Abstract
CD4 T-cell help is required during the generation and maintenance of effective antitumor CD8 T cell–mediated immunity. The goal of this study was to determine whether HER-2/neu–specific CD8 T-cell immunity could be elicited using HER-2/neu–derived MHC class II “helper” peptides, which contain encompassed HLA-A2–binding motifs. Nineteen HLA-A2 patients with HER-2/neu–overexpressing cancers received a vaccine preparation consisting of putative HER-2/neu helper peptides p369–384, p688–703, and p971–984. Contained within these sequences are the HLA-A2–binding motifs p369–377, p689–697, and p971–979. After vaccination, the mean peptide-specific T-cell precursor frequency to the HLA-A2 peptides increased in the majority of patients. In addition, the peptide-specific T cells were able to lyse tumors. The responses were long-lived and detectable for more than 1 year after the final vaccination in select patients. These results demonstrate that HER-2/neu MHC class II epitopes containing encompassed MHC class I epitopes are able to induce long-lasting HER-2–specific IFN-γ–producing CD8 T cells.
Introduction
The cytolytic CD8 T cell is generally thought to be the major mediator of antitumor immunity. Many tumor antigens have been discovered within the last decade, from which multiple MHC class I–restricted epitopes have been identified (1). One such tumor antigen that is overexpressed on several cancers, including breast and ovarian cancers, is HER-2/neu, the gene product of erbB2/neu proto-oncogene (2–4). Using both predictive and MHC elution techniques, it has been possible to identify several MHC class I–restricted epitopes of HER-2/neu for use in immune-based cancer therapies (5–7).
Antitumor immunization strategies have taken many forms, including the use of whole cell-, peptide-, protein-, and DNA-based vaccines. Peptide-based vaccines are attractive over other forms because peptides are (a) easily constructed, (b) chemically stable, (c) free of contaminating substances such as bacterial pathogens, and (d) devoid of oncogenic potential. Although some successful immunization has been achieved using MHC class I–restricted peptide-based vaccines (8, 9), many studies with native, unmodified peptide resulted in no response or only low-level responses (10–13). For example, Pass and colleagues demonstrated generation of peptide-specific precursors to the gp100209–217 in five of six melanoma patients following immunization (8). In contrast, in parallel studies from the same group it was observed that only two of seven and zero of seven patients had detectable precursors to gp100280–288 or MART-127–35, respectively, after immunization (8). Immunity to MHC class I peptides can be augmented by adding “help” in the form of CD4 T-helper cells. CD4 T-cell responses are essential, both in order to extend the life of the antitumor CD8 T cells and to promote the accumulation of antigen-presenting cells at the tumor site (14).
The necessity of CD4 T-cell help to generate and sustain the MHC class I–restricted CD8 T-cell responses has led to the use of universal, nonspecific MHC class II–restricted epitopes such as PADRE in clinical vaccination trials (15, 16). Although responses to the universal MHC class II–restricted epitopes are typically increased, the responses to the tumor antigen epitopes usually have been limited. We have hypothesized that increased immunogenicity to MHC class I–restricted epitopes may be achieved by immunizing with MHC class II–restricted epitopes derived from the same protein (12, 17).
In this study we evaluated whether active immunization with HER-2/neu helper peptide epitopes, each containing putative HLA-A2 MHC class I epitopes, would generate both CD4 and CD8 T-cell peptide and protein responses in vivo. In addition, we questioned whether HER-2/neu peptide–specific T cells, if they could be elicited, could recognize naturally processed and presented tumors. Finally, we questioned at what level immunity is elicited and how long immunity would last, as the ultimate goal is to generate long-term protective immunity against de novo formation or recurrence of tumor.
Methods
Clinical trial.
Between August 1996 and August 1998, 19 patients with breast or ovarian cancer were enrolled in a phase I HER-2/neu peptide–based vaccine trial approved by the University of Washington’s Human Subjects Division and the United States Food and Drug Administration. Eligibility was dependent upon subjects (a) being diagnosed with stage III/IV breast or ovarian cancer and having been treated for their primary and metastatic disease according to recommended disease-appropriate standards with surgery, chemotherapy, radiation therapy, or combined modality, (b) having a white blood cell count greater than 3.5 dl/ml, (c) showing HER-2/neu protein overexpression in the primary tumor or metastasis, (d) being off immunosuppressive drugs and chemotherapy for at least 30 days before enrolling, and (e) being HLA-A2 positive. Patients were tested for immune competence responsiveness to a minimum of two of seven recall antigens by skin testing with Multitest CMI (Pasteur Merieux Connaught Labs, Institut Merieux, Lyon, France). All patients signed a protocol-specific consent and received monthly vaccinations with three 15–amino acid (15-aa) HER-2/neu–derived peptides, p369–p384, p688–p703, and p971–p984, containing within each the putative HLA-A2–binding motifs p369–p377 (6), p689–p697 (7), and p971–p979 (18). Five hundred micrograms of each peptide (1.5 μg total peptide dose) were solubilized in 10 mM sodium acetate (pH 4.0) and admixed with 125 μg rhuGM-CSF (kindly supplied by Immunex Corp., Seattle, Washington, USA) as an adjuvant. The vaccine preparation was divided into two intradermal injections administered to the same draining lymph node site monthly for 6 months. Subjects underwent peripheral blood draws or a leukapheresis before and 30 days after each vaccination for immunologic monitoring.
Materials.
