Abstract
Peroxynitrite, a cytotoxic oxidant formed from nitric oxide (NO) and superoxide, induces DNA strand breakage, which activates the nuclear enzyme poly(ADP-ribose) synthase (PARS; EC 2.4.2.30). The cellular function of PARS was determined in fibroblast lines from PARS knockout animals (PARS−/−) and corresponding wild-type animals (PARS+/+), with the aid of the lipophilic PARS inhibitor 5-iodo-6-amino-1,2-benzopyrone (INH2BP). We investigated the role of PARS in peroxynitrite-induced fibroblast injury in vitro and also in the development of arthritis in vivo. Exposure of embryonic fibroblasts from the PARS+/+ animals to peroxynitrite caused DNA single-stand breakage and PARS activation and caused an acute suppression of mitochondrial respiration. INH2BP protected the PARS+/+ cells against the suppression of mitochondrial respiration in response to peroxynitrite (50–100 μM). Similarly to PARS inhibition with INH2BP, the PARS−/− cells were protected against peroxynitrite-induced injury. The protection against cellular injury by PARS−/− phenotype or INH2BP waned when cells were challenged with higher concentrations of the oxidant. Inhibition of PARS by INH2BP or by PARS−/− phenotype reduced inducible nitric-oxide synthase (iNOS; EC 1.14.13.39) mRNA levels and inhibited production of NO in immunostimulated cells. INH2BP had no peroxynitrite scavenging or hydroxyl radical scavenging effects, and it exerted no additional (nonspecific) effects in the PARS−/− cells. In collagen-induced arthritis, significant staining for nitrotyrosine, a marker of peroxynitrite formation, was found in the inflamed joints. Oral treatment with INH2BP (0.5 g/kg, daily), starting at the onset of arthritis (day 25), delayed the development of the clinical signs at days 26–35 and improved histological status in the knee and paw. Our data demonstrate that deletion of PARS by genetic manipulation or pharmacological inhibition of PARS protects against oxidant-induced cellular injury in vitro and exhibits anti-inflammatory effects in vivo.
Keywords: nitric oxide, superoxide, inflammation, inducible nitric-oxide synthase, DNA single-strand break
Nitric oxide (NO), superoxide, and their cytotoxic reaction product peroxynitrite (ONOO−) are terminal mediators of cellular injury in various forms of inflammation. In vitro studies employing conventional inhibitors of the nuclear enzyme poly(ADP-ribose) synthase (PARS; EC 2.4.2.30) suggested that the oxidative injury in response to oxy radicals and peroxynitrite is related to DNA single-strand breakage and consequent activation of PARS (1, 2). Massive ADP-ribosylation of nuclear proteins by PARS then results in cellular energy depletion and injury, reminiscent of necrosis (1–3). However, objections can be raised against the conclusions of these studies, because the commonly used relatively high concentrations of PARS inhibitors (e.g., nicotinamide and benzamide analogs), have additional effects as free radical scavengers, and have short cellular residence time (4–6).
More recently a potent pharmacologically active inhibitor of PARS, the lipophilic 6-iodo-5-amino-1,2-benzopyrone (INH2BP), was developed (7, 8). Moreover, a genetically engineered mouse line that lacks PARS is now available: a fibroblast cell line from these animals can be used for in vitro investigations (9). These tools allow a direct testing of the role of PARS. The present work was designed to elucidate (i) whether inhibition of PARS by INH2BP protects against cellular oxidant injury triggered by peroxynitrite, a cytotoxic oxidant produced by the reaction of superoxide and NO (10–14); (ii) whether the PARS−/− cells are protected against cellular injury compared with the PARS+/+ cells; (iii) whether the PARS−/− phenotype or pharmacological inhibition of PARS affects NO production in response to immunostimulation; and (iv) whether INH2BP exerts any oxidant scavenging or nonspecific effects. In addition, to elucidate the role of the peroxynitrite-PARS pathway in the pathogenesis of inflammation, we evaluated (v) whether inhibition of PARS with INH2BP affects the course of collagen-induced arthritis.
