Abstract
HIV type 1 (HIV-1) not only directly kills infected CD4+ T cells but also induces immunosuppression of uninfected T cells. Two immunosuppressive proteins, interferon α (IFNα) and extracellular Tat, mediate this process because specific antibodies against these proteins prevent generation of suppressor cells in HIV-1-infected peripheral blood mononuclear cell cultures. Furthermore, the production of C-C chemokines in response to immune cell activation, initially enhanced by IFNα and Tat, ultimately is inhibited by these proteins in parallel with their induction of immunosuppression. The clinical corollary is the immunosuppression of uninfected T cells and the decline in C-C chemokine release found at advanced stages of HIV-1 infection paralleling rising levels of IFNα and extracellular Tat. We, therefore, suggest that IFNα and Tat may be critical targets for anti-AIDS strategies.
The progression toward AIDS of HIV type 1 (HIV-1)-infected individuals is marked by a progressive decline of CD4+ T cells caused not only by lysis of infected cells after their activation (1) but also and more significantly by loss of the cellular immune response (2) as evidenced by anergy (3) and apoptosis of uninfected T cells (4). The view of some investigators is that the CD4+ T cell decline is caused chiefly, if not solely, by extensive HIV-1 replication with direct lysis of infected cells followed by a rapid CD4+ T cell replacement (5). Recent findings (6) involving T cell telomere measurement, however, do not support the steady-state interpretation but on the contrary indicate that “the major damage the virus does to the immune system is not distal but proximal in the CD4+ T cell life cycle” (6). This assumption is also favored by a series of experimental results (2–4, 7) that showed that the decline in CD4+ T cells also importantly involves immunosuppression and apoptosis of uninfected T cells, thereby challenging the view of a simple killing of infected T cells by the virus and the rapid replacement of these cells (5).
Immunosuppression of uninfected cells may be initiated in vitro by CD4+ T cell anergy (8), which in HIV-1 infection may be caused by an effect of the HIV-1 gp120 on these cells (7, 9, 10). This effect should occur in vivo when the viral load is high enough to yield locally an amount of free gp120 sufficient to bind to antigen-stimulated, uninfected CD4+ T cells (9). Anergy is associated with dysregulation of cytokines, including lack of interleukin (IL) 2 (11, 12) and IL-12 (13) and overproduction of interferon (IFN) γ (13, 14) and IFNα occurring at an early stage of HIV-1 infection (15). IFNα is produced chiefly by macrophages and dendritic cells (16), and although it may exhibit antiviral effects, it also acts as a cytokine during cell activation; at low concentrations, it induces T cell proliferation (17) and differentiation (18, 19), whereas at higher concentrations it induces an antiproliferative effect (20, 21). In immunodeficient patients, serum levels of IFNα are elevated (22, 23), although the half-life of this cytokine is only a few minutes (24).
Like gp120, the HIV-1 transactivator Tat protein is produced by infected cells and released into the extracellular compartment during acute infection (25, 26). There is now evidence for extracellular Tat involvement in HIV-induced immune suppression. Thus, extracellular Tat may act as a viral toxin on uninfected T cells (27) and trigger immunosuppression (28, 29) and apoptosis of these uninfected cells (30, 31).
Recently, the proinflammatory C-C chemokines were found to exhibit potent anti-HIV-1 effects (32). This discovery and the independent discovery by Feng et al. (33) that chemokine receptors are the second receptor for T-tropic HIV-1 initiated a series of studies to determine the role of these cytokines and their receptor in the prevention or control of HIV-1 infection (34–36).
In the present report, we show that, during acute HIV-1 infection, immunosuppression of uninfected T cells is governed by the cytokine IFNα and by the HIV-1 Tat protein and that the early production of C-C chemokines by immune cells in response to activation depends on IFNα and Tat and markedly declines as T cell immunosuppression progresses in patients at an advanced stage of infection.
MATERIALS AND METHODS
Viral Proteins.
HIV-1 Tat protein was prepared either by DEAE chromatography followed by gel filtration or by solubilization in 6 M guanidine/HCl-containing buffer followed by chromatography on nickel chelate agarose (NTA, Qiagen, Chatsworth, CA). Tat cDNA expression vectors were derived from HTLVIIIB, pCV1. Recombinant gp160 from HIV-1 LAI was a gift from Institut Mérieux (Lyon, France).
Cytokines and Activators.
Recombinant IFNγ and IFNα (IFNα2b) were from Biosidus (Buenos Aires); recombinant IL-2 was from Roussel-UCLAF; phytohemagglutinin (PHA)-P was from Difco; lipopolysaccharide (LPS) and Staphylococcus enterotoxic B (SEB) were from Sigma; tuberculin purified protein derivative (PPD) and tetanus toxoid (TT) were from Institut Mérieux.
Antibodies (Abs).