The following peptides used in this study, either for immunization or in vitro use, were HLA-A2 flu matrix peptide (pFlu), GILGFVFTL (19); HLA-A2 cytomegalovirus (CMV) peptide, NLVPMVATV (20); and HER-2/neu peptides, p369-384, KIFGSLAFLPESFDGDPA (21), p688-703, RRLLQETELVEPLTPS (21), p971-984, ELVSEFSRMARDPQ (21), p369-377, KIFGSLAFL (6), p689-697, RLLQETELV (7), and p971-979, ELVSEFSRM (18). All peptides used for in vitro immunological monitoring were manufactured either by United Biochemical Inc. (Seattle, Washington, USA) or Multiple Peptide Systems Inc. (San Diego, California, USA), and all were greater than 95% pure as assessed by HPLC and mass-spectrometric analysis. Peptides used in vaccine preparations were manufactured by Multiple Peptide Systems (kindly provided by Corixa Corp., Seattle, Washington, USA) and approved for use in humans. Ficoll-Hypaque was purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). RPMI-1640, HBSS, and PBS were purchased from Life Technologies (Rockville, Maryland, USA) and EHAA-120 from Biofluids (Rockville, Maryland, USA). [3H] thymidine and [51Cr] sodium chromate were purchased from NEN Life Science Products Inc. (Boston, Massachusetts, USA), human AB+ serum from Valley Biomedical Inc. (Winchester, Virginia, USA), sterile nitrocellulose-backed microfiltration 96-well plates from Millipore Corp. (Bedford, Massachusetts, USA), and streptavidin-alkaline phosphatase and AP-colorimetric reagents were from Bio-Rad Laboratories Inc. (Hercules, California, USA). Purified anti–IFN-γ (clone number 1-D1K) and biotin-conjugated anti–IFN-γ (clone number 7-B6-1) were purchased from Mabtech AB (Nacka, Sweden). Recombinant HER-2/neu protein domains (intracellular domain [ICD] and extracellular domain [ECD]) were provided by Corixa Corp. HLA testing was performed by the Puget Sound Blood Bank (Seattle, Washington, USA).
Cell lines.
Epstein-Barr virus–transformed (EBV-transformed) lymphoblastoid cells (BLCLs) were produced from PBMCs using culture supernatant from the EBV-producing B95-8 cell line (American Type Culture Collection, Manassas, Virginia, USA). HLA-A2+ BLCLs stably expressing human HER-2/neu were a kind gift from Steve Fling of Corixa Corp. The HER-2/neu–overexpressing cell lines SKOV3 and SKOV3-A2 and BLCLs were maintained in RPMI-1640 with L-glutamine, penicillin, streptomycin, 2-mercaptoethanol, and 10% FCS. The SKOV3-A2 tumor cells are the same as SKOV3 tumor cells, except they are stably transfected with a vector encoding HLA-A2 (22).
Preparation of PBMCs.
PBMCs were obtained either by leukapheresis or 180–250 ml blood draws and isolated by density gradient centrifugation as described previously (21). Cells were analyzed immediately or aliquoted and cryopreserved in liquid nitrogen in freezing media (90% FBS and 10% dimethylsulfoxide) at a cell density of 25–50 × 106 cells/ml.
T-cell proliferation assays.
HER-2/neu–specific T-cell proliferative responses were measured at base line, before each vaccination, and at the end of the study. T-cell proliferation was assessed using a modified limiting dilution assay designed for detecting low-frequency lymphocyte precursors based on Poisson distribution as described previously (21, 23). Data is reported as a stimulation index (SI), which is the mean of 24 experimental wells/mean of 24 no-antigen wells. An age-matched control population of 30 volunteer blood donors was analyzed similarly (data not shown). No volunteer donor had a response to HER-2/neu proteins or peptides. The mean and 3 SDs of the volunteer donor responses to all antigens (SI of 1.98) established a base line, therefore an SI greater than two was considered consistent with an immunized response.
Enzyme-linked immunosorbent spot assay.
A 10-day enzyme-linked immunosorbent spot (ELIspot) assay was used to determine precursor frequencies of peptide-specific CD8 T lymphocytes as described previously (24). Briefly, on day 1, 2.5 ×105 PBMCs/well were plated into 96-well plates in six-well replicates in 200 μl of RPMI-1640 containing L-glutamine, penicillin, streptomycin, and 10% AB serum (T-cell medium) in the presence or absence of 10 μg/ml peptide antigen or 0.5 U/ml tetanus toxoid. The cells were incubated at 37°C at 5% CO2. On day 5, IL-2 was added to 10 U/ml. On day 8, 2.5 × 105/well irradiated autologous PBMCs and 10 μg/ml antigens were added. Also on day 8, nitrocellulose-backed 96-well plates (NC-plates) were coated with 10 μg/ml anti–IFN-γ Ab in PBS at 50 μl/well. On day 9 the NC-plate was washed three times with PBS and blocked for 2 hours with PBS containing 2% BSA, followed by three washes with PBS. On day 9, the cells were gently resuspended, pooled, centrifuged, and the media was replaced. The cells were transferred into the NC-plate in a volume of 100 μl/well in T-cell medium. The NC-plate was incubated at 37°C for a further 20–24 hours followed by washing three times using PBS containing 0.05% Tween-20. The plate was then incubated for 2.5 hours at room temperature in 50 μl/well PBS containing 5 μg/ml biotinylated anti–IFN-γ Ab, washed three times with PBS, and further incubated with 100 μl/well streptavidin-alkaline phosphatase at a dilution of 1:1,000 in PBS for 2 hours at room temperature. After washing three times in PBS, the plate was incubated with 100 μl/well AP-colorimetric substrate for 20–30 minutes, rinsed with cool tap water, and allowed to dry completely. Resultant spots were then enumerated using a dissecting microscope. Precursor frequencies were calculated by subtracting the mean number of spots obtained from the no-antigen control wells from the mean number obtained in the experimental wells. Statistical analysis was performed using the Student’s t test (Microsoft Excel 97; Microsoft Inc., Bellevue, Washington, USA). Precursor frequencies to viral peptide antigens were also enumerated from peripheral blood from four HLA-A2+ healthy, volunteer individuals for comparison purposes. Assay validation was established in preliminary studies using the HLA-A2, pFlu peptide over a PBMC range of 1.0–3.5 × 105 cells and also with the use of IFN-γ–coated polystyrene beads (24). These preliminary studies demonstrated that the assay is linear and precise between 2.0 and 3.5 × 105 PBMCs/well, has a detection limit of 1:100,000, and has a detection efficiency of 93%. The attributes of this assay, such as the limit of detection, are consistent with previously reported ELIspot methods (25, 26). The background number of spots per well, in the absence of antigen, was 10 ± 1 (mean ± SEM, n = 180). A positive response was defined as a precursor frequency that was both significantly (P < 0.05) greater than the mean of control no-antigen wells and detectable (i.e., >1:100,000). Although the ELIspot assay is sensitive and suitable for detecting low-level responses to vaccination (8, 13, 25), it is currently unknown if the calculated precursor frequencies represent actual numbers of antigen-specific cytolytic T cells in the peripheral blood.
