLO scanning system (Carl Zeiss Jena, Jena, Germany) using the same 40X oil objective and a Coherent Chameleon near infrared tunable Ti:Sapphire laser (Coherent, Santa Clara, CA, cholesterol data only). Isolated OHCs at each developmental time point were processed for immunohistochemistry simultaneously to reduce processing variability.
Specific precautions to ensure comparability of fluorescence levels across specimens and developmental time points were as follows: (1) OHCs labeled with each fluorophore were imaged under identical conditions and settings. Detector and pinhole settings for each fluorophore were determined by optimizing the signal (to ensure that saturation did not occur) for adult specimens (prestin, spectrin) or immature specimens (F-actin), whichever exhibited the greater fluorescence during preliminary experiments. Imaging settings for filipin fluorescence were determined by optimizing signal settings associated with randomly selected OHCs from adult (P21+) and immature (P0) specimens. (2) To eliminate fluorophore bleaching as a source of artifact, OHCs were located using transmitted light only and were imaged no more than twice. (3) To control for potential slow drifts in laser output during the few days over which measurements were made, specimens were examined in random order. (4) At least two dishes, constituting a large number of cells, were examined at each time point (Table I).
TABLE I.
OHCs Examined in Each Exeriment
| P0 | P3 | P6 | P9 | P12 | P15 | P18 | P21+ | |
|---|---|---|---|---|---|---|---|---|
| Stiffness | 0 | 5 | 16 | 21 | 19 | 8 | 12 | 13 |
| Prestin | 27 | 74 | 57 | 115 | 89 | 78 | 66 | 57 |
| F-actin | 31 | 64 | 114 | 104 | 115 | 125 | 83 | 155 |
| Spectrin | 34 | 40 | 69 | 68 | 57 | 64 | 78 | 74 |
| Cholesterol | 43 | 61 | 77 | 61 | 75 | 56 | 68 | 61 |
Intensity profiles were generated from linear cross-sections taken at 25 (apical), 50, and 75% (basal) of total cell length. The profile peak averages (arbitrary units) were plotted as a function of postnatal age. For prestin fluorescence, the basal-most tips of isolated OHCs were also profiled. Intensity profiles were generated using Image J 1.33u (National Institutes of Health, Bethesda, MD) (see Fig. 3a). Profile peaks were analyzed using analysis of variance tests (ANOVA). Post-hoc t-tests were performed when significant differences were detected within each data set. Table I shows the number of OHCs examined in each experiment. Three dimensional reconstructions of prestin-labeled OHCs were generated from image stacks obtained using proprietary Zeiss software (Carl Zeiss, Jena, Germany) (see Figs. 3b–3d). Correlation coefficients were calculated from normalized fluorescence intensity and specific stiffness data sets using Microsoft Excel.
Fig. 3.
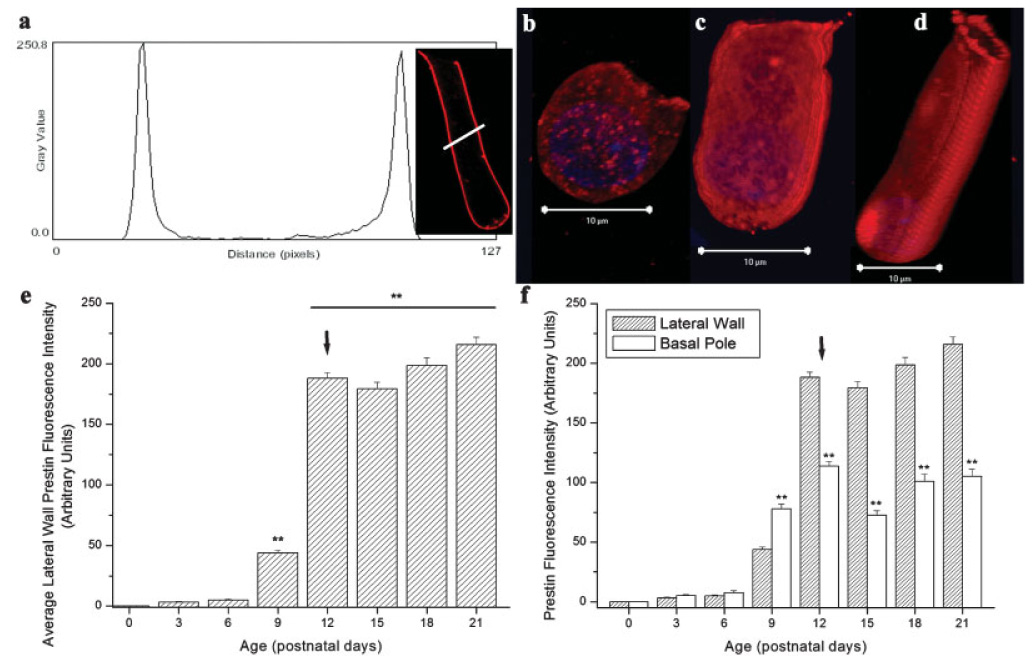
Lateral wall prestin accumulation in developing OHCs. (a) Representative prestin fluorescence intensity profile (P21). (b–d) 3-D reconstructions of P6, P9, and P12 prestin-labeled isolated OHCs, respectively. (e) Significant differences in lateral wall prestin accumulation, measured by alterations in fluorescence intensity, were observed in developing OHCs (F(7,571) = 640.1, P < 0.01). Lateral wall prestin accumulation was greatest immediately before hearing onset (P9–P12). After hearing onset (P12), the OHC lateral wall contained significantly more prestin than before hearing onset (P9). (f) Comparison of average lateral wall and basal pole prestin labeling during postnatal development. At and after the onset of hearing, prestin was significantly reduced in the basal pole. Arrow = hearing onset, ** = P < 0.01.
Transmission Electron Microscopy
The absence of SSC specific antibodies or markers mandated that SSC content be examined using transmission electron microscopy. Protocols for processing cochlear tissue were identical to a previous study investigating SSC in gerbil OHCs [Weaver and Schweitzer, 1994]. Blocks containing individual turns of cochlear tissue from developing (P0–P12) gerbils were sectioned to less than 1000 Å using a diamond knife and placed onto copper grids. Grids were stained with 4% uranyl acetate in methanol followed by 0.1% lead citrate. Micrographs were taken using a JEOL transmission electron microscope operated at 60 kV.
RESULTS
Postnatal Modifications of OHC Specific Stiffness
Isolated OHC specific stiffness varied significantly before (≤P9) and after (≥P12) the onset of hearing (F(7,80) = 13.1, P < 0.001). A dramatic decrease in specific stiffness was observed before (P6–P9) the onset of hearing (Fig. 2). Specifically a three fold decrease in specific stiffness was observed between P3 and P9 (from 48.48 ± 14.2 to 15.42 ± 1.33 N µm/km). This significant decrease in OHC specific stiffness occurs immediately before the onset of hearing (P < 0.001). In contrast, at hearing onset (P12), there was an increase in specific stiffness relative to P9 (from 15.42 ± 1.33 to 53.57 ± 7.99 N µm/km, P < 0.001). After P12 no significant differences in OHC specific stiffness were observed.
Fig. 2.