The results of the current study support the role of PARS activation in the peroxynitrite-mediated cellular oxidant injury and inflammation, and they demonstrate that either deletion of PARS or its selective inhibition by INH2BP protects against inflammatory cell injury.
METHODS
Cell Culture.
Mouse embryo fibroblasts from the PARS−/− mouse and fibroblasts from the corresponding wild-type controls (9) were grown in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. Cells were cultured in 96-well plates or in 12-well plates until 90% confluence. Cells were exposed to peroxynitrite (25–1000 μM) with or without a 10-min pretreatment with INH2BP (100 μM). For immunostimulation, cells were exposed to bacterial lipopolysaccharide (LPS; Escherichia coli, O111:B4, 10 μg/ml) and murine interferon γ (IFN-γ, 50 units/ml) for 2–48 h in the presence or absence of INH2BP (50–100 μM). INH2BP was synthesized as described (7, 8).
Determination of DNA Single-Strand Breaks and Measurement of Cellular PARS Activity.
At 10 min after peroxynitrite exposure, the formation of DNA single-strand breaks in double-stranded DNA was determined by the alkaline unwinding method as previously described (11, 12). PARS activity was measured 10 min after peroxynitrite exposure, using radiolabeled NAD+ as described, in digitonin-permeabilized cells (11, 12).
Measurement of Mitochondrial Respiration and Cellular NAD+ Levels.
At 60 min after peroxynitrite exposure or 48 h after immunostimulation, respiration was assessed by the mitochondrial-dependent reduction of MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to formazan as described (12). In addition, in some experiments, cellular NAD+ levels were determined by using HPLC (12, 13).
Measurement of Nitrite or Nitrite/Nitrate Production, Inducible NO Synthase (iNOS; EC 1.14.13.39) mRNA, and iNOS Protein Expression by Immunostimulated Cells.
Nitrite in culture supernatants at 24 h after stimulation was measured by the Griess reaction as described (13). For the determination of total nitrite/nitrate concentrations, nitrate was reduced to nitrite by incubation with nitrate reductase (13). After cells had been exposed to LPS/IFN-γ in the presence or absence of INH2BP (100 μM) for 1–24 h, Northern blotting for iNOS mRNA and Western blotting for iNOS protein, using a primary rabbit anti-mouse iNOS antibody (Upstate Biotechnology, Lake Placid, NY), were performed as described (14). The activity of iNOS in cell homogenates was determined by the measurement of the calcium-independent conversion of l-arginine to l-citrulline (13).
Effect of INH2BP on Peroxynitrite- and Hydrogen Peroxide-Induced Oxidation of Dihydrorhodamine 123.
In a separate set of studies, the effect of INH2BP and the conventional PARS inhibitor 3-aminobenzamide (100 μM to 3 mM) on the peroxynitrite-mediated oxidation of dihydrorhodamine 123 was studied in vitro. These studies were performed in phosphate-buffered saline (PBS) containing 100 μM diethylenepentaacetic acid (DTPA), pH 7.4. The oxidation of dihydrorhodamine 123 by peroxynitrite (1 μM) or hydrogen peroxide (1 μM) plus 25 μg/ml horseradish peroxidase in the presence of various concentrations of the PARS inhibitors was measured by the change in absorbance at 500 nm (ɛ = 78,000 M−1⋅cm−1) after 30-min incubation at 37°C (15).
Induction of Collagen-Induced Arthritis, and Detection of Nitrotyrosine in the Inflamed Joints.
