Abstract
The mismatch negativity (MMN), a component of event-related potentials (ERPs), is assumed to reflect a pre-attentive auditory discrimination process. Although an involvement of hippocampal structures in deviance detection was shown in animal experiments, invasive recordings in humans have not been able to provide such an evidence so far. In the current study, ERPs were recorded from intrahippocampal and scalp electrodes in 16 epilepsy patients. Stimulation consisted of trains of six tones, with one tone deviating in duration (100 vs. 50 ms). In the rhinal cortex, ERPs elicited by deviants were larger in amplitude than those of standards (around 200 ms). The rhinal activation was succeeded by a long-lasting hippocampal ERP component (around 350 ms). However, in contrast to the rhinal activation, hippocampal activation was also elicited by the 1st stimuli of the train and might, therefore, be related more to salience detection than to deviance detection. The current study provides evidence that the MMN is part of a multistage comparison process and that the rhinal cortex is part of its underlying cortical network.
Keywords: auditory evoked potentials, attention, electrocorticography
Introduction
The mismatch negativity (MMN) is one of the most intensely investigated components of event-related potentials (ERPs). It is thought to reflect a pre-attentive auditory discrimination process, as it is elicited by discernible changes in an otherwise uniform auditory stimulation, in absence of directed attention. It is assumed that the MMN results from a comparison process between a memory trace of the repeated and the incoming deviant sound (Näätänen, 2001). For the elicitation of an MMN, the memory trace of the standards must be established and in an active state (Cowan et al., 1993).
Disruptions of this function have been described for various neuropsychiatric diseases, e.g. schizophrenia (first reported by Shelley et al., 1991) or Alzheimer's disease (first reported by Pekkonen et al., 1994). For a better understanding of pathological processes leading to an attenuation (or delay) of the MMN, more detailed knowledge about the generating structures of the MMN would be helpful. Recent studies have revealed a cortical network of mainly bilateral temporal, but also frontal and parietal structures, involved in the generation of the MMN (for overview: Rosburg et al., 2005). Furthermore, animal data revealed deviance related activity already on the level of the medial geniculate body and the colliculus inferior (Csepe et al., 1993), as well as of the nonprimary thalamocortical pathways (Kraus et al., 1994), suggesting a multistage comparison process involved in auditory deviance detection. From a theoretical point of view, an involvement of the hippocampus in the generation (or modulation) of the MMN appears to be likely, as the hippocampus is assumed to act as comparator and novelty detection system (Sokolov et al., 1960; Vinogradova, 2001). The role of the hippocampus in novelty detection is stressed by studies showing that hippocampal lesions resulted in a diminution of the novelty related cortical activity (P3a), but also in reduced autonomic skin responses to novel stimuli (Knight, 1996). The MMN and P3a are often observed in immediate succession in passive oddball paradigms (Escara et al., 2000). However, it is open whether MMN and P3a share functional and/or anatomical properties although the temporal lobe sources of the neuromagnetic MMN and P3a were found to be located in relatively close vicinity (Alho et al., 1998). It is also not clarified whether the hippocampus is directly involved in sound deviance detection or whether hippocampal deviance related activity reflects an hierarchically higher order process.
Positive findings for hippocampal contribution to MMN generation originate from animal research. Research was initiated by Valeria Csepe and co-workers who reported on a deviance related activity in the hippocampus of cats, occurring at a latency range of 30-50 ms and thus coinciding with MMN-like activity recorded over the auditory cortices (Csepe et al., 1987; 1988). Other researchers did not detect this early hippocampal activity consistently across their experimental animals, but a later deviance-related negativity between 100-170 ms which, however, was regarded as correlate of the P3a (Ruusuvirta et al., 1995b). In a study of this group on rabbits, early deviance related activity was detected in the hippocampus, but it did not exhibit an MMN-like dependence on standard stimuli (Ruusuvirta et al., 1995a) and might, therefore, not be regarded as an analogue of the MMN.
While animal studies are helpful in many aspects, it is often difficult to draw direct conclusion from animal data on human data because of the tremendous cortico-anatomical differences between species. The contribution of the hippocampus in the generation of human MMN (and any other ERP component) is difficult to investigate by scalp EEG because of the closed field structure of the hippocampus (Klee and Rall, 1977). Therefore, invasive recordings offer the nearly exclusive possibility to investigate hippocampal functions with high temporal resolution. However, in contrast to animal studies, invasive recordings in humans have given no evidence of an hippocampal involvement in MMN generation. Kropotov and co-workers investigated the MMN on frequency deviants in the hippocampus of three patients with obsessive compulsive disorder (Kropotov et al., 1995) and fourteen patients with epilepsy and Parkinson's disease (Kropotov et al., 2000). In both studies, an MMN was observed above the auditory association cortex, but not in the hippocampus. Other studies recorded strong deviance related hippocampal ERPs (P3b) in active oddball paradigms, but no systematic deviance related ERP when subjects were ignoring the stimuli (McCarthy et al., 1989; Halgren et al., 1995). Also, a lesion study proposed a minor role of the hippocampus in preattentive discrimination processes. Patients with hippocampal lesions did not exhibit a reduced MMN (Alain et al., 1998). However, two recent ERP studies showed that the hippocampus was activated by uniform auditory stimulation after long interstimulus intervals (ISIs) (Grunwald et al., 2003; Boutros et al., 2005).
Taken together, animal and human data are diverging and empirical evidence for an involvement of the human hippocampus in MMN generation is still missing, maybe owing to the limited number of conducted studies by means of invasive recordings. The aim of the current study was to evaluate ERPs invasively recorded from the hippocampus and the rhinal cortex (Fig. 1) in a passive oddball paradigm. The stimulation consisted of trains of six tones, with one deviating in its duration. Sound deviance was expected to result in a stronger hippocampal response as compared to standard tones. As auditory stimuli were found to elicit hippocampal activity which was suppressed by stimulus repetition (Grunwald et al., 2003; Boutros et al., 2005), an analysis of this response decrement by stimulus repetition was regarded as helpful for an understanding of the hippocampal ERPs and dissociate effects of sound deviance from effects of sound salience. Intracranial data were compared with simultaneously recorded data from the scalp. Finally, all data were screened for a possible occurrence of long-term habituation, as previous studies have shown a response decrease of the MMN within experiments (McGee et al., 2001; Rosburg et al., 2004).
Figure 1.