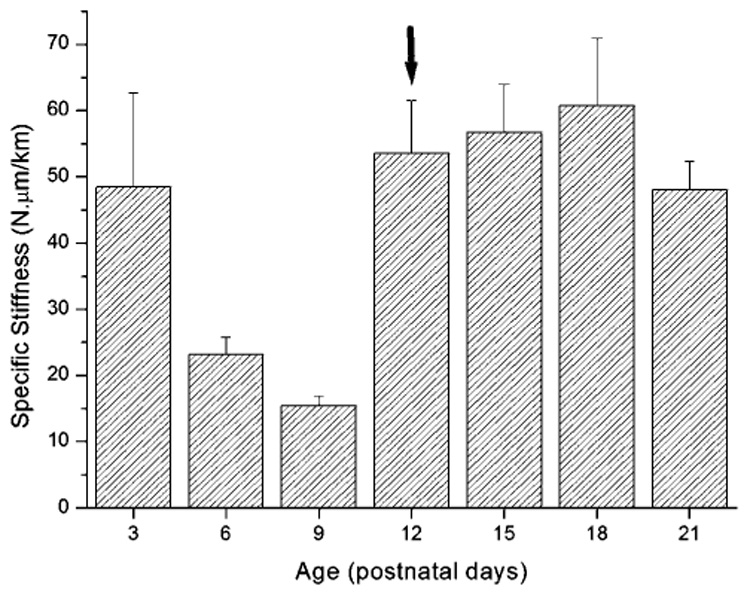
OHC specific stiffness differs before and after the onset of hearing. Arrow = hearing onset, ** = P < 0.01.
Modification of PM Prestin and Cholesterol Content during Lateral Wall Formation
Prestin was not detected in the OHC lateral wall at birth (P0), although occasional weak label in the cytoplasm, suggestive of low levels of protein synthesis, was observed. As described in the Methods, lateral wall fluorescence intensity profiles were analyzed to determine variations in lateral wall prestin accumulation during postnatal development (Fig. 3a). During the first week of life (P0–P6), prestin was difficult to detect in the lateral wall of isolated OHCs. Prestin label rapidly increased in the OHC lateral wall during postnatal development (Figs. 3b–3e, F(7,571) = 640.1, P < 0.01). Between P9 and P12, a four fold increase in lateral wall prestin label was observed (from 46.76 ± 2.25 to 188.1 ± 4.56, arbitrary units) during this time. This increase in lateral wall prestin incorporation occurs immediately before the onset of hearing (Fig. 3e). Three dimensional reconstructions of isolated OHCs at P6, P12 and P21 also indicated random incorporation of prestin aggregates into the OHC lateral wall during this period (Figs. 3b–3d).
Intensity profiles indicated prestin label was present in all regions of the OHC lateral wall, including the basal pole (Fig. 3f). In contrast to previous reports [Belyantseva et al., 2000a; Weber et al., 2002; Winter et al., 2006], prestin label was reliably observed in the basal pole of mature isolated OHCs (Figs. 3b–3d, and 3f). A previous report also describes prestin label is present throughout the lateral wall [Yu et al., 2006]. Label in the basal pole was, however, significantly reduced relative to the total prestin label in the lateral wall (Fig. 3f).
Unesterfied cholesterol in isolated postnatal OHCs was predominantly localized to the cytoplasmic compartment (Figs. 4a–4c). Although postnatal alterations in cytoplasmic cholesterol distribution occurred (Fig. 4d), there is no precedent for cytoplasmic cholesterol to alter cellular mechanics.
Fig. 4.
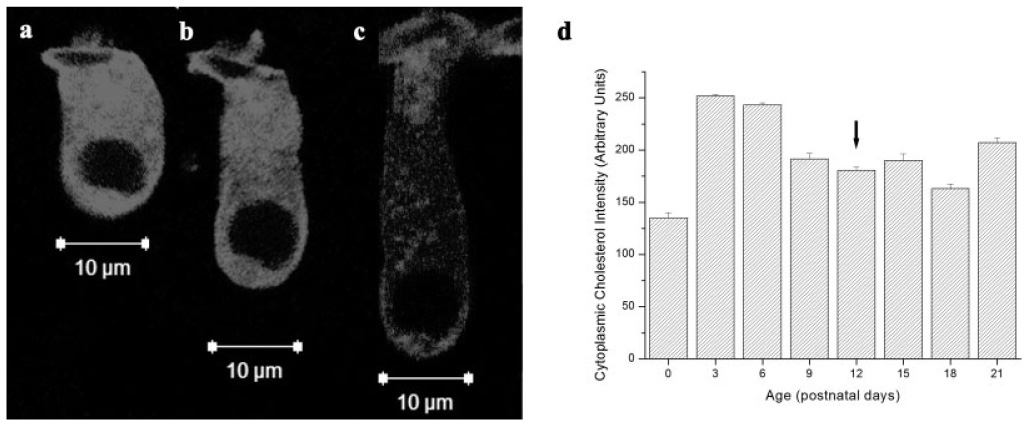
Cytoplasmic cholesterol content varies in developing OHCs. (a–c) Filipin-labeled cholesterol in three representative OHCs from P6, P12, and P18 cochleae, respectively. (d) Cytoplasmic cholesterol content varied during postnatal development. Arrow = hearing onset.
Modification of F-Actin and Spectrin During Lateral Wall Development
In contrast to prestin, F-actin was detected in the OHC lateral wall throughout development. Lateral wall F-actin labeling varied significantly during the postnatal period (Fig. 5d). A significant reduction in lateral wall F-actin incorporation was observed throughout the early postnatal period (Fig. 5d, F(7,764) = 162.3, P < 0.01). By P3, the dense F-actin network present at birth was significantly reduced (t(128) = 3.83, P < 0.01). This initial dense F-actin network was progressively reduced until P21. Transient regional differences in lateral wall F-actin label occurring during postnatal development were not observed in mature OHCs (P21, data not shown).
Fig. 5.
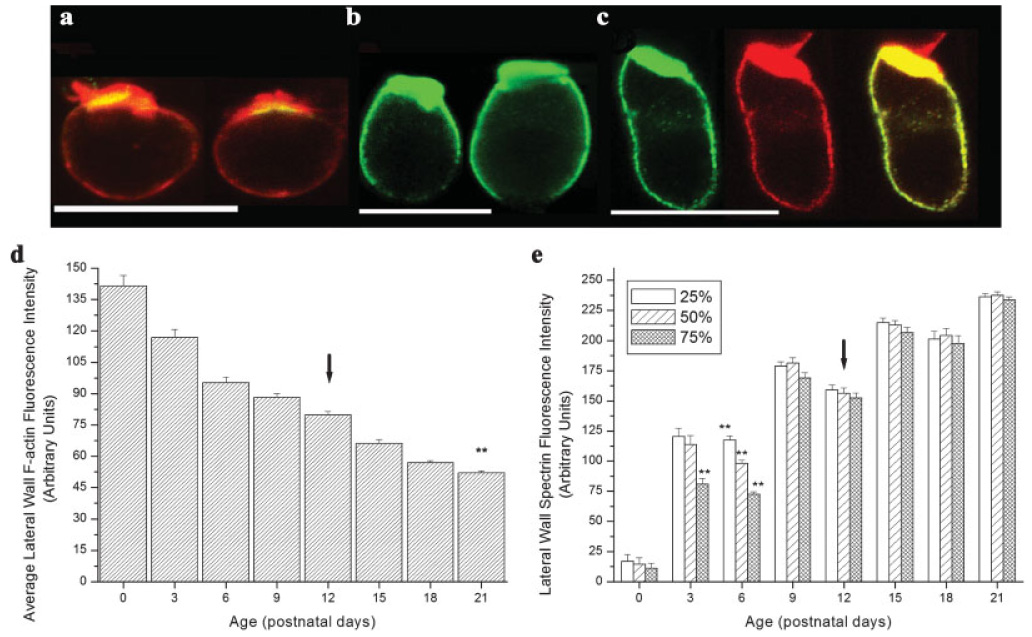
Alterations in lateral wall F-actin and spectrin accumulation during postnatal development. (a) F-actin is reduced in the OHC lateral wall during development (F(7,764) = 162.3, P < 0.01). Immature OHCs (P0–P18) have more lateral wall F-actin than mature OHCs (P21). (b) Increases in lateral wall spectrin accumulation occur between P0 and P21. Significant regional differences in lateral wall spectrin labeling were observed at P3 and P6 (F(2,117) = 10.75, P < 0.01, F(2,204) = 56.37, P < 0.01, respectively). (c–e) F-actin (red) and spectrin (green) labeling in isolated P0, P6 (spectrin only) and, P9 OHCs. Regions of F-actin and spectrin colocalization are depicted as yellow. By P9 F-actin and spectrin were present throughout the OHC lateral wall. Arrow = hearing onset, ** = P < 0.001. Scale bars = 10 µm.