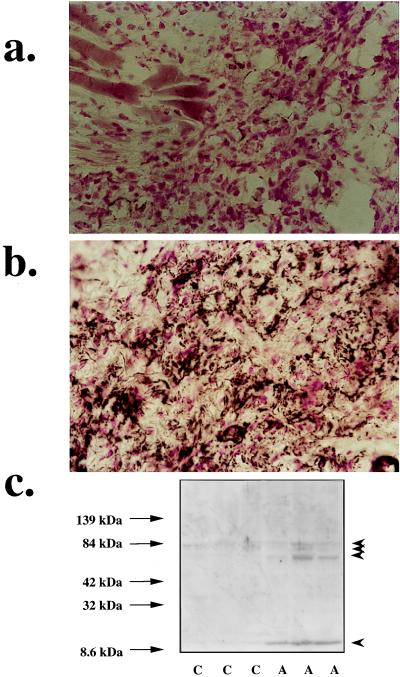
Male DBA/1J mice (9 weeks, The Jackson Laboratory) were used for these studies. Chicken type II collagen (CII) was dissolved in 0.01 M acetic acid at a concentration of 2 mg/ml by stirring overnight at 4°C. Dissolved CII was frozen at −70°C until use. Complete Freund’s adjuvant (CFA) was prepared by the addition of Mycobacterium tuberculosis H37Ra at a concentration of 2 mg/ml. Before injection, CII was emulsified with an equal volume of CFA. Collagen-induced arthritis was induced as previously described (16). On day 1, mice were injected intradermally at the base of the tail with 100 μl of the emulsion (containing 100 μg of CII). On day 21, a second injection of CII in CFA was administered. At Day 35, joints were taken, embedded in M1 medium, and snap frozen in liquid nitrogen. Cryostat sections (6 μm) were cut with a microtome equipped with a carbide steel knife. Joint sections were analyzed for the presence of nitrotyrosine, an indicator of peroxynitrite, by immunohistochemistry as described, using a primary anti-nitrotyrosine antibody (Upstate Biotechnology) (17). In control experiment, sections were incubated in the presence of 10 mM nitrotyrosine. This intervention eliminated the nitrotyrosine staining presented in Fig. 4.
Figure 4.
Nitrotyrosine immunostaining in the paw of a control mouse (a) and the paw of a mouse at 35 days of collagen-induced arthritis (b). (×64.) (c) Nitrotyrosine Western blots from paw extracts of control mice (lanes C) and mice after 35 days of collagen-induced arthritis (lanes A) are shown. Note the marked increase in nitrotyrosine staining in the paws in arthritis. Also, note the increased tyrosine nitration of several proteins (indicated with arrowheads): three proteins (approximately 60–80 kDa) and a low molecular mass protein or protein fragment (approximately 10 kDa). Representative pictures or gels of n = 3 experiments are shown.
In another set of studies, aqueous joint extracts were prepared from control animals and from animals at 35 days of arthritis as described (18), by homogenization in a lysis buffer in the presence of protease inhibitors. Extracts were analyzed for the presence of nitrated proteins by using Western blotting, as described (19).
Induction of Collagen-Induced Arthritis and Its Suppression by INH2BP.
In another set of experiments, PARS was inhibited in the animals with INH2BP. Animals were treated either with vehicle (n = 10) or with INH2BP (n = 6) (0.5 g/kg orally) every 24 h, starting from day 25. Experiments with 14C-labeled INH2BP have established that the dosage regimen used in this study provides adequate tissue uptake of the PARS inhibitor (8). Mice were evaluated daily for arthritis by using a macroscopic scoring system: 0 = no signs of arthritis; 1 = swelling and/or redness of the paw or one digit; 2 = two joints involved; 3 = more than two joints involved; and 4 = severe arthritis of the entire paw and digits (16). Arthritic index for each mouse was calculated by adding the four scores of individual paws. At day 35, animals were sacrificed while they were under anesthesia, and paws and knees were removed and fixed for histological examination, which was done by an investigator blinded for the treatment regime.
Data Analysis and Presentation.