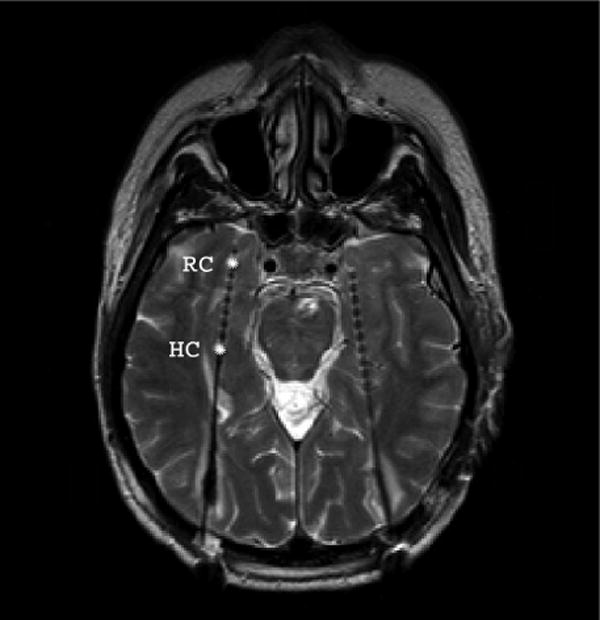
Magnetic resonance image of a single subject. Implanted electrodes become observable as small artifacts. Each electrode contained ten cylindrical contacts of a nickel-chromium alloy (2.5 mm) every 4 mm. The electrodes in the rhinal cortex (RC) and hippocampus (HC), selected for the analysis, are marked by asterisks.
Methods
Subjects
Total study group consisted of 33 epilepsy patients, undergoing presurgical evaluation with depth electrodes in the mesial temporal lobe. Data of patients with bilateral Ammon's horn sclerosis (AHS) (n = 3) or an unilateral implantation on the side of the AHS were excluded (n = 7). 7 other data sets were excluded for the following reasons: in 3 recordings, more than 50% of the trials were affected by spike activity. In 2 subjects, depth electrodes were not placed exactly in the target region, and 2 other subjects were excluded because they did not exhibit an MMN signal at the Cz electrode, leaving the possibility that they were not able to discriminate the standard and the deviant sound. The final sample consisted of 16 patients (9 males, average age 33.5 years, range 24 to 45). 4 of these patients had been included in a previous study (Rosburg et al., 2006). All patients were on anticonvulsive medication at the time of recording: 7 patients received levetiracetam (1000-3500 mg), 7 patients lamotrigine (300-600 mg), 2 patients pregabalin (150-300 mg), 2 patients carbamazepine (1000-1200 mg), 1 patient each oxcarbazepine (1500 mg), phenytoin (250 mg) and gabapentin (2800 mg). 10 patients were medicated additionally by benzodiazepines. Patients gave written consent after being thoroughly informed about the purpose of the study. The study was approved by the ethics committee of the University of Bonn.
Stimulation
Subjects were binaurally stimulated by earphones with 100 trains of six tones (five standards and one deviant). Standards and deviants were identical in pitch (1000 Hz) and intensity (50 dB above hearing level), but deviants were longer in tone duration (standards: 50 ms; deviants: 100 ms, including 5 ms rise and fall time each). A deviant occurred with equal chance at position 3, 4 or 5 of the train. The tones were separated by 981 ms and the trains by 5000 ms. Subjects were instructed to sit relaxed on their chair and watched a movie of their own choice during recordings, with the sound switched off. The subjects were neither informed about the occurrence of the tone deviance, nor was a response required to the deviance. Attention was not actively controlled.
Recording and data analysis
All patients included in the study had at least one catheter-like, 1 mm thick silastic depth electrode, implanted via the longitudinal axis of the medial temporal lobe from an occipital approach (van Roost et al., 1998). These electrodes had 10 cylindrical platinum contacts of 2.5 mm every 4 mm. Electrodes were implanted with the most anterior contacts reaching the rhinal cortex and the more posterior contacts targeting the hippocampal body (Fig. 1). The stereotactic localization of the electrodes was based on computer tomography scans. Electrophysiological data were recorded at a sampling rate of 1000 points/s in a quiet environment, with mastoids as references. Recordings were simultaneously obtained from depth electrodes and six scalp electrodes (Cz, C5, C6, T5, T6, Oz). Impedance of scalp electrodes was kept below 5 kΩ.
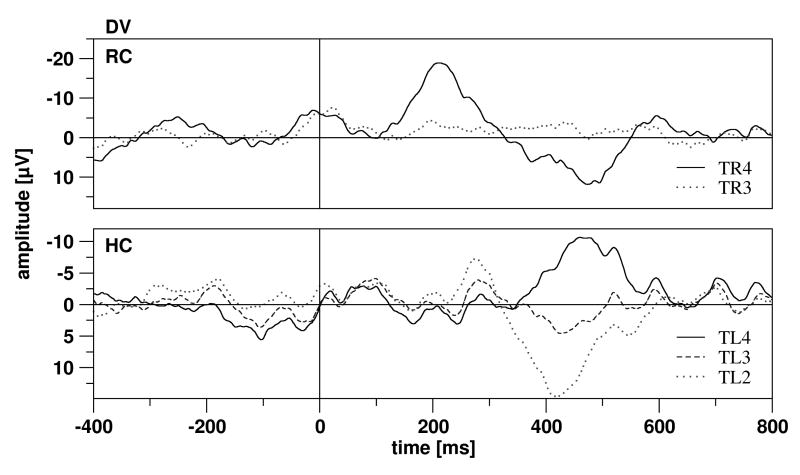
As the MMN in scalp recordings is maximal at fronto-central electrodes, analysis of scalp data focused on data from Cz. Previous studies (Grunwald et al., 2003; Boutros et al., 2005) on sensory gating let us expect differential low frequency signals in the rhinal cortex and hippocampus. The rhinal and the hippocampal contact exhibiting the strongest (positive or negative) response on either the 1st tone of the train or on the deviating sound were selected for further investigation. Contacts located in the rhinal cortex and hippocampus were differentiated anatomically, on basis of magnetic resonance imaging (MRI) scans, obtained in the clinical post-operative routine. All 16 patients included had electrode contacts in the hippocampus, while the rhinal cortex was penetrated by the depth electrode in 12 patients. Data of neighboring electrodes are exemplarily described in order to provide information about the spatial distribution of the ERPs (Fig. 2).