Lateral wall spectrin labeling was also altered before and after the onset of hearing. Unlike F-actin, spectrin was restricted to the OHC cuticular plate at birth (Fig. 5b). Spectrin progressively accumulated in the OHC lateral wall before the onset of hearing. Label was observed to extend from the cuticular plate towards the basal pole between P3 and P9 (Figs. 5a–5c). Figure 5e shows significant regional differences in spectrin labeling between apical, middle, and basal lateral wall regions of P3 and P6 isolated OHCs (F(2,117) = 10.75, P < 0.01, F(2,204) = 56.37, P < 0.01, respectively). Specifically, at P3 spectrin in the basal most region of the lateral wall was reduced relative to apical and middle regions of the lateral wall (P < 0.01). By P6 significant differences were observed between all three regions of the lateral wall (P < 0.01). Spectrin label was present throughout the lateral wall by P9 (Figs. 5c and 5e). Despite the uniform presence of spectrin in the OHC lateral wall by P9, an increase in total spectrin fluorescence intensity was observed after the onset of hearing suggesting the continued elaboration of the OHC lateral wall.
Modification of SSC Content During Lateral Wall Development
SSC were absent from the OHC lateral wall at birth (Fig. 6a). Although no SSC were observed at P3, one fractionated cisternal layer was associated with the OHC lateral wall at P6. These SSC were present in the apical region of P6 OHCs, just below cuticular plate-associated tight junctions (Fig. 6b), and extended down the lateral wall into the subnuclear region (Fig. 6c). One complete layer of cisterns was present in P9 OHCs. Unlike P6 OHCs, cisterns from P9 OHCs tapered off at the perinuclear region. By the onset of hearing (P12), no more than one complete layer of cisterns was present along the OHC lateral wall (Fig. 6d). As for P9 OHC, no cisternae were present in the perinuclear region of P12 OHCs.
Fig. 6.
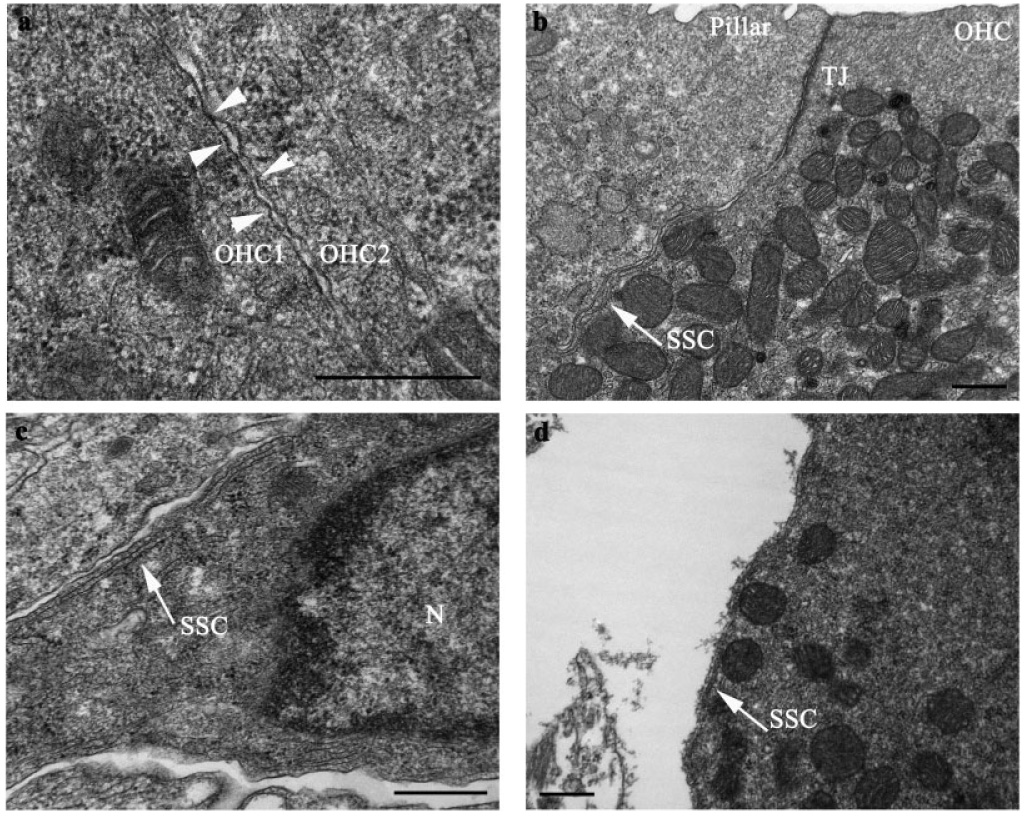
Postnatal alterations in subsurface cisternae. (a) Absence of cisterns in two adjacent OHCs at birth (P0). Lateral wall indicated by arrowheads. (b) One noncontiguous layer of cisterns in a P6 OHC. Cisterns were present just below the tight junctions associated with apical membrane of the OHC, as indicated by arrows. (c) Subsurface cisternae in the subnuclear region of a P6 OHC. (d) One layer of cisterns in a P12 OHC. Outer hair cell (OHC), tight junction (TJ), subsurface cisternae (SSC), nucleus (N), arrow heads = lateral wall, scale bars = 500 nm.
Correlated Alterations in Specific Stiffness and Lateral Wall Component Formation
Temporal changes in specific stiffness and lateral wall content (prestin, F-actin, spectrin, SSC, and cytoplasmic cholesterol) are plotted as a function of postnatal age in Fig. 7. Positive correlations were observed between specific stiffness and each lateral constituent during various stages of postnatal development. A steady decrease in OHC specific stiffness was observed before the onset of hearing (P3–P9). This decrease was highly correlated (r = 0.999) with F-actin removal from the OHC lateral wall (Fig. 7b). A moderate correlation (r = 0.774) between cytplasmic cholesterol content and specific stiffness also occurred before hearing onset (Fig. 7e). Before the onset of hearing, prestin, spectrin and SSC addition to the lateral wall were negatively correlated with specific stiffness (Figs. 7a, 7c, and 7d). Around the onset of hearing (P9–P15), a marked increase in lateral wall prestin label occurred. This increase was highly correlated (r = 0.992) with increased specific stiffness around hearing onset (Fig. 7a). Positive correlations between specific stiffness, spectrin and SSC content were meager at this time (r = 0.39, 0.68, respectively). After hearing onset, weak positive correlations were observed between specific stiffness, F-actin and SSC content (r = 0.528, 0.67, respectively).