Generation of antigen-specific T-cell lines and clones.
Antigen-specific T-cell lines and clones were generated by culturing 25 × 106 PBMCs in T25 tissue-culture flasks in 20 ml of T-cell medium. For the generation of HER-2/neu–specific T-cell lines, PBMCs were cultured in 1 μM each of the HER-2/neu 9-aa peptides, p369–377, p689–697, and p971–979. For generation of p369–377-specific clones, p369–377 peptide was added to the flasks to 1 μm. The flasks were incubated at 37°C and 5% CO2. On day 3 and every other subsequent day, IL-2 was added to 5 U/ml. On day 10, in vitro stimulation (IVS) was performed with peptide-pulsed, irradiated autologous PBMCs. The cultures were further incubated for an additional 10 days with periodic IL-2 administration. After the second IVS, the antigen-specific T-cell lines were examined for cytolytic activity as described below and in some cases were cloned. For cloning, bulk cultures were diluted to achieve approximately 0.3 viable cells/200 μl and plated onto flat-bottom 96-well plates in complete medium. Peptide-pulsed, irradiated autologous PBMCs (2.0 × 105) were added to each well in the presence of 50 U/ml IL-2. The wells were then tested for lytic activity in a [51Cr]-release assay using 50 μl of cells from each well after 14 days. Positive wells were identified as those having specific activity of 5% or greater. The positive wells were transferred to new 96-well plates and subsequently restimulated with peptide-pulsed, irradiated autologous BLCLs. The cultures were eventually expanded and carried using IL-2 and peptide-pulsed, irradiated autologous BLCLs.
[51Cr]-release assays.
Cytolytic activity was measured using standard [51Cr]-release assays. Effector cells were plated into 96-well plates at various effector-to-target (E/T) cell ratios. Targets used were either peptide- or protein-pulsed BLCL or the human HER-2/neu–overexpressing tumor cell lines, SKOV3 and SKOV3-A2. Targets were labeled with 200 μCi 51Cr for 1–2 hours at 37°C. BLCLs were labeled simultaneously with 10 μM peptide. Before mixing with effectors, the targets were washed two times with medium and resuspended to 1,000 targets/100 μl. The reaction was carried out for 4 hours at 37°C, after which the plates were centrifuged and 50 μl of medium from each well was assayed for [51Cr] content in a scintillation counter. The percentage of specific activity was calculated using the following equation: percentage of specific lysis = (sample well release – basal release) / (detergent release – basal release).
Results
Patients with advanced-stage HER-2/neu–overexpressing cancers can be safely immunized with peptide-based vaccines.
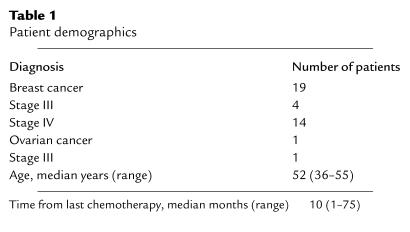
Nineteen subjects were enrolled on trial (Table 1). The median age was 52 years (range 36–55), and the median time from last chemotherapy was 10 months (range 1–75). Fourteen subjects received six vaccinations, two received four vaccinations, two received three vaccinations, and one received one vaccination. Postvaccination data are presented on 18 subjects who received more than one vaccine. At enrollment, 18 subjects had positive-recall antigen testing. The one subject who was anergic received one vaccine. This subject withdrew from the study because of worsening asthma. Toxicity was assessed using the National Cancer Institute Common Toxicity Criteria. Among all 19 subjects, there was one grade 2 skin rash characterized by a mild chronic urticaria that did not require treatment and resolved after completion of the vaccination series. Five subjects were followed a median of 12 months (range 7–17) after completing six vaccines.
Table 1.
Patient demographics
Patients immunized with 15-aa HER-2/neu peptides develop HER-2/neu peptide– and protein–specific T-cell proliferative responses.
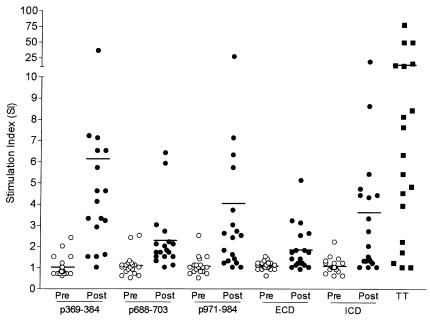
T-cell proliferative responses were measured against 15-aa HER-2/neu peptides and the ECD and ICD proteins before, during, and after the vaccination series. As shown in Figure 1, before immunization proliferative responses were detected to p369–384 in 2 of 19 subjects (mean SI 1.0, range 0.6–2.4), to p688–703 in 2 of 19 subjects (mean SI 1.1, range 0.5–2.5), and to p971–984 in 1 of 19 subjects (mean SI 1.1, range 0.5–2.5).