Figure 2.
Exemplary ERP data of two different subjects in response to the deviant tone. Top: For the rhinal cortex in subject 172, contact TR4 was selected for the analysis; contact TR3 was located anterior to the rhinal cortex. The ERPs at TR3 and TR4 exhibited a steep gradient, suggesting a local generation of the potential. Bottom: For the hippocampal cortex in subject 158, contact TL4 was selected, the contacts TL2 and TL3 were located further anterior (with TL2 most anterior). The hippocampal component (HC1) exhibited a phase reversal. Subject 158 had no contacts in the rhinal cortex.
For scalp recordings, all trials with more than 75 μV at Cz were excluded as artifacts. For intracranial recordings, trials with artifacts or interictal spike activity were removed on the basis of visual inspection. Only recordings with at least 80 artifact free trials were included. EEG segments of 1200 ms duration with a 400 ms prestimulus period as baseline were averaged for the first, second and sixth stimuli of the train (ERPS1, ERPS2, ERPS6, all stimuli at these positions were standards), as well as for all standard stimuli at positions 2 to 5 (ERPSTD) and for all deviants (ERPDV). For the investigation of habituation effects, sub-averages were calculated for each ERP (trial 1-40: ERPn.1; trial 21-60: ERPn.2, trial 41-80: ERPn.3). ERPs were filtered from 1-20 Hz with a slope of 12 dB/oct and baseline corrected. Amplitudes were determined for consecutive 50 ms windows from 0 to 800 ms (as areas in μVms) and as peak amplitudes for the N100 and P200, in the latency ranges from 70-135 and 125-250 ms respectively. As stimulus repetition normally leads to amplitude decreases of N100 and P200, and those components have different polarities, effects on peaks were described instead of providing the effects on amplitudes of time windows. Besides the surface peaks, the peaks of the rhinal and hippocampal components were determined post-hoc, using the following nomenclature: component RH1 peaking at the rhinal electrode between 110 and 310 ms with negative polarity; component RH2 peaking at the rhinal electrode between 250 and 500 ms with positive polarity; and component HC1 peaking at the hippocampal electrode between 210 and 510 ms. Because of the low signal-to-noise ratio in ERPS2 and ERPS6, these peaks were determined only for ERPS1, ERPSTD and ERPDV. Analyses of all EEG data were performed by Brain Vision Analyzer 1.05 (Brain Products, Munich, Germany).
Amplitudes were compared by means of an analysis of variance (ANOVA) with TONE REPETITION (ERPS1 vs. ERPS2 vs. ERPS6) or DEVIANCE (ERPSTD vs. ERPDV) as factors for repeated measurement. Effects of HABITUATION were tested in an additional repeated measurement ANOVA for each ERP separately (ERPn.1 vs. ERPn.2 vs. ERPn.3). The level of significance was set at p < 0.01 in order to reduce the possibility of false positive findings. P values p < 0.05 for certain time windows were only taken into consideration if the comparison of preceding or succeeding time windows revealed p values below 0.01. A Greenhouse-Geisser correction was performed where necessary, as indicated by the citation of ε values. Paired t-tests were applied for post-hoc testing of significant differences in the ANOVA. Statistics were performed by SPSS 12.0.
Results
Exemplary rhinal and hippocampal ERP data in response to the deviant tone and data of neighboring electrodes are depicted in Figure 2. Rhinal ERPs were characterized by a negative component followed by a positive component, hippocampal ERPs by a positive component. Steep gradients from a rhinal or hippocampal contact to the neighboring contacts outside the structure in question were more consistently observed than phase reversals. The depicted examples show a steep gradient for the rhinal contact (Fig. 2 top) and a phase reversal for the hippocampal contact (Fig. 2 bottom).
Effects of sound deviance
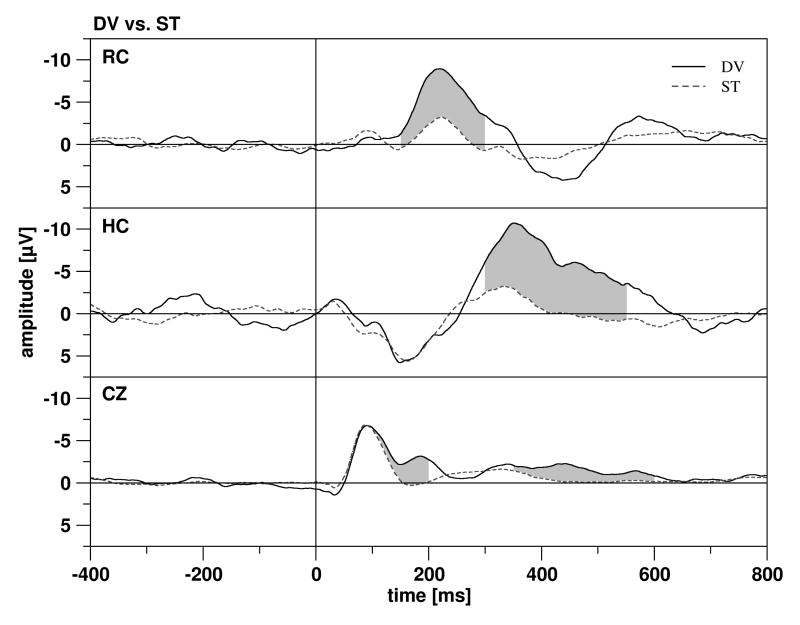
On the scalp (Cz), an MMN-effect (with deviants being more negative than standards) was found in the time windows 100-150 ms and 150-200 ms. But the ERP elicited by deviants (ERPDV) was also more negative than the ERP of standards (ERPSTD) between 350 and 600 ms. Thus, at the scalp the reaction to sound deviance was characterized by the occurrence of an MMN and a later negativity, whereas a P3a (often following an MMN) was not observed in the current sample (Fig. 3). Slight, but significant differences in the initial time window 0-50 ms might be caused by a sub-optimal baseline (see Table 1 for all significant effects of sound deviance).
Figure 3.
The grand means of the ERPs in the rhinal cortex (RC, top), hippocampus (HC, middle) and at Cz (bottom). ERPs elicited by the deviant tone are depicted by solid lines, the ERPs elicited by the standard by dotted lines. Significant differences between the two ERPs are marked by grey shading.
Table 1.