Sheep and horse anti-IFNα Abs (titers >104) were a gift from C. Chany (Hôpital Saint-Vincent de Paul, Paris). Mouse neutralizing anti-HIV-1 Tat sera (titers >4 × 103) were produced in our laboratory. Control sera from nonimmunized sheep, horse, and mouse as sheep anti-tetanus serum (titer > 500 Lf/ml, where Lf is limit flocculation unit) were a gift from Institut Pasteur (Paris). Anti-p24 monoclonal murine Abs were from Intracell (Cambridge, MA). Murine anti-CD3 Abs were purchased from Ortho Diagnostics.
Virus Stock.
HIV-1 consisted of the supernatant (SN) of a permanent cell culture infected with a laboratory nonsyncytium-inducing virus isolate (strain Z96, reverse transcriptase activity = 800 × 103 cpm/ml) or, in some experiments, the SN of cultures of peripheral blood mononuclear cells (PBMC) from primary isolate (AUD) with normal activated PBMC (reverse transcriptase activity > 300 × 103 cpm/ml). Inactivated HIV-1 was obtained by incubation at 56°C for 2 h.
Cells.
PBMC were isolated on Ficoll/Hypaque from heparinized blood. Monocytes elutriated by counter current from PBMC were used as antigen-presenting cells (APC) or cultured at 2.5 × 106/ml in Teflon Life cell bags (Baxter, Deerfield, IL) at 37°C in Iscove’s modified Dulbecco’s medium containing fetal calf serum (10%) and granulocyte/macrophage colony-stimulating factor (50 ng/ml). Monocytes differentiated into macrophages (CD14+, CD1a−) harvested at day 6. Alternatively, monocytes were cultured in RPMI 1640 medium containing fetal calf serum (10%) and granulocyte/macrophage colony-stimulating factor (50 ng/ml) plus IL-4 (1,000 units/ml) (Roussel-UCLAF). At day 6, these cells showed typical differentiated dendritic morphology (CD14−, CD1a+). Characterization of purified APC was performed by using flow cytometry.
Cell Activation.
PBMCs (106 cell/ml) were activated with PHA-P (3 μg/ml) or anti-CD3 Abs (1/100) in the presence of IL-2 or were stimulated by superantigen SEB (1 μg/ml) in RPMI 1640 medium containing 10% human serum AB. Purified APC (macrophages or dendritic cells; 8 × 104 cells/well) were activated by lipopolysaccharide (100 ng/ml), PHA (3 μg/ml), IFNγ (300 units/ml), PPD/TT (PPD 1,000 units/ml and TT 1,000 Lf/ml) or anti-CD3 Abs (1/100).
Generation of Suppressor Cells by HIV-1 Infection.
Forty eight-hour PHA-activated PBMC were pelleted and incubated at room temperature for 90 min with 1 ml of a 1:10 dilution of virus stock (nonsyncytium-inducing strain Z96 or primary isolate AUD) and washed twice. These cells were cultured for 6 days in medium supplemented with IL-2 (100 units/ml) in the presence of specific (experimental samples) or irrelevant (control samples) Abs. PBMC also were incubated with either 1 ml of medium (uninfected samples), heat-inactivated HIV-1, or HIV-1 gp160 and cultured in similar conditions for 6 days. All of these 6-day cultured cells were irradiated (10,000 rad) before suppressor cell activity assay.
IFNα Treatment.
Recombinant IFNα was added (0.03–15 nM) to the culture medium on APC or PBMC at the initiation of activation.
Tat Pretreatment.
PBMC, macrophages, or dendritic cells were incubated in serum-free medium with (experimental) or without (control) Tat at various concentrations (50–1000 nM) for 2–3 h at 37°C before activation.
T Cell Proliferation.
T cell proliferation was measured by 3H-thymidine incorporation assay. T cell proliferation was assayed: (i) to evaluate immune cell response in SEB-activated PBMC from seronegative individuals cultured for 3 days in the presence of HIV-1 or IFNα or after Tat pretreatment—results were given as direct cpm or percentage of T cell proliferation calculated as cpm in treated cells-to-cpm in nontreated cells × 100; (ii) to test suppressor cell activity in 6-day cultured PBMC—autologous PBMC were stimulated by SEB and cultured for 3 days in the presence of these cells after irradiation (test cultures) (T), reference cultures (R) were SEB-stimulated PBMC, and results were expressed as the ratio of cpm T-to-cpm R × 100; and (iii) to measure immune cell response in 48-h PHA-activated PBMC from seropositive individuals. Results were given as proliferation index = cpm of activated cells-to-cpm of resting cells.
Titration of C-C Chemokines.
Macrophage inflammatory protein (MIP)-1α, MIP-1β, and RANTES (MMR) produced by activated cells were measured by ELISA (kits from R & D Systems) in culture SN of PBMC after 48 h and of APC after 24 h.
Titration of IFNα.
IFNα was assayed in culture SN by the standard biological test by using MDBK cells and vesicular stomatitis virus (37).
RESULTS
Induction of Suppressor T Cells by HIV-1.
