Abstract
Background
In cigarette smokers, the most commonly-reported areas of brain activation during visual cigarette cue exposure are: the prefrontal, anterior cingulate, and visual cortices. We sought to determine changes in brain activity in response to cigarette cues when smokers actively resist craving.
Methods
Forty-two tobacco dependent smokers underwent functional magnetic resonance imaging, during which they were presented with videotaped cues. Three cue presentation conditions were tested: cigarette cues with subjects allowing themselves to crave (cigarette cue crave), cigarette cues with the instruction to resist craving (cigarette cue resist), and matched neutral cues.
Results
Activation was found in the cigarette cue resist (compared to the cigarette cue crave) condition in the left dorsal anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and precuneus. Lower MR signal for the cigarette cue resist condition was found in the cuneus bilaterally, left lateral occipital gyrus, and right post-central gyrus. These relative activations and deactivations were more robust when the cigarette cue resist condition was compared to the neutral cue condition.
Conclusions
Suppressing craving during cigarette cue exposure involves activation of limbic (and related) brain regions and deactivation of primary sensory and motor cortices.
Keywords: Nicotine Dependence, functional magnetic resonance imaging, cue-induced cigarette craving, cingulate cortex, visual cortex
Introduction
Cigarette craving is associated with relapse in smokers attempting to maintain abstinence (Killen and Fortmann 1997; Niaura et al 1989; Swan et al 1996; Catley et al 2000). In tobacco-dependent smokers, craving increases naturally over the minutes and hours following the last cigarette (Schuh and Stitzer 1995; Jarvik et al 2000), and can be elicited readily in the laboratory using cigarette-related cues (Carter and Tiffany 1999; Due et al 2002; Brody et al 2002; McClernon et al 2005). The neurobiology of both craving (Nestler 2002; Weiss 2005) and the ability to resist craving (Modell and Mountz 1992; Stormark et al 1995) are growing areas of interest, because medications that inhibit craving or enhance the ability to resist craving may prove useful for the treatment of tobacco dependence (a condition with a relatively low long-term treatment response rate (Fiore et al 2000; Jorenby et al 1999)).
Several research groups have examined regional brain activation associated with presentation of cigarette-related cues, and correlations between brain activity and cigarette craving. The most commonly-reported areas of activation during presentation of visual cigarette-related (compared to neutral) cues are: the medial (Lee et al 2005; McClernon et al 2005) and lateral (Due et al 2002; Lee et al 2005) frontal cortex, anterior cingulate cortex (ACC) (Lee et al 2005; Smolka et al 2005; Wilson et al 2005; Due et al 2002; Brody et al 2002; McClernon et al 2005), precuneus (Lee et al 2005), cuneus (Smolka et al 2005; Wilson et al 2005), and occipital cortex (Wilson et al 2005; Lee et al 2005). The most commonly-reported regions found to have positive correlations between activity and cigarette craving are: the lateral prefrontal cortex (PFC) (McClernon et al 2005; Brody et al 2002) and ACC (McClernon et al 2005; Zubieta et al 2005). Recently, researchers have begun to use cues and functional brain imaging to examine situations that mimic more complex real life smoking situations (e.g., (Wilson et al 2005)).
In addition to functional imaging studies of cue-induced craving, studies of cognitive reappraisal and cognitive modulation of emotion provide insight into brain mechanisms of resisting the urge to smoke, since these functions may be elicited during exposure to cues with craving resistance. Prior research has demonstrated both activation of the dorsal ACC (Kalisch et al 2006; Ray et al 2005; Ochsner et al 2004; Phillips et al 2003), PFC (Ray et al 2005; Pessoa et al 2002) and other brain regions associated with emotion (Ray et al 2005; Ochsner et al 2004; Pessoa et al 2002) and deactivation of the visual system (Pessoa et al 2002) during active reappraisal and modulation of emotional responses to cues. Mental effort has also been linked to activation of the dorsal ACC (Paus et al 1998). Based on these studies and the imaging studies cited above, we hypothesized that the dorsal ACC, PFC, and related regions would be activated and that the visual system would be deactivated when smokers resist craving during cigarette-related cue exposure.
In the present study, we used functional magnetic resonance imaging (fMRI) to examine brain function in smokers facing a situation that they commonly encounter, especially if they are trying to quit smoking, namely resisting the urge to smoke while being exposed to cigarette-related cues.
Method
Subjects
Forty-two otherwise healthy smokers (> 15 cigarettes/day), who met DSM-IV criteria for Nicotine Dependence, were recruited through local newspaper and internet advertisements. Subjects underwent telephone and in-person screening. For the telephone screening, medical, psychiatric, and substance abuse histories were obtained without personal identifiers. In-person screening was performed by a primary study investigator (A.L.B. or R.E.O.), and included screening questions from the Structured Clinical Interview for DSM-IV (SCID) (First et al 1995), and administration of the Smoker’s Profile, Fagerström Test for Nicotine Dependence (FTND) (Fagerström 1978; Heatherton et al 1991), Urge to Smoke (UTS) Scale (Jarvik et al 2000; Brody et al 2002), and Hamilton Depression (HAM-D) (Hamilton 1967) and Anxiety (HAM-A) (Hamilton 1969) rating scales. Exhaled carbon monoxide (CO) levels were measured at the time of initial screening to verify recent smoking (an exhaled CO of > 8 ppm) using a MicroSmokerlyzer (Bedfont Scientific Ltd, Kent, UK). Smokers in this study were treatment-seeking, and received standardized treatment following this fMRI protocol with practical group counseling, bupropion HCl, or placebo.
Exclusion criteria included: (1) history of any Axis I psychiatric diagnosis other than Nicotine Dependence as determined by screening questions from the SCID, (2) medical conditions that might affect brain function, (3) current use of medications that could alter brain function, and (4) pregnancy (because of the medication trial following this fMRI study). Subjects with recreational alcohol (≤ 1 drink per day), drug (< one use per week), or caffeine (≤ two cups of coffee per day or the equivalent) use not meeting criteria for abuse/dependence were allowed to participate, but were instructed to abstain for 24 h prior to scanning (72 h for marijuana, verified by urine toxicology screen, if subjects reported illicit drug use within the past year).
fMRI Procedure
Within one week of the in-person screening, subjects underwent fMRI scanning. They were instructed to smoke their usual morning cigarette(s) prior to testing which began at 7AM. At that time, subjects were interviewed to ensure that they had smoked that morning, and smoking was verified with an exhaled CO measurement in the same manner as described above. Scanning began at 7:15AM and the data of interest were obtained about 10 min later, so that these tobacco-dependent participants would be abstinent for 25 mins, and would be expected to have some craving (Schuh and Stitzer 1995), but would still be responsive to cigarette-related cues.