Significant effects of sound deviance on mean amplitudes
| Time window | Cz | Rhinal cortex | Hippocampus |
|---|---|---|---|
| 000-050 | F 1,15 = 18.322, p < 0.001 | ||
| 050-100 | |||
| 100-150 | F 1,15 = 10.281, p = 0.006 | ||
| 150-200 | F 1,15 = 52.579, p < 0.001 | F 1,15 = 5.485, p = 0.039 | |
| 200-250 | F 1,15 = 10.060, p = 0.009 | ||
| 250-300 | F 1,15 = 9.104, p = 0.012 | ||
| 300-350 | F 1,15 = 23.547, p < 0.001 | ||
| 350-400 | F 1,15 = 5.500, p = 0.033 | F 1,15 = 17.184, p < 0.001 | |
| 400-450 | F 1,15 = 36.423, p < 0.001 | F 1,15 = 13.190, p = 0.001 | |
| 450-500 | F 1,15 = 38.511, p < 0.001 | F 1,15 = 13.435, p = 0.002 | |
| 500-550 | F 1,15 = 7.588, p = 0.015 | F 1,15 = 5.587, p = 0.032 | |
| 550-600 | F 1,15 = 17.704, p < 0.001 | ||
| 600-650 | |||
| 650-700 | |||
| 700-750 | |||
| 750-800 |
In the rhinal cortex (RC), the ERP was characterized by two components: a negative component with a latency of about 200 ms (RH1) and a positive component with a latency of about 400 ms (RH2) (Fig. 3). Significant differences between ERPDV and ERPSTD were observed for the earlier of the two components (time windows from 150-300 ms), with ERPDV being more negative than ERPSTD. Thus, at rhinal contacts, significant differences between ERPDV and ERPSTD occurred shortly after the MMN at Cz (peak latency at Cz: 182 ms; at rhinal contacts: 209 ms, as obtained from grand average data). There was some tendency for a longer latency of the rhinal components, elicited by deviants, as compared to standards (RH1: t 11 = 2.332, p = 0.040; RH2: t 11 = 2.219, p = 0.048).
In the hippocampus (HC), the ERP was characterized by a large negative component with a latency of about 300-400 ms (HC1) (Fig. 3). Differences between ERPDV and ERPSTD were observed for this component between 300 and 500 ms, with ERPDV being more negative than ERPSTD. HC1 for deviants peaked significantly later than for standards (t 15 = 3.465, p = 0.003). Descriptive data of rhinal and hippocampal peaks are provided in Table 2.
Table 2.
Mean latencies and amplitudes for the rhinal and hippocampal components
| ERPS1 | ERPSTD | ERPDV | ||
|---|---|---|---|---|
| RH1 | Latency | 211.1 (42.2) | 218.5 (18.7) | 242.9 (36.6) |
| Amplitude | −11.9 (5.3) | −3.9 (2.0) | −11.3 (5.0) | |
| RH2 | Latency | 367.1 (64.9) | 362.2 (59.9) | 409.1 (65.5) |
| Amplitude | 10.2 (6.2) | 3.9 (1.9) | 8.5 (5.4) | |
| HC1 | Latency | 292.8 (61.0) | 316.9 (66.2) | 354.2 (65.7) |
| Amplitude | −19.7 (12.2) | −5.5 (3.4) | −15.5 (7.2) |
Effects of sound repetition
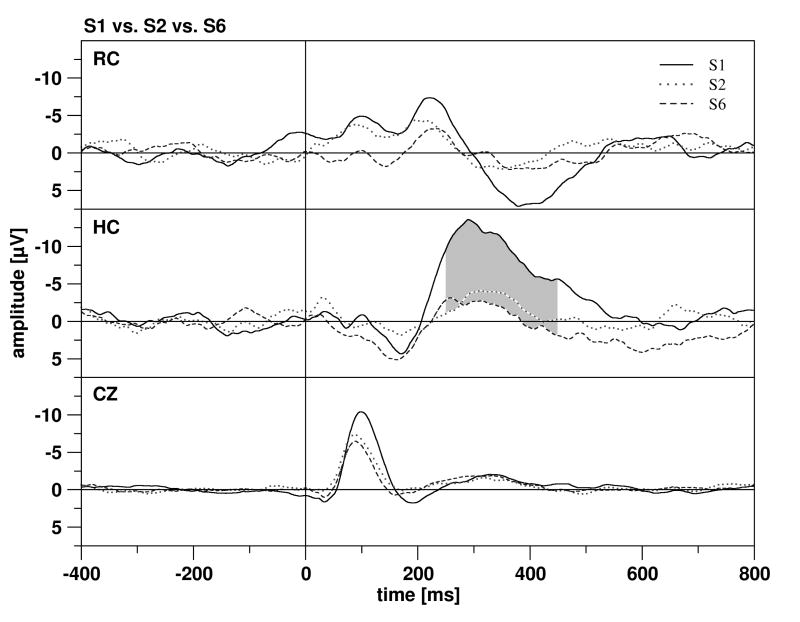
In scalp recordings, standard tones elicited a pronounced N100, but a relatively weak P200 (for grand averages see Fig. 4). For the N100, stimulus repetition resulted in a significant amplitude (F 2,30 = 19.993, p < 0.001, ε = 0.648) and latency decrease (F 2,30 = 7.336, p = 0.003). P200 amplitudes were also reduced by stimulus repetition (F 2,30 = 7.001, p = 0.008, ε = 0.729), while differences in P200 latency were not significant (F 2,30 = 2.634, n.s.). The negative deflection following the P200 (200-400 ms) was unaffected by stimulus repetition. In the rhinal cortex, no significant differences between ERP elicted by the 1st stimulus (ERPS1), by the 2nd stimulus (ERPS2) and by the 6th stimulus of the train (ERPS6) were observed. However, the difference in the time window 400-450 ms missed the significance level of 0.01 minimally (F 2,22 = 5.535, p = 0.011). In the hippocampus, effects of stimulus repetition were more pronounced. Differences between ERPS1, ERPS2 and ERPS6 were detected between 250-450 ms, with ERPS1 being more negative than ERPS2 and ERPS6 (250-300 ms: F 2,30 = 9.343, p < 0.001; 300-350 ms: F 2,30 = 7.969, p = 0.002; 350-400 ms: F 2,30 = 5.154, p = 0.012; 400-450 ms: F 2,30 = 4.280, p = 0.040, ε = 0.691).
Figure 4.
