Abstract
HIV-1–infected patients treated early with combination antiretrovirals respond favorably, but not all maintain viral suppression and improved HIV-specific Th function. To understand if genetic factors contribute to this variation, we prospectively evaluated over 18 months 21 early-treated patients stratified by alleles of class II haplotypes. All seven subjects with the DRB1*13-DQB1*06 haplotype, but only 21% of other subjects, maintained virus suppression at every posttreatment measurement. Following HIV-1 p24 antigen stimulation, PBMCs from patients with this haplotype demonstrated higher mean lymphoproliferation and IFN-γ secretion than did cells from patients with other haplotypes. Two DRB1*13-restricted Gag epitope regions were identified, a promiscuous one that bound its putative restriction element with nanomolar affinity, and another that mapped to a highly conserved region. These findings suggest that class II molecules, particularly the DRB1*13 haplotype, have an important impact on virologic and immunologic responses. The advantage of the haplotype may relate to selection of key HIV-1 Th1 epitopes in highly conserved regions with avid binding to class II molecules. Eliciting responses to the promiscuous epitope region may be beneficial in vaccine strategies.
Introduction
Th cells are crucial in maintaining humoral and cytotoxic immune responses. In HIV-1 infection, the ability to detect HIV-1 Gag-specific CD4+ T cells correlates with reduced plasma viremia, which supports the role of Th cells in immune defense against HIV-1 infection (1). However, in most persons with HIV-1 infection, CD4+ T-cell number is diminished and antigen-specific Th function is impaired (2–4). CD4+ T cells secreting IFN-γ in vitro in response to HIV-1 Gag may be found in HIV-1–infected subjects (5), but the frequency of these cells during chronic HIV-1 infection and prolonged treatment do not approach those recognizing other viral antigens such as cytomegalovirus (CMV). Moreover, detection of HIV-1–specific Th memory cells by lymphoproliferation to HIV-1 antigens in vitro is uncommon (6), except in persons with long-term nonprogressive infection and those receiving potent combination therapy, particularly early in infection (1, 7). Even among early-treated patients, we have noted variability in the magnitude and durability of their HIV-1–specific Th responses, which are primarily directed to Gag epitopes (7). The interval between infection and initiation of treatment and the level of viral suppression are potential factors that may determine the maintenance of Th responses. However, the extent to which these and other factors contribute to the persistence of Th responses have not been delineated.
Host genetic factors influence disease progression, including genetic polymorphisms within the HLA class I and II loci, genes encoding transporters associated with antigen processing (TAPs), and the chemokine receptors that serve as HIV-1 coreceptors (8–15). MHC class II molecules specifically present antigen to CD4+ Th cells, and the genes encoding them are extremely polymorphic, particularly in the peptide-binding groove. This renders them highly specific in the epitopes they bind. In a study of 375 seroconverters aggregated from three cohorts, HLA DRB1*13-DQB1*06 was the only class II haplotype associated with a trend toward increased duration of AIDS-free time (9). In addition, inheritance of DRB1*13 alleles has been associated with long-term survival among children with vertically transmitted HIV-1 infection (11). Thus, the properties of class II molecules, and particularly HLA DRB1*13-DQB1*06, influence HIV-1 disease outcome. However, the role of HLA DRB1*13-DQB1*06 and other class II molecules in shaping virologic and immunologic responses among subjects receiving antiretroviral therapy is not known. We investigated previously the effects of early antiretroviral therapy in patients with newly diagnosed HIV-1 infection. We observed a preponderance of DRB1*13-DQB1*06 among subjects with the most robust and sustained HIV-1–specific Th1 responses. Based on these findings and the established critical role of CD4+ Th function in HIV-1 infection, in this investigation we hypothesized that HLA class II polymorphisms may influence virologic and HIV-specific Th responses in early-treated patients.
Thus, we conducted a prospective longitudinal analysis in 21 patients with early HIV-1 infection receiving combination antiretroviral therapy to investigate the association of DRB1*13-DQB1*06 (and other HLA class II haplotypes present in four or more subjects) with viral suppression and rebound, CD4+ T-cell counts, and HIV-1 Gag-specific T-cell responses. Our findings indicate that maintenance of durable virus suppression and HIV-specific Th response among early-treated patients is linked to inheritance of the DRB1*13-DQB1*06 haplotype and the use of these molecules in HIV-1 antigen presentation.
Methods
Study population.
Twenty-one adults with early HIV-1 infection were recruited and enrolled by clinicians at the University of Washington (UW) Primary HIV Infection Clinic between 1996 and 1998. The UW Human Subject Review Board approved all aspects of the investigation. Details for study eligibility have been reported previously (7). The duration of infection was defined as the time since the onset of signs and/or symptoms, suggestive of acute retroviral syndrome. In the absence of signs and/or symptoms the duration of infection was estimated as the midpoint between previously documented negative enzyme immunoassay (EIA) results within the past 180 days and the recent positive confirmatory test. The subjects received open-label therapy with 800 mg indinavir administered by mouth every 8 hours, 200 mg zidovudine administered by mouth every 8 hours (or stavudine), and 150 mg lamivudine administered by mouth twice a day immediately after diagnosis of infection. They underwent clinical evaluation and venipuncture for virological studies weekly for the first month and then every 4 weeks for 1 year. Anticoagulated blood for immunological studies was obtained one to two times before treatment and then approximately every 1–3 months thereafter.
Eighteen HIV-1–infected patients with long-term nonprogressive disease were recruited through the UW AIDS Vaccine Evaluation Unit and followed at every 3- to 6-month intervals. Subjects were categorized as long-term nonprogressors according to the following criteria: HIV-infected for greater than or equal to 10 years in the absence of antiretroviral therapy, no history of opportunistic infections, two base-line CD4 counts, either both above 600 cells/mm3 or greater than 500 cells/mm3, with an upward sloping trend. Additionally, the CD4 count must never have dropped below 500 cells/mm3.
Virological and T-cell subset analyses.
Plasma HIV-1 RNA was determined by quantitative branched-DNA assay (bDNA; Chiron Corp., Emeryville, California, USA) and ultrasensitive RT-PCR assay (Roche Molecular Systems, Branchburg, New Jersey, USA). Levels were expressed as copies per milliliter, and the lower levels of sensitivity were 500 copies/ml (bDNA assay) and 50 copies/ml (RT-PCR assay) (16). We report viral levels measured by the bDNA assay when there are more than 500 copies/ml and by the ultrasensitive RT-PCR assay when the bDNA assay was less than or equal to 500 copies/ml. Absolute blood CD4+ T-cell count was measured by consensus flow-cytometry methodology (17).
HLA typing.
Class I alleles at the A and B loci and class II alleles at the DRB1 and DQB1 loci were identified by the PCR, using sequence-specific primers (SSP) (Micro SSP DNA Typing Kit; One Lambda Inc., Canoga Park, California, USA). These assays were performed according to the manufacturer’s instructions by the Puget Sound Blood Center Immunogenetics Laboratory (Seattle, Washington, USA).
Lymphocyte proliferation assays.
Lymphocyte proliferation assays (LPA) were performed as described previously (7). In brief, fresh PBMCs suspended in RPMI with 10% heat-inactivated human serum (R-10 HS; Biocell Laboratories Inc., Rancho Dominguez, California, USA), L-glutamine (1 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml), were plated at 105 cells/well in 96-well round-bottomed plates. Quadruplicate wells contained no antigen, 5 μg/ml baculovirus-expressed recombinant HIV-1LAI p24, and 0.15 μg/ml baculovirus control protein (Protein Sciences Inc., Meriden, Connecticut, USA). On day 6, 1 μCi of 3H-thymidine (NEN Life Science Products Inc., Boston, Massachusetts, USA) was delivered to each well, and cells were washed and harvested 18 hours later. Radioactive thymidine incorporation was measured using a Packard TopCount (Packard Instrument Inc., Meriden, Connecticut, USA) and expressed as cpm. The stimulation index (SI) was calculated as the mean cpm of stimulated cultures divided by mean cpm of unstimulated cultures. Responses with both SI > 4.0 and mean cpm > 2,000 were considered positive, based on comparisons in our laboratory with in vitro lymphoproliferation to these proteins by PBMCs from HIV-uninfected controls. Positive responses typically were in the range of 5,000 to 50,000 cpm.
Analysis of cytokine production.