Functional scanning was performed with a 1.5 T Magnetom Sonata scanner (Siemens AG, Erlangen, Germany) with echo–planar imaging capability, using a gradient echo, echo–planar acquisition sequence in which the repetition time was 2.5 sec, echo time was 45 msec, flip angle was 80 degrees, matrix image was 128 by 64, field of view was 40 by 20 cm, and in-plane resolution was 3 mm. Sixteen slices, each 4 mm thick, with a 1-mm gap between slices were obtained every 2.5 sec for 45 sec while the subjects were exposed to each cigarette-related and neutral cue, and during control (resting state with a blank screen) periods. High-resolution spin–echo echo-planar scans (128 by 256 matrix; in-plane resolution, 1.5 mm; repetition time, 4000 msec; echo time, 54 msec; four excitations), acquired in the same plane as the functional scans, were acquired with bandwidth matched to that of the functional studies. The spatial distortions of the functional and high resolution spin echo EPI scans were held in common in order to facilitate the subsequent spatial normalization procedure.
Cue Presentation and Craving Monitoring
Videotaped cues were developed based on work by our group (Brody et al 2002; Conklin and Tiffany 2001) and others. Twenty-seven video cues with different scenarios (eighteen cigarette and nine neutral) were used for this study. These cues were filmed from the first person point of view and were 45 sec in length. They were intended to present generic situations and were filmed with a professional actor and actress. Cigarette cue videos presented smoking in a variety of situations (e.g., writing a letter, standing outside of a building, driving), with the first 10 to 15 sec of each video being preparation to smoke (taking out a cigarette and lighter and preparing to light the cigarette) and the remainder of the video being actual cigarette smoking (with the burn of the cigarette upon being lit and exhaled cigarette smoke being visible). Neutral cue videos were filmed in similar settings, but did not include smoking or cigarette paraphernalia.
For presentation of videos, MRI-compatible goggles were fitted to the subject’s head, using a headphone/microphone headset (MRVision 2000 Ultra, Resonance Technology, Northridge, CA). These goggles provide a 1024x768 digital image with a 30 degree field of view. The headphones provided stereo sound with noise cancellation and 30 db attenuation while the microphone utilized active noise cancellation. Each participant was positioned on the scanner bed and his/her head fixed in place using adjustable tabs in the coil to minimize movement.
Prior to scanning, participants were shown how to provide ratings of their craving using an optically-isolated USB interface, which consisted of a five-button (1=low to 5=high) response box (Rowland Institute at Harvard, Cambridge, MA). Subjects provided ratings of their craving immediately following each cue presentation. Cabling for the response box and videos was passed though an RF and magnetically shielded panel (MRA Inc., Washington, PA) into a control room where Pentium notebook computers were used to play the video cues and record button presses.
Prior to fMRI scanning, subjects were instructed to allow themselves to crave cigarettes during the cue exposure unless instructed to resist such craving. During scanning, subjects were presented with three runs of three cue videos each (three conditions). Each run consisted of two cigarette cue videos and one neutral cue video. For the pair of videotaped cigarette cues, subjects were either given no instructions and allowed themselves to crave (cigarette cue crave) or were instructed to resist craving (cigarette cue resist). The resist instruction was stated as “during the next video clip, try to resist any feelings of craving for cigarettes.” The brief instruction to resist craving was intended to mimic real life situations in which a smoker sees cigarette cues and attempts to resist craving. For the neutral cue videos, subjects were given no instruction. The order of cue conditions was a randomized Latin square design, so that each condition (cigarette cue crave, cigarette cue resist, and neutral cue) appeared in a balanced serial order, with six separate orders of cue presentation. Subjects underwent a brief semi-structured interview after the fMRI scan, and were asked (1) if the cigarette cues induced craving and (2) what strategy they employed to resist craving.
fMRI/Statistical Analysis
For the MRI scans, brain extraction was performed manually using MEDx 3.3 (Sensor Systems Inc., Sterling, VA) by removing structures outside of the brain. These extractions were then used to form an “average brain” template that was used later for co-registration (Woods et al 1999).
FMRI analysis was performed using the functional magnetic imaging of the brain (FMRIB) Software Library ([FSL]; www.fmrib.ox.ac.uk/fsl) (Smith et al 2004). Because this was a multi-session (multiple repetitions of stimuli presentation during a single fMRI session) multi-subject experiment, we used three levels of analysis hierarchically within the FEAT (FMRI Expert Analysis Tool, version 5.1) part of FMRIB, with the first level analyzing the data from each session (each series of cue presentations) within each subject, the second analyzing across sessions within each subject, and the third analyzing across the group of subjects.
First-level analysis used three primary explanatory variables (cigarette cue crave, cigarette cue resist, and neutral cue conditions). MR signal from the time spent on instructions and craving ratings was excluded from study analyses. Six contrasts were tested: cigarette cue crave minus neutral cue, neutral cue minus cigarette cue crave, cigarette cue resist minus neutral cue, neutral cue minus cigarette cue resist, cigarette cue crave minus cigarette cue resist, and cigarette cue resist minus cigarette cue crave. Prior to cluster analysis, the voxels were threshholded at Z > 2.3, with final cluster thresholding performed at p = 0.01. This cluster thresholding provides correction for multiple comparisons (Forman et al 1995).
Second and third level analyses were performed using FEAT’s FLAME (FMRIB’s Local Analysis of Mixed Effects) tool in order to estimate the inter-session and inter-subject random-effects component of the mixed effects variance, with the third level analysis carried out six times (once for each contrast), using the same statistical thresholds as for the first level analysis. These statistical thresholds are similar or identical to those of other published activation studies using FSL (Smith et al 2002; Parry et al 2003; Gobel et al 2004; Osterbauer et al 2005; Iannetti et al 2005).
Correlation analysis also was performed to examine the relationship between self-reported craving ratings and brain activity across the three conditions. First-level analysis was done using each session’s scan as input data. Two explanatory variables were used, with the first one consisting of the rating scale data, and the second consisting of mean data (showing no variability for craving scores). A double-gamma HRF convolution and temporal filtering were used for both explanatory variables. Two contrasts were performed (rating scale minus mean data, and mean minus rating scale), with a Z threshold of 2.3 and a cluster threshold of p = 0.01. Higher level analyses were then performed in the same manner as described above.
Results
Participants were adults (mean age 38.0 years ± standard deviation 12.4 years; 12 females, 30 males), who smoked an average of 23.3 (± 8.2) cigarettes per day and had a mean 24.1 (± 24.5) pack-year smoking history. FTND scores were moderately high (5.7 ± 1.7), and HAM-D and HAM-A scores were low (1.9 ± 2.4 and 2.3 ± 2.7, respectively). Exhaled CO levels were 21.1 ± 10.6 parts per million (ppm) at the time of screening and 15.2 ± 7.6 ppm at the time of scanning, consistent with the study protocol.
Subjects rated their craving as mild to moderate (2.4 ± 1.3, on the 1 to 5 scale) during the neutral condition, and significantly higher during the cigarette cue crave (2.9 ± 1.4) and cigarette cue resist (3.0 ± 1.3) conditions (paired Student’s t-tests, p < .0001 for both contrasts). Differences in craving severity between the cigarette cue crave and cigarette cue resist conditions were not significant (paired Student’s t-tests, p = .45). In the semi-structured interview following the scans, seven subjects reported that the videos did not affect their craving levels, while the remaining subjects reported mild to moderate changes in craving in response to the cigarette-related cues. In describing strategies used to resist craving, 74% of subjects reported that they tried to distract themselves with thoughts unrelated to smoking or to ignore thoughts about smoking. The remaining 26% were unable to verbalize the strategy that they used. Eight subjects spontaneously reported that the distraction strategy was similar to the one they used when trying to resist the urge to smoke in natural settings.