The grand means of the ERPs in the rhinal cortex (RC, top), hippocampus (HC, middle) and at Cz (bottom). ERPs elicited by the 1st tone are depicted by solid lines, the ERPs elicited by the 2nd tone by dotted lines, and the ERPs elicited by the 6th tone by dashed lines. Significant differences between the ERPs are marked by grey shading.
Effects of long-term habituation
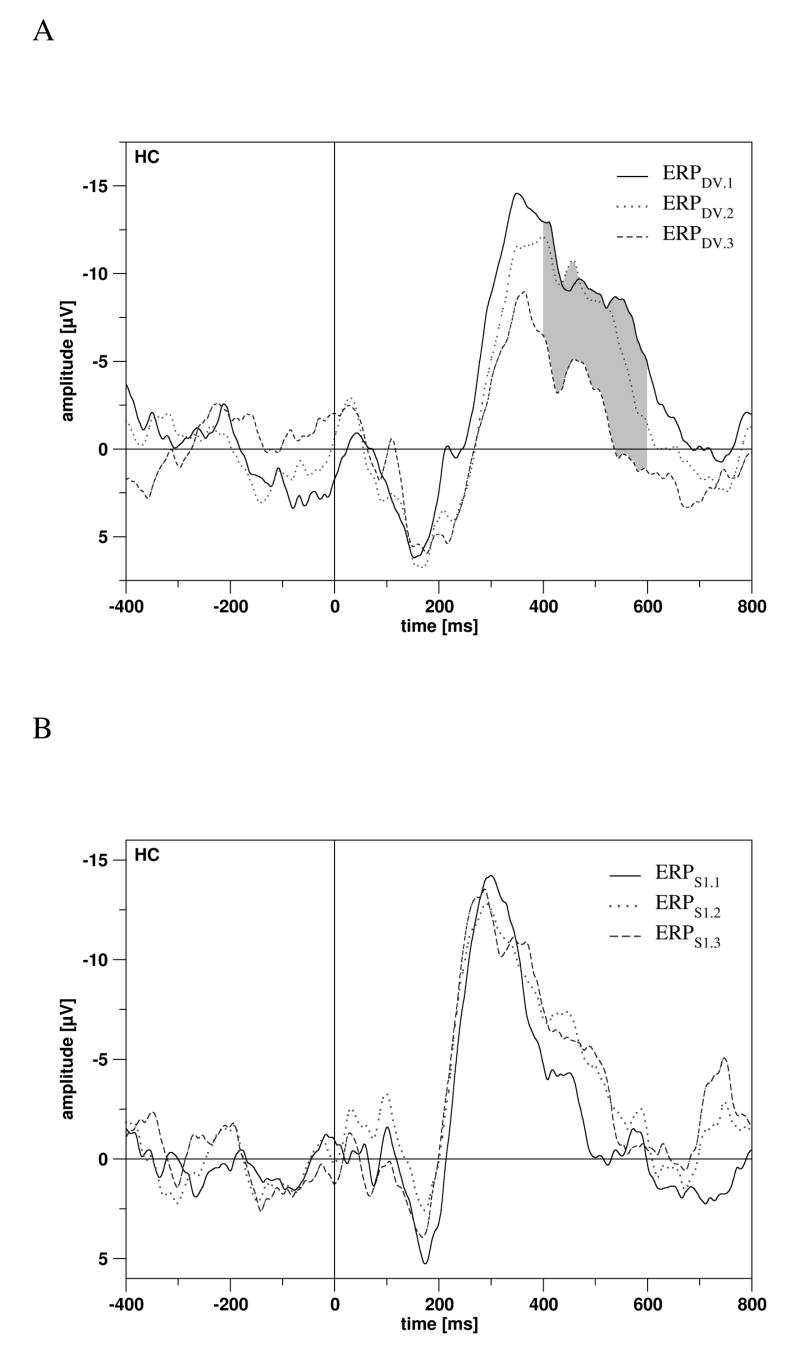
In scalp recordings, no significant effects of habituation were observed. In rhinal recordings, a response increase during the experiment was observed for ERPS1 for two very early time windows (0-50 ms: F 2,22 = 3.878, p = 0.036; 50-100 ms: F 2,22 = 8.231, p = 0.002). In hippocampal recordings, only ERPDV was affected by HABITUATION. In the time window from 400-600 ms, amplitudes decreased (400-450 ms: F 2,30 = 8.541, p = 0.001; 450-500 ms: F 2,30 = 4.417, p = 0.021; 500-550 ms: F 2,30 = 5.075, p = 0.013; 550-600 ms: F 2,30 = 5.131, p = 0.012; Fig. 5A). Of note, the latter effects were not observed for ERPS1 (Fig. 5B) and ERPSTD.
Figure 5.
A: Long-term habituation effects on ERPs elicited by the deviant tone, for trials 1-40 (ERPDV.1), for trials 21-60 (ERPDV.2, dotted line), and for trials 41-80 (ERPDV.3, dashed line) in the hippocampal cortex (HC). Significant effects of habituation are marked by grey shading. B: The corresponding figure for ERPs elicited by the 1st tone (ERPS1). Effects of habituation on ERPS1 were not significant (n.s.).
Discussion
The current study provides evidence for an involvement of hippocampal structures in pre-attentive discrimination processes in humans. Both in the rhinal cortex and hippocampus, the amplitudes of ERP components elicited by deviants exceeded those of standard tones. The amplitudes of these ERP components were relatively small and amounted to about 90 % less, as compared to active oddball paradigms (Halgren et al., 1995; Grunwald et al., 1999). For the hippocampal activity elicited by deviants, a response decrease was observed within the course of the experiment. Both factors (small amplitudes and habituation) might explain why previous studies (McCarthy et al., 1989; Halgren et al., 1995; Kropotov et al., 1995; 2000) have not been successful in detecting this kind of activity. However, also other factors might possibly have contributed to these negative findings, e.g. the usage of clicks as stimulus material (McCarthy et al, 1989), because an MMN cannot be elicited when stimuli are too short (Paavilainen et al., 1993).