For the in vitro studies, all values in the figures and text are expressed as mean ± SEM of n observations, where n represents the number of wells studied (6–9 wells from two or three independent experiments). Data sets were examined by one- and two-way analysis of variance, and individual group means were then compared with Student’s unpaired t test. For the arthritis studies, Mann–Whitney U test (two-tailed, independent) was used to compare medians of the arthritic indices (16). Values in for the in vitro studies are presented as incidences (%), or medians. A P value less than 0.05 was considered statistically significant.
RESULTS
Role of PARS Activation in the Peroxynitrite-Mediated Inhibition of Mitochondrial Respiration in Fibroblasts.
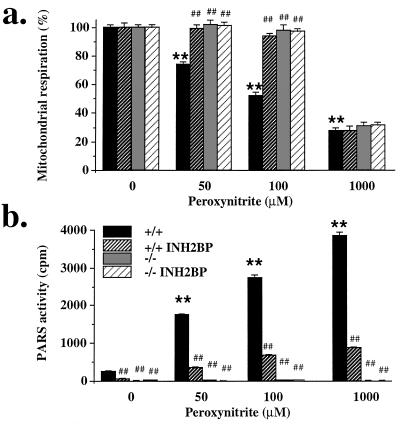
Exposure of wild-type (PARS+/+) fibroblasts to peroxynitrite (50–1000 μM) caused a dose-dependent suppression of the mitochondrial respiration at 1 h (Fig. 1a). In addition, peroxynitrite dose-dependently increased the percentage of single-strand breaks of the DNA in these cells. For instance, at 100 μM peroxynitrite, the percentage of single-strand breaks increased from 3% ± 2% to 28% ± 2% (P < 0.01) (n = 6). Peroxynitrite also caused a dose-dependent activation of PARS (Fig. 1b), with some basal PARS activity detectable in unstimulated wild-type cells (Fig. 1b). INH2BP prevented PARS activation in response to peroxynitrite (Fig. 1b), without affecting the extent of DNA single-strand breakage (not shown). Pharmacological inhibition of PARS caused a significant protection against the peroxynitrite-induced suppression of mitochondrial respiration at 50 μM, 100 μM (Fig. 1a) and 250 μM (not shown) peroxynitrite. However, when cells were exposed to very high concentrations of peroxynitrite (1000 μM), the suppression of mitochondrial respiration could no longer be prevented by pharmacological inhibition of PARS, indicating nonspecific cellular damage (Fig. 1a).
Figure 1.
Effect of peroxynitrite (50–1000 μM) on mitochondrial respiration (a) and PARS activity (b) in PARS+/+ and PARS−/− fibroblasts: effect of INH2BP (100 μM) in both cell types. ∗ and ∗∗ represent significant effects of peroxynitrite in PARS+/+ cells compared with unstimulated controls (P < 0.05, P < 0.01, respectively), # and ## represent significant effect of INH2BP in the presence of peroxynitrite (P < 0.05 and P < 0.01, respectively); n = 6–9.