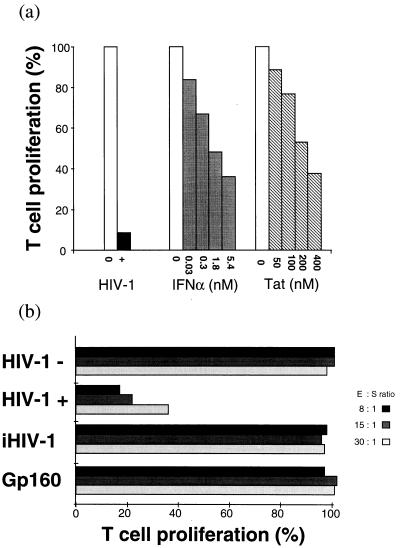
PBMC from seronegative individuals, when activated in the presence of HIV-1, exhibit a marked inhibition of their cellular immune response (Fig. 1a). A similar T cell suppression of antigen-stimulated PBMC may also be induced by IFNα and Tat protein in a dose-dependent manner (Fig. 1a). Furthermore, when the PBMC initially were activated by PHA and then infected by HIV-1, their T cell proliferation was inhibited. In the HIV-1-infected but not in uninfected PBMC cultures, suppressor T cells were generated after 6 ± 1 days. Generation of suppressor cells in these cultures was assessed by demonstrating that T cell proliferation of SEB-stimulated autologous PBMC was inhibited in the presence of the 6-day cultured cells, tested after their irradiation (Fig. 1b). The suppressor activity was dose-dependent and associated with the uninfected CD8+ T cell subpopulation (not shown). It should be noted that: (i) suppressor cells were not generated when activated PBMC were incubated with noninfectious heat-inactivated HIV-1 or with HIV-1 Env gp160 (Fig. 1b) and (ii) the suppressor cell activity was not mediated by contaminating HIV-1 because the culture SN of the washed irradiated test cells did not contain HIV-1 and long term cocultures of irradiated test cells with normal PBMC did not yield viral particles as detected by both reverse transcriptase activity and p24 ELISA in the culture SN.
Figure 1.
HIV-1-induced immunosuppression. (a) Inhibition of T cell response induced by HIV-1, IFNα, or Tat. Fresh PBMC from seronegative donors were SEB-activated and cultured for 3 days in the absence (□) or the presence (▪) of HIV-1 (nonsyncytium-inducing strain Z96) or with varying concentrations of IFNα (IFNα2b, Biosidus). ░⃞, Fresh PBMC were pretreated at 37°C for 2 h in HL-1 medium in the absence (□) or the presence (▧) of varying concentrations of HIV-1 Tat protein. Tat-pretreated PBMC then were cultured for 3 days in a medium containing human AB serum and SEB (Right). T cell proliferation of treated PBMC (T) was compared with that of untreated PBMC (R), and results are expressed as T:R × 100. (b) HIV-1-induced generation of suppressor cells. Preparation of suppressor cells: 48-h PHA-activated PBMC infected by HIV-1 and cultured for 6 days in a medium supplemented with IL-2 (100 units/ml). Cell samples also were incubated with either heat-inactivated HIV-1 (iHIV-1) or HIV-1 gp160 and cultured in similar conditions. The assay for generation of suppressor cells was carried out by culturing for 3 days fresh effector autologous PBMC (E) stimulated by SEB in the presence (experimental samples) or the absence (control samples) of the 6-day cultured cells, tested after irradiation at varying effector/suppressor ratios. The suppressor cell activity was evaluated by T cell proliferation of effector PBMC (E) measured by 3H-thymidine incorporation. T cell proliferation in test cells (T) was compared with that of SEB-stimulated PBMC cultures used as reference cultures (R), and results are expressed as T:R × 100.
Prevention of HIV-1-Induced Suppressor T Cells by Anti-IFNα and Tat Abs.
The generation of suppressor T cells was prevented when HIV-1-infected PBMC were cultured in the presence of anti-IFNα and/or anti-Tat Abs but not that of irrelevant control Abs (Table 1). The inhibition of suppressor cell activity generally was observed when both anti-IFNα and anti-Tat Abs were combined, and it was independent of the PBMC donor (Table 1). In most experiments, the anti-IFNα Abs alone were effective but in some anti-Tat Abs significantly contributed to the block of suppressor cell formation. The different patterns shown in Table 1 may reflect experimental variability in the kinetics of production of the early synthesized Tat protein known to be released after acute HIV infection of T cells (25).
Table 1.