Figure 1.
Patients immunized with a 15-aa HER-2/neu peptide–based vaccine develop HER-2/neu peptide–specific and protein–specific T-cell proliferation responses. Shown are the preimmunization (open circles) and maximal postimmunization (filled circles) proliferative responses (SI) for the HER-2/neu peptides, p369–384, p688–703, p971–984, and the HER-2/neu protein domains, ECD and ICD. For comparison, the maximal responses to tetanus toxoid (TT) are shown. Each symbol represents a measurement from a single unique subject, calculated from 24 replicates. The solid lines indicate the mean SI for the group.
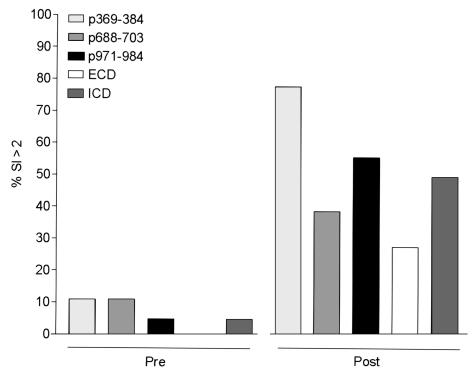
After vaccination, 14 of 18 (83%) subjects had proliferative responses to at least one of the 15-aa HER-2/neu peptides contained within their vaccine formulations (Figure 2). After immunization, proliferative responses were detected to p369–384 in 14 of 18 subjects (mean SI 6.4, range 1.0–35.6), to p688–p703 in 7 of 18 subjects (mean SI 2.4, range 1.0–6.4), and to p971–984 in 10 of 19 subjects (mean SI 4.2, range 1.0–26.1) (Figure 1). The differences in means between the preimmunization responses and the maximal postimmunization responses were significant for all the peptides (p369–384, P = 0.003; p688–703, P = 0.001; p971–984, P = 0.02). Overall, new immunity was generated to p369–384, p688–703, and p971–984 helper peptides in 67%, 33%, and 50% of subjects, respectively (Figure 2). As a comparison, the mean maximal response to tetanus toxoid in the patient population was an SI of 14.8 (range 1.0–76.8) (Figure 1).
Figure 2.
The majority of patients could be immunized to HER-2/neu. Data are shown as the percentage of the population before immunization and after immunization that had a positive proliferation response (SI > 2) to each of the peptides in the vaccine, p369–384 (light gray bar), p688–703 (gray bar), p971–984 (filled bar), as well as to the HER-2 protein domains, ECD (open bar) and ICD (dark gray bar). The mean (range) preimmunization SI of patients considered as having a positive response to p369–384 was 2.4 (one patient only), to p688–703 was 2.5 (2.4–2.5), to p971–984 was 2.5 (one patient only), to ECD (no patients), and to ICD was 2.2 (one patient only). The mean (range) postimmunization SI of patients considered as having a positive response to p369–384 was 7.5 (2.9–35.6), to p688–703 was 3.5 (2.1–6.4), to p971–984 was 6.2 (2.4–26.1), to ECD was 3.3 (2.5–5.1), and to ICD was 6.2 (2.7–18).
Patients immunized with peptides also developed responses to the naturally processed and presented HER-2/neu protein.
As shown in Figure 1, before immunization proliferative responses were detected to the ECD in 0 of 19 subjects (mean SI 1.1, range 0.6–1.5) and to the ICD in 1 of 19 (mean SI 1.1, range 0.6–2.2). After immunization, proliferative responses were detected to ECD in 5 of 18 subjects (mean SI 1.9, range 1.0–5.1) and to ICD in 9 of 18 subjects (mean SI 3.9, range 1–18). The differences in the mean postimmunization responses were significantly higher than the mean preimmunization SIs for both proteins (ECD, P = 0.001; ICD, P = 0.004). Overall, new immunity was generated to ECD protein in 28% and to ICD protein in 50% of subjects.
Patients immunized with 15-aa HER-2/neu peptides increase T-cell precursors to the HLA-A2 9-aa peptide epitopes contained within the longer peptide sequences of the vaccine peptides.
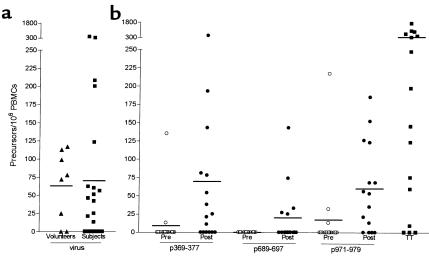
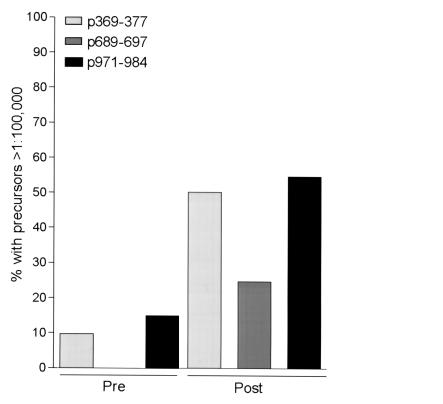
Generation of HER-2/neu T-cell precursors to the 9-aa peptides, p369–377, p689–697, and p971–979, were evaluated in patients using an IFN-γ–based ELIspot (Figure 3). Figure 3a demonstrates that study patients had similar levels of viral-specific T-cell precursors compared with the levels of viral-specific precursors detected in a cohort of HLA-A2 volunteers. Before vaccination, IFN-γ–producing CD8 T-cell responses, defined as HER-2/neu peptide-specific precursors/106 PBMCs, were detectable to p369–377 in 2 of 15 (mean 12, range 0135), to p689–697 in 0/15 (mean 0, range 0-0) and to p971–979 in 3/15 (mean 21, range 0–217) subjects. As shown in Figure 3, after immunization CD8 T-cell responses were detected to p369–377 in 10 of 15 subjects (mean 75, range 0–471), to p688–703 in 5 of 15 subjects (mean 20, range 0–143), and to p971–984 in 12 of 15 subjects (mean 63, range 0–185). Overall, new CD8 T-cell immunity was generated to p369–377 in 62%, to p689–697 in 31%, and to p971–979 in 54% of subjects (Figure 4). The pre- and postimmunization responses to tetanus toxoid and the HLA-A2 peptides from influenza virus and CMV were not significantly changed (P > 0.05) as a result of vaccination with HER-2/neu peptides (data not shown).