PBMCs were incubated at 2 × 106 cells/ml in 24-well plates in the presence of HIV-1LAI p24 (5 μg/ml) or baculovirus control protein (0.15 μg/ml) (Protein Sciences Inc.). Secretion of IFN-γ in cell-culture supernatants harvested after 6 days was measured with ELISA (Endogen Inc., Woburn, Massachusetts, USA), according to the manufacturer’s instructions. All samples, standards, and controls were run in duplicate, and the lower limit of detection of the assay was 0.4 pg/ml.
Synthetic HIV-1 peptides.
Synthetic 20-mer peptides overlapping by 10 amino acids (aa) and corresponding to gene products from the HIV-1 subtype B (HXB2) were obtained through the NIH AIDS Research and Reference Reagent Program (Bethesda, Maryland, USA). The 10- and 11-mer peptide truncations for fine mapping of epitopes and a panel of 100 overlapping peptides, 15 aa in length and overlapping by 4 aa, spanning the entire HIV-1 p24 (HXB2) for use in the enzyme-linked immunosorbent spot (ELISPOT) assay, were synthesized by Chiron Corp. The peptides were reconstituted at a concentration of 2 mg/ml in 100% DMSO (Sigma Chemical Co., St. Louis, Missouri, USA) and were used at a final concentration of 5 μg/ml unless stated otherwise.
CD4+ T-cell cloning, epitope mapping, and cytotoxic T-lymphocyte activity.
Freshly isolated PBMCs suspended in R-10 HS were incubated at 4 × 106 cells/ml in 24-well plates for 14 days in the presence of 5 μg/ml HIV-1LAI p24. After day 6, the wells were provided with fresh media and recombinant IL-2 (Chiron Corp.) at a final concentration of 10 U/ml twice weekly. On day 14, CD4+ T cells were positively selected using anti-CD4 Ab-coated microbeads (Miltenyi Biotec, Auburn, California, USA). Cells were plated at 0.5 cells/well in round-bottomed 96-well plates with γ−irradiated allogeneic PBMCs (50,000 cells) and Epstein-Barr virus-transformed (EBV-transformed) B-lymphoblastoid cell lines (B-LCL) (10,000 cells) feeders, 30 ng/ml OKT3 (Ortho Diagnostic System, Raritan, New Jersey, USA), and 50 U/ml recombinant IL-2 in a total volume of 200 μl for 2 weeks. Fresh medium and IL-2 were provided on day 7. Cells demonstrating growth after 2 weeks were screened for HIV-1 p24 antigen-specific proliferation in a thymidine-incorporation assay, as described above, and wells containing HIV-1–specific proliferation were expanded into CD4+ T-cell clones (18). Epitope mapping and specific HLA restriction analyses were performed using autologous or HLA-mismatched allogeneic B-LCL pulsed with Gag p24 and overlapping 20-mer peptides as stimulator cells.
Clones were screened for HIV-1–specific cytotoxic T-lymphocyte (CTL) activity using a 51Cr-release assay (7) against autologous EBV-transformed B-LCLs pulsed with the whole protein and the recognized peptide at an effector-to-target (E/T) ratio of 20:1. Results were expressed as percentage of specific lysis, determined as (mean experimental cpm – mean spontaneous cpm)/(mean maximal cpm – mean spontaneous cpm) × 100. Spontaneous lysis was less than 20% of maximal lysis.
Ab blocking of proliferation.
Monoclonal Abs L243, B7/221, and SPV-L3, recognizing HLA-DR, -DP, and -DQ framework determinants, respectively, were used in blocking experiments as described previously (19). The supernatants were generated from hybridoma cell lines secreting L243, B7/221 (American Type Culture Collection, Rockville, Maryland, USA), and SPV-L3 (provided by H. Yssel, DNAX Research Institute, Palo Alto, California, USA) and used at 1:4 final dilution. These concentrations inhibit proliferation of CD4+ T-cell clones restricted at relevant class II loci by greater than 80% and at irrelevant HLA by less than 15%.
Affinity purification of HLA-DR molecules.
Class II molecules were purified by affinity chromatography, essentially as described previously (20), using the mAb LB3.1 coupled to Sepharose CL-4B beads. Lysates were filtered twice through two precolumns of inactivated Sepharose CL-4B and protein A-Sepharose, and then passed over the anti-DR column. The anti-DR column was then washed with 10-column volumes of 10 mM Tris-HCl, pH 8.0, in 1% Nonidet P-40 (NP-40), PBS, 2-column volumes of PBS, and 2-column volumes of PBS containing 0.4% n-octylglucoside. Finally, DR molecules were eluted with 50 mM diethylamine in 0.15 M NaC1 containing 0.4% n-octylglucoside, pH 11.5. A 1/25 volume of 2.0 M Tris, pH 6.8, was added to the eluate to reduce the pH to approximately 8.0. The eluate was then concentrated by centrifugation in Centriprep 30 concentrators (Millipore Corp., Bedford, Massachusetts, USA).
Class II peptide-binding assays.
Peptide-binding assays were performed as described previously (21, 22). Specifically, purified human HLA class II molecules (5–500 nM) were incubated with various unlabeled peptide inhibitors and 1–10 nM 125I-radiolabeled probe peptide for 48 hours in PBS containing 0.05% NP-40 in the presence of a protease-inhibitor cocktail. The final concentrations of protease inhibitors (Calbiochem-Novabiochem International Inc., La Jolla, California, USA) were: 1 mM PMSF, 1.3 nM 1.10 phenanthrolene, 73 μM pepstatin A, 8 mM EDTA, 6 mM N-ethylmaleimide, and 200 μM N alpha-p-tosyl-L-lysine chloromethyl ketone (TLCK). Final detergent concentration in the incubation mixture was 0.05% NP-40. Assays were performed at pH 7.0, except for DRB1*0301 and DRB4*0101, which were performed at pH 4.5 and 5.0, respectively (22). Class II peptide complexes were separated from free peptide by gel filtration on TSK200 columns (TosoHaas 16215; TosoHaas, Montgomeryville, Pennsylvania, USA) and the fraction of bound peptide calculated as described previously (22).
Radiolabeled peptides were iodinated using the chloramine-T method (22). The radiolabeled probe peptides used were HA Y307-319 (sequence YPKYVKQNTLKLAT; DRB1*0101), TT 830-843 (sequence QYIKANSKFIGITE; DRB5*0101, DRB1*1101, DRB1*0701, DRB1*0802, DRB1*0901), MBP Y85-100 (sequence PVVHFFKNIVTPRTPPY; DRB1*1501), MT 65 kDa Y3-13 with Y7 substituted with F (sequence YKTIAFDEEARR; DRB1*0301), a non-natural peptide (peptide 717.01, sequence YARFQSQTTLKQKT; DRB1*0401, DRB1*0405), a non-natural peptide (peptide 717.10, sequence YARFQRQTTLKAAA; DRB4*0101), a naturally processed peptide (sequence EALIHQLKINPYVLS) (21, 23) of unknown origin eluted from a DRB1*1201 + C1R cell line, integrin β3 Y24-37 (sequence YAWASDEALPLGSPR; DRB3*0101) (24), and an S836→A analog of TT 830-843 (sequence QYIKANAKFIGITE) for DRB1*1302.
In preliminary experiments, each DR preparation was titered in the presence of a fixed amount of the appropriate radiolabeled peptide to determine the concentration of class II molecules necessary to bind 10–20% of the total radioactivity. All subsequent inhibition and direct-binding assays were performed using these class II concentrations. Inhibitor peptides were tested typically at concentrations ranging from 120 μg/ml to 1.2 ng/ml in two to four completely independent experiments. Under conditions where [label] < [MHC] and IC50 ≥ [MHC], the measured IC50 values are reasonable approximations of true Kd values. Peptides were classified as binders for each DR molecule for which its binding capacity was less than or equal to 1,000 nM.
IFN-γ ELISPOT assay.