Prevention of HIV-1-induced suppressor cell generation by anti-IFNα and anti-Tat Abs
| Donor | Exp. | Control Abs | T cell
proliferation, %
|
||
|---|---|---|---|---|---|
| Antibodies
to
| |||||
| IFNα | Tat | IFNα + Tat | |||
| A | 1 | 12 | ND | ND | 117 |
| 2 | 25 | 121 | 19 | 104 | |
| 3 | 20 | 90 | 14 | 99 | |
| B | 1 | 8 | 109 | 8 | 74 |
| C | 1 | 26 | 101 | 63 | 101 |
| 2 | 17 | 12 | ND | 85 | |
| 3 | 28 | 26 | 19 | 84 | |
| D | 1 | 59 | 94 | 67 | 84 |
| 2 | 37 | 103 | 6 | 86 | |
| E | 1 | 16 | ND | ND | 100 |
| 2 | 55 | ND | ND | 88 | |
| 3 | 67 | ND | ND | 104 | |
| F | 1 | 25 | 93 | 6 | 88 |
| 2 | 17 | 96 | 16 | 83 | |
| G | 1 | 26 | 24 | 17 | 106 |
| 2 | 11 | 104 | 87 | 107 | |
| 3 | 34 | 114 | 107 | 107 | |
| H | 1 | 35 | ND | ND | 87 |
| 2 | 41 | 122 | 31 | 114 | |
| I | 1 | 26 | 90 | 60 | 98 |
| J | 1 | 28 | 95 | 51 | 91 |
| K | 1 | 10 | 17 | 7 | 118 |
| L | 1 | 9 | ND | ND | 92 |
PHA-activated PBMC from seronegative subjects were infected by HIV-1 and cultured in the presence of specific (experimental samples) or irrelevant (control samples) Abs. To test suppressor activity in these cultures, SEF-stimulated PBMC were cultured in the presence (test cultures) (T) or the absence (reference cultures) (R) of 6-day cultured cells after their irradiation. T cell proliferation of experimental or control test cultures (T) was compared with that of reference cultures (R), and results are expressed as T:R × 100. In these assays, T cell proliferation of SEB-stimulated PBMC cocultured with uninfected cultured cells was identical to that of reference cultures (100%). ND, not done.
Secretion of C-C Chemokines, an Early Effector of the Cellular Response to Activation.
The effects of HIV-1-induced immunosuppression on C-C chemokine production by activated immunocytes next were assessed in this study. In our experience, assays of human sera and plasma for C-C chemokines are unreliable (because C-C chemokines aggregate, bind to carbohydrate moieties present in blood, and are released from contaminating platelets). The production of MIP-1α, MIP-1β, and RANTES by blood-derived cells can, however, be measured in PBMC culture systems. When cultured in monolayers without activation for 24–48 h, differentiated macrophages (38) and dendritic cells (39) of seronegative donors released background levels of chemokines in the culture SN (<1 × 103 pg/ml) as measured by ELISA (Table 2A). After 24-h activation by lipopolysaccharide, PHA, IFNγ, or PPD/TT, these APC monolayers (8 × 104 cells/200-μl well) released large amounts of MIP-1α and MIP-1β, in the range of 10–50 × 103 pg/ml SN but minimal quantities of RANTES (<0.5 × 103 pg/ml; Table 2A). The C-C chemokine production by macrophages was significant by 6 h after activation, peaked at 24 h, and declined markedly afterward (not shown). In the same way, PBMC activated by different stimulants (PHA, PPD, TT, and SEB) released significant amounts of C-C chemokines. In the cohort of seronegative subjects studied, the release of MIP-1α, MIP-1β, and RANTES by 48-h PHA-activated PBMC (2 × 106 pg/ml) varied from 5 to 50 × 103 pg/ml of SN in contrast to resting PBMC, which was <1 × 103 pg/ml. It is noteworthy that: (i) C-C chemokines secretion was an early cellular response, starting 12 ± 6 h after PBMC activation and before T cell proliferation; after it peaked at 48 h, the release declined to basal levels (1–3 × 103 pg/ml); (ii) variation in the release of each of the three chemokines in one individual exhibited the same trend, as if cellular activation induced a coordinated cellular response; and (iii) IFNα production by PBMC roughly paralleled the levels of C-C chemokines (not shown).
Table 2.
C-C chemokine production in APC in response to cell activation
| A Activator |
Macrophages
|
Dendritic
Cells
|
||
|---|---|---|---|---|
| MIP-1α (pg/ml) | MIP-1β (pg/ml) | MIP-1α (pg/ml) | MIP-1β (pg/ml) | |
| None | <1,000 | <1,000 | <1,000 | <1,000 |
| LPS | 12,100 | 17,100 | 16,000 | 19,400 |
| PHA | 31,900 | 24,300 | 22,800 | 23,100 |
| IFN γ | 5,100 | 1,600 | <1,000 | 1,100 |
| PPD/TT | 50,200 | 45,000 | 22,600 | 17,900 |
| B IFNα, nM |
MIP-1α,
pg/ml
|
|||||
|---|---|---|---|---|---|---|
| Macrophages
|
Dendritic cells
|
|||||
| No activation | PHA | LPS | No activation | PHA | LPS | |
| 0 | <1,000 | 16,200 | 38,700 | <1,000 | 11,700 | 9,600 |
| 0.03 | <1,000 | 19,100 | — | <1,000 | 12,500 | — |
| 0.3 | <1,000 | 25,600 | 59,500 | <1,000 | 16,600 | — |
| 3 | <1,000 | 36,400 | 57,600 | 1,000 | 15,100 | 13,600 |
| 15 | <1,000 | 33,300 | 71,900 | 1,300 | — | — |
| C HIV-1-Tat, nM |
MIP-1β, pg/ml | |||||
|---|---|---|---|---|---|---|
| 0 | <1,000 | 11,200 | 12,400 | <1,000 | 7,000 | 16,700 |
| 50 | <1,000 | 20,600 | — | <1,000 | 4,000 | — |
| 500 | 1,400 | 34,000 | 31,100 | 1,200 | 8,500 | 35,500 |
| 1000 | 3,400 | — | 31,600 | 5,500 | 10,000 | 28,300 |
(A) Monolayers of purified macrophages and dendritic cells (8 × 104 in 200 μl/well) were cultured in HL-1 medium containing 3% of human AB serum for 24 h in the presence of various activators. Activators were lipopolysaccharide (LPS) (100 ng/ml); PHA-P DIFCO (3 μg/ml); IFNγ (300 units/ml), and PPD/TT (PPD at 1,000 units/ml/TT at 1,000 Lf/ml). (B) Increasing concentrations of IFNα (recombinant IFNα2b; Biosidus) were added to the culture medium. (C) APC were incubated for 2 h at 37°C in HL-1 medium containing increasing concentrations of HIV-1 Tat protein before activation. Control samples were treated in the same conditions without activator. MIP-1α, MIP-1β, and RANTES production in the culture SN was measured by ELISA, and results were expressed as program per milliliter. For each activation, 3–20 experiments were performed using cells from different seronegative donors. A wide variation of chemokine secretion dependent on donor, mode of activation, kinetics, and culture conditions was found. Results from representative experiments are shown in A–C. RANTES production by APC not exceeding background levels (<1 pg/ml) is not represented.