Hippocampal response
Both salient stimuli (1st stimuli of the train and deviants) elicited a similar response in the hippocampus (HC1), with peak latencies differing by 60 ms. This latency difference is likely to be explained by the fact that deviants cannot be perceived as deviants until at least 50 ms of stimulation, thus their salience becomes later apparent as the salience of the 1st stimuli of the trains. As the hippocampal response was elicited by salient stimuli in general and not by deviants alone, it is more likely to be regarded as a P3a which has been proposed to be a central marker of the orienting response (Squires et al., 1975). Thus, the HC1 is probably not genuinely related to the MMN and might be regarded as an equivalent to the late hippocampal response (“N130”) observed in animal experiments (Ruusuvirta et al., 1995a). A hippocampal generation of the P3a is in line with lesion studies (Knight, 1996) and intracranial recordings (Dowman et al., 2007), but it should be mentioned that others questioned a hippocampal generation (Halgren et al., 1995, p. 244).
Habituation has been described for the P3a (Polich and McIsaac, 1994), but P3 activity habituates much slower than the orienting response itself (Donchin et al., 1984). In the current study, the HC1 component elicited by deviants exhibited habituation during the course of the experiment, while the MMN (as measured at the scalp) remained stable. This dissociation also supports the notion that the HC1 component is not directly linked to the MMN. In contrast to deviants, the HC1 component elicited by 1st stimuli did not decrease during the experiment. This might possibly be explained by the fact that subjects were not aware of the absolute length of the experiment (number of trains), whereas subjects could have been sure that the train contained six stimuli and that one stimulus would be deviating, once it had started. Furthermore, the subjective temporal uncertainty was larger for the intertrain interval than for the interstimulus interval because of its longer duration.
Rhinal response
Differences between standards and deviants were observed for the early rhinal component RH1, peaking shortly after the MMN at the scalp. In contrast to deviants, 1st stimuli of the train did not elicit a stronger RH1 component than the succeeding standards (S2 and S6). Thus, the stronger RH1 response to deviants is more likely related to sound deviance than to stimulus salience. Both, the scalp MMN and the deviance related RH1 did not habituate during the experiment. Thus, there is also no apparent dissociation between MMN and RH1 component with regard to habituation, as it was observed for hippocampal activity.
To our knowledge, the rhinal cortex has not been explicitly investigated in any other study applying a passive oddball paradigm as yet. However, also the time point of deviance related activity is different from any up to now obtained invasive (animal) data. In the study of Csepe et al. (1988), deviance related activity in the hippocampus coincided with or even preceded activity in secondary auditory cortices which is assumed to reflect the generating neocortical structure of the MMN in the temporal lobe (Kropotov et al., 2000; Pincze et al., 2001). In contrast, we revealed deviance related activity in the rhinal cortex shortly after the scalp MMN.
The morphology and timing of the rhinal and hippocampal ERPs is apparently different, as well as their sensitivity to novelty and sound deviance. Thus, the rhinal cortex and the hippocampus appear to have different functional roles in auditory deviance detection, with the rhinal cortex being more closely associated to mismatch detection. However, the exact functional significance of the rhinal activation warrants further investigation. On basis of the temporal order of deviance related activity, it might be speculated that the information of sound deviance goes from the auditory cortex via the rhinal cortex to the hippocampus, especially since the rhinal cortex generally supplies the major input to the hippocampus (Amaral, 1987). However, it has to be stressed that a direct investigation of the coupling of these regions was not within the scope of the current study.
Generation of the rhinal and hippocampal ERP components
The rhinal and hippocampal ERPs are assumed to be generated locally, as the ERPs in these two regions considerably differed in their morphology which would not have been the case when they resulted from volume conduction. It has also to be stated that the rhinal and hippocampal ERPs were not reflected in scalp ERPs and vice versa. Phase reversals at contacts neighboring the rhinal cortex and hippocampus were observed in some, but not all subjects, probably due to electrode placement, with few electrode contacts outside the target regions of the rhinal cortex and hippocampus, but partly also due to the relatively small amplitudes of the observed ERPs. However, in most recordings, ERPs exhibited relatively steep gradients from one contact to another, again supporting the hypothesis of a local generation.
Scalp recordings
In scalp recordings, no ERP components with a similar time characteristic as the rhinal and hippocampal activity were observed. Besides the MMN, a deviance related negativity between 350 and 600 ms was observed. To our knowledge, this later negativity has not been systematically investigated as yet. In many previous studies, this negativity was not evaluable because ISIs shorter than 500 ms were used. However, it was apparent in some MMN studies on healthy subjects (e.g. Jacobsen & Schröger 2003) and can, therefore, not be regarded as a characteristic of epilepsy patients. The negativity probably does not represent a reorienting negativity (RON, Schröger & Wolff, 1998), although it occurs in the same time range, because the RON is always preceded by a P3a. Finally, it is also unlikely that it represents a pattern-related MMN, as all possible patterns (trains with deviants at the 3rd, 4th and 5th position) were equally distributed. Of note, high density recordings of this late deviance-related negativity revealed a fronto-central distribution, similar to those of an N100 (own unpublished data). Further studies are needed to evaluate the functional significance of this late deviance related ERP component.
Limitations of the study
The lack of further experimental variation and low spatial resolution of the implanted electrodes might be regarded as possible shortcomings of the current study. However, one should keep in mind that the implantation of electrodes in patients always has to follow solely clinical considerations and all recordings take place in a primarily clinical setting.
Furthermore, attention effects were not actively controlled in the current study and it cannot be fully clarified to what extent the observed rhinal and hippocampal potentials depend on attention. However, in active oddball paradigms, ERPs elicited by deviants (targets) are characterized by a strong P3b (for the comparison of passive and active oddball ERP data see e.g. Alain and Woods, 1997) and no P3 was obvious in scalp ERPs of our study, strongly suggesting that subjects were actually not paying attention to the deviants and that the observed rhinal and hippocampal ERP components were elicited by sound deviance in absence of directed attention.
All subjects included in the study were epileptic patients and, in consequence, under anti-convulsive medication. Both factors might have influenced the latencies and amplitudes of ERP components. The impact of chronic anti-convulsive medication on ERPs has not been systematically investigated as yet, but impairment of reaction velocity is a core side effect of this kind of medication and a down slowing of ERP components has been described for medicated epilepsy patients (Drake et al., 1986; Triantafyllou et al., 1992; Verleger et al., 1997). However, the peak latency of the MMN observed in the current sample does not differ notably from MMN latencies reported for healthy subjects when the same kind of deviance was applied (Todd et al., 2000).