Cryopreserved PBMCs obtained 24 weeks after treatment were incubated in R-10 HS at 4 × 106 cells/ml in 24-well plates for 14 days in the presence of 5 μg/ml HIV-1LAI p24. After day 6, the wells were provided with fresh media and recombinant IL-2 (Chiron Corp.) at a final concentration of 10 U/ml twice weekly. On day 14 cells were plated in 96-well hydrophobic PVDF-backed plates (Millipore Corp.) previously coated with 50 μl of 10 μg/ml anti–IFN-γ mAb (1-D1K, mouse IgG1; Mabtech AB, Nacka, Sweden) overnight at 4°C. Cells were added to the wells at 2 × 104 cells/well in 100 μl of R10 in duplicate. HIV-1–specific peptides and control peptides (pool of 5 irrelevant peptides derived from actin and a highly conserved region of HLA class I α-chain precursor) were added to the wells at a final concentration of 2 μg/ml. Cells stimulated with control peptides served as the positive control. Plates were incubated overnight (16–20 hours) at 37°C in 5% CO2, washed with PBS-T (PBS + 0.05% Tween-20), and incubated at room temperature for 2 hours with biotinylated anti–IFN-γ mAb at 1 μg/ml (7-B6-1, mouse IgG1; Mabtech AB). The avidin-biotinylated enzyme complex (ABC) from the Vectastain ABC Elite Kit (PK-6100;Vector Laboratories, Burlingame, California, USA) was added at room temperature for 1 hour, followed by the Vectastain AEC peroxidase substrate. Spot-forming cells (SFCs) were counted using Immunospot (Cellular Technology Ltd., Cleveland, Ohio, USA) and are expressed as SFCs per 105 input cells. The number of specific IFN-γ–secreting cells was calculated by subtracting the SFCs in the negative control wells. Responses were considered positive if the number of SFCs was greater than or equal to 20 SFCs per 105 cells after subtraction of SFCs in the negative control wells, and twofold those in the negative control wells. An estimate of the overall response to all epitopes within HIV-1 p24 was obtained from the total of SFCs after stimulation with 100 overlapping 15-mer peptides spanning the entire p24 region in four pools of 25 peptides each. Recognition of specific epitopes was identified by using a matrix of ten peptide pools each consisting of ten peptides such that any two pools had only one peptide in common between them.
Statistical methods.
All class II haplotypes present in 4 or more subjects (DRB1*03, 04, 07, 11, and 13) were screened for association with virologic and immunologic responses. The Bonferroni test was used to adjust P values for multiple comparisons. Base-line viral loads and CD4+ T-cell counts were compared using t tests for differences in the means of the log-transformed values. Follow-up data were analyzed, taking into consideration all data points and using generalized estimating equations (GEE) (25, 26) to accommodate possible correlation due to repeated observations from the same individuals. Decay and growth slopes were calculated as the coefficient of the time variable in GEE regression models, and differences between groups were tested based on the significance of interaction of time with the covariate of interest. GEE allow us to make full use of all the longitudinal data collected, rather than just choosing specific time points for cross-sectional analysis. Adjustments for effects of chemokine receptor polymorphisms (Δ-32 mutation and 59029 GG promoter polymorphism) was done in the analysis by including these as adjustment covariates of virologic and immunologic responses. Kaplan-Meier curves were used to describe the distribution of time until loss of viral suppression, and tests for differences in these survival functions were performed using both the log rank test and Fisher exact test.
Results
Study population.
The study population consisted of 21 males (17 Caucasians, two African Americans, and two Hispanics) with early HIV-1 infection who enrolled and maintained follow-up in the open-label treatment trial consisting of indinavir, lamivudine, and zidovudine (or stavudine) for at least 24 weeks. The median duration of follow-up was 78 weeks (range 32–78 weeks). Two patients discontinued the study at 32 and 40 weeks, thus extended follow-up for these patients is not available. The mean age of the study participants was 31 years (range 20–38), and 95% of the patients experienced a symptomatic illness during acute infection. The median duration of infection at study entry was 43 days (range 9–137). The mean base-line plasma HIV-1 RNA was 4.471 log copies/ml and the mean CD4+ T-cell count was 651 cells/mm3.
The frequencies of HLA class II alleles in our study participants were broadly comparable with the allele frequencies in the US Caucasian population (data not shown). Thirty-three percent (7 of 21) of the patients had the HLA DRB1*13-DQB1*06 haplotype, which is higher but not significantly different (P = 0.266) from the reported frequency of 21% among US Caucasians (27). Four of the seven patients inherited the DRB1*1302 allele, two inherited the DRB1*1301 allele, and one co-inherited DRB1*1302 and 1301 alleles.
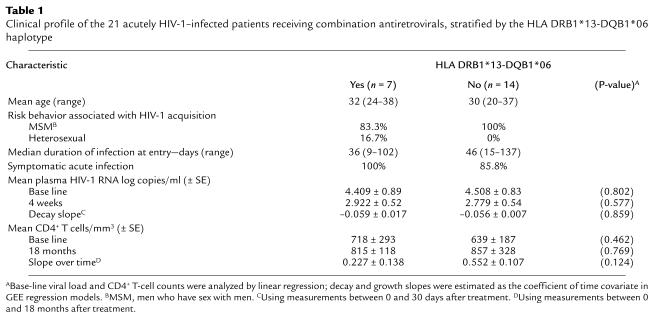
As shown in Table 1, the age, risk behavior, incidence of symptomatic infection, and the median time between acquisition of infection and study entry were comparable between the subjects stratified by the presence or absence of the DRB1*13-DQB1*06 haplotype. Compliance with the treatment regimen was similar among patients in each stratum, as determined by a clinician-administered questionnaire at each visit (data not shown). The initial mean (± SE) log10 plasma HIV-1 RNA levels (copies/ml) measured before treatment were analogous among those with and without HLA DRB1*13-DQB1*06 (4.4 ± 0.89 and 4.5 ± 0.83, respectively; P = 0.802) (Table 1). The initial mean (± SE) CD4+ T-cell counts were 718 (± 293) and 639 (± 187) cells/mm3 among patients with and without HLA DRB1*13-DQB1*06, respectively (P = 0.462) (Table 1).
Table 1.
Clinical profile of the 21 acutely HIV-1–infected patients receiving combination antiretrovirals, stratified by the HLA DRB1*13-DQB1*06 haplotype
Class I alleles associated with slow disease progression, HLA B27 and B57, were uncommon in the study population (n = 0 and n = 1, respectively). It is of note that none of the subjects with the DRB1*13-DQB1*06 haplotype co-inherited these alleles, thus excluding the contributory effect of these alleles to the superior virologic and immunologic responses in these patients. Analysis of inheritance of gene-encoding transporters associated with antigen processing (TAP alleles) that facilitate peptide delivery to class I molecules was beyond the scope of this study but of interest in future studies.
Induction of viral suppression with antiretroviral therapy.
Early treatment led to a reduction in plasma viremia to undetectable levels (<50 HIV-1 RNA copies/ml) in all patients, and this occurred at a median of 20 weeks. The sharpest decay in plasma viremia occurs typically during the first 4 weeks of treatment, and the viral load at the end of this time period may reflect long-term outcome (28). Therefore, to determine if inheritance of the DRB1*13-DQB1*06 was associated with virologic response to therapy, we compared the mean log10 plasma HIV-1 RNA levels (copies/ml) among the groups at the end of 4 weeks of therapy as well as the rate of decay over this time period. As shown in Table 1, there were no significant differences in the plasma viral load at 4 weeks among the DRB1*13-DQB1*06–positive and –negative subjects (P = 0.577). The viral load decay measured over the first 4 weeks of therapy was also comparable among patients stratified by the presence or absence of the DRB1*13-DQB1*06 haplotype (P = 0.859) (Table 1). Similarly, no significant difference in the initial viral load or the plasma viral load decay was observed when patients were stratified by the presence or absence of other class II haplotypes present in four or more subjects (DRB1*03, 04, 07 and 11; data not shown). Thus, the initial viral load and induction of HIV-1 suppression with treatment were not associated with differences in inheritance of these HLA class II haplotypes.
Alterations in CD4+ T-cell counts following antiretroviral therapy.
To determine if inheritance of HLA DRB1*13-DQB1*06 was associated with differences in CD4+ T-cell response to therapy, we stratified patients by inheritance of this haplotype and analyzed alterations in their CD4+ T cell counts over time. The mean CD4+ T-cell slopes using measurements between 0 and 18 months after treatment were comparable among all groups (P = 0.124, stratified by DRB1*13-DQB1*06 haplotype) (Table 1). CD4+ T-cell counts measured over the treatment period rose in all patients, with a mean increase of 0.4 cells/mm3 per day. After 18 months of therapy, no difference was observed in the CD4+ T-cell counts among patients stratified by the DRB1*13-DQB1*06 haplotype (Table 1). Thus, improvement in CD4+ T-cell counts occurred with early therapy and was unrelated to the presence or absence of the DRB1*13-DQB1*06 haplotype. Similarly, improvement in CD4+ T-cell counts was unrelated to inheritance of other class II haplotypes described above (data not shown).