Fig. 7.
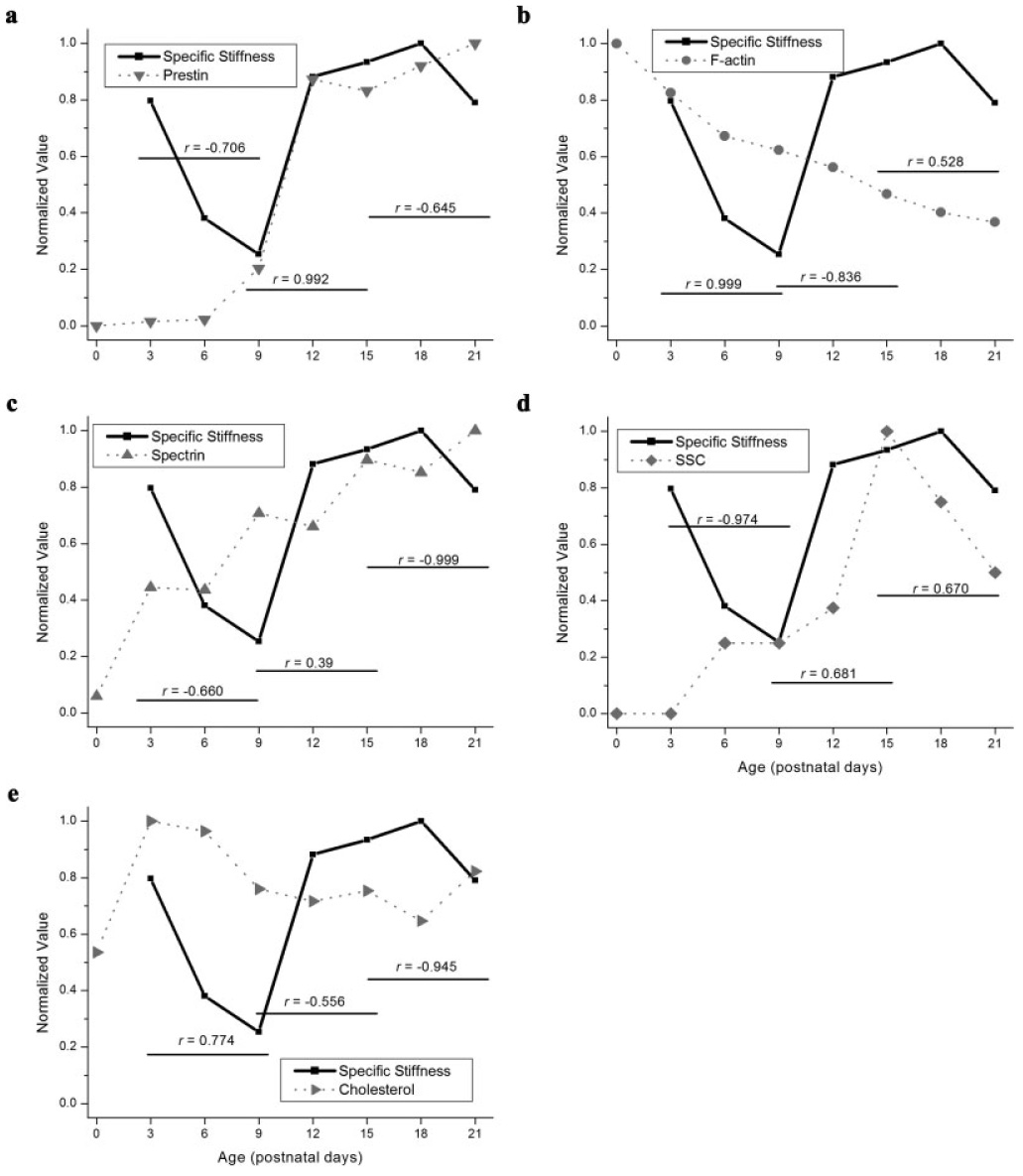
Correlated alterations in OHC specific stiffness (N·µm/km) and lateral wall protein accumulation, subsurface cisternae distribution and cytoplasmic cholesterol before (P3–P9), during (P9–P15), and after (P15–P21) hearing onset. (a) Correlated alterations in average lateral wall prestin label and specific stiffness. (b) Correlated alterations in average lateral wall F-actin label and specific stiffness. (c) Alterations in average lateral wall spectrin label and specific stiffness. (d) Alterations in number of SSC and specific stiffness. (e) Correlated alterations in cytoplasmic cholesterol labeling and specific stiffness.
DISCUSSION
OHC electromotile length change is crucial for establishing and maintaining a sharply-tuned mammalian cochlea. The unique mechanical properties of cochlear OHCs permit the effective transmission of electromotile force to the organ of Corti. The highly-specialized OHC lateral wall has frequently been hypothesized to mediate the mechanical properties of mature OHCs. No other protein structures capable of contributing to OHC axial stiffness have been described [Pack and Slepecky, 1995; Vago et al., 1996; Hallworth et al., 2000; Jensen-Smith et al., 2003]. This report correlates the maturation of OHC specific stiffness before and after the onset of hearing with the accumulation of specific proteins and structures in the trilaminate OHC lateral wall. This data is crucial for interpreting experimental results suggesting that biochemical modification of certain OHC structures may influence OHC motility and therefore cochlear transduction.
Influence of PM Content on OHC Specific Stiffness
Our OHC reconstructions indicate that the motor-protein prestin is enriched in the mature OHC lateral wall. Consistent with previous reports, we observed an increase in lateral wall prestin label after P6 [Belyantseva et al., 2000a; Weber et al., 2002]. The largest increase in lateral wall prestin label occurred between P9 and P12, immediately before the onset of hearing, and was correlated with increased specific stiffness (Fig. 7a). This increase in specific stiffness was highly correlated (r = 0.992) with the marked increase in lateral wall prestin label that occurs between P9 and P15 (Fig. 7a). Prestin content was not correlated with specific stiffness before or after hearing onset.
In contrast to previous reports [Belyantseva et al., 2000a; Weber et al., 2002; Winter et al., 2006], prestin was reliably detected (albeit significantly reduced) in the basal pole, and close to the apex, of isolated OHCs (Figs. 3b–3d, and 3f). This discrepancy is likely due to variations in OHC preparations between these reports. Specifically, frozen sectioned organ of Corti rarely contains intact OHCs in a given section. Therefore low levels of protein accumulation in the PM may be below the level of detection due to an artifactual decrease in total protein available for labeling. An underestimate of basal pole prestin content is also likely to occur when imaging intact organ of Corti because fluorophore excitation and emission will decrease several tens of micrometers below of surface of a preparation (OHC base) relative to the top of the preparation (OHC apex). This report uses isolated OHCs which reduces the required imaging depth to that of a healthy individual OHC, 8–10 µm. Indeed prestin was recently described in the basal pole of isolated adult mouse, guinea pig and rat OHCs [Yu et al., 2006]. Our observation need not be incompatible with previous reports indicating that prestin-related functions (non-linear capacitance, gating currents and motility amplitudes) are significantly reduced [Hallworth et al., 1993; Huang and Santos-Sacchi, 1993; Takahashi and Santos-Sacchi, 2001] or absent [Dallos et al., 1991; Kalinec et al., 1992; Gale and Ashmore, 1997; Santos-Sacchi et al., 1998; Wada et al., 2001] from the basal pole of isolated OHCs. The estimated size of the prestin monomer is much smaller than the 10 nm particles described in the OHC lateral wall [Gulley and Reese, 1977; Kalinec et al., 1992], suggesting functional prestin (i.e. prestin capable of undergoing electromotile shape change) is an oligomer. Indeed several lines of evidence suggest functional prestin exists as a higher-order oligomer [Dong and Iwasa, 2004; Navaratnam et al., 2005; Zheng et al., 2005; Wu et al., 2007]. Unfortunately, such reports suggesting prestin exclusion from the basal pole have not attempted to discriminate between nonfunctional prestin monomers and functional prestin complexes. If functional prestin oligomers were absent from the basal pole, non-linear capacitance and gating currents, the key electrical signatures of electromotility, would also be absent despite the presence of monomeric prestin label.