Figure 3.
Patients immunized with a 15-aa HER-2/neu vaccine increase T-cell precursors to the encompassed HLA-A2 9-aa peptides. (a) Combined ELIspot responses (precursors/106 PBMCs) to HLA-A2–binding epitopes of influenza and CMV virus are shown. Data are from normal HLA-A2 volunteers (triangles) and study subjects (squares), with the mean delineated by a bar. (b) Preimmunization and maximal postimmunization ELIspot responses (precursors/106 PBMCs) to the HLA-A2 HER-2/neu peptides, p369–377, p689–697, p971–979, in subjects are shown. For comparison, the maximal responses to TT are shown. Each symbol represents a measurement from a single unique subject, calculated on six replicates.
Figure 4.
The majority of patients could be immunized to HLA-A2 HER-2/neu peptides. Data are shown as the percentage of the population before immunization and after immunization that had a detectable ELIspot response to each of the HER-2/neu, HLA-A2 peptides, p369–377 (light gray bar), p689–697 (dark gray bar), p971–979 (filled bar). The mean (range) preimmunization HER-2/neu–specific precursor frequency of patients considered as having a positive response to p369–377 was 125 (32–217, two patients only), to p689–697 (no patients), and to p971–979 was 74 (13–135, two patients only). The mean (range) maximal postimmunization HER-2/neu–specific precursor frequency of patients considered as having a positive response to p369–377 was 111 (11–417), to p689–697 was 60 (25–143), and to p971–979 was 97 (13–185).
Peptide-specific T cells can lyse HLA-matched cells expressing HER-2/neu protein.
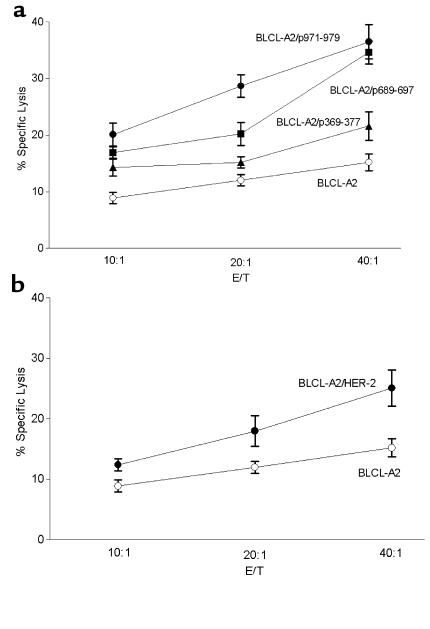
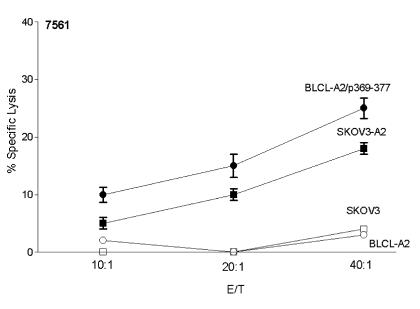
Previous studies of peptide immunization indicate peptide-specific T cells may not have the capacity to lyse tumors (9, 27, 28). Thus, we assessed whether HER-2/neu peptide–specific T cells could lyse HLA-A2, HER-2/neu–expressing cell lines. As an example, Figure 5 demonstrates cytolytic activity against an HLA-A2 BLCLs transfected with HER-2/neu in a breast cancer patient after immunization. Postimmunization precursor frequencies in this representative subject after vaccination were 81, 27, and 56 precursors/106 PBMCs to p369–377, p689–697, and p971–979, respectively. The patient had no pre-existing peptide-specific T-cell precursors before immunization. A representative T-cell line established on this patient demonstrated a 40:1 E/T ratio, 22% lysis to p369–377, 35% lysis to p689–697, and 37% lysis to p971–979 (Figure 5). Furthermore, peptide-specific T cells were able to lyse HLA-A2+ BLCLs expressing HER-2/neu protein (25% at 40:1 E/T). In an additional example, 21 CD8 peptide-specific T-cell clones were generated from an ovarian cancer patient, 0756, after vaccination (29). Shown in Figure 6 is the cytolytic activity of a representative peptide-specific clone against p369–377–loaded HLA-A2 BLCLs (25% at 40:1 E/T) or tumor cells expressing both HER-2/neu and HLA-A2 (18% at 40:1).
Figure 5.