The extent of DNA single-strand breakage in the PARS−/− cells was similar to the DNA single-strand breakage in the PARS+/+ controls. For instance, at 100 μM peroxynitrite, the percentage of single-strand breaks increased from 3% ± 2% to 31% ± 4% in these cells (P < 0.01) (n = 6). When the cellular responses in the fibroblast line derived from the PARS−/− mice were compared with the response in the corresponding wild-type cells, the results were similar to what we have observed with the pharmacological inhibitor, INH2BP. Cells from the PARS−/− mice were protected against peroxynitrite-induced suppression of mitochondrial respiration (without adding INH2BP) (Fig. 1). This protection diminished when extremely high peroxynitrite concentrations were used (e.g., 1000 μM). The PARS−/− cells were also protected against the peroxynitrite-induced suppression of cellular NAD+ levels. For instance, 100 μM peroxynitrite caused a complete depletion of cellular NAD+ in the wild-type cells from (8.2 ± 1.6 to 0.1 ± 0.1 nmol/mg of protein; P < 0.01, n = 3); whereas NAD+ was well maintained in the PARS−/− cells (control: 9.9 ± 0.5 nmol/mg of protein; after 100 μM peroxynitrite exposure: 5.0 ± 0.9 nmol/mg of protein). Even at very high concentrations of peroxynitrite, where no protection against the suppression of mitochondrial respiration was provided in the PARS−/− phenotype, cellular NAD+ levels in the PARS−/− cells were relatively maintained. For instance, in response to 1000 μM peroxynitrite, cellular NAD+ was 5.1 ± 0.9 nmol/mg of protein in the PARS−/− cells (n = 3). These results suggest that PARS activation plays an important role in the cellular injury at low to intermediate concentrations of peroxynitrite. However, at extremely high oxidant concentrations, overwhelming PARS-independent mechanisms of cytotoxicity become activated. This latter finding is in accordance with observations in pancreatic islet cells, macrophages, and endothelial cells, where extremely high concentrations of oxidants caused massive cytotoxicity, which was no longer preventable by pharmacological inhibition of PARS (12, 15, 18).
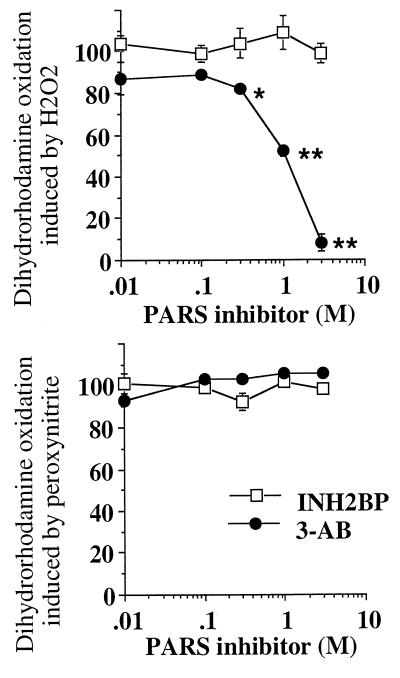
To directly investigate any potential scavenging effect of INH2BP, in vitro studies were performed with INH2BP and 3-aminobenzamide, a prototypical PARS inhibitor, in an assay that utilizes the peroxynitrite- or hydrogen peroxide-induced oxidation of dihydrorhodamine 123. The results showed that INH2BP does not inhibit the peroxynitrite- or hydrogen peroxide-induced oxidation, whereas [and in line with previous studies (4–6)], 3-aminobenzamide dose-dependently inhibited the oxidation of dihydrorhodamine induced by hydrogen peroxide, but not by peroxynitrite (Fig. 2). These observations, coupled with the finding that in the PARS−/− cells, which resisted the suppression of mitochondrial respiration at low to intermediate concentrations of peroxynitrite, INH2BP did not provide any nonspecific additional protection (Fig. 1a), indicate that INH2BP does not act as a scavenger of peroxynitrite.
Figure 2.
Effect of INH2BP and 3-aminobenzamide on the oxidation of dihydrorhodamine 123 by hydrogen peroxide (Upper) or peroxynitrite (Lower). ∗ and ∗∗ represent significant inhibition of dihydrorhodamine oxidation by 3-aminobenzamide in response to hydrogen peroxide (P < 0.05, P < 0.01, respectively; n = 6).
Role of PARS in the Regulation of NO Production in Response to Immunostimulation.