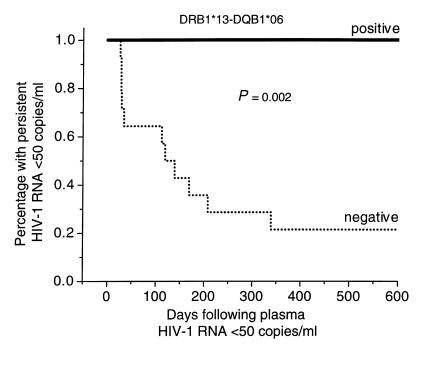
Maintenance of viral suppression during combination antiretroviral therapy.
Since detection of plasma HIV-1 RNA during treatment indicates ongoing viral replication and may predict long-term outcome (29), patients were evaluated for their ability to maintain suppression of plasma viremia (<50 HIV-1 RNA copies/ml) after initial decay (Figure 1). Viral load measurements were obtained once a month during the first year and every 3 months thereafter over the period of study (18 months). These determinations were of comparable frequency among patients stratified by inheritance of the DRB1*13-DQB1*06 haplotype. Eleven patients experienced intermittent low-level plasma viremia (range 51–1200 HIV-1 RNA copies/ml) at a median of two time points (range 1–4) over the course of the study. It is of note that once viral suppression to less than 50 HIV-1 RNA copies/ml was achieved, all seven (100%) subjects with the DRB1*13-DQB1*06 haplotype maintained suppression at all time points for the duration of follow-up (Figure 1). By contrast, only 3 of 14 (21%) without the DRB1*13 haplotype continued to maintain full suppression at 18 months (P = 0.002, log rank test; P = 0.001, Fisher exact test) (Figure 1). Thus, subjects who inherit the DRB1*13-DQB1*06 haplotype appear to have more durable virologic responses to this combination antiretroviral regimen, which occurs independent of both base-line and early posttreatment viral load, and CD4+ T-cell count rises. By contrast, virologic suppression was comparable when patients were stratified by inheritance of DRB1*03, 04, 07, and 11 haplotypes (P = 0.66, 0.09, 1.0, and 0.3, respectively; Fisher exact test).
Figure 1.
Kaplan-Meier curves display the probability of maintaining plasma viremia at less than 50 copies/ml once viral suppression is achieved. Breakthrough viremia is the end point. Patients are stratified by the presence or absence of the DRB1*13-DQB1*06 haplotype.
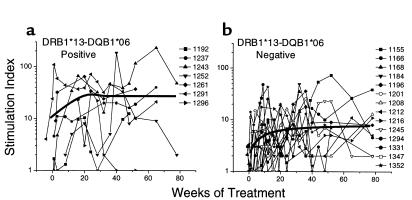
Factors associated with maintenance of HIV-1 Gag-specific T-cell proliferative responses.
Before therapy, HIV-1 p24-specific lymphoproliferative responses were noted in PBMCs from only two patients. However, treatment was associated with induction of detectable HIV-1 p24-specific lymphoproliferative responses in all patients at varying time points (Figure 2). For example, 14 of 18 (77.8%) patients had responses at 12–16 weeks after treatment, and 15 of 19 (78.9%) patients maintained responses at 12–18 months after treatment.
Figure 2.
Lymphoproliferative stimulation indices to HIV-1 p24 antigen after antiretroviral treatment in 21 early HIV-1–infected patients, stratified by the presence (a) or absence (b) of the HLA DRB1*13-DQB1*06 haplotype. Measurements are linearly interpolated between observations. The thin lines indicate individual patient responses, and the heavy line represents the Loess curve denoting the median pathway for the measurements for the groups.
Despite the detection of Gag-specific Th responses, there was a wide variation in the level of these responses. We first hypothesized that persons with consistently stronger responses may be those who initiated therapy very early after acute infection and that those who delayed treatment may have less robust responses. To test this possibility, we assessed the relationship between the posttreatment immune response (using measurements between 50 days and 18 months after treatment, adjusting for days of treatment) and the duration of infection before initiation of treatment (ranging from 9 to 137 days) employing GEE-type regression analysis. The unit change in log p24 SI for each additional day of infection before treatment was 0.0018 ± 0.0015 (P = 0.21) over the duration of follow-up. Thus, the magnitude of the HIV-1 p24-specific proliferative response during the posttreatment period did not depend upon the duration of infection before initiation of therapy.
We considered the possibility that inheritance of the HLA DRB1*13-DQB1*06 haplotype may be associated with the induction of stronger HIV-1 p24-specific proliferative responses. As shown in Figure 2, patients with the DRB1*13-DQB1*06 haplotype (Figure 2a) developed more vigorous and durable responses compared with those without the haplotype (Figure 2b). Using all measurements between 50 days and 18 months after treatment, the mean p24 lymphoproliferative response (SI) was 11.97 in the former group and 4.63 in the latter (P = 0.005) (Table 2). By contrast, the log p24 antigen-specific responses over time were comparable when patients were stratified by the inheritance of DRB1*03, 04, 07, and 11 haplotypes (P = 0.84, 0.87, 0.81, and 0.18).
Table 2.
Lymphoproliferative responses and IFN-γ secretion after p24 antigen stimulation in 21 acutely HIV-1–infected patients receiving combination antiretrovirals, stratified by the HLA DRB1*13-DQB1*06 haplotype
Secretion of IFN-γ by HIV-1 Gag-specific T cells.
To determine if HIV-1 Gag-specific T-cell responses were associated with the secretion of antiviral cytokines, we stimulated PBMCs in vitro with HIV-1 p24 antigen and measured IFN-γ secretion in the culture supernatants. We have shown previously that CD4+ T cells are the predominant cells among PBMCs secreting IFN-γ after stimulation with the recombinant HIV-1 p24 soluble protein (7). The levels of IFN-γ secretion from these cells after p24 antigen stimulation did not correlate with the duration of infection before commencing treatment (coefficient, 0.0078 ± 0.0051; P = 0.126). Thus, the overall magnitude of the Th response measured by proliferation and IFN-γ secretion was not dependent upon the rapidity of treatment initiation.
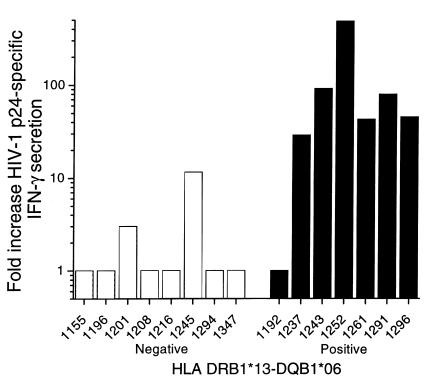
When results were stratified on the basis of inheritance of the DRB1*13-DQB1*06 haplotype, higher levels of IFN-γ secretion were observed in the DRB1*13-DQB1*06–positive subjects (mean of 43.4-fold over background) compared with levels in the DRB1*13-DQB1*06–negative subjects (1.5-fold over background, P < 0.001) (Table 2, Figure 3). The levels ranged from 3.0 to 3,881 pg/ml in the DRB1*13-DQB1*06–positive subjects compared with 0–1703 pg/ml among the DRB1*13-DQB1*06–negative subjects.
Figure 3.
Secretion of IFN-γ by PBMCs stimulated with HIV-1 p24 antigen. The bars represent the ratio of IFN-γ secretion in antigen-stimulated wells to unstimulated control wells in 16 early-treated patients stratified for the presence or absence of the HLA DRB1*13-DQB1*06 haplotype.
Analysis of CD4+ T-cell clones from HLA DRB1*13-DQB1*06–positive subjects: epitope specificity, HLA restriction, cytokine secretion.