In comparing the cigarette cue crave with neutral cue exposure, higher MR signal was found during the cigarette cue crave condition in secondary visual processing centers on the left (cuneus, lingual gyrus, and lateral occipital gyrus), the supramarginal gyrus bilaterally (extending to the lateral occipital gyrus on the right) and left angular gyrus (Table 1 and Fig. 1). There were no voxels with significantly lower MR signal for the cigarette cue crave (compared to the neutral) condition.
Table 1.
Cigarette Cue Crave Versus Neutral Cues
| Regions | No. of Voxels in Cluster | x, y, z Talairach Coordinates | Cluster Z Score | P | |
|---|---|---|---|---|---|
| Cigarette > Neutral | |||||
| Retrosplenial area | L | 537 | −12, −60, 4 | 4.53 | 2.26e-08 |
| Cuneus | L | −14, −70, 12 | 4.29 | ||
| −4, −70, 6 | 4.08 | ||||
| Precuneus | L | −14, −74, 26 | 3.89 | ||
| −10, −86, 34 | 3.36 | ||||
| Lingual gyrus | L | −6, −72, −16 | 3.57 | ||
| Supramarginal gyrus | R | 183 | 54, −62, 20 | 4.29 | 0.002 |
| 54, −66, 20 | 3.99 | ||||
| Lateral occipital gyrus | R | 36, −82, −10 | 4.16 | ||
| 28, −82, −10 | 3.62 | ||||
| 44, −84, 12 | 3.30 | ||||
| Supramarginal gyrus | L | 171 | −50, −48, 20 | 4.39 | 0.003 |
| −46, −62, 28 | 3.57 | ||||
| −54, −52, 28 | 3.38 | ||||
| Angular gyrus | L | −46, −74, 12 | 4.10 | ||
| −40, −70, 14 | 3.65 | ||||
| −52, −70, 14 | 2.97 | ||||
| Cigarette < Neutral | |||||
| No Activations | |||||
L = left; R = right
Figure 1.
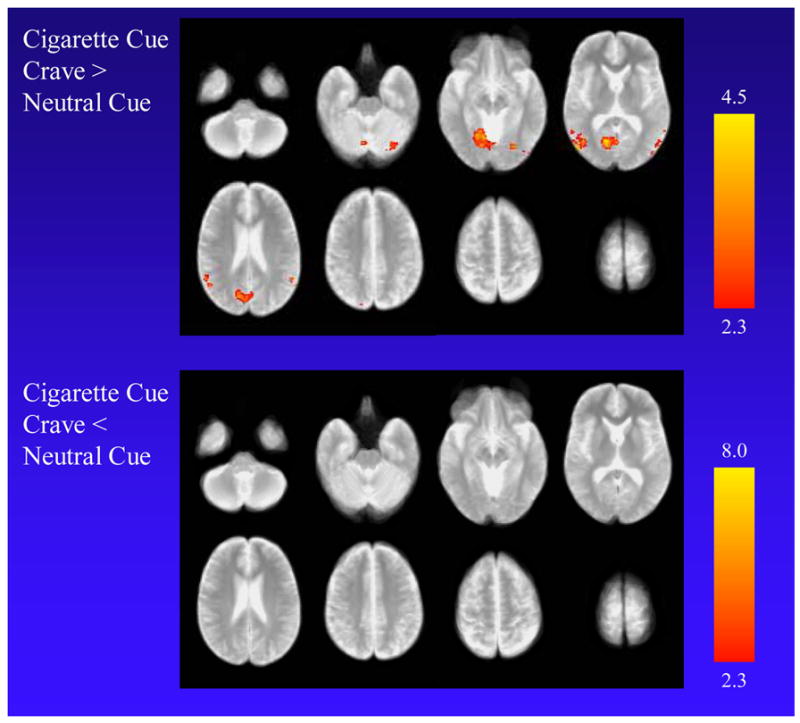
Functional MRI findings when cigarette smokers (n = 42) were exposed to cigarette-related cues and allowed themselves to crave (cigarette cue crave condition) compared to the neutral cue condition. The top panel shows higher MR signal for the cigarette cue crave condition in the left cuneus, lingual gyrus, and lateral occipital gyrus, the supramarginal gyrus bilaterally, and the left angular gyrus. The bottom panel shows no voxels with significantly lower MR signal for the cigarette cue crave condition. Scaling for Z scores is presented on the right.
For the comparison of the cigarette cue resist with the neutral cue exposure, higher MR signal was found in the cigarette cue resist condition in the posterior cingulate cortex (PCC) extending to the precuneus and retrosplenial area bilaterally, medial aspect of the superior frontal gyrus and dorsal anterior cingulate cortex (ACC) spanning the midline, left angular gyrus, and supramarginal gyri bilaterally (Table 2 and Fig. 2). Lower MR signal was found in the cigarette cue resist condition in the cuneus and post-central gyri bilaterally and right pre-central gyrus.
Table 2.
Cigarette Cue Resist vs. Neutral Cues
| Regions | No. of Voxels in Cluster | x, y, z Talairach Coordinates | Cluster Z Score | P | |
|---|---|---|---|---|---|
| Cigarette Resist > Neutral | |||||
| Posterior cingulate cortex | L | 1780 | −6, −56, 34 | 5.43 | 1.07e-22 |
| −18, −68, 20 | 4.86 | ||||
| Precuneus | B | 16, −70, 20 | 5.26 | ||
| −8, −70, 28 | 4.83 | ||||
| Retrosplenial area | B | −14, −58, 6 | 4.99 | ||
| 10, −56, 6 | 4.64 | ||||
| Superior frontal gyrus | B | 395 | 0, 28, 26 | 3.85 | 1.79e-07 |
| −6, 50, 34 | 3.76 | ||||
| 0, 44, 8 | 3.73 | ||||
| −2, 34, 28 | 3.68 | ||||
| −4, 36, 28 | 3.65 | ||||
| Dorsal anterior cingulate cortex | L | −4, 10, 34 | 3.64 | ||
| Angular gyrus | L | 348 | −46, −76, 26 | 4.89 | 7.75e-07 |
| −48, −64, 26 | 4.09 | ||||
| −36, −78, 26 | 3.98 | ||||
| −38, −86, 26 | 3.74 | ||||
| Supramarginal gyrus | L | −56, −52, 28 | 4.35 | ||
| −52, −62, 26 | 4.00 | ||||
| Supramarginal gyrus | R | 274 | 42, −62, 26 | 3.71 | 1.23e-05 |
| 48, −72, 26 | 3.50 | ||||
| 54, −62, 20 | 3.49 | ||||
| 48, −64, 28 | 3.41 | ||||
| 42, −78, 28 | 3.28 | ||||
| 50, −58, 34 | 3.26 | ||||
| Cigarette Resist < Neutral | |||||
| Post-central gyrus | R | 705 | 36, −48, 50 | 5.07 | 1.14e-11 |
| 46, −32, 42 | 5.07 | ||||
| 50, −32, 42 | 5.05 | ||||
| 32, −54, 56 | 4.25 | ||||
| 40, −42, 48 | 4.14 | ||||
| 44, −36, 20 | 3.81 | ||||
| Cuneus | B | 676 | 10, −94, 12 | 4.95 | 2.58e-11 |
| −12, −92, 12 | 4.87 | ||||
| 6, −96, 20 | 4.60 | ||||
| 0, −86, 6 | 4.46 | ||||
| −2, −90, 12 | 4.26 | ||||
| −18, −90, 28 | 3.62 | ||||
| Post-central gyrus | L | 642 | −36, −48, 50 | 5.03 | 6.87e-11 |
| −38, −38, 42 | 4.96 | ||||
| −24, −58, 56 | 4.55 | ||||
| −52, −30, 34 | 4.47 | ||||
| −26, −54, 50 | 4.17 | ||||
| −52, −26, 28 | 4.04 | ||||
| Pre-central gyrus | R | 126 | 22, −20, 56 | 3.90 | 0.007 |
| 18, −22, 64 | 3.65 | ||||
| 36, −18, 56 | 2.54 | ||||
B = bilateral; L = left; R = right
Figure 2.