Conclusion
In the current study, an involvement of hippocampal and parahippocampal structures in the pre-attentive processing of sound deviance was revealed by direct measurements in the human hippocampus. The rhinal activation was related to sound deviance, while the hippocampal activity might be referred more generally to stimulus salience, as also stimuli presented after long ISIs (1st stimuli) elicited this kind of activity. As the MMN was not found to be reduced in patients with hippocampal lesions at short ISIs (Alain et al., 1998) and in patients with Alzheimer's disease only at long ISIs (Pekkonen et al., 1994), the hippocampus might not represent an essential part of the MMN system, but might facilitate the detection of sound deviance when sound discrimination becomes more difficult. The effects of selective lesions of the rhinal cortex on MMN generation have yet to be tested, but might be more serious than hippocampal lesions.
Acknowledgments
Supported by the NIH under grant number R01 MH063476.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alain C, Woods DL. Attention Modulates Auditory Pattern Memory As Indexed By Event-Related Brain Potentials. Psychophysiology. 1997;34:534–546. doi: 10.1111/j.1469-8986.1997.tb01740.x. [DOI] [PubMed] [Google Scholar]
- Alain C, Woods DL, Knight RT. A Distributed Cortical Network for Auditory Sensory Memory in Humans. Brain Res. 1998;812:23–37. doi: 10.1016/s0006-8993(98)00851-8. [DOI] [PubMed] [Google Scholar]
- Alho K, Winkler I, Escera C, Huotilainen M, Virtanen J, Jääskeläinen IP, Pekkonen E, Ilmoniemi RJ. Processing of Novel Sounds and Frequency Changes in the Human Auditory Cortex: Magnetoencephalographic Recordings. Psychophysiology. 1998;35:211–224. [PubMed] [Google Scholar]
- Amaral DG. Memory: anatomical organization of candidate brain regions. In: Plum F, Mountcastle V, editors. Higher functions of the brain Handbook of Physiology Part 1 Am Physiol Soc. Washington DC: 1987. pp. 194–211. [Google Scholar]
- Boutros NN, Trautner P, Rosburg T, Korzyukov O, Grunwald T, Schaller C, Elger CE, Kurthen M. Sensory Gating in the Human Hippocampal and Rhinal Regions. Clin Neurophysiol. 2005;116:1967–1974. doi: 10.1016/j.clinph.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cowan N, Winkler I, Teder W, Näätänen R. Memory Prerequisites of Mismatch Negativity in the Auditory Event-Related Potential (ERP) J Exp Psychol Learn Mem Cogn. 1993;19:909–921. doi: 10.1037//0278-7393.19.4.909. [DOI] [PubMed] [Google Scholar]
- Csepe V, Karmos G, Molnar M. Evoked Potential Correlates of Stimulus Deviance During Wakefulness and Sleep in Cat--Animal Model of Mismatch Negativity. Electroencephalogr Clin Neurophysiol. 1987;66:571–578. doi: 10.1016/0013-4694(87)90103-9. [DOI] [PubMed] [Google Scholar]
- Csepe V, Karmos G, Molnar M. Subcortical evoked potential correlates of sensory mismatch process in cats. In: Bajic M, editor. Advances in the biosciences. Vol. 70. Pergamon Press; Oxford: 1988. pp. 43–46. [Google Scholar]
- Csepe V, Karmos G, Molnar M. Animal model of mismatch negativity. In: Heinze HJ, Münte F, Mangun GR, editors. New developments in event-related potentials. Birkhäuser; Boston: 1993. pp. 29–33. [Google Scholar]
- Donchin E, Heffley E, Hillyard SA, Loveless N, Maltzman I, Ohman A, Rosler F, Ruchkin D, Siddle D. Cognition and Event-Related Potentials. II. The Orienting Reflex and P300. Ann N Y Acad Sci. 1984;425:39–57. doi: 10.1111/j.1749-6632.1984.tb23522.x. [DOI] [PubMed] [Google Scholar]
- Dowman R, Darcey T, Barkan H, Thadani V, Roberts D. Human Intracranially-Recorded Cortical Responses Evoked By Painful Electrical Stimulation of the Sural Nerve. Neuroimage. 2007;34:743–763. doi: 10.1016/j.neuroimage.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Drake ME, Jr, Burgess RJ, Gelety TJ, Ford CE, Brown ME. Long-Latency Auditory Event-Related Potentials in Epilepsy. Clin Electroencephalogr. 1986;17:10–13. [PubMed] [Google Scholar]
- Escera C, Alho K, Schröger E, Winkler I. Involuntary Attention and Distractibility As Evaluated With Event-Related Brain Potentials. Audiol Neurootol. 2000;5:151–166. doi: 10.1159/000013877. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Beck H, Lehnertz K, Blumcke I, Pezer N, Kutas M, Kurthen M, Karakas HM, Van Roost D, Wiestler OD, Elger CE. Limbic P300s in Temporal Lobe Epilepsy With and Without Ammon's Horn Sclerosis. Eur J Neurosci. 1999;11:1899–1906. doi: 10.1046/j.1460-9568.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Boutros NN, Pezer N, von Oertzen J, Fernandez G, Schaller C, Elger CE. Neuronal Substrates of Sensory Gating Within the Human Brain. Biol Psychiatry. 2003;53:511–519. doi: 10.1016/s0006-3223(02)01673-6. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal JP, Biraben A. Intracerebral Potentials to Rare Target and Distractor Auditory and Visual Stimuli. II. Medial, Lateral and Posterior Temporal Lobe. Electroencephalogr Clin Neurophysiol. 1995;94:229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Jacobsen T, Schröger E. Measuring Duration Mismatch Negativity. Clin Neurophysiol. 2003;114:1133–1143. doi: 10.1016/s1388-2457(03)00043-9. [DOI] [PubMed] [Google Scholar]
- Klee M, Rall W. Computed Potentials of Cortically Arranged Populations of Neurons. J Neurophysiol. 1977;40:647–666. doi: 10.1152/jn.1977.40.3.647. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of Human Hippocampal Region to Novelty Detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T, Littman T, Nicol T, King C. Nonprimary Auditory Thalamic Representation of Acoustic Change. J Neurophysiol. 1994;72:1270–1277. doi: 10.1152/jn.1994.72.3.1270. [DOI] [PubMed] [Google Scholar]