In view of the fact that very little cytoplasm separates the nucleus from the basal PM of gerbil OHCs, prestin located in the synthetic machinery could, therefore appear as positive labeling in the basal pole. This is unlikely as there was no evidence for prestin in the upper cytoplasmic region surrounding the nucleus. Furthermore, Wada et al. [Wada et al., 2001], using hypo-osmotic challenge, observed that guinea pig lateral wall stiffness was constant from the basal-most tip to the middle/upper region of the lateral wall. This result is perplexing if prestin and the SSC were absent from the basal pole, as no compensatory changes in the actin-spectrin cortical lattice have been described in this region [Ekstrom von Lubitz, 1981; Furness and Hackney, 1990; Forge, 1991; Forge et al., 1993; Weaver and Schweitzer, 1994; Lutz and Schweitzer, 1995]. Finally, substantial redistribution of prestin into the OHC basal pole due to enzymatic digestion is unlikely as all previous experiments describing an absence or reduction in prestin-related functions in the basal pole were also conducted in isolated OHCs. Our results therefore support the presence of prestin in the basal pole of the OHC lateral wall.
PM cholesterol distribution is likely to be extremely important for mechanically coupling prestin molecules to changes in OHC length [Holley, 1996]. Indeed in a recent report, artificial depletion of cholesterol from prestin-transfected HEK cells decreases prestin coupling in the PM [Sturm et al., 2007]. Our data indicated cholesterol is predominately localized in the cytoplasm of developing and mature gerbil OHCs. Although some cholesterol has been observed in mature gerbil OHCs [Forge, 1991], our data are consistent with Nguygen and Brownell [Nguyen and Brownell, 1998] who also described low levels of cholesterol in the lateral wall of isolated mature guinea pig OHCs. A moderate correlation (r = 0.774) between cytplasmic cholesterol content and specific stiffness before hearing onset occurred (Fig. 7e). This result is likely artificial as there is no data to indicate cytoplasmic cholesterol can alter cellular mechanics. Likewise, the observed substantial correlation between F-actin removal and decreasing specific stiffness (r = 0.999) suggests that F-actin is the predominating factor determining OHC stiffness at this time. Although artificially increasing lateral wall cholesterol distribution has been shown to increase the stiffness of isolated OHCs [Nguyen and Brownell, 1998], there is no clear precedent for cytoplasmic cholesterol to alter cellular mechanics.
Influence of F-Actin on OHC Specific Stiffness
A steady decrease in OHC specific stiffness was observed before the onset of hearing (P3–P9). This decrease was highly correlated (r = 0.999) with F-actin removal from the OHC lateral wall (Fig. 7b). Before hearing onset, F-actin is the primary lateral wall constituent. It is therefore not surprising that removal of F-actin from the lateral wall was highly correlated with decreased OHC specific stiffness between P3 and P9. Although this is the first quantitative description of F-actin reduction in the lateral wall, others have described a qualitatively similar reduction [Kuhn and Vater, 1996; Vago et al., 1996]. No substantial positive correlations between F-actin labeling and specific stiffness were observed during or after the onset of hearing.
Influence of Spectrin and Cortical Lattice Formation on Specific Stiffness
This report provides the first description of postnatal alterations in lateral wall spectrin content. Spectrin is not present in the lateral wall at birth and, unlike prestin, is incorporated into the PM basalward from the cuticular plate, as if a specific structure were being assembled in accumulative fashion. We failed to observe strong positive correlations between spectrin accumulation and OHC specific stiffness before or after hearing onset. Our observed increase in total lateral wall spectrin fluorescence intensity after P9 suggests cortical lattice remodeling may still be underway after even after the onset of hearing. Recall, however, it is the F-actin spectrin cortical lattice that is predicted to alter OHC mechanics, not necessarily actin or spectrin independently. Determining when the actin-spectrin cortical lattice forms is a difficult task. Ultimately, the actin-spectrin cortical lattice can only alter OHC mechanics if it is attached to the PM. Pillar structures observed between the PM and actin-spectrin cortical lattice are the only proteins poised to perform this task [Lim et al., 1989; Arnold and Anniko, 1990; Forge, 1991; Pujol et al., 1991; Weaver et al., 1993; Zine and Schweitzer, 1997]. Sparse, widely-separated pillar structures first appear in gerbil OHCs between P8 and P10 [Ito et al., 1995; Souter et al., 1995; Forge et al., 1997]. Pillar content increases between P10 and P12 but mature levels are not reached until P16 [Souter et al., 1995]. Clearly the capacity of the actin-spectrin cortical lattice to alter OHC mechanical properties is limited before the onset of hearing.
Influence of the SSC on OHC Specific Stiffness
Figure 7d displays correlated alterations in SSC content and specific stiffness (SSC values were averaged from the current report; [Weaver and Schweitzer, 1994; Souter et al., 1995; He, 1997]). By the onset of hearing (P12), gerbil OHCs have one (current report, [Weaver and Schweitzer, 1994; He, 1997]) to two [Souter et al., 1995] cisternal layers. By P15 two to four additional cisternal layers are added to the lateral wall [Weaver and Schweitzer, 1994]. This is followed by a reduction in cistern number between P15 and P21. Meager positive correlations were observed between SSC content and specific stiffness around and after hearing onset (r = 0.681, 0.67, respectively).
Although, salicylate-induced vesticulation and dilation of the SSC are associated with an increase in lateral wall stiffness, as measured by micropipette aspiration [Lue and Brownell, 1999], it is unclear if this increase in stiffness is mediated by salicylates actions on the SSC or the PM. Our failure to observe substantial positive correlations between SSC number and specific stiffness, coupled with previously described cistern variability within and between species [Weaver and Schweitzer, 1994; Lutz and Schweitzer, 1995] shed significant doubt on the ability of the cisterns to significantly influence OHC mechanical properties.
OHC Stiffness Change Before and After Hearing Onset
We have already observed that lateral wall F-actin content exerts the primary influence on OHC stiffness before hearing onset (P3–P9), as no other structure or protein undergoes significant appropriate change during this period. Spectrin, prestin and the SSC are insufficiently elaborated to compensate for this dramatic loss of F-actin.
OHC specific stiffness increased significantly around the onset of hearing, between P9 and P15. Adding substantial quantities of any protein to the lateral wall is predicted to increase OHC specific stiffness. This increase in specific stiffness was highly correlated with rapid prestin accumulation in the OHC lateral wall (Fig. 7a). Our conclusion that prestin incorporation dominates changes in OHC specific stiffness around the onset of hearing is in accordance with previous reports stressing the importance of a substantial amount of PM stiffness for the expression of somatic motility [Holley and Ashmore, 1988a; Tolomeo et al., 1996; He et al., 2003]. Although one report suggests the cortical lattice may contribute 65–70% of the OHCs stiffness [Oghalai et al., 1998], the PM must be within the same order of magnitude or stiffer than the cortical lattice (and/or the SSC) or the energy associated with voltage-driven conformational changes of prestin would be stored as strain energy in the PM [He et al., 2006]. OHC specific stiffness remained constant after P12. With the exception of the SSC, all developmental alterations in lateral wall protein content are essentially complete by this time. Therefore OHC specific stiffness is likely a function of the newly formed composite OHC lateral wall shortly after hearing onset.