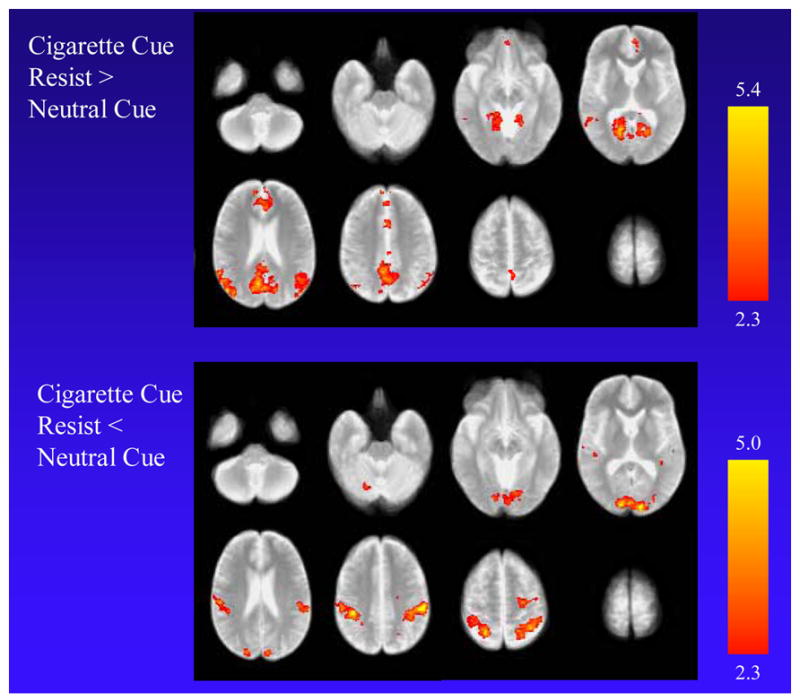
Functional MRI findings when smokers were exposed to cigarette-related cues and resisted craving (cigarette cue resist condition) compared to the neutral cue condition. The top panel shows higher MR signal for the cigarette cue resist condition in the posterior cingulate cortex extending to the precuneus and retrosplenial area bilaterally, the medial aspect of the superior frontal gyrus and anterior cingulate cortex spanning the midline, the left angular gyrus, and the supramarginal gyrus bilaterally. The bottom panel shows lower MR signal for the cigarette cue resist condition in the cuneus and post-central gyri bilaterally, and the right pre-central gyrus.
In directly contrasting the cigarette cue resist with the cigarette cue crave condition, higher MR signal was found in the cigarette cue resist condition in the left ACC, medial superior frontal gyrus, precuneus, and PCC (Table 3 and Fig. 3). Lower MR signal for the cigarette cue resist condition was found in the cuneus and lateral occipital gyri bilaterally, left middle temporal gyrus, and right post-central gyrus. Analysis of the subgroup of subjects that used distraction as a strategy to resist cue-induced craving revealed almost identical areas of activation and deactivation to the overall group, except that the size of the lower MR signal in the cuneus was considerably larger (5031 voxels).
Table 3.
Cigarette Cue Resist versus Cigarette Cue Crave
| Regions | No. of Voxels in Cluster | x, y, z Talairach Coordinates | Cluster Z Score | P | |
|---|---|---|---|---|---|
| Cigarette Cue Resist > Cigarette Cue | |||||
|
| |||||
| Posterior cingulate cortex | L | 233 | −6, −66, 28 | 4.15 | 3.86e-05 |
| −16, −58, 20 | 3.84 | ||||
| −6, −48, 34 | 3.75 | ||||
| Precuneus | L | −6, −62, 34 | 3.78 | ||
| −10, −52, 42 | 3.50 | ||||
| −18, −68, 20 | 3.16 | ||||
| Superior frontal gyrus (medial) | L | 119 | −6, 34, 28 | 3.56 | 0.008 |
| −10, 34, 28 | 3.49 | ||||
| Perigenual anterior cingulate cortex | L | −8, 30, 12 | 3.53 | ||
| −4, 20, 12 | 3.38 | ||||
| Anterior cingulate cortex (dorsal) | L | −16, 24, 20 | 3.16 | ||
| Cigarette Cue Resist < Cigarette Cue | |||||
| Cuneus | L | 3134 | −10, −96, 12 | 5.50 | 5.93e-35 |
| −4, −82, 6 | 5.25 | ||||
| −8, −80, 6 | 5.19 | ||||
| −4, −96, 12 | 5.11 | ||||
| Middle temporal gyrus | L | −50, −68, 0 | 5.16 | ||
| Lateral occipital gyrus | L | −42, −76, −8 | 4.86 | ||
| Post-central gyrus | R | 164 | 22, −52, 50 | 4.36 | 0.0008 |
| 32, −54, 56 | 3.94 | ||||
| 38, −52, 56 | 3.92 | ||||
| 36, −48, 56 | 3.49 | ||||
| 26, −52, 64 | 3.16 | ||||
| 22, −65, 48 | 3.16 | ||||
L = left; R = right
Figure 3.