Peptide-specific T cells isolated from a breast cancer patient after immunization can lyse HLA-A2 cells overexpressing HER-2/neu protein. PBMCs from a representative patient, 0107, were examined for cytolytic activity against BLCL-A2 alone (open circles), peptide-loaded A2-BLCLs (filled symbols), or HER-2/neu–expressing BLCL-A2 (open squares) at three different E/T ratios. The peptides (p369–377, p689–697, p971–979) used to pulse the BLCL-A2 were the HLA-A2–binding peptides encompassed in the 15-aa HER-2/neu vaccine. Each point represents the mean of three replicates.
Figure 6.
Peptide-specific T-cell clones isolated from an ovarian cancer patient after immunization can lyse HLA-A2+ tumor cells overexpressing HER-2/neu protein. A p369–377–specific clone was examined for cytolytic activity against BLCL-A2 alone (open circles), p369–377-loaded BLCL-A2 (filled circles), or the HER-2/neu–overexpressing tumor cells, SKOV3 (open squares) and SKOV3-A2 (filled squares). SKOV3-A2 are SKOV3 cells stably expressing HLA-A2. Each point represents the mean of three replicates (± SEM). The absence of errors indicates a standard of the mean less than 1%.
Patients immunized with 15-aa HER-2/neu peptides maintain HER-2/neu immune responses after immunizations have ended.
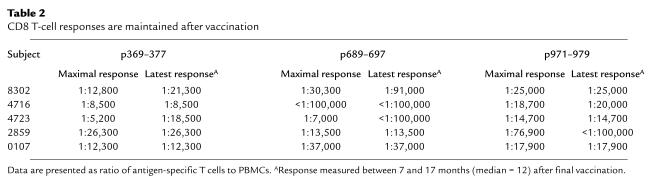
To determine if CD8 T-cell responses were maintained after active immunization, five patients were followed between 7 and 17 months after the end of vaccination. All five of the patients maintained responses to two or more the HLA-A2 9-aa epitopes contained within their vaccine peptides (Table 2). At a median of 12 months after the last vaccination, the mean (n = 5 patients) precursor frequency, expressed as peptide-specific precursors/106 PBMCs, to p369–377 was 68 (range 38–118), to p689–697 was 22 (range 0–74), and to p971-979 was 43 (range 0–68).
Table 2.
CD8 T-cell responses are maintained after vaccination
Discussion
The cytolytic T-cell response is believed to be the critical immune effector arm in mediating potential antitumor immunity. Thus, studies of tumor immunity primarily have focused on the identification of MHC class I epitopes for tumor-associated antigens such as MAGE-1 (30), NY-ESO-1 (31), HER-2/neu (6, 7), tyrosinase (32), and gp-100 (33). In in vitro experiments, these epitopes, when presented in the context of MHC class I, activate CD8 T cells that can directly lyse tumors. Therefore, several clinical trials have been conducted assessing the feasibility and efficacy of cancer vaccination with peptide-based MHC class I peptides to generate tumor-specific CD8 T cells. Several problems have been identified with MHC class I peptide vaccination, including the inability to generate (a) peptide-specific precursors that directly recognize naturally processed antigen, (b) a significant precursor frequency, and (c) long-lasting immunity.
The requirement of CD4 help to initiate and sustain a CD8 response is well established and has led to the development of antitumor vaccines that attempt to induce both T-cell subsets (1, 34). In the absence of defined tumor-antigen MHC class II epitopes needed to activate CD4 T cells, immunization strategies have been employed combining tumor antigen MHC class I epitopes with universally recognized MHC class II epitopes such as PADRE (35) and the promiscuous epitopes of tetanus toxoid (36). Although immunity to the helper epitopes is usually robust, responses to the antigen of interest have been limited. For example, Brander and colleagues reported that inclusion of the promiscuous tetanus epitope, p30, into a vaccine formulation containing an HIV HLA-A2 peptide epitope did not result in immunization to the HIV epitope but significantly reactivated the memory response to the tetanus peptide (37). Similarly, PADRE was unable to significantly induce immunity to two human papilloma virus–derived, HLA-A2 peptides in a phase I clinical trial (16). In contrast to these strategies, providing CD4 help within the same antigenic background has been successfully used to boost CD8 responses (12, 17). In fact, immunizing with an MHC class II epitope that encompasses an MHC class I–binding motif within its natural sequence resulted in effective immunity in a murine lymphocyte choriomeningitis virus model (17). Previous investigations by our group identified putative T-helper epitopes of the HER-2/neu protein that contained HLA-A2 motifs (2). By providing HER-2/neu-specific MHC class II and MHC class I epitopes simultaneously, we hoped to overcome the problems associated by immunizing with MHC class I epitopes alone.
The peptide-specific T cells that were generated in vivo in the present study were able to lyse tumor cells. The inability of peptide-specific T cells generated by vaccination to directly recognize naturally processed and presented antigen has been reported for some MHC class I epitopes, including those derived from HER-2/neu (9) and MART-1 (28). Peptide-specific T cells that do not respond to endogenous antigen may be detected after immunization if target cells (e.g., tumor cells) do not naturally process or present the epitope in sufficient quantities to stimulate recognition. Alternatively, immunization with excessive quantities of class I–restricted peptide may result in the generation of peptide-specific T cells that are of low affinity and would not be activated by the level of naturally occurring peptide in tumor cells. Vaccinating with longer peptides encompassing class I motifs, as in the present study, may allow processing of the peptide and presentation in class I MHC at levels that more closely mimic those present on tumors.