Although we have discussed OHC specific stiffness as a consequence of the concerted actions of the various components of the OHC lateral wall, positive hydrostatic pressure is another likely component of OHC stiffness [Brownell, 1990]. Specifically, OHCs must possess a requisite amount of turgor pressure to exhibit electromotile length change [Brownell, 1990; Shehata et al., 1991]. Water permeability increases in developing rat OHCs between P12 and P15 [Belyantseva et al., 2000b]. Although this increase in water permeability could lead to developmental alterations in OHC turgor, two lines of evidence limit the possibility for potential alterations in turgor pressure to mediate developmental alterations in OHC stiffness. First, water permeability peaks after the onset of hearing (P12–P15), well after the greatest increase in OHC stiffness (P9–P12). Secondly, immature OHCs (P3–P9) contain few aquaporin channels [Belyantseva et al., 2000b] and therefore have little to no osmotic water permeability before the onset of hearing. Although our data indicates substantial OHC stiffness before the onset of hearing, if turgor pressure is the primary mediator of OHC stiffness, these immature cells should be less turgid and therefore less stiff than their mature counterparts.
We conclude, as previously stated, that lateral wall F-actin is the major determinant of OHC passive stiffness before the onset of hearing, and that, at and after the onset of hearing, lateral wall prestin predominates. Thus modulation of OHC specific stiffness in the mature OHC is likely to be predominately mediated via alterations in the motor-protein prestin. This notion is supported by a recent report describing an increase membrane tension, as measured by membrane tether formation force, in prestin-transfected HEK cells [Zhang et al., in press]. OHC stiffness modulation may also occur via phosphorylation and dephosphorylation of prestin [Deak et al., 2005]. The actin-spectrin cortical lattice, on the other hand, is likely to be crucial for directing prestin-mediated OHC shape change into the longitudinal direction for optimal augmentation of the structures within the organ of Corti [Kalinec et al., 1992; Lim and Kalinec, 1998; Batta et al., 2003). Full characterization of the mechanical properties of sensory and support cells will undoubtedly facilitate our understanding of the processes mediating permanent cochlear damage due to acoustic trauma. These results permit future studies directed at producing methods to protect and/or regenerate functional hair cells in an attempt to maintain or reestablish the delicate mechanical properties of the mammalian cochlea necessary for a sharply-tuned mammalian cochlea.
ACKNOWLEDGMENTS
We thank Rebecca Van Winkle, Alex Bien and Karen Bovard for their technical assistance and Dr. Jing Zheng (Northwestern University, Evantson, IL) for donation of the prestin-specific antibody.
Contract grant sponsor: National Center for Research Resources, NIH; Contract grant number: 1 C06 RR17417-01; Contract grant sponsor: NSF—Nebraska EPSCoR; Contract grant number: EPS-0346476 (CFD 47.076); Contract grant sponsor: NIH; Contract grant number: DC02053; Contract grant sponsor: Deafness Research Foundation.
REFERENCES
- Arnold W, Anniko M. Structurally based new functional interpretations of the subsurface cisternal network in human outer hair cells. Acta Otolaryngol. 1990;109(34):213–220. doi: 10.3109/00016489009107436. [DOI] [PubMed] [Google Scholar]
- Ashmore JF. A fast motile response in guinea-pig outer hair cells: The cellular basis of the cochlear amplifier. J Physiol. 1987;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batta TJ, Panyi G, Gaspar R, Sziklai I. Active and passive behaviour in the regulation of stiffness of the lateral wall in outer hair cells of the guinea-pig. Pflugers Arch. 2003;447(3):328–336. doi: 10.1007/s00424-003-1186-9. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Adler HJ, Curi R, Frolenkov GI, Kachar B. Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J. Neurosci. 2000a;20(24):RC116. doi: 10.1523/JNEUROSCI.20-24-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Frolenkov GI, Wade JB, Mammano F, Kachar B. Water permeability of cochlear outer hair cells: Characterization and relationship to electromotility. J Neurosci. 2000b;20(24):8996–9003. doi: 10.1523/JNEUROSCI.20-24-08996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear Hear. 1990;11(2):82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Brownell WE, Spector AA, Raphael RM, Popel AS. Micro- and nanomechanics of the cochlear outer hair cell. Annu Rev Biomed Eng. 2001;3:169–194. doi: 10.1146/annurev.bioeng.3.1.169. [DOI] [PubMed] [Google Scholar]
- Dallos P, Evans BN, Hallworth R. Nature of the motor element in electrokinetic shape changes of cochlear outer hair cells. Nature. 1991;350(6314):155–157. doi: 10.1038/350155a0. [DOI] [PubMed] [Google Scholar]
- Dallos P, He DZ, Lin X, Sziklai I, Mehta S, Evans BN. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci. 1997;17(6):2212–2226. doi: 10.1523/JNEUROSCI.17-06-02212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak L, Zheng J, Orem A, Du GG, Aguinaga S, Matsuda K, Dallos P. Effects of cyclic nucleotides on the function of prestin. J Physiol. 2005;563(Part 2):483–496. doi: 10.1113/jphysiol.2004.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XX, Iwasa KH. Tension sensitivity of prestin: Comparison with the membrane motor in outer hair cells. Biophys J. 2004;86(2):1201–1208. doi: 10.1016/S0006-3495(04)74194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom von Lubitz DK. Subsurface tubular system in the outer sensory cells of the rat cochlea. Cell Tissue Res. 1981;220(4):787–795. doi: 10.1007/BF00210462. [DOI] [PubMed] [Google Scholar]
- Evans BN, Dallos P. Stereocilia displacement induced somatic motility of cochlear outer hair cells. Proc Natl Acad Sci USA. 1993;90(18):8347–8351. doi: 10.1073/pnas.90.18.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A. Structural features of the lateral walls in mammalian cochlear outer hair cells. Cell Tissue Res. 1991;265(3):473–483. doi: 10.1007/BF00340870. [DOI] [PubMed] [Google Scholar]
- Forge A, Zajic G, Li L, Nevill G, Schacht J. Structural variability of the sub-surface cisternae in intact, isolated outer hair cells shown by fluorescent labelling of intracellular membranes and freeze-fracture. Hear Res. 1993;64(2):175–183. doi: 10.1016/0378-5955(93)90003-j. [DOI] [PubMed] [Google Scholar]
- Forge A, Souter M, Denman-Johnson K. Structural development of sensory cells in the ear. Semin Cell Dev Biol. 1997;8(3):225–237. doi: 10.1006/scdb.1997.0147. [DOI] [PubMed] [Google Scholar]