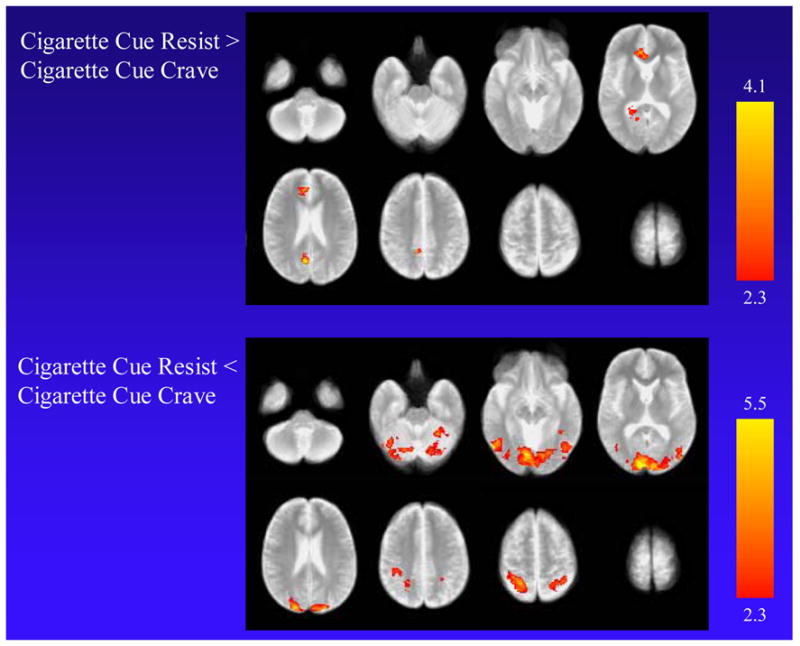
Functional MRI findings when smokers were exposed to cigarette-related cues and resisted craving (cigarette cue resist condition) compared to cigarette-related cues and allowing craving (cigarette cue crave condition). The top panel shows higher MR signal for the cigarette cue resist condition in the left anterior cingulate cortex, precuneus, and posterior cingulate cortex. The bottom panel shows lower MR signal for the cigarette cue resist condition in the cuneus and lateral occipital gyri bilaterally, left middle temporal gyrus, and right post-central gyrus.
Examination of associations between Urge to Smoke Scale ratings and MR signal revealed positive correlations for the medial aspect of the superior frontal gyrus, supramarginal gyrus, precuneus, inferior frontal gyrus/anterior insula, and corpus callosum bilaterally, along with the left pre-central gyrus, putamen, and middle frontal gyrus and right lingual gyrus extending to the fusiform gyrus. Negative correlations were found for the cuneus bilaterally; left occipital gyrus, anterior temporal lobe, post-central gyrus, and insula; and right angular gyrus (Table 4 and Fig. 4).
Table 4.
Correlations between Cigarette Craving and MR Signal Across All Study Conditions
| Regions | No. of Voxels in Cluster | x, y, z Talairach Coordinates | Cluster Z Score | P | |
|---|---|---|---|---|---|
| Positive Correlations | |||||
| Anterior cingulate cortex/Superior frontal gyrus | B | 778 | −4, 12, 42 | 4.79 | 1.72e-10 |
| −2, 4, 50 | 4.77 | ||||
| −4, 30, 28 | 4.39 | ||||
| −10, 26, 28 | 4.21 | ||||
| 0, 28, 28 | 4.10 | ||||
| 0, 30, 28 | 3.97 | ||||
| Supramarginal gyrus | L | 640 | −48, −58, 42 | 5.09 | 4.5e-09 |
| −42, −54, 42 | 4.90 | ||||
| −42, −58, 50 | 4.79 | ||||
| −36, −74, 42 | 4.74 | ||||
| −52, −52, 42 | 4.39 | ||||
| Pre-central gyrus | L | −44, −28, 56 | 4.56 | ||
| Precuneus | B | 619 | 2, −74, 42 | 5.27 | 7.54e-09 |
| −8, −74, 42 | 4.90 | ||||
| −10, −70, 34 | 4.71 | ||||
| −14, −66, 28 | 3.98 | ||||
| −12, −62, 34 | 3.26 | ||||
| Inferior frontal gyrus/anterior insula | L | 581 | −46, 14, 6 | 4.74 | 1.95e-08 |
| −30, 20, 6 | 4.63 | ||||
| −38, 10, 6 | 4.45 | ||||
| −48, 12, 6 | 4.39 | ||||
| −34, 6, 12 | 4.34 | ||||
| Putamen | −22, 4, 6 | 4.03 | |||
| Middle frontal gyrus | L | 562 | −46, 20, 34 | 4.69 | 5.96e-08 |
| −40, 20, 42 | 4.43 | ||||
| −42, 40, 20 | 4.02 | ||||
| −42, 14, 50 | 3.97 | ||||
| −36, 48, 12 | 3.95 | ||||
| −3, 32, 42 | 3.75 | ||||
| Inferior frontal gyrus/anterior insula | R | 522 | 42, 10, 6 | 4.71 | 5.96e-08 |
| 38, 10, 6 | 4.30 | ||||
| 32, 10, 4 | 4.15 | ||||
| 48, 10, 6 | 3.96 | ||||
| 32, 16, 12 | 3.85 | ||||
| 28, 16, 12 | 3.66 | ||||
| Corpus callosum | B | 351 | 2, −26, 28 | 5.05 | 1.04e-05 |
| −2, −36, 28 | 4.86 | ||||
| Posterior cingulate cortex | B | −2, −32, 34 | 4.83 | ||
| −5, −32, 34 | 4.80 | ||||
| 0, −32, 28 | 4.79 | ||||
| Supramarginal gyrus | R | 247 | 34, −58, 42 | 4.24 | 0.0003 |
| 32, −62, 42 | 4.23 | ||||
| 42, −56, 50 | 4.20 | ||||
| 46, −56, 42 | 3.98 | ||||
| 46, −60, 42 | 3.91 | ||||
| 54, −52, 34 | 3.56 | ||||
| Lingual gyrus | R | 215 | 12, −54, −16 | 3.89 | 0.0008 |
| 10, −46, −8 | 3.17 | ||||
| Fusiform gyrus | R | 30, −62, −22 | 3.65 | ||
| 28, −52, −22 | 3.58 | ||||
| 30, −56, −22 | 3.48 | ||||
| 16, −48, −16 | 3.41 | ||||
| Negative Correlation | |||||
| Occipital gyrus | L | 1869 | −42, −70, −2 | 4.92 | 2.95e-20 |
| Cuneus | B | −10, −96, 20 | 4.79 | ||
| 8, −100, 12 | 4.41 | ||||
| −16, −98, 18 | 4.34 | ||||
| Angular gyrus | R | 46, −70, 6 | 4.54 | ||
| 48, −64, 6 | 4.34 | ||||
| Anterior temporal lobe (Heschl’s gyrus) | L | 167 | −50, −18, 6 | 4.01 | 0.004 |
| Post-central gyrus | L | −58, −22, 12 | 3.84 | ||
| −58, −32, 12 | 3.25 | ||||
B = bilateral; L = left; R = right
Figure 4.