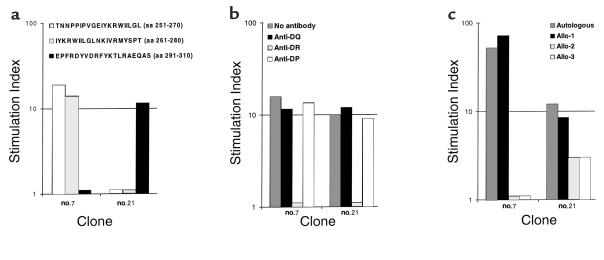
To determine the epitope specificity and HLA restriction of the HIV-1 Gag-specific lymphoproliferative response, we established Gag-specific CD4+ T-cell clones from two subjects with the DRB1*13-DQB1*06 haplotype (patient 1291: DRB1*0701, 1302, and DQB1*02, 06; and patient 1243: DRB1*03011, 1301, and DQB1*02, 06). One of two clones (number 7) from patient 1291 recognized sequential p24 peptides spanning aa 251–270 (TNNPPIPVGEIYKRWIILGL, Gag 26), and aa 261–280 (IYKRWIILGLNKIVRMYSPT, Gag 27), and the remaining clone (number 21) recognized the 20-mer spanning aa 291–310 (EPFRDYVDRFYKTLRAEQAS, Gag 30) (Figure 4a). (Immune response against Gag 30 was further mapped in an ELISPOT assay using PBMCs from patient 1291 to aa 295–309 DYVDRFYKTLRAEQA; data not shown.) Peptide-specific proliferation of both clones was inhibited more than 90% by anti-DR Ab, but not by anti-DP or anti-DQ Abs, indicating HLA DR as the restriction element (Figure 4b). Both clones proliferated when stimulated with peptide-pulsed autologous B-LCL (DRB1*1302, 0701) and partially mismatched B-LCL (DRB1*0101, 1302), but not with other partially mismatched (DRB1*0408, 0701) and completely mismatched B-LCL (DRB1*04, 08) (Figure 4c). These results indicate that recognition of the p24 peptide by the clones was restricted by DR molecules expressed on DRB1*1302 B-LCL. It is of note that these clones were tested for cytolytic activity in a chromium-release assay using autologous B-LCL targets pulsed with the specific peptides (Gag 26, 27, and 30) and HIV-1 p24 protein. Neither clone exhibited significant Gag-specific lysis greater than that against the control targets.
Figure 4.
HIV-1 p24–specific CD4+ T-cell clones from subject 1291 map to epitopes between aa 251–270 (Gag 26) and aa 291–310 (Gag 30). (a) Clone number 7 proliferates when stimulated with sequential peptides spanning HIV-1 p24 amino acids (aa) 251–270 (TNNPPIPVGEIYKRWIILGL) and aa 261–280 (IYKRWIILGLNKIVRMYSPT), but not with HIV-1 p24 peptide spanning aa 291–310 (EPFRDYVDRFYKTLRAEQAS). Clone number 21 proliferates when stimulated with HIV-1 p24 peptide spanning aa 291–310 (EPFRDYVDRFYKTLRAEQAS), but not with HIV-1 p24 peptides spanning aa 251–270 (TNNPPIPVGEIYKRWIILGL) and aa 261–280 (IYKRWIILGLNKIVRMYSPT). (b) Peptide-specific lymphoproliferation of clones 7 and 21 is inhibited by anti-DR but not anti-DQ and anti-DP Ab. (c) Proliferation of clones 7 and 21 is stimulated with peptide-pulsed autologous (DRB1*1302, 0701), partially mismatched allo-1 (DRB1*0101, 1302), but not allo-2 (DRB1*0408, 0701), and completely mismatched allo-3 (DRB1*04, 08) B-LCL.
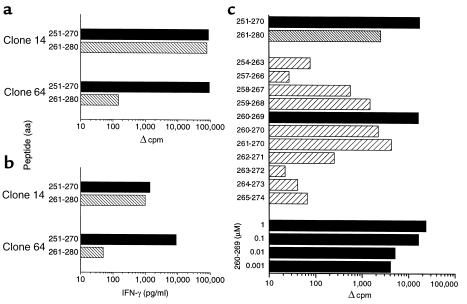
Three CD4+ T-cell clones from patient 1243 also recognized the HIV-1 p24 sequential peptides Gag 26 and Gag 27 (Figure 5a, depicting one representative clone, number 14). Another two clones from patient 1243 recognized the p24 peptide Gag 26, but not Gag 27 (Figure 5a, depicting one representative clone, number 64). The clones secreted high levels of IFN-γ secretion consistent with a Th1 phenotype (Figure 5b). Examining truncated peptides within the aa 251–274 region, peptide spanning aa 260–269 (EIYKRWIILG) stimulated the strongest lymphoproliferation by clone number 14. Moreover, this peptide (aa 260–269) was recognized by the clone at concentration as low as 0.001 μΜ in a dose-response assay using serial tenfold dilutions (Figure 5c). Clone number 64 was mapped to aa 251–265 (TNNPPIPVGEIYKRW) using truncated peptides in an ELISPOT assay (data not shown). Our results suggest that there are T-cell specificities recognizing two distinct epitopes in the region 251–270 (Gag 26) and one in the region 291–310 (Gag 30). The epitopes/epitope regions are: (a) TNNPPIPVGEIYKRW, (b) EIYKRWIILG, and (c) DYVDRFYKTLRAEQA, with an aromatic residue at position 1 (I, I, Y, respectively) and enrichment of polar basic residues at positions 4–6 (G, R, R, respectively).
Figure 5.
HIV-1 p24-specific CD4+ T-cell clones from subject 1243 map to two epitopes between aa 251–270 (Gag 26) and have a Th1 phenotype. (a) Clone number 14 proliferates when stimulated with peptides spanning HIV-1 p24 sequential aa 251–270 (TNNPPIPVGEIYKRWIILGL) and aa 261–280 (IYKRWIILGLNKIVRMYSPT). Clone number 64 proliferates when stimulated with HIV-1 p24 peptide spanning aa 251–270 (TNNPPIPVGEIYKRWIILGL), but not with HIV-1 p24 peptide spanning aa 261–280 (IYKRWIILGLNKIVRMYSPT). Data are expressed as Δ cpm, equal to mean cpm in antigen-stimulated cultures minus the mean cpm in medium alone, which was less than 500 cpm in each case. (b) Clone number 14 secretes high levels of IFN-γ when stimulated with peptides spanning HIV-1 p24 sequential aa 251–270 (TNNPPIPVGEIYKRWIILGL) and aa 261–280 (IYKRWIILGLNKIVRMYSPT). Clone number 64 secretes high levels of IFN-γ when stimulated with HIV-1 p24 peptide spanning aa 251–270 (TNNPPIPVGEIYKRWIILGL), but not with HIV-1 p24 peptide aa 261–280 (IYKRWIILGLNKIVRMYSPT). IFN-γ secretion (picograms per milliliter) is depicted as the amount secreted in antigen-stimulated cultures minus the amount in medium alone, which was less than 50 pg/ml. (c) Clone number 14 maps to epitope spanning aa 260–269. Proliferative responses were measured to 20-mer peptides spanning HIV-1 p24 sequential aa 251–270 and 261–280 and 10-mer peptides spanning aa 254–274 at 1 μM. The 10-mer peptide aa 260–269 was used at tenfold serial dilutions between 1.0 μM and 0.001 μM. Data are expressed as Δ cpm, equal to mean cpm in antigen-stimulated cultures minus the mean cpm in medium alone, which was less than 500 cpm in each case.
HLA DR–binding capacity of the Gag epitope region (aa 251–270).
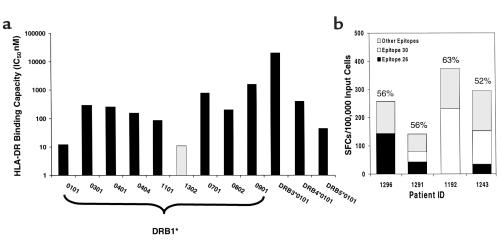
To determine the affinity of Gag 26 (aa 251–270) to class II molecules, the binding capacity of the region was analyzed in detail to various HLA-DR molecules. The peptide bound to ten of the 12 DR molecules tested at an IC50 < 1000 nM (median, 223 nM) (Figure 6a). Moreover, the highest affinity binding (11 nM) was observed to the peptide’s putative restriction element, DRB1*1302. Similar binding properties would be predicted for 1301 as for 1302, since the two molecules differ only at position 86 (glycine to valine change), and this does not appear to impact the binding capacity (30, 31). Thus, the high-affinity binding to DRB1*13 may account for the strong Gag-specific Th responses in patients with the DRB1*13-DQB1*06 haplotype.
Figure 6.