C-C Chemokines Secretion by PMBC from HIV-1-Infected Individuals.
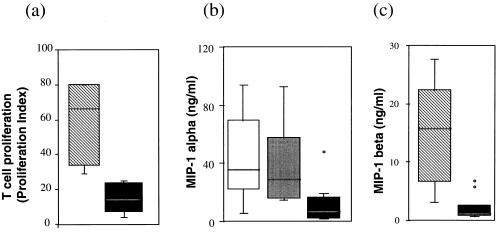
C-C chemokine production by PBMC of infected persons varied according to the stage of HIV-1 infection. The T cells of patients with low CD4+ T cell count (<250 cells/mm3) exhibit a diminished T cell proliferation (Fig. 2a) and a markedly reduced production of MIP-1α (Fig. 2b) compared with asymptomatic seropositive individuals using equivalent numbers of T cells. This latter group corresponds either to patients at an early stage of infection or to nonprogressors, individuals who are HIV-1-positive for at least 8 years, who are still asymptomatic, and who have >500 CD4+ T cells/mm3. The mean and median of MIP-1α production by activated PBMC from patients with low CD4+ T cell count (3 and 7 × 103 pg/ml of SN, respectively) were markedly lower than those of asymptomatic seropositive individuals at early stage of infection (36 and 29.7 × 103 pg/ml, respectively) or nonprogressors (43 and 35 × 103 pg/ml, respectively). As expected, MIP-1β production presented the same pattern as MIP-1α (Fig. 2c). These results clearly show that the inhibition of immune cell activation, characterizing advanced stages of HIV-1 infection, is associated not only with inhibition of T cell proliferation (Fig. 2a) as a measure of immunosuppression and with loss of IL-2 production (11, 12) but also with a decline in chemokine production. The latter result could be anticipated because anti-HIV-1 C-C chemokines production occurred only as an effector response to immune cell activation. These results are not sufficient to conclude that the production of the C-C chemokines is a causative correlate for nonprogression, but they are, nevertheless, clearly consistent with this role.
Figure 2.
Box plot analysis of T cell proliferation (a) MIP-1α (b) and MIP-1β (c) production in PBMC from seropositive individuals. PHA-activated fresh PBMC were cultured for 48 h, and T cell proliferation was evaluated by a 3H-thymidine incorporation test. MIP-1α and MIP-1β were measured in the culture SN by ELISA. Shown are groups of seropositive individuals with: CD4+ T cell counts >500 per mm3 (▧); with CD4+ T cells <250 per mm3 (▪); seropositive at an early stage (░⃞); and nonprogressors (□). Horizontal bars = median and vertical bars = SD. The box limits correspond to the medium 50% percentage of each group.
Effect of IFNα and Tat on C-C Chemokine Secretion.