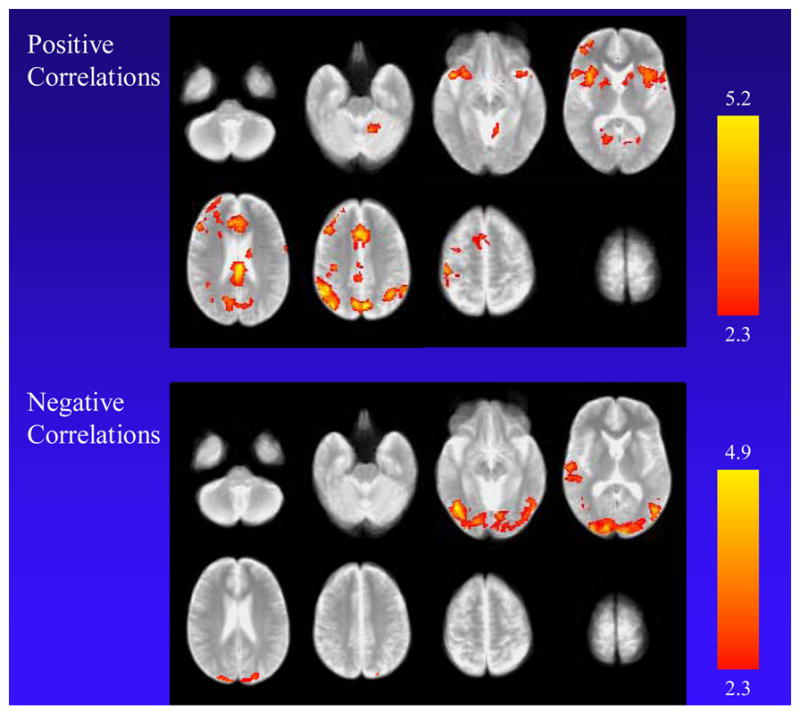
Positive and negative correlations between Urge to Smoke Scale score (craving) and MR signal across all three study conditions. The top panel shows regions with positive correlations, including the medial aspect of the superior frontal gyrus, supramarginal gyrus, precuneus, and inferior frontal gyrus/anterior insula bilaterally, along with the left pre-central gyrus, putamen, and middle frontal gyrus and right lingual gyrus extending to the fusiform gyrus. Negative correlations were found for the cuneus bilaterally; left occipital gyrus, anterior temporal lobe, post-central gyrus, and insula; and right angular gyrus.
Discussion
Treatment seeking cigarette smokers have heightened craving when exposed to cigarette-related cues in a laboratory setting, regardless of whether or not they attempt to resist the urge to smoke. The extent of this heightened craving was consistent with prior functional brain imaging research demonstrating cigarette cue-induced craving (Brody et al 2002; McBride et al 2006; Wilson et al 2005). However, patterns of regional brain activation (and deactivation) were clearly different depending on whether or not subjects attempted to resist craving.
Consistent with prior research (Smolka et al 2005; Wilson et al 2005; Lee et al 2005), exposure to visual cigarette cues when subjects allowed themselves to crave (compared with the neutral cue condition) activated primary (left lateral occipital gyrus) and secondary (left cuneus, precuneus, and lingual and angular gyri, and bilateral supramarginal gyri) visual processing centers that also are activated during heightened visual attention (Makino et al 2004; Servos et al 2002; Roland and Gulyas 1995) and recognition of familiar objects (Sugiura et al 2005) and memories (Yonelinas et al 2005). The retrosplenial cortex also showed activation in this analysis, possibly related to its role in memory formation (Ranganath et al 2005), (including recall of autobiographical (Steinvorth et al 2005) and emotionally salient events (Maddock 1999)). While these results do overlap with regions found to activate in previous studies, we did not find activation in the prefrontal cortex or ACC, as has been reported previously when comparing cigarette-related with neutral cue states, possibly because subjects in this study may have experienced greater arousal when asked to resist craving with corresponding lower levels of arousal when not asked to resist.
For the cigarette cue resist compared to the neutral cue condition, activation was also found in secondary visual processing centers (bilateral precuneus, left angular gyrus, and bilateral supramarginal gyri) and retrosplenial cortex (bilaterally), but these clusters were larger than those found in the preceding analysis. This analysis also revealed activation of the dorsal ACC (a region associated with response conflict (Liu et al 2004), decision-making (Paulus et al 2005; Rushworth et al 2004; Turk et al 2004), regulation of anxiety-related behavior (Kalin et al 2005), and planning (Lazeron et al 2000)) and the PCC (a region associated with responses to anxiety-provoking video (Fredrikson et al 1997), and recognition of words in an emotionally negative context (Maratos et al 2001)). Deactivations were found with resisting craving in the sensorimotor cortices (bilateral post-central and right pre-central gyri) and cuneus bilaterally.
For the central analysis of this study comparing responses to the cigarette cues with versus without resisting cigarette craving, greater MR signal was found with resisting craving in regions involved in decision-making/planning (left dorsal and perigenual ACC) and attentional motivation (left PCC), along with a secondary visual processing center (left precuneus). Lower MR signal was found in a visual processing center (left cuneus) and in motor cortex (right post-central gyrus). The dorsal ACC activation and visual cortical deactivation found here are consistent with examinations of brain function during cognitive reappraisal and cognitive modulation of emotion (Pessoa et al 2002; Ochsner et al 2004; Ray et al 2005; Kalisch et al 2006). Engagement of the ACC, which is implicated in conflict avoidance and attentional control (Barch et al 2001; Braver et al 2001; Liu et al 2004), may reflect the active direction of attention away from the hypersalient smoking stimuli as an effortful process that is contrary to automatic patterns of attention. Taken together, these findings suggest that actively suppressing the urge to smoke involves a redistribution of resources from sensory and motor areas to limbic (and related) brain areas.
While results here are in agreement with prior work, two aspects of the central study comparison were surprising, namely that subjects reported slightly (and non-significantly) more craving and that brain activation (particularly in the ACC and PCC) was greater when subjects were actively trying to resist the urge to smoke than when they allowed themselves to crave. These findings may be partly accounted for by the “white bear” effect, where subjects paradoxically think more strongly about a topic that they are instructed to suppress (Wegner et al 1987; Enticott and Gold 2002). While control instructions, such as asking subjects to actively try to crave during the cigarette cue crave presentation or to resist craving during the neutral cue presentation, would control for the intention aspects of the results, the present study sought to simulate real-life situations. And indeed, subjects did report that study conditions mimicked naturally-occurring situations.
Craving levels positively correlated with MR signal in the same decision-making (dorsal ACC), attentional processing (PCC), sensorimotor (left pre-central gyrus) and secondary visual processing (precuneus, right lingual and fusiform gyri, and bilateral supramarginal gyri) regions as in the preceding analyses, as well as regions that mediate (Kimbrell et al 1999; Goldin et al 2005; Kuchinke et al 2005) and interpret (Menon et al 2000; Kesler-West et al 2001; Drexler et al 2000) emotional stimuli (anterior insula and inferior frontal gyri bilaterally) and a region associated with sustained attention and episodic memory (left middle frontal gyrus (Cabeza and Nyberg 2000)). Negative correlations were found in visual and auditory processing centers, as well as left motor cortex.