- Kropotov JD, Alho K, Näätänen R, Ponomarev VA, Kropotova OV, Anichkov AD, Nechaev VB. Human Auditory-Cortex Mechanisms of Preattentive Sound Discrimination. Neurosci Lett. 2000;280:87–90. doi: 10.1016/s0304-3940(00)00765-5. [DOI] [PubMed] [Google Scholar]
- Kropotov JD, Näätänen R, Sevostianov AV, Alho K, Reinikainen K, Kropotova OV. Mismatch Negativity to Auditory Stimulus Change Recorded Directly from the Human Temporal Cortex. Psychophysiology. 1995;32:418–422. doi: 10.1111/j.1469-8986.1995.tb01226.x. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC, Williamson PD, Spencer DD. Task-Dependent Field Potentials in Human Hippocampal Formation. J Neurosci. 1989;9:4253–4268. doi: 10.1523/JNEUROSCI.09-12-04253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee TJ, King C, Tremblay K, Nicol TG, Cunningham J, Kraus N. Long-Term Habituation of the Speech-Elicited Mismatch Negativity. Psychophysiology. 2001;38:653–658. [PubMed] [Google Scholar]
- Näätänen R. The Perception of Speech Sounds By the Human Brain As Reflected By the Mismatch Negativity (MMN) and Its Magnetic Equivalent (MMNm) Psychophysiology. 2001;38:1–21. doi: 10.1017/s0048577201000208. [DOI] [PubMed] [Google Scholar]
- Paavilainen P, Jiang D, Lavikainen J, Näätänen R. Stimulus Duration and the Sensory Memory Trace: An Event-Related Potential Study. Biol Psychol. 1993;35:139–152. doi: 10.1016/0301-0511(93)90010-6. [DOI] [PubMed] [Google Scholar]
- Pekkonen E, Jousmäki V, Könönen M, Reinikainen K, Partanen J. Auditory Sensory Memory Impairment in Alzheimer's Disease: An Event-Related Potential Study. Neuroreport. 1994;5:2537–2540. doi: 10.1097/00001756-199412000-00033. [DOI] [PubMed] [Google Scholar]
- Pincze Z, Lakatos P, Rajkai C, Ulbert I, Karmos G. Separation of Mismatch Negativity and the N1 Wave in the Auditory Cortex of the Cat: A Topographic Study. Clin Neurophysiol. 2001;112:778–784. doi: 10.1016/s1388-2457(01)00509-0. [DOI] [PubMed] [Google Scholar]
- Polich J, McIsaac HK. Comparison of Auditory P300 Habituation from Active and Passive Conditions. Int J Psychophysiol. 1994;17:25–34. doi: 10.1016/0167-8760(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Marinou V, Haueisen J, Smesny S, Sauer H. Effects of Lorazepam On the Neuromagnetic Mismatch Negativity (MMNm) and Auditory Evoked Field Component N100m. Neuropsychopharmacology. 2004;29:1723–1733. doi: 10.1038/sj.npp.1300477. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Trautner P, Boutros NN, Korzyukov OA, Schaller C, Elger CE, Kurthen M. Habituation of Auditory Evoked Potentials in Intracranial and Extracranial Recordings. Psychophysiology. 2006;43:137–144. doi: 10.1111/j.1469-8986.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Trautner P, Dietl T, Korzyukov OA, Boutros NN, Schaller C, Elger CE, Kurthen M. Subdural Recordings of the Mismatch Negativity (MMN) in Patients With Focal Epilepsy. Brain. 2005;128:819–828. doi: 10.1093/brain/awh442. [DOI] [PubMed] [Google Scholar]
- Ruusuvirta T, Korhonen T, Penttonen M, Arikoski J. Hippocampal Evoked Potentials to Pitch Deviances in an Auditory Oddball Situation in the Rabbit: No Human Mismatch-Like Dependence On Standard Stimuli. Neurosci Lett. 1995a;185:123–126. doi: 10.1016/0304-3940(94)11240-j. [DOI] [PubMed] [Google Scholar]
- Ruusuvirta T, Korhonen T, Penttonen M, Arikoski J, Kivirikko K. Hippocampal Event-Related Potentials to Pitch Deviances in an Auditory Oddball Situation in the Cat: Experiment I. Int J Psychophysiol. 1995b;20:33–39. doi: 10.1016/0167-8760(95)00024-m. [DOI] [PubMed] [Google Scholar]
- Schröger E, Wolff C. Attentional Orienting and Reorienting Is Indicated By Human Event-Related Brain Potentials. Neuroreport. 1998;9:3355–3358. doi: 10.1097/00001756-199810260-00003. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch Negativity: An Index of a Preattentive Processing Deficit in Schizophrenia. Biol Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Nervous Model of Stimulus and the Orienting Reflex. Voprosy Psichologii. 1960;4:128–137. [in Russian] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two Varieties of Long-Latency Positive Waves Evoked By Unpredictable Auditory Stimuli in Man. Electroencephalogr Clin Neurophysiol. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Todd J, Michie PT, Budd TW, Rock D, Jablensky AV. Auditory Sensory Memory in Schizophrenia: Inadequate Trace Formation? Psychiatry Res. 2000;96:99–115. doi: 10.1016/s0165-1781(00)00205-5. [DOI] [PubMed] [Google Scholar]
- Triantafyllou NI, Zalonis I, Kokotis P, Anthracopoulos M, Siafacas A, Malliara S, Hamburger HL, Papageorgiou C. Cognition in Epilepsy: A Multichannel Event Related Potential (P300) Study. Acta Neurol Scand. 1992;86:462–465. doi: 10.1111/j.1600-0404.1992.tb05124.x. [DOI] [PubMed] [Google Scholar]
- Van Roost D, Solymosi L, Schramm J, van Oosterwyck B, Elger CE. Depth Electrode Implantation in the Length Axis of the Hippocampus for the Presurgical Evaluation of Medial Temporal Lobe Epilepsy: A Computed Tomography-Based Stereotactic Insertion Technique and Its Accuracy. Neurosurgery. 1998;43:819–826. doi: 10.1097/00006123-199810000-00058. [DOI] [PubMed] [Google Scholar]
- Verleger R, Lefebre C, Wieschemeyer R, Kompf D. Event-Related Potentials Suggest Slowing of Brain Processes in Generalized Epilepsy and Alterations of Visual Processing in Patients With Partial Seizures. Brain Res Cogn Brain Res. 1997;5:205–219. doi: 10.1016/s0926-6410(96)00071-7. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus As Comparator: Role of the Two Input and Two Output Systems of the Hippocampus in Selection and Registration of Information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]