(a) Gag region aa 251–270 (TNNPPIPVGEIYKRWIILGL, Gag 26) containing two DRB1*13–restricted epitopes binds with high affinity to DRB1*1302. The bars represent the binding capacity (IC50 nM) of the peptide for common DRB1, DRB3, DRB4, and DRB5 alleles. (b) Contribution of the responses to epitope regions Gag 26 and Gag 30 in comparison with the total response to all epitopes in HIV-1 p24 in four early-infected subjects. Cells from HIV-1 p24–stimulated cell lines were screened in an ELISPOT assay with four pools of 25 15-mer peptides. The sum of the SFCs in these wells represents the total activity detected to all epitopes in HIV-1 p24. Specific activity against 15-mers corresponding to Gag 26 (aa 251–265, aa 255–269) and Gag 30 (aa 291–305) was determined using a peptide matrix. SFCs are expressed per 100,000 input cells.
We sought to determine the contribution of the recognition of the peptide epitope regions, Gag 26 and Gag 30, to the overall Th response. PBMCs from four early-infected patients with the DRB1*13 haplotype were stimulated with HIV-1 p24 protein for 2 weeks and then tested for IFN-γ secretion by ELISPOT assay for recognition of these epitopes in comparison with the recognition of all other overlapping peptides spanning the p24 region. In all four subjects, responses were detected against one or both the epitope regions (Figure 6b). The sum of the responses to the two epitope regions constituted 52–63% of the total response detected against all overlapping peptides spanning the p24 region (Figure 6b). These findings provide evidence that T cells recognizing these epitope regions constitute the majority of the total response to the p24 region.
HLA DRB1*13 frequency and HIV-1 Gag-specific responses among long-term nonprogressors.
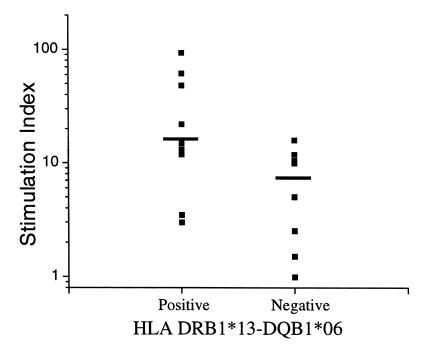
To assess whether subjects with the DRB1*13 haplotype may more effectively control viral replication and have slower disease progression in the absence of antiretroviral therapy, our evaluation was extended to a separate study population of HIV-1–infected long-term nonprogressors (LTNPs). It is of note that 9 of 18 (50%) LTNPs inherited the DRB1*13-DQB1*06 haplotype, a frequency higher than the 21% observed in the general population (P = 0.012). The median duration of follow-up was 18 months (range 2–30 months). Lymphoproliferative assays (median of 3; range 2–7) were performed in 17 patients over the course of follow-up (1 of the 18 subjects discontinued the study after enrollment). When patients were stratified based on inheritance of the DRB1*13 haplotype, the duration of follow-up and the frequency of assays were comparable in both groups. As shown in Figure 7, those with the DRB1*13 haplotype exhibited more vigorous HIV-1 p24-specific lymphoproliferative responses compared with those without the haplotype (SI = 14.15 vs. 3.83, P = 0.0037, comparing log SI of all measurements). These data suggest that the haplotype is associated with superior immunologic responses among subjects who exhibit delayed HIV-1 disease progression in the absence of therapy.
Figure 7.
Lymphoproliferative stimulation indices to HIV-1 p24 region in 17 subjects with long-term nonprogressive disease, stratified by the presence or absence of the DRB1*13 haplotype. Each symbol represents the median of all measurements for an individual. The horizontal line represents the median for the group.
Discussion
Initiation of aggressive antiretroviral treatment during early infection, rather than after disease progression, preserves HIV-1–specific Th responses in conjunction with suppression of plasma viremia (1, 32, 33). Yet it remains unclear how soon treatment must be initiated to achieve this result. Surprisingly, we observed similar rates of plasma virus decay and detection and maintenance of HIV-1–specific Th activities regardless of when treatment was instituted, which ranged from 9 to 137 days from acquisition of HIV-1 infection. This suggests that postponing therapy for a few weeks after diagnosis of acute HIV-1 infection may be less critical in maintaining the repertoire of antigen-specific CD4+ T cells.
While the early virologic responses to therapy were similar, maintenance of suppression of viremia and HIV-specific T-cell help varied over the subsequent months in this study group, and our results indicate that other host factors become important in sustaining these responses. In particular, patients who inherit HLA DRB1*13-DQB1*06 have a greater likelihood of controlling HIV-1 replication, consistently exhibiting less than 50 HIV-1 RNA copies/ml in plasma and maintaining Gag-specific CD4+ Th activities throughout the study period. Most patients instituted therapy before reaching their viral set point, which may explain in part why any association between the inheritance of the DRB1*13-DQB1*06 haplotype and the initial pretreatment viral load was not detected. The differences in virus suppression among patients stratified by inheritance of DRB1*13-DQB1*06 are unlikely to relate to emergence of drug-resistant strains in those lacking this haplotype, since detection of viremia was transient and did not require changes in the treatment regimen (34). Medication adherence and the frequency of viral load measurements were comparable in both groups of patients, hence the difference in virus suppression was not attributable to these factors. In future studies, it will be important to determine if the maintenance of plasma HIV-1 suppression in association with inheritance of the DRB1*13-DQB1*06 haplotype extends to substantial lowering of HIV-1 reservoirs in resting CD4+ T cells and other target cells.
Based on our results, the superior virus suppression in subjects with the DRB1*13-DQB1*06 haplotype is best explained by effective control of viral replication through sustained T-cell help. Despite initiation of therapy early in infection, our patients demonstrated considerable variability in the magnitude and the durability of Gag-specific CD4+ T-cell responses over time. Inheritance of the HLA DRB1*13-DQB1*06 haplotype was the host factor most strongly predictive of these helper activities. After stimulation with HIV-1 p24 antigen, PBMCs from patients with this haplotype demonstrated robust lymphoproliferation and secreted high levels of IFN-γ, suggesting a Th1-type cytokine profile. Thus, based on these findings, we hypothesize that sustained levels of HIV-specific CD4+ T cells in patients inheriting HLA DRB1*13-DQB1*06 may control small bursts of HIV-1 replication, and this may occur through release of antiviral cytokines and maintenance of help for cytotoxic responses. This is consistent with the general paradigm that T-cell help is critical in controlling viral infections, including HIV-1 (18, 35–37).
The association of DRB1*13-DQB1*06 with improved virologic responses and slower disease progression suggests that the molecular interaction of these class II molecules and their cognate HIV-1 epitopes with the CD4+ T-cell receptor (TCR) may be more efficient in stimulating T-cell help, which is supported by the following line of evidence. The Gag 251-270 peptide, recognized by T-cell clones from patients with persistently strong CD4+ T-cell responses, binds with high affinity to its restricting element, DRB1*13. High-affinity binding generally correlates with an increased density of peptide-MHC complexes on the antigen-presenting cell and an increased duration of TCR signaling. These are essential mechanisms for CD40L upregulation, which then preferentially induces Th1 responses (38–41). In addition, high-affinity binding leads to superior immunogenicity, hence eliciting stronger lymphoproliferation and a more robust CD4+ T-cell response. Thus, naive T cells differentiating from the high-affinity interaction in subjects with the DRB1*13-DQB1*06 haplotype may evolve predominantly into CD4+ T cells producing high levels of Th1 cytokines and demonstrating higher p24-specific lymphoproliferative responses. Importantly, the highly avid epitope binding to DRB1*13 may be sufficient to sustain strong Gag-specific T-cell help in the setting of combination antiretroviral therapy, despite lower levels of HIV-1 antigen as a result of markedly reduced viral replication. Hence, the memory immune response may more effectively curtail viral replication in these subjects with the DRB1*13-DQB1*06 haplotype.
High-affinity binding of Gag 251-270 peptide was also observed for DRB1*01 (Figure 6). We predict these patients may also develop robust Th1 responses. In this study, however, the number of patients with this haplotype was too small to examine this hypothesis. It is of note that binding studies were performed with the DRB1*1302 allele, but not with the DRB1*1301 allele, due to lack of availability of purified DRB1*1301 molecules. However, the two alleles differ only by the presence of glycine (*1302) or valine (*1301) at position 86 of the DRβ chain. DRB1*0401 and 0404 also differ from each other by the presence of glycine or valine at position 86 and yet demonstrate similar binding capacity to Gag 251-270 peptide. Thus, our data would predict similar avid binding for the DRB1*1301 allele as observed for the 1302 allele. Also, strong HIV-1–specific proliferation and IFN-γ responses were detected in patients with either DRB1*1301 or DRB1*1302 alleles.