- Fridberger A, de Monvel JB. Sound-induced differential motion within the hearing organ. Nat Neurosci. 2003;6(5):446–448. doi: 10.1038/nn1047. [DOI] [PubMed] [Google Scholar]
- Frolenkov GI, Mammano F, Belyantseva IA, Coling D, Kachar B. Two distinct Ca(2+)-dependent signaling pathways regulate the motor output of cochlear outer hair cells. J Neurosci. 2000;20(16):5940–5948. doi: 10.1523/JNEUROSCI.20-16-05940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Hackney CM. Comparative ultrastructure of subsurface cisternae in inner and outer hair cells of the guinea pig cochlea. Eur Arch Otorhinolaryngol. 1990;247(1):12–15. doi: 10.1007/BF00240941. [DOI] [PubMed] [Google Scholar]
- Gale JE, Ashmore JF. The outer hair cell motor in membrane patches. Pflugers Arch. 1997;434(3):267–271. doi: 10.1007/s004240050395. [DOI] [PubMed] [Google Scholar]
- Gulley RL, Reese TS. Regional specialization of the hair cell plasmalemma in the organ of corti. Anat Rec. 1977;189(1):109–123. doi: 10.1002/ar.1091890108. [DOI] [PubMed] [Google Scholar]
- Hallworth R. Passive compliance and active force generation in the guinea pig outer hair cell. J Neurophysiol. 1995;74(6):2319–2328. doi: 10.1152/jn.1995.74.6.2319. [DOI] [PubMed] [Google Scholar]
- Hallworth R. Modulation of outer hair cell compliance and force by agents that affect hearing. Hear Res. 1997a;114(12):204–212. doi: 10.1016/s0378-5955(97)00167-6. [DOI] [PubMed] [Google Scholar]
- Hallworth R. Outer hair cell stiffness and force and their modulation by agents that affect hearing. In: Lewis ER, Long GR, Lyon RF, Narris PM, Steele CR, Hecht-Poinar E, editors. Diversity in Auditory Mechanics. Singapore: World Scientific; 1997b. pp. 524–530. [Google Scholar]
- Hallworth R, Evans BN, Dallos P. The location and mechanism of electromotility in guinea pig outer hair cells. J Neurophysiol. 1993;70(2):549–558. doi: 10.1152/jn.1993.70.2.549. [DOI] [PubMed] [Google Scholar]
- Hallworth R, McCoy M, Polan-Curtain J. Tubulin expression in the developing and adult gerbil organ of Corti. Hear Res. 2000;139(12):31–41. doi: 10.1016/s0378-5955(99)00165-3. [DOI] [PubMed] [Google Scholar]
- He DZ. Relationship between the development of outer hair cell electromotility and efferent innervation: A study in cultured organ of corti of neonatal gerbils. J Neurosci. 1997;17(10):3634–3643. doi: 10.1523/JNEUROSCI.17-10-03634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DZ, Dallos P. Somatic stiffness of cochlear outer hair cells is voltage-dependent. Proc Natl Acad Sci USA. 1999;96(14):8223–8228. doi: 10.1073/pnas.96.14.8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DZ, Jia S, Dallos P. Prestin and the dynamic stiffness of cochlear outer hair cells. J Neurosci. 2003;23(27):9089–9096. doi: 10.1523/JNEUROSCI.23-27-09089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DZ, Zheng J, Kalinec F, Kakehata S, Santos Sacchi J. Tuning in to the amazing outer hair cell: Membrane wizardry with a twist and shout. J Membr Biol. 2006;209(23):119–134. doi: 10.1007/s00232-005-0833-9. [DOI] [PubMed] [Google Scholar]
- Holley M. Outer hair cell motility. In: Dallos PP, Popper AN, Fay RR, editors. The Cochlea. New York: Springer-Verlag; 1996. pp. 386–434. [Google Scholar]
- Holley MC, Ashmore JF. A cytoskeletal spring in cochlear outer hair cells. Nature. 1988a;335(6191):635–637. doi: 10.1038/335635a0. [DOI] [PubMed] [Google Scholar]
- Holley MC, Ashmore JF. On the mechanism of a high-frequency force generator in outer hair cells isolated from the guinea pig cochlea. Proc R Soc London B Biol Sci. 1988b;232(1269):413–429. doi: 10.1098/rspb.1988.0004. [DOI] [PubMed] [Google Scholar]
- Huang G, Santos-Sacchi J. Mapping the distribution of the outer hair cell motility voltage sensor by electrical amputation. Biophys J. 1993;65(5):2228–2236. doi: 10.1016/S0006-3495(93)81248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Spicer SS, Schulte BA. Cytological changes related to maturation of the organ of Corti and opening of Corti’s tunnel. Hear Res. 1995;88(12):107–123. doi: 10.1016/0378-5955(95)00106-e. [DOI] [PubMed] [Google Scholar]
- Jensen-Smith HC, Eley J, Steyger PS, Luduena RF, Hallworth R. Cell type-specific reduction of β tubulin isotypes synthesized in the developing gerbil organ of Corti. J Neurocytol. 2003;32(2):185–197. doi: 10.1023/b:neur.0000005602.18713.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B, Brownell WE, Altschuler R, Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986;322(6077):365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kalinec F, Holley MC, Iwasa KH, Lim DJ, Kachar B. A membrane-based force generation mechanism in auditory sensory cells. Proc Natl Acad Sci USA. 1992;89(18):8671–8675. doi: 10.1073/pnas.89.18.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec F, Zhang M, Urrutia R, Kalinec G. Rho GTPases mediate the regulation of cochlear outer hair cell motility by acetylcholine. J Biol Chem. 2000;275(36):28000–28005. doi: 10.1074/jbc.M004917200. [DOI] [PubMed] [Google Scholar]
- Kuhn B, Vater M. The early postnatal development of F-actin patterns in the organ of Corti of the gerbil (Meriones unguiculatus) and the horseshoe bat (Rhinolophus rouxi) Hear Res. 1996;99(12):47–70. doi: 10.1016/s0378-5955(96)00087-1. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419(6904):300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Lim DJ, Kalinec F. Cell and molecular basis of hearing. Kidney Int Suppl. 1998;65:S104–S113. [PubMed] [Google Scholar]
- Lim DJ, Hanamure Y, Ohashi Y. Structural organization of the outer hair cell wall. Acta Otolaryngol. 1989;107(56):398–405. doi: 10.3109/00016488909127529. [DOI] [PubMed] [Google Scholar]
- Lue AJ, Brownell WE. Salicylate induced changes in outer hair cell lateral wall stiffness. Hear Res. 1999;135(12):163–168. doi: 10.1016/s0378-5955(99)00102-1. [DOI] [PubMed] [Google Scholar]
- Lutz C, Schweitzer L. Longitudinal and radial differences in the subsurface cisternal system in the gerbil cochlea. Hear Res. 1995;84(12):12–8. doi: 10.1016/0378-5955(95)00008-r. [DOI] [PubMed] [Google Scholar]
- Navaratnam D, Bai JP, Samaranayake H, Santos-Sacchi J. N-terminal mediated homo-multimerization of prestin, the outer hair cell motor protein. Biophys J. 2005;89(5):3345–3352. doi: 10.1529/biophysj.105.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Brownell WE. Contribution of membrane cholesterol to outer hair cell lateral wall stiffness. Otolaryngol Head Neck Surg. 1998;119(1):14–20. doi: 10.1016/S0194-5998(98)70167-6. [DOI] [PubMed] [Google Scholar]