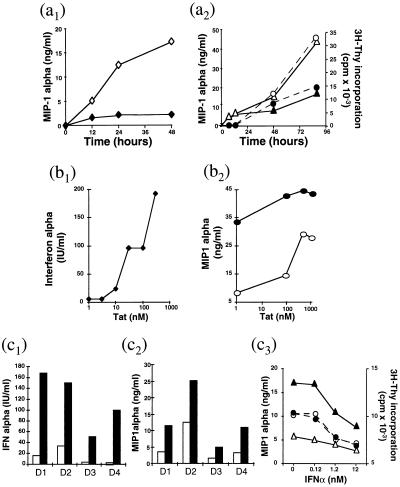
IFNα appears first to act as a costimulus for more C-C chemokine release by activated APC (differentiated macrophages and dendritic cells) because addition of IFNα to the culture medium at concentrations of >0.03 nM significantly increased chemokine production by these cells (Table 2B). Furthermore, chemokine release by PHA-activated PBMC was reduced markedly in the presence of sheep anti-IFNα (neutralizing endogenous release of the cytokine) but not in the presence of irrelevant control Abs (Fig. 3a1). However, activation of PBMC in the presence of exogenous IFNα at higher concentrations, which inhibited T cell proliferation (immunosuppression), reduced chemokine secretion (Fig. 3a2). The inhibition of chemokine production paralleled the lack of T cell response to activation. These effects of exogenous IFNα on PBMC were dose-dependent from 0.1 to 12 nM, and they occurred progressively as a function of time. The dual contribution of IFNα in the physiologic regulation of chemokines production by PBMC, namely, an initial increase but ultimate suppression of chemokine secretion, may correspond to the known biological properties of this cytokine. It is known that IFNα acts as an inducer of immune cell differentiation (18, 19), which we show results in increasing chemokine secretion chiefly from activated T cells and APC. However, at higher concentrations, IFNα is an inhibitor of T cell activation/proliferation (20), thereby ultimately reducing chemokine secretion.
Figure 3.
Effect of IFNα and Tat on the regulation of C-C chemokines production. (a) Effect of IFNα on immune cells. (a1) Kinetics of MIP-1α production by PBMC from a seronegative donor, cultured in the presence of sheep anti-IFNα (—⧫—) or control serum (—⋄—) at 1:200 dilution. (a2) Kinetics of T cell proliferation (—○— —•—) and of MIP-1α (—▵— —▴—) production in PBMC from a representative donor, cultured in the absence (—○— —▵—) or the presence (—•— —▴—) of recombinant IFNα at 10 nM. Each experiment was repeated at least three times. MIP-1α levels were measured by ELISA; cell proliferation by 3H-thymidine incorporation and results were expressed as cpm. (b) Effect of HIV-1 Tat on macrophages. (b1 and b2) Monolayers of purified macrophages were pretreated for 3 h at 37°C in HL-1 medium with varying concentrations of Tat protein. Tat pretreated cells were then cultured in the presence of PHA-P for 24 h. (b1) SN were collected for IFNα titration (41). (b2) Cells were cultured in the presence (—•—) or the absence (—○—) of IFNα (12.5 nM), and SN was collected after 24 h for MIP-1α titration. (c) Effect of Tat on PBMC. PBMC from seronegative donors were pretreated for 3 h with (▪) or without (□) Tat (concentration 200 nM) before activation with anti-CD3 Abs and cultured for 3 days in the presence of IL-2. SN were tested in c1 for IFNα and in c2 for MIP-1α production. (c3) SN of cells pretreated with (—•— —▴—) or without (—○— —▵—) Tat protein were tested for MIP-1α production (—▵— —▴—) and for T cell proliferation (—○— —•—). D1, D2, D3, and D4 = donors 1, 2, 3, and 4.
The HIV-1 Tat protein markedly enhances IFNα secretion by macrophages (Fig. 3b1), and this is associated with a marked increase of IFNα mRNA in the cells as detected by PCR by using IFNα universal and IFNαa4 primers (not shown). Tat also modulates chemokine production by immune cells. MIP-1α and MIP-1β secretion by activated APC is enhanced by pretreatment of the cells with Tat in a dose-dependent manner (50–1000 nM) (Table 2C), and this effect is augmented by IFNα (Fig. 3b2). The Tat effect on C-C chemokine production is mediated partly by IFNα because it is antagonized partially by neutralizing anti-IFN Abs (data not shown). The increased production of IFNα and C-C chemokines also was found with Tat-pretreated activated PBMC (Fig. 3 c1 and c2), and this also was dose-dependent (from 50 to 500 nM). Here again, the Tat effect on C-C chemokine production seemed not to be totally mediated by the increase of IFNα because the effect still was found, although at a reduced level, in PBMC cultured in the presence of specific IFNα neutralizing Abs. In contrast, the chemokine production by Tat-pretreated PBMC was down-regulated when exogenous IFNα was present at concentrations that induce suppressive effects on T cell proliferation (Fig. 3c3).
DISCUSSION AND CONCLUSION
In AIDS, CD4+ cell decline derives not only from direct killing as inferred from mathematical models (5) but chiefly from the loss of immune response of uninfected T cells after activation. In this report, we describe results related to the molecular mechanisms of the HIV-1-induced suppression of the uninfected T cells involving IFNα and Tat and to the consequent decline of anti-HIV-1 C-C chemokines release during infection. We first found that IFNα and extracellular Tat were responsible for the generation of suppressor T cells in HIV-1-infected PBMC (Fig. 1b).