The majority of patients in the current study generated viral-like levels of HER-2/neu–specific CD8 T-cell precursors. Immunization with MHC class I cancer vaccines often has resulted in undetectable or low-level immune responses. For example, Pass and colleagues reported that only two of seven (29%) melanoma patients vaccinated with the gp100 HLA-A2–binding motif, g208, developed detectable peptide-specific precursors (8). In a parallel study, they also observed no detectable response in another cohort vaccinated against the HLA-A2 MART-127–35 epitope (8). Our success at generating high levels of peptide-specific precursors to HER-2/neu HLA-A2 peptides was most likely due to the patient population selected, having either low-level or nondetectable disease and an excellent performance status. Scheibenbogen and colleagues have demonstrated that the presence of antigen-specific immune reactivity in melanoma patients can be correlated with disease being in remission (38). Our goal, like that in infectious disease, is to develop vaccination strategies that prevent disease rather then treat disease. The inability of vaccines to eradicate actively growing tumors has been clearly shown in animal models (39). In our study 86% of patients generated increased frequencies of HER-2/neu-specific CD8 T cells. The mean HER-2/neu (all peptides) precursor frequency of 49/106 PBMCs was very similar and within one SD to the mean viral (flu and CMV) precursor frequency of 65/106 PBMCs. Furthermore, the levels of viral precursors measured in the present study are consistent with those observed by Scheibenbogen in a cohort of melanoma and noncancer-bearing patients (38). These findings together raise the important question as to whether levels of cancer immunity that are similar to viral immunity would be sufficient to protect against cancer relapse. This question can be answered only in the context of clinical studies designed to correlate level of immunity generated after immunization with protection from cancer relapse or development of disease.
Our vaccination strategy also resulted in persistent peptide-specific T-cell precursors. Over the past decade, immunization with CD8 T cell–inducing epitopes was associated with only short-lived responses (40). However, strategies are being developed to lengthen the duration of the response. It is likely that our strategy resulted in long-lived CD8 T-cell responses due to the concurrent activation of CD4 T cells. Both murine antiviral and antitumor models have clearly established the important role of CD4 T cells in maintaining a persistent CD8 T cell response (14, 34, 41). Using a different strategy, Stewart and Rosenberg have found that gp100-specific CD8 T-cell responses are long-lived in melanoma patients immunized with a modified gp100 MHC class I–binding peptide (42).
In summary, the primary use of cancer vaccines is most likely to prevent, rather than eradicate, malignancy. In this study immunization of cancer patients against HER-2/neu was demonstrated with a peptide-based vaccine consisting of helper T-cell epitopes, each containing an HLA-A2 motif. Active immunization resulted in the generation of both CD4 and CD8 T-cell immunity. The resulting peptide-specific T-cell precursors recognized naturally processed HER-2/neu protein and the immunity was long-lived.
Acknowledgments
We would like to express our gratitude to the patients who participated in this study and to the oncologists who referred their patients to us. This work is supported for K.L. Knutson by a fellowship from the Department of Defense Breast Cancer Program and for M.L. Disis by grants from the NIH, the National Cancer Institute (R01 CA75163), and the Department of Defense Breast Cancer Program. Patient care was conducted through the Clinical Research Center Facility at the University of Washington, which is supported through the NIH grant MO1-RR-00037. We are grateful for the expert nursing care and protocol coordination by Lynne Fitzsimmons and Donna Davis.
References
- 1.Wang RF, Rosenberg SA. Human tumor antigens for cancer vaccine development. Immunol Rev. 1999; 170:85–100. doi: 10.1111/j.1600-065x.1999.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 2.Disis ML, Cheever MA. HER-2/neu oncogenic protein: issues in vaccine development. Crit Rev Immunol. 1998; 18:37–45. doi: 10.1615/critrevimmunol.v18.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Gusterson BA, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992; 10:1049–1056. doi: 10.1200/JCO.1992.10.7.1049. [DOI] [PubMed] [Google Scholar]
- 5.Disis ML, Smith JW, Murphy AE, Chen W, Cheever MA. In vitro generation of human cytolytic T-cells specific for peptides derived from the HER-2/neu protooncogene protein. Cancer Res. 1994; 54:1071–1076. [PubMed] [Google Scholar]
- 6.Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995; 181:2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peoples GE, et al. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci USA. 1995; 92:432–436. doi: 10.1073/pnas.92.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pass HA, Schwarz SL, Wunderlich JR, Rosenberg SA. Immunization of patients with melanoma peptide vaccines: immunologic assessment using the ELISPOT assay. Cancer J Sci Am. 1998; 4:316–323. [PubMed] [Google Scholar]
- 9.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369-377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998; 58:4902–4908. [PubMed] [Google Scholar]