Our hypothesis that persons inheriting DRB1*13-DQB1*06 may more effectively control HIV-1 infection is further supported by findings in other cohort studies. For example, the DRB1*13-DQB1*06 haplotype was found in 50% (9 of 18) of patients with untreated, long-term nonprogressive infection enrolled in our longitudinal study. This frequency is significantly higher than the 21.6% reported previously (27) in the US Caucasian population (P = 0.012). In addition, PBMCs from LTNPs with the DRB1*13 haplotype showed significantly higher p24-specific Th responses (P = 0.0037). The haplotype was recently reported to be the only DRB1-DQB1 haplotype that showed a trend for independent association with protection against development of AIDS (P = 0.07) (9). In a multicenter cross-sectional study, 36.7% of 30 children surviving more than 8 years had one or more of the HLA-DR13 alleles, versus none of 14 rapidly progressing children who died within 2 years (P = 0.009, Haldane RR = 17.1) (11). These data suggest that the haplotype influences disease progression among both treated and untreated patients.
Besides the HLA DRB1*13 haplotype, other class II haplotypes (DRB1*03, 04, 07, and 11) present in four or more subjects were analyzed for an association with the virologic or immunologic response to therapy. However, no significant difference in these responses was observed when patients were stratified by inheritance of these haplotypes. Genes encoding polymorphisms in the CCR5 coreceptor reported to be associated with slower disease progression (CCR5 Δ32 allele or 59029 G/G promoter polymorphism) were also analyzed, but did not account for the observed difference in the virologic and immunologic responses among those with and without the DRB1*13 haplotype. AIDS-free time in HIV-1 infection has also been related to inheritance of numerous class I alleles, alone or in conjunction with transporter protein variants. Since we did not examine polymorphisms in TAP (42), we cannot exclude the influence of these on the virological and immunological responses in our study. However, it is of note that none of the patients with the DRB1*13-DQB1*06 haplotype co-inherited the B27 or the B57 allele. Thus, the protective effects of DRB1*13-DQB1*06 haplotype in our study appeared to be independent of the effect of other HLA class I alleles associated with slow disease progression (43, 44). Alleles associated with rapid disease progression, A24 (10), DRB1*12-DQB1*03 (9), and homozygosity at alleles A and B (12) were present in only small numbers of patients in our group (n = 1–2) and could not be assessed conclusively in our study.
We defined two DRB1*13-restricted epitopes within the aa 251–270, one of which (aa 261–270) overlapped with a 22-mer (aa 263–284) reported previously to be recognized in two LTNPs (1). HLA-restriction data was not reported in that study, therefore it cannot be said conclusively whether the epitope was the same as that defined in our study. It is of note that HLA-binding studies to aa region 251–270 revealed that this area may be promiscuous in its binding to various DRB1, 3, 4, and 5 alleles. The Gag peptide spanning aa 251–270 bound 10 of 12 of the most common DR types worldwide, with binding affinity below the 1,000-nM threshold associated previously with immunogenicity for HLA-DR–restricted epitopes (21). These data suggest that direct immunization of subjects with non-DRB1*13 class II haplotypes with such a highly cross-reactive peptide may focus the immune response on a potentially protective epitope region and overcome inefficient liberation of the epitopic region from the whole protein.
A second DRB1*13 epitope region, aa 291–310, overlapped with previously described epitopes known to evoke in vitro T-cell proliferative responses in HIV-infected donors (45–47). Notably, this region is located in a highly conserved epitope domain (aa 287–309) that has similar amino acid sequences in primary isolates of HIV-1 subtypes A to G (48). It is structurally similar among HIV-1, simian immunodeficiency viruses (SIV), and feline immunodeficiency viruses (FIV) (49). In addition, overlapping epitopes for cytotoxic T cells, helper T cells, and B cells were recently identified in mice immunized with this Gag 23-mer peptide (aa 287–309) (50). These findings lend support for the potential broad utility of priming and boosting T-cell immunity by inserting these DRB1*13-restricting peptides in preventive as well as therapeutic vaccines.
In our study both DRB1*1301 and DRB1*1302 alleles were associated with superior virus suppression and HIV-1 p24–specific Th1 responses. The small patient numbers did not permit separate analysis of these alleles. The two alleles differ only by the presence of glycine (*1302) or valine (*1301) at position 86 of the DRβ chain, and there is a large overlap in the epitopes restricted by these alleles (31). It remains possible that DRB1*13 is a marker for a linked gene that confers protection against HIV-1 disease progression, such as the DQB1*06 alleles or the HLA DRB3*0101 and *0301 with which the DRB1*1301 and 1302 alleles, respectively, are in strong linkage disequilibrium. We believe that this is less likely for two reasons. First, transcription of the DRB3 gene is at least fivefold lower than that of the DRB1 gene; hence, the contribution of the DRB3 gene product to the total expression of cell surface HLA-DR is minor (51). Second, we were unable to define any DQ-restricted Gag epitopes among these patients.
It is notable that DRB1*1301 and 1302 alleles are associated with protection against other infectious diseases. Both alleles protect against persistent HBV infection (52) and against development of cervical carcinoma related to human papilloma virus (53). DRB1*1302 is also associated with protection against severe Plasmodium falciparum malaria in Gambian children (54). All the microorganisms associated with these diseases are complex, with numerous potential epitopes with different MHC restriction elements. Thus, our findings together with these just discussed, suggest that certain class II–restricted responses may be effective in protection against disease caused by these important pathogens and a crucial consideration in HIV-1 vaccine design.
In summary, this study provides evidence that inheritance of the HLA DRB1*13-DQB1*06 haplotype is associated with an improved therapeutic outcome in early-treated patients with HIV-1 infection. The haplotype may exert its protective effects by presenting crucial epitopes with avid class II binding in highly conserved regions of HIV-1 that may be potentially important targets for HIV-1 vaccines. It is likely that the more potent HIV-1–specific cellular responses observed in patients inheriting this haplotype contribute to improved control of the virus and do so even in the absence of therapy. Whether patients with the DRB1*13-DQB1*06 haplotype are suitable candidates for possible withdrawal of therapy remains to be clarified in future studies. We speculate that the strength of the Th1 response in addition to the HLA type may be important in the selection of patients for drug withdrawal and in the maintenance of viral suppression. Most importantly, genetic polymorphisms within the class II locus may play a dominant role in influencing disease progression even among those receiving aggressive antiretroviral therapy during acute infection.
Acknowledgments
We thank G. Nepom and S. Self for helpful discussions and critical comments and N. Russell for advice on the ELISPOT assay; T. Shea and C. Stevens for patient recruitment; D. Brown for technical assistance; D. Hughes and L. Xie for data management; C. Alef and A. Cerna for assistance with preparation of the manuscript; Glaxo-Wellcome and Merck Pharmaceuticals for provision of drugs; and the study participants for their time and effort. This work was supported by NIH grants AI-41535, AI-01411, AI-30731, POICA-76466, and AI-27757.