Increasing titers of circulating IFNα are associated with the progressive lack of cellular immune response (immunosuppression) found in HIV-1-infected patients (29). Consistent with this finding are results in both primates and humans. (i) IFNα is elevated in sera of macaques with simian immunodeficiency virus-induced AIDS but not in asymptomatic HIV-1-infected chimps nor in macaques infected with nonpathogenic simian immunodeficiency virus strains (40). (ii) IL-2 secretion is another effector component of the cellular immune response by PBMC, and we have shown previously (11) that the decline in IL-2 secretion from activated T cells derived from AIDS patients is repaired partially in the presence of anti-IFNα Abs (11). (iii) High levels of circulating IFNα in AIDS patients are associated with a poor prognosis (41).
In HIV-1-infected cells, Tat induces T cell hyperactivation (42), which results in viral replication and direct cell lysis (1), whereas in uninfected T cells, extracellular Tat, as a viral toxin (27), contributes to their immunosuppression (Fig. 1a) (28, 29) partly because of its enhancing effect on IFNα production (Fig. 3b1).
C-C chemokines normally are released by immune cells of infected patients as long as these cells respond to immune activation. This is the case in subjects at early stages of infection and in nonprogressors (Fig. 2 b and c). However, release of C-C chemokines declined at late stages of infection when proliferation of uninfected T cells was inhibited. This decline was anticipated by our in vitro data showing that IFNα inhibited the immune response of antigen-stimulated PBMC (Fig. 1a) in the same manner as sera from patients at advanced stages of infection (21). In effect, the other components of the immune response, including IL-2 production (11, 12) and cytotoxic T lymphocyte(s) activity (18, 19), also markedly declined at these late stages. The inhibition of both C-C chemokine release and cytotoxic T lymphocyte activity, two anti-HIV-1-specific effector components of the cellular response, should result in the absence of immune control of infected cells (27) with a consequent increased viral replication (43, 44) and release of Tat (25, 26). Tat released by infected cells should result in an amplification of the immunosuppression of uninfected cells because Tat enhances IFNα production (Fig. 3b) and is immunosuppressive itself (Fig. 1a) (28, 29).
Evolution toward AIDS is preceded by an initial asymptomatic phase in which the CD4+ T cell count is normal or near normal and viral isolates (usually nonsyncytium-inducing ) slowly replicate. At this stage of infection, circulating IFNα is not found (22, 23) and extracellular Tat release is negligible (25, 26). Furthermore, the activated PBMC still exhibit a normal cellular response (Fig. 2) (11, 43, 44). We suggest that the host anti-HIV-1-specific effector weapons, namely, C-C chemokines and cytotoxic T lymphocytes, account for the early control of HIV production and low viral load and should be the primary immune parameters to be induced with an HIV vaccine (27). By contrast, during late stages of HIV infection, there is a dramatic decline of CD4+ T cells and the predominant viral phenotypes are mainly SI. As expected from the high titers of circulating IFNα and the increase in release of extracellular Tat, the cellular immune response of these patients is lacking. As a result, the absence of anti-HIV-1 effector cytotoxic T lymphocyte and of C-C chemokines results in the lack of control of virus replication and immune collapse (27).
We thank A. Astgen, M. Fouchard, and H. Lecoq for their skillful technical assistance and E. Gueorguieva and C. Péchenet for their excellent editorial assistance. This work was supported in part by a grant from SIDACTION (France) and a fellowship from Association de Recherche sur le Sida to A.L. and V.C.
ABBREVIATIONS
- Abs
antibodies
- APC
antigen-presenting cells
- HIV-1
HIV type 1
- IFN
interferon
- IL
interleukin
- MIP
macrophage inflammatory protein
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- PPD
purified protein derivative
- SEB
Staphylococcus enterotoxic B
- SN
supernatant
- Tat
transactivation of transcription
- TT
tetanus toxoid
References
- 1.Zagury D, Bernard J, Leibowitch J, Safai B, Groopman J E, Feldman M, Sarngadharan M G, Gallo R C. Science. 1984;226:449–451. doi: 10.1126/science.6208607. [DOI] [PubMed] [Google Scholar]
- 2.Clerici M, Stocks N I, Zajac R A, Boswell R N, Lucey D R, Via C S, Shearer G M. J Clin Invest. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oyaizu N, Chirmule N, Kalyanaraman V S, Hall W W, Pahwa R, Shuster M, Pahwa S. Proc Natl Acad Sci USA. 1990;87:2379–2383. doi: 10.1073/pnas.87.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ameisen J C, Capron A. Immunol Today. 1991;4:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 5.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Nature (London) 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 6.Wolthers K C, Bea G, Wisman A, Otto S A, de Roda Husman A M, Schaft N, de Wolf F, Goudsmit J, Coutinho R A, van der Zee A G, et al. Science. 1996;274:1543–1547. doi: 10.1126/science.274.5292.1543. [DOI] [PubMed] [Google Scholar]