- 10.Rosenberg SA, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998; 4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeger E, et al. Generation of cytotoxic T-cell responses with synthetic melanoma-associated peptides in vivo: implications for tumor vaccines with melanoma-associated antigens. Int J Cancer. 1996; 66:162–169. doi: 10.1002/(SICI)1097-0215(19960410)66:2<162::AID-IJC4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Widmann C, Romero P, Maryanski JL, Corradin G, Valmori D. T helper epitopes enhance the cytotoxic response of mice immunized with MHC class I-restricted malaria peptides. J Immunol Methods. 1992; 155:95–99. doi: 10.1016/0022-1759(92)90275-x. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JJ, et al. Evaluation of CD8(+) T-cell frequencies by the Elispot assay in healthy individuals and in patients with metastatic melanoma immunized with tyrosinase peptide. Int J Cancer. 2000; 87:391–398. doi: 10.1002/1097-0215(20000801)87:3<391::aid-ijc13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Hung K, et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998; 188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber JS, et al. A phase I trial of an HLA-A1 restricted MAGE-3 epitope peptide with incomplete Freund’s adjuvant in patients with resected high-risk melanoma. J Immunother. 1999; 22:431–440. doi: 10.1097/00002371-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Ressing ME, et al. Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma. J Immunother. 2000; 23:255–266. doi: 10.1097/00002371-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Fayolle C, Deriaud E, Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol. 1991; 147:4069–4073. [PubMed] [Google Scholar]
- 18.Ioannides CG, et al. Cytotoxic T cells isolated from ovarian malignant ascites recognize a peptide derived from the HER-2/neu proto-oncogene. Cell Immunol. 1993; 151:225–234. doi: 10.1006/cimm.1993.1233. [DOI] [PubMed] [Google Scholar]
- 19.Sauma SY, et al. Recognition by HLA-A2-restricted cytotoxic T lymphocytes of endogenously generated and exogenously provided synthetic peptide analogues of the influenza A virus matrix protein. Hum Immunol. 1993; 37:252–258. doi: 10.1016/0198-8859(93)90508-x. [DOI] [PubMed] [Google Scholar]
- 20.Engstrand M, et al. Characterization of CMVpp65-specific CD8+ T lymphocytes using MHC tetramers in kidney transplant patients and healthy participants. Transplantation. 2000; 69:2243–2250. doi: 10.1097/00007890-200006150-00005. [DOI] [PubMed] [Google Scholar]
- 21.Disis ML, Grabstein KH, Sleath PR, Cheever MA. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res. 1999; 5:1289–1297. [PubMed] [Google Scholar]
- 22.Disis ML, et al. Immunity to the HER-2/neu oncogenic protein. Ciba Found Symp. 1994; 187:198–207. doi: 10.1002/9780470514672.ch13. [DOI] [PubMed] [Google Scholar]
- 23.Reece JC, Geysen HM, Rodda SJ. Mapping the major human T helper epitopes of tetanus toxin. The emerging picture. J Immunol. 1993; 151:6175–6184. [PubMed] [Google Scholar]
- 24.McCutcheon M, et al. A sensitive ELISPOT assay to detect low-frequency human T lymphocytes. J Immunol Methods. 1997; 210:149–166. doi: 10.1016/s0022-1759(97)00182-8. [DOI] [PubMed] [Google Scholar]
- 25.Schmittel A, Keilholz U, Scheibenbogen C. Evaluation of the interferon-gamma ELISPOT-assay for quantification of peptide specific T lymphocytes from peripheral blood. J Immunol Methods. 1997; 210:167–174. doi: 10.1016/s0022-1759(97)00184-1. [DOI] [PubMed] [Google Scholar]
- 26.Asai T, Storkus WJ, Whiteside TL. Evaluation of the modified ELISPOT assay for gamma interferon production in cancer patients receiving antitumor vaccines. Clin Diagn Lab Immunol. 1999; 7:145–154. doi: 10.1128/cdli.7.2.145-154.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawashima I, et al. The multi-epitope approach for immunotherapy for cancer: identification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors. Hum Immunol. 1998; 59:1–14. doi: 10.1016/s0198-8859(97)00255-3. [DOI] [PubMed] [Google Scholar]
- 28.Rivoltini L, et al. Induction of tumor-reactive CTL from peripheral blood and tumor-infiltrating lymphocytes of melanoma patients by in vitro stimulation with an immunodominant peptide of the human melanoma antigen MART-1. J Immunol. 1995; 154:2257–2265. [PubMed] [Google Scholar]
- 29.Knutson KL, Crosby P, Disis ML. Isolation of HER-2/neu (HER2) specific T cell clones from a cancer patient following immunization with a HER2 peptide vaccine. Proceedings of the American Association for Cancer Research. 1999; 40:310. (Abstr.) [Google Scholar]
- 30.Traversari C, et al. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992; 176:1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jager E, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998; 187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfel T, et al. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994; 24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 33.Kawakami Y, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995; 154:3961–3968. [PubMed] [Google Scholar]
- 34.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998; 188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander J, et al. The optimization of helper T lymphocyte (HTL) function in vaccine development. Immunol Res. 1998; 18:79–92. doi: 10.1007/BF02788751. [DOI] [PubMed] [Google Scholar]
- 36.Panina-Bordignon P, et al. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989; 19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 37.Brander C, Corradin G, Hasler T, Pichler WJ. Peptide immunization in humans: a combined CD8+/CD4+ T cell-targeted vaccine restimulates the memory CD4 T cell response but fails to induce cytotoxic T lymphocytes (CTL) Clin Exp Immunol. 1996; 105:18–25. doi: 10.1046/j.1365-2249.1996.d01-725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheibenbogen C, et al. Analysis of the T cell response to tumor and viral peptide antigens by an IFNgamma-ELISPOT assay. Int J Cancer. 1997; 71:932–936. doi: 10.1002/(sici)1097-0215(19970611)71:6<932::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Cheever MA, Chen W. Therapy with cultured T cells: principles revisited. Immunol Rev. 1997; 157:177–194. doi: 10.1111/j.1600-065x.1997.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 40.Nehete PN, Arlinghaus RB, Sastry KJ. Use of helper T cell-inducing peptides from conserved regions in HIV-1 env in a noncovalent mixture with a CTL-inducing V3-loop peptide for in vivo induction of long-lasting systemic CTL response. Viral Immunol. 1994; 7:189–197. doi: 10.1089/vim.1994.7.189. [DOI] [PubMed] [Google Scholar]
- 41.Greenberg PD, Cheever MA, Fefer A. Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1+2-lymphocytes. J Exp Med. 1981; 154:952–963. doi: 10.1084/jem.154.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart JH, Rosenberg SA. Long-term survival of anti-tumor lymphocytes generated by vaccination of patients with melanoma with a peptide vaccine. J Immunother. 2000; 23:401–404. doi: 10.1097/00002371-200007000-00002. [DOI] [PubMed] [Google Scholar]