One limitation of this study was the absence of a non-smoking control group exposed to the same cues as the smokers studied here. However, in our prior work (Brody et al 2002), non-smokers demonstrated neither cigarette craving nor changes in mood/anxiety associated with presentation of cigarette-related cues. Also, since the primary analysis here was the examination of cigarette cue exposure with and without resisting craving, the use of non-smoking control subjects would not be expected to help in the central interpretation of this study. The main strengths of this report include a relatively large sample size for a study of this type, the control of cue presentation through specialized MR-compatible equipment, and the fact that cues (e.g.- video of chore performance with or without smoking) and states (allowing oneself to crave versus resisting craving while watching similar cues) were matched closely.
In conclusion, we report significant differences in brain function when treatment-seeking smokers are exposed to cigarette-related cues and are actively resisting craving versus allowing themselves to crave. During craving resistance, activation was found in brain regions involved in decision-making, regulation of anxiety-related behaviors, and heightened attention, while deactivation was found in primary visual and motor cortices. Similar activations and deactivations were found when comparing the cigarette cue resistance to the neutral cue condition. Additionally, exposure to cigarette cues without craving resistance was found to activate visual processing centers when compared with neutral cue exposure. These results identify regions that may mediate the effects of existing Tobacco Dependence treatments and that may be targets for medication development. For example, enhanced catecholiminergic (Passerin et al 2000; Schweimer et al 2005), acetylcholinergic (Jacobsen et al 2004; Gozzi et al 2006; Choi et al 2006), and cannabinoid (Mathew et al 1997; Mathew et al 1999; O'Leary et al 2002) neurotransmission activate the ACC, including activation during effortful decision-making (Schweimer et al 2005), while enhanced GABA (Passerin et al 2000; Mintzer et al 2001) neurotransmission diminishes ACC activation. Currently available medications for Tobacco Dependence, such as the catecholamine reuptake blocker bupropion HCl (Ascher et al 1995; Horst and Preskorn 1998) and nicotine replacement (acetylcholine agonist) therapy may exert their therapeutic effects at least partly through enhancement of ACC activation with concomitant improvement in the ability to resist craving, while medications that alter cannabinoid and GABAergic neurotransmission are currently under investigation as potential treatments for Tobacco Dependence. Thus, the present study may help elucidate the brain mediation of existing Tobacco Dependence treatments and suggests potential neurotransmitter system targets for medication development.
Acknowledgments
Supported by the National Institute on Drug Abuse (A.L.B. [R01s DA15059 and DA20872] and E.D.L. [R01 DA14093]), a Department of Veterans Affairs (VA) Type I Merit Review Award (A.L.B.), the Tobacco-Related Disease Research Program (A.L.B. [11RT-0024] and E.D.L. [10RT-0091]), and a National Alliance for Research in Schizophrenia and Depression Independent Investigator Award (A.L.B.). The authors would like to thank Darryl Stallworth for technical assistance in performing functional magnetic resonance imaging scans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- 2.Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: Effects of response modality and processing domain. Cerebral Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- 3.Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: Effects of frequency, inhibition and errors. Cerebral Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- 4.Brody AL, Mandelkern MA, London ED, Childress AR, Bota RG, Ho ML, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 5.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 6.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 7.Catley D, O'Connell KA, Shiffman S. Absentminded lapses during smoking cessation. Psychol Addict Behav. 2000;14:73–76. doi: 10.1037//0893-164x.14.1.73. [DOI] [PubMed] [Google Scholar]
- 8.Choi JK, Mandeville JB, Chen YI, Kim YR, Jenkins BG. High resolution spatial mapping of nicotine action using pharmacologic magnetic resonance imaging. Synapse. 2006;60:152–157. doi: 10.1002/syn.20284. [DOI] [PubMed] [Google Scholar]
- 9.Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Experimental and Clinical Psychopharmacology. 2001;9:399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- 10.Drexler K, Schweitzer JB, Quinn CK, Gross R, Ely TD, Muhammad F, et al. Neural activity related to anger in cocaine-dependent men: a possible link to violence and relapse. Am J Addict. 2000;9:331–339. doi: 10.1080/105504900750047382. [DOI] [PubMed] [Google Scholar]
- 11.Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 12.Enticott PG, Gold RS. Contrasting the ironic monitoring and motivational explanations of postsuppressional rebound. Psychol Rep. 2002;90:447–450. doi: 10.2466/pr0.2002.90.2.447. [DOI] [PubMed] [Google Scholar]
- 13.Fagerström KO. Measuring the degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 14.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2000. Treating Tobacco Use and Dependence. [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-I/P, version 2.0) 1995. [Google Scholar]
- 16.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 17.Fredrikson M, Fischer H, Wik G. Cerebral blood flow during anxiety provocation. J Clin Psychiatry. 1997;58(Suppl 16):16–21. [PubMed] [Google Scholar]
- 18.Gobel SM, Johansen-Berg H, Behrens T, Rushworth MF. Response-selection-related parietal activation during number comparison. J Cogn Neurosci. 2004;16:1536–1551. doi: 10.1162/0898929042568442. [DOI] [PubMed] [Google Scholar]
- 19.Goldin PR, Hutcherson CA, Ochsner KN, Glover GH, Gabrieli JD, Gross JJ. The neural bases of amusement and sadness: a comparison of block contrast and subject-specific emotion intensity regression approaches. Neuroimage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Gozzi A, Schwarz A, Reese T, Bertani S, Crestan V, Bifone A. Region-specific effects of nicotine on brain activity: a pharmacological MRI study in the drug-naive rat. Neuropsychopharmacology. 2006;31:1690–1703. doi: 10.1038/sj.npp.1300955. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton M. Diagnosis and rating of anxiety. Br J Psychiatry. 1969;3:76–79. [Google Scholar]
- 23.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 24.Horst WD, Preskorn SH. Mechanisms of action and clinical characteristics of three atypical antidepressants: venlafaxine, nefazodone, bupropion. J Affect Dis. 1998;51:237–254. doi: 10.1016/s0165-0327(98)00222-5. [DOI] [PubMed] [Google Scholar]
- 25.Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, et al. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci. 2005;102:18195–18200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen LK, D'Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- 28.Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. NEJM. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- 29.Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain Regions Associated with the Expression and Contextual Regulation of Anxiety in Primates. Biol Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalisch R, Wiech K, Critchley HD, Dolan RJ. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30:1458–1466. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Kesler-West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ, et al. Neural substrates of facial emotion processing using fMRI. Brain Res Cogn Brain Res. 2001;11:213–226. doi: 10.1016/s0926-6410(00)00073-2. [DOI] [PubMed] [Google Scholar]