- 7.Zagury J F, Chams V, Zagury D, Lachgar A, Bizzini B, Burny A, Feldman M. Cell Death Diff. 1995;2:23–32. [PubMed] [Google Scholar]
- 8.Lombardi G, Sidhu S, Batchelor R, Lechler R. Science. 1994;264:1587–1589. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 9.Clayton L K, Siech M, Pious D A, Reinherz E L. Nature (London) 1989;339:548–551. doi: 10.1038/339548a0. [DOI] [PubMed] [Google Scholar]
- 10.Zagury J F, Bernard J, Achour A, Astgen A, Lachgar A, Fall L S, Carelli C, Issing W, M’Bika J P, Picard O, et al. Proc Natl Acad Sci USA. 1993;90:7573–7577. doi: 10.1073/pnas.90.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zagury D, Gagne I, Reveil B, Bernard J, Zagury J F, Saimot A G, Sarin P S, Gallo R C. Lancet. 1985;2:449. doi: 10.1016/s0140-6736(85)92770-9. [DOI] [PubMed] [Google Scholar]
- 12.Clerici M, Lucey D R, Berzofsky J A, Pinto L A, Wynn T A, Blatt S P, Dolan M J, Hendrix C W, Wolf S F, Shearer G M. Science. 1993;262:1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 13.Caruso A, Canaris A D, Licenziati S, Cantalamessa A, Folghera S, Lonati M A, de Panfilis G, Garotta G, Turano A. J Acquir Immune Defic Syndr. 1995;10:462–470. doi: 10.1097/00042560-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Capobianchi M R, Ameglio F, Fei P C, Castilletti C, Mercuri F, Fais S, Dianzani F. AIDS Res Hum Retroviruses. 1993;10:957–962. doi: 10.1089/aid.1993.9.957. [DOI] [PubMed] [Google Scholar]
- 15.Orenstein J M. Lancet. 1983;i:284. [Google Scholar]
- 16.Ferbas J J, Toso J F, Logar A J, Navratil J S, Rinaldo C R., Jr J Immunol. 1994;152:4649–4662. [PubMed] [Google Scholar]
- 17.Tough D F, Borrow P, Sprent J. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 18.Belardelli F, Gresser I. Immunol Today. 1997;17:369–372. doi: 10.1016/0167-5699(96)10027-X. [DOI] [PubMed] [Google Scholar]
- 19.Von Hoegen P, Zawatzky R, Shirrmacher V. Cell Immunol. 1990;126:80–90. doi: 10.1016/0008-8749(90)90302-8. [DOI] [PubMed] [Google Scholar]
- 20.Brouty-Boye D, Cheng Y S E, Chen L B. Cancer Res. 1981;41:4174–4184. [PubMed] [Google Scholar]
- 21.Lachgar A, Bizzini B. Biomed Pharmacother. 1994;48:73–77. doi: 10.1016/0753-3322(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 22.Ambrus J L, Poiesz B J, Lillie M A, Stadler I, Di Berardino L A, Chadha K C. Am J Med. 1989;87:405–407. doi: 10.1016/s0002-9343(89)80822-8. [DOI] [PubMed] [Google Scholar]
- 23.Francis M L, Meltzer M S, Gendelman H E. AIDS Res Hum Retroviruses. 1992;2:199–237. doi: 10.1089/aid.1992.8.199. [DOI] [PubMed] [Google Scholar]
- 24.Ho M. Jpn J Exp Med. 1967;37:169–182. [PubMed] [Google Scholar]
- 25.Ensoli B, Barillari G, Salahuddin S Z, Gallo R C, Wong-Staal F. Nature (London) 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 26.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang H K, Brady J N, Gallo R C. Nature (London) 1994;371:674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- 27.Zagury D. Nat Med. 1997;3:156–157. doi: 10.1038/nm0297-156. [DOI] [PubMed] [Google Scholar]
- 28.Viscidi R P, Mayur K, Lederman H M, Frankel A D. Science. 1989;246:1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- 29.Zagury J F, Chams V, Lachgar A, Carcagno M, Rappaport J, Bizzini B, Burny A. Cell Pharmacol AIDS Sci. 1996;3:123–128. [Google Scholar]
- 30.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 31.Westendrop M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Nature (London) 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 32.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 33.Feng Y, Broder C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 34.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 35.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 36.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 37.Rubinstein S, Familletti P C, Pestka S. J Virol. 1981;37:755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreesen R, Scheibenbogen C, Brugger W, Krause S, Meerpohl H G, Leser H G, Engler H, Lohr G W. Cancer Res. 1990;50:7450–7456. [PubMed] [Google Scholar]
- 39.Sallusto F, Lanzaveechia A. J Exp Med. 1994;179:1109. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tovey M G, Lebon P, Meyer F. J Interferon Res. 1993;12:S62. doi: 10.1089/jir.1994.14.287. [DOI] [PubMed] [Google Scholar]
- 41.Gringeri A, Santagostino E, Cusini M, Muca-Perja M, Marinoni A, Mannucci P M, Burny A, Criscuolo M, Lu W, Andrieru J M, et al. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:55–67. doi: 10.1097/00042560-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Ott M, Emiliani S, VanLint C, Herbein G, Lovett J, Chirmule N, McCloskey T, Pahwa S, Verdin E. Science. 1997;275:1481–1485. doi: 10.1126/science.275.5305.1481. [DOI] [PubMed] [Google Scholar]
- 43.Goulder P J, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, et al. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 44.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, et al. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]