- Nuttall AL, Ren T. Electromotile hearing: Evidence from basilar membrane motion and otoacoustic emissions. Hear Res. 1995;92(12):170–177. doi: 10.1016/0378-5955(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Oghalai JS, Patel AA, Nakagawa T, Brownell WE. Fluorescence-imaged microdeformation of the outer hair cell lateral wall. J Neurosci. 1998;18(1):48–58. doi: 10.1523/JNEUROSCI.18-01-00048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack AK, Slepecky NB. Cytoskeletal and calcium-binding proteins in the mammalian organ of Corti: Cell type-specific proteins displaying longitudinal and radial gradients. Hear Res. 1995;91(12):119–135. doi: 10.1016/0378-5955(95)00173-5. [DOI] [PubMed] [Google Scholar]
- Pujol R, Zajic G, Dulon D, Raphael Y, Altschuler RA, Schacht J. First appearance and development of motile properties in outer hair cells isolated from guinea-pig cochlea. Hear Res. 1991;57(1):129–141. doi: 10.1016/0378-5955(91)90082-k. [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC. Application of a commercially-manufactured Doppler-shift laser velocimeter to the measurement of basilar-membrane vibration. Hear Res. 1991;51(2):215–230. doi: 10.1016/0378-5955(91)90038-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Kakehata S, Kikuchi T, Katori Y, Takasaka T. Density of motility-related charge in the outer hair cell of the guinea pig is inversely related to best frequency. Neurosci Lett. 1998;256(3):155–158. doi: 10.1016/s0304-3940(98)00788-5. [DOI] [PubMed] [Google Scholar]
- Scherer MP, Gummer AW. Impedance analysis of the organ of corti with magnetically actuated probes. Biophys J. 2004;87(2):1378–1391. doi: 10.1529/biophysj.103.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata WE, Brownell WE, Dieler R. Effects of salicylate on shape, electromotility and membrane characteristics of isolated outer hair cells from guinea pig cochlea. Acta Otolaryngol. 1991;111(4):707–718. doi: 10.3109/00016489109138403. [DOI] [PubMed] [Google Scholar]
- Slepecky N. Structure of the mammalian cochlea. In: Dallos PP, Popper AN, Fay RR, editors. The Cochlea. New York: Springer-Verlag; 1996. pp. 44–129. [Google Scholar]
- Souter M, Nevill G, Forge A. Postnatal development of membrane specialisations of gerbil outer hair cells. Hear Res. 1995;91(12):43–62. doi: 10.1016/0378-5955(95)00163-8. [DOI] [PubMed] [Google Scholar]
- Souter M, Nevill G, Forge A. Postnatal maturation of the organ of Corti in gerbils: Morphology and physiological responses. J Comp Neurol. 1997;386(4):635–651. [PubMed] [Google Scholar]
- Sturm AK, Rajagopalan L, Yoo D, Brownell WE, Pereira FA. Functional expression and microdomain localization of prestin in cultured cells. Otolaryngol Head Neck Surg. 2007;136(3):434–439. doi: 10.1016/j.otohns.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Santos-Sacchi J. Non-uniform mapping of stress-induced, motility-related charge movement in the outer hair cell plasma membrane. Pflugers Arch. 2001;441(4):506–513. doi: 10.1007/s004240000455. [DOI] [PubMed] [Google Scholar]
- Tolomeo JA, Steele CR, Holley MC. Mechanical properties of the lateral cortex of mammalian auditory outer hair cells. Biophys J. 1996;71(1):421–429. doi: 10.1016/S0006-3495(96)79244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago P, Ripoll C, Tournebize R, Lenoir M. Distribution of actin and tubulin in outer hair cells isolated from developing rat cochlea: A quantitative study. Eur J Cell Biol. 1996;69(4):308–315. [PubMed] [Google Scholar]
- Wada H, Usukura H, Takeuchi S, Sugawara M, Kakehata S, Ikeda K. Distribution of protein motors along the lateral wall of the outer hair cell. Hear Res. 2001;162(12):10–18. doi: 10.1016/s0378-5955(01)00355-0. [DOI] [PubMed] [Google Scholar]
- Wada H, Usukura H, Sugawara M, Katori Y, Kakehata S, Ikeda K, Kobayashi T. Relationship between the local stiffness of the outer hair cell along the cell axis and its ultrastructure observed by atomic force microscopy. Hear Res. 2003;177(12):61–70. doi: 10.1016/s0378-5955(02)00798-0. [DOI] [PubMed] [Google Scholar]
- Weaver SP, Schweitzer L. Development of gerbil outer hair cells after the onset of cochlear function: An ultrastructural study. Hear Res. 1994;72(12):44–52. doi: 10.1016/0378-5955(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Weaver SP, Hoffpauir J, Schweitzer L. Actin distribution along the lateral wall of gerbil outer hair cells. Brain Res Bull. 1993;31(12):225–228. doi: 10.1016/0361-9230(93)90029-b. [DOI] [PubMed] [Google Scholar]
- Weber T, Zimmermann U, Winter H, Mack A, Kopschall I, Rohbock K, Zenner HP, Knipper M. Thyroid hormone is a critical determinant for the regulation of the cochlear motor protein prestin. Proc Natl Acad Sci USA. 2002;99(5):2901–2906. doi: 10.1073/pnas.052609899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Braig C, Zimmermann U, Geisler HS, Franzer JT, Weber T, Ley M, Engel J, Knirsch M, Bauer K, Christ S, Walsh EJ, McGee J, Kopschall I, Rohbock K, Knipper M. Thyroid hormone receptors TRα1 and TRβ differentially regulate gene expression of Kcnq4 and prestin during final differentiation of outer hair cells. J Cell Sci. 2006;119(Part 14):2975–2984. doi: 10.1242/jcs.03013. [DOI] [PubMed] [Google Scholar]
- Wu X, Currall B, Yamashita T, Parker LL, Hallworth R, Zuo J. Prestin-prestin and prestin-GLUT5 interactions in HEK293T cells. Dev Neurobiol. 2007;67(4):483–497. doi: 10.1002/dneu.20357. [DOI] [PubMed] [Google Scholar]
- Yu N, Zhu ML, Zhao HB. Prestin is expressed on the whole outer hair cell basolateral surface. Brain Res. 2006;1095(1):51–58. doi: 10.1016/j.brainres.2006.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenskaya A, Boutet de Monvel J, Pesen D, Radmacher M, Hoh JH, Ulfendahl M. Evidence for a highly elastic shell-core organization of cochlear outer hair cells by local membrane indentation. Biophys J. 2005;88(4):2982–2993. doi: 10.1529/biophysj.104.052225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Kalinec GM, Urrutia R, Billadeau DD, Kalinec F. ROCK-dependent and ROCK-independent control of cochlear outer hair cell electromotility. J Biol Chem. 2003;278(37):35644–35650. doi: 10.1074/jbc.M301668200. [DOI] [PubMed] [Google Scholar]
- Zhang R, Qian F, Rajagopalan L, Pereira FA, Brownell WE, Anvari B. Prestin modulates mechanics and electromechanical force of the plasma membrane. Biophys J. doi: 10.1529/biophysj.107.107573. in press: doi:10.1529/bio-physj. 107.107573. April 27, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Du GG, Matsuda K, Orem A, Aguinaga S, Deak L, Navarrete E, Madison LD, Dallos P. The C-terminus of prestin influences nonlinear capacitance and plasma membrane targeting. J Cell Sci. 2005;118(Part 13):2987–2996. doi: 10.1242/jcs.02431. [DOI] [PubMed] [Google Scholar]
- Zine A, Schweitzer L. Localization of proteins associated with the outer hair cell plasma membrane in the gerbil cochlea. Neuroscience. 1997;80(4):1247–1254. doi: 10.1016/s0306-4522(97)00163-2. [DOI] [PubMed] [Google Scholar]