- 32.Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharm. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- 33.Kimbrell TA, George MS, Parekh PI, Ketter TA, Podell DM, Danielson AL, et al. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biol Psychiatry. 1999;46:454–465. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- 34.Kuchinke L, Jacobs AM, Grubich C, Vo ML, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: An fMRI study. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 35.Lazeron RH, Rombouts SA, Machielsen WC, Scheltens P, Witter MP, Uylings HB, et al. Visualizing brain activation during planning: the tower of London test adapted for functional MR imaging. AJNR Am J Neuroradiol. 2000;21:1407–1414. [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Appl Psychophysiol Biofeedback. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22:1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 38.Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- 39.Makino Y, Yokosawa K, Takeda Y, Kumada T. Visual search and memory search engage extensive overlapping cerebral cortices: an fMRI study. Neuroimage. 2004;23:525–533. doi: 10.1016/j.neuroimage.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 40.Maratos EJ, Dolan RJ, Morris JS, Henson RN, Rugg MD. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39:910–920. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- 41.Mathew RJ, Wilson WH, Chiu NY, Turkington TG, DeGrado TR, Coleman RE. Regional cerebral blood flow and depersonalization after tetrahydrocannabinol administration. Acta Psychiatr Scand. 1999;100:67–75. doi: 10.1111/j.1600-0447.1999.tb10916.x. [DOI] [PubMed] [Google Scholar]
- 42.Mathew RJ, Wilson WH, Coleman RE, Turkington TG, DeGrado TR. Marijuana intoxication and brain activation in marijuana smokers. Life Sci. 1997;60:2075–2089. doi: 10.1016/s0024-3205(97)00195-1. [DOI] [PubMed] [Google Scholar]
- 43.McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of Expectancy and Abstinence on the Neural Response to Smoking Cues in Cigarette Smokers: an fMRI Study. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- 44.McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menon V, White CD, Eliez S, Glover GH, Reiss AL. Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Hum Brain Mapp. 2000;11:117–129. doi: 10.1002/1097-0193(200010)11:2<117::AID-HBM50>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mintzer MZ, Griffiths RR, Contoreggi C, Kimes AS, London ED, Ernst M. Effects of triazolam on brain activity during episodic memory encoding: a PET study. Neuropsychopharmacology. 2001;25:744–756. doi: 10.1016/S0893-133X(01)00280-9. [DOI] [PubMed] [Google Scholar]
- 47.Modell JG, Mountz JM. Effect of haloperidol on craving and impaired control following alcohol consumption in alcoholic subjects. Ann N Y Acad Sci. 1992;654:492–495. doi: 10.1111/j.1749-6632.1992.tb26010.x. [DOI] [PubMed] [Google Scholar]
- 48.Nestler EJ. From neurobiology to treatment: progress against addiction. Nat Neurosci. 2002;5(Suppl):1076–1079. doi: 10.1038/nn945. [DOI] [PubMed] [Google Scholar]
- 49.Niaura R, Abrams DB, Monti PM, Pedraza M. Reactivity to high risk situations and smoking cessation outcome. J Substance Abuse. 1989;1:393–405. [PubMed] [Google Scholar]
- 50.O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, et al. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26:802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 51.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 52.Osterbauer RA, Matthews PM, Jenkinson M, Beckmann CF, Hansen PC, Calvert GA. Color of scents: chromatic stimuli modulate odor responses in the human brain. J Neurophysiol. 2005;93:3434–3441. doi: 10.1152/jn.00555.2004. [DOI] [PubMed] [Google Scholar]
- 53.Parry AM, Scott RB, Palace J, Smith S, Matthews PM. Potentially adaptive functional changes in cognitive processing for patients with multiple sclerosis and their acute modulation by rivastigmine. Brain. 2003;126:2750–2760. doi: 10.1093/brain/awg284. [DOI] [PubMed] [Google Scholar]
- 54.Passerin AM, Cano G, Rabin BS, Delano BA, Napier JL, Sved AF. Role of locus coeruleus in foot shock-evoked Fos expression in rat brain. Neuroscience. 2000;101:1071–1082. doi: 10.1016/s0306-4522(00)00372-9. [DOI] [PubMed] [Google Scholar]
- 55.Paulus MP, Feinstein JS, Leland D, Simmons AN. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage. 2005;25:607–615. doi: 10.1016/j.neuroimage.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 56.Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 57.Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 58.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 59.Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005 doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- 60.Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn Affect Behav Neurosci. 2005;5:156–168. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- 61.Roland PE, Gulyas B. Visual memory, visual imagery, and visual recognition of large field patterns by the human brain: functional anatomy by positron emission tomography. Cereb Cortex. 1995;5:79–93. doi: 10.1093/cercor/5.1.79. [DOI] [PubMed] [Google Scholar]
- 62.Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacology (Berl) 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- 64.Schweimer J, Saft S, Hauber W. Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behav Neurosci. 2005;119:1687–1692. doi: 10.1037/0735-7044.119.6.1687. [DOI] [PubMed] [Google Scholar]
- 65.Servos P, Osu R, Santi A, Kawato M. The neural substrates of biological motion perception: an fMRI study. Cereb Cortex. 2002;12:772–782. doi: 10.1093/cercor/12.7.772. [DOI] [PubMed] [Google Scholar]
- 66.Smith KA, Ploghaus A, Cowen PJ, McCleery JM, Goodwin GM, Smith S, et al. Cerebellar responses during anticipation of noxious stimuli in subjects recovered from depression. Functional magnetic resonance imaging study. Br J Psychiatry. 2002;181:411–415. doi: 10.1192/bjp.181.5.411. [DOI] [PubMed] [Google Scholar]
- 67.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 68.Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2005:1–12. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- 69.Steinvorth S, Corkina S, Halgren E. Ecphory of autobiographical memories: An fMRI study of recent and remote memory retrieval. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stormark KM, Laberg JC, Bjerland T, Nordby H, Hugdahl K. Autonomic cued reactivity in alcoholics: the effect of olfactory stimuli. Addict Behav. 1995;20:571–584. doi: 10.1016/0306-4603(95)00017-7. [DOI] [PubMed] [Google Scholar]
- 71.Sugiura M, Shah NJ, Zilles K, Fink GR. Cortical representations of personally familiar objects and places: functional organization of the human posterior cingulate cortex. J Cogn Neurosci. 2005;17:183–198. doi: 10.1162/0898929053124956. [DOI] [PubMed] [Google Scholar]
- 72.Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of relapse in smokers. Addict Behav. 1996;21:481–490. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 73.Turk DJ, Banfield JF, Walling BR, Heatherton TF, Grafton ST, Handy TC, et al. From facial cue to dinner for two: the neural substrates of personal choice. Neuroimage. 2004;22:1281–1290. doi: 10.1016/j.neuroimage.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 74.Wegner DM, Schneider DJ, Carter SR, III, White TL. Paradoxical effects of thought suppression. J Pers Soc Psychol. 1987;53:5–13. doi: 10.1037//0022-3514.53.1.5. [DOI] [PubMed] [Google Scholar]
- 75.Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob Res. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woods RP, Dapretto M, Sicotte NL, Toga AW, Mazziotta JC. Creation and use of a Talairach-compatible atlas for accurate, automated, nonlinear intersubject registration, and analysis of functional imaging data. Hum Brain Mapp. 1999;8:73–79. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<73::AID-HBM1>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zubieta JK, Heitzeg MM, Xu Y, Koeppe RA, Ni L, Guthrie S, et al. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry. 2005;162:567–577. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]