References
- 1.Rosenberg ES, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997; 278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 2.Shearer G, Payne S, Joseph L, Biddison W. Functional T lymphocyte immune deficiency in a population of homosexual men who do not exhibit symptoms of acquired immune deficiency syndrome. J Clin Invest. 1984; 74:496–506. doi: 10.1172/JCI111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane H, et al. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985; 313:79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- 4.Miedema F, et al. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men: HIV affects the immune system before CD4+ T cell depletion occurs. J Clin Invest. 1988; 82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitcher CJ, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999; 5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 6.Musey LK, et al. Early and persistent human immunodeficiency virus type 1 (HIV-1)-specific T helper dysfunction in blood and lymph nodes following acute HIV-1 infection. J Infect Dis. 1999; 180:278–284. doi: 10.1086/314868. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra U, et al. Effect of combination antiretroviral therapy on T-cell immunity in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000; 181:121–131. doi: 10.1086/315202. [DOI] [PubMed] [Google Scholar]
- 8.Cohen OJ, Kinter A, Fauci AS. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997; 159:31–48. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 9.Keet IP, et al. Consistent associations of HLA class I and II and transporter gene products with progression of human immunodeficiency virus type 1 infection in homosexual men. J Infect Dis. 1999; 180:299–309. doi: 10.1086/314862. [DOI] [PubMed] [Google Scholar]
- 10.Kaslow RA, et al. A1, Cw7, B8, DR3 HLA antigen combination associated with rapid decline of T-helper lymphocytes in HIV-1 infection. A report from the Multicenter AIDS Cohort Study. Lancet. 1990; 335:927–930. doi: 10.1016/0140-6736(90)90995-h. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, et al. Influence of HLA alleles on the rate of progression of vertically transmitted HIV infection in children: association of several HLA-DR13 alleles with long-term survivorship and the potential association of HLA-A*2301 with rapid progression to AIDS. Long-Term Survivor Study. Hum Immunol. 1997; 55:154–162. doi: 10.1016/s0198-8859(97)00092-x. [DOI] [PubMed] [Google Scholar]
- 12.Carrington M, et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999; 283:1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 13.Dean M, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996; 273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996; 2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 15.McDermott DH, et al. CCR5 promoter polymorphism and HIV-1 disease progression. Multicenter AIDS Cohort Study (MACS) Lancet. 1998; 352:866–870. doi: 10.1016/s0140-6736(98)04158-0. [DOI] [PubMed] [Google Scholar]
- 16.Dewar RL, et al. Application of branched DNA signal amplification to monitor human immunodeficiency virus type 1 burden in human plasma. J Infect Dis. 1994; 170:1172–1179. doi: 10.1093/infdis/170.5.1172. [DOI] [PubMed] [Google Scholar]
- 17.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996; 125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 18.Walter EA, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995; 333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 19.Koelle DM, et al. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol. 1994; 68:2803–2810. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demotz S, et al. Self peptide requirement for class II major histocompatibility complex allorecognition. Proc Natl Acad Sci USA. 1991; 88:8730–8734. doi: 10.1073/pnas.88.19.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Southwood S, et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 1998; 160:3363–3373. [PubMed] [Google Scholar]
- 22.Sidney J, et al. The measurement of MHC/peptide interactions by gel infiltration. In Current protocols in immunology. JE Coligan, AM Kruisbeek, DH Margulies, EM Shevach, and W Strober, editors Greene Publishing Associates Inc and John Wiley and Sons Inc New York, New York, USA. 1998;18:3.1–3. 19. [Google Scholar]
- 23.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Pool sequencing of natural HLA-DR, DQ, and DP ligands reveals detailed peptide motifs, constraints of processing, and general rules. Immunogenetics. 1994; 39:230–242. doi: 10.1007/BF00188785. [DOI] [PubMed] [Google Scholar]
- 24.Wu S, Maslanka K, Gorski J. An integrin polymorphism that defines reactivity with alloantibodies generates an anchor for MHC class II peptide binding: a model for unidirectional alloimmune responses. J Immunol. 1997; 158:3221–3226. [PubMed] [Google Scholar]
- 25.Zeger SL, Diggle PJ. Semiparametric models for longitudinal data with application to CD4 cell numbers in HIV seroconverters. Biometrics. 1994; 50:689–699. [PubMed] [Google Scholar]
- 26.Lipsitz SR, Fitzmaurice GM, Orav EJ, Laird NM. Performance of generalized estimating equations in practical situations. Biometrics. 1994; 50:270–278. [PubMed] [Google Scholar]
- 27.Sintasath DM, et al. Relative HLA-DRB1*13 allele frequencies and DRB3 associations of unrelated individuals from five US populations. Hum Immunol. 1999; 60:1001–1010. doi: 10.1016/s0198-8859(99)00085-3. [DOI] [PubMed] [Google Scholar]
- 28.Raboud JM, et al. Suppression of plasma virus load below the detection limit of a human immunodeficiency virus kit is associated with longer virologic response than suppression below the limit of quantitation. J Infect Dis. 1999; 180:1347–1350. doi: 10.1086/314998. [DOI] [PubMed] [Google Scholar]
- 29.Raboud JM, et al. Suppression of plasma viral load below 20 copies/ml is required to achieve a long-term response to therapy. AIDS. 1998; 12:1619–1624. doi: 10.1097/00002030-199813000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Verreck FA, et al. Natural peptides isolated from Gly86/Val86-containing variants of HLA-DR1, -DR11, -DR13, and -DR52. Immunogenetics. 1996; 43:392–397. doi: 10.1007/BF02199809. [DOI] [PubMed] [Google Scholar]
- 31.Davenport MP, et al. Naturally processed peptides from two disease-resistance-associated HLA-DR13 alleles show related sequence motifs and the effects of the dimorphism at position 86 of the HLA-DR beta chain. Proc Natl Acad Sci USA. 1995; 92:6567–6571. doi: 10.1073/pnas.92.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Autran B, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997; 277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 33.Lederman MM, et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998; 178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 34.Grossman Z, et al. Ongoing HIV dissemination during HAART. Nat Med. 1999; 5:1099–1104. doi: 10.1038/13410. [DOI] [PubMed] [Google Scholar]
- 35.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994; 68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994; 68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone MB. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995; 212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 38.Murray JS, Pfeiffer C, Madri J, Bottomly K. Major histocompatibility complex (MHC) control of CD4 T cell subset activation. II. A single peptide induces either humoral or cell-mediated responses in mice of distinct MHC genotype. Eur J Immunol. 1992; 22:559–565. doi: 10.1002/eji.1830220239. [DOI] [PubMed] [Google Scholar]
- 39.Pfeiffer C, et al. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995; 181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schountz T, Kasselman JP, Martinson FA, Brown L, Murray JS. MHC genotype controls the capacity of ligand density to switch T helper (Th)-1/Th-2 priming in vivo. J Immunol. 1996; 157:3893–3901. [PubMed] [Google Scholar]
- 41.Chaturvedi P, Yu Q, Southwood S, Sette A, Singh B. Peptide analogs with different affinites for MHC alter the cytokine profile of T helper cells. Int Immunol. 1996; 8:745–755. doi: 10.1093/intimm/8.5.745. [DOI] [PubMed] [Google Scholar]
- 42.Rozemuller EH, et al. Sequencing-based typing reveals new insight in HLA-DPA1 polymorphism. Tissue Antigens. 1995; 45:57–62. doi: 10.1111/j.1399-0039.1995.tb02415.x. [DOI] [PubMed] [Google Scholar]
- 43.McNeil AJ, et al. Association of HLA types A1-B8-DR3 and B27 with rapid and slow progression of HIV disease. QJM. 1996; 89:177–185. doi: 10.1093/qjmed/89.3.177. [DOI] [PubMed] [Google Scholar]
- 44.Goulder PJ, et al. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res Hum Retroviruses. 1996; 12:1691–1698. doi: 10.1089/aid.1996.12.1691. [DOI] [PubMed] [Google Scholar]
- 45.Wahren B, et al. HIV-1 peptides induce a proliferative response in lymphocytes from infected persons. J Acquir Immune Defic Syndr. 1989; 2:448–456. [PubMed] [Google Scholar]
- 46.Schrier RD, et al. T cell recognition of HIV synthetic peptides in a natural infection. J Immunol. 1989; 142:1166–1176. [PubMed] [Google Scholar]
- 47.Adams SL, Biti RA, Stewart GJ. T-cell response to HIV in natural infection: optimized culture conditions for detecting responses to gag peptides. J Acquir Immune Defic Syndr Hum Retrovirol. 1997; 15:257–263. doi: 10.1097/00042560-199708010-00002. [DOI] [PubMed] [Google Scholar]
- 48.Louwagie J, et al. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993; 7:769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Nishino Y, et al. Major core proteins, p24s, of human, simian, and feline immunodeficiency viruses are partly expressed on the surface of the virus-infected cells. Vaccine. 1992; 10:677–683. doi: 10.1016/0264-410x(92)90089-3. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura Y, et al. A chain section containing epitopes for cytotoxic T, B and helper T cells within a highly conserved region found in the human immunodeficiency virus type 1 Gag protein. Vaccine. 1997; 15:489–496. doi: 10.1016/s0264-410x(96)00224-1. [DOI] [PubMed] [Google Scholar]
- 51.Berdoz J, et al. Constitutive and induced expression of the individual HLA-DR beta and alpha chain loci in different cell types. J Immunol. 1987; 139:1336–1341. [PubMed] [Google Scholar]
- 52.Thursz MR, et al. Association between an MHC class II allele and clearance of hepatitis B virus in the Gambia. N Engl J Med. 1995; 332:1065–1069. doi: 10.1056/NEJM199504203321604. [DOI] [PubMed] [Google Scholar]
- 53.Apple RJ, et al. HLA DR-DQ associations with cervical carcinoma show papillomavirus-type specificity. Nat Genet. 1994; 6:157–162. doi: 10.1038/ng0294-157. [DOI] [PubMed] [Google Scholar]
- 54.Hill AV, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991; 352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]