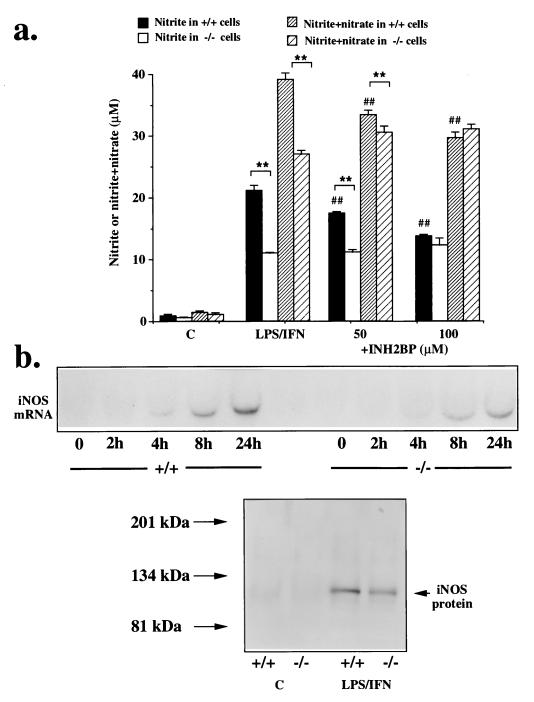
Stimulation of the cells with LPS and interferon-γ induced the production of nitrite and nitrate (oxidation products of NO, produced by iNOS) in the fibroblasts, as measured at 24 h. There was a significantly lower nitrite and nitrate production in response to immunostimulation in the PARS−/− cells, compared with wild-type controls. INH2BP (50–100 μM) caused a dose-dependent inhibition of nitrite and nitrate production in the wild-type cells, lowering it to the level found in the PARS−/− cells (Fig. 3a). INH2BP, however, did not inhibit NO production in the PARS−/− cells (Fig. 3a). Similar differences in the NO production persisted at 48 h after immunostimulation (not shown).
Figure 3.
(a) Concentrations of nitrite (solid bars) or nitrite and nitrate (hatched bars), in PARS+/+ and PARS−/− fibroblasts immunostimulated with LPS/IFN-γ for 24 h; effect of INH2BP (50–100 μM). ∗∗ represents significant effects of immunostimulation compared with unstimulated controls (P < 0.01); ## represents significant effect of INH2BP in the PARS+/+ cells (P < 0.01); n = 6–9. (b) Comparison of the level of iNOS mRNA (at 2, 8, 12 and 24 h) and iNOS protein (at 12 h) expression in PARS+/+ and PARS−/− cells in response to stimulation with IFN-γ and LPS. Representative blots of n = 3 or 4 experiments are shown.
In the PARS−/− cells, there was a significantly lower expression of iNOS, as indicated by lower amounts of iNOS steady-state mRNA and iNOS protein levels (Fig. 3b). The mRNA for iNOS was (2.8 ± 0.7)-fold higher at 8 h after immunostimulation and (4.6 ± 1.9)-fold higher at 24 h after immunostimulation in the PARS+/+ cells than in the PARS−/− cells (n = 3; P < 0.01). Direct measurements of iNOS activity in immunostimulated cells confirmed these results: at 12 h after immunostimulation, calcium-independent iNOS activity amounted to 306 ± 39 nmol/min per mg of protein in PARS+/+ cells, 95 ± 11 nmol/min per mg of protein in INH2BP-pretreated PARS+/+ cells, 90 ± 24 nmol/min per mg of protein in PARS−/− cells, and 76 ± 38 nmol/min per mg of protein in INH2BP-pretreated PARS−/− cells (n = 6). These findings are consistent with the view that PARS is involved in the process of iNOS induction (see Discussion). The lack of effect of INH2BP in the PARS−/− cells reiterates that this agent does not exert PARS-independent cellular actions.
At 48 h after immunostimulation, changes in mitochondrial respiration in PARS+/+ and PARS−/− cells were also compared. There was a 46% ± 6% inhibition of the respiration in response to LPS/IFN-γ in the PARS+/+ cells (P < 0.01, n = 12), whereas in the PARS−/− cells, no suppression of mitochondrial respiration was observed: the respiration amounted to 114% ± 8% of the unstimulated controls (n = 12, P < 0.01).
Effect of INH2BP in the Development of Collagen-Induced Arthritis.
NO synthase inhibitors and superoxide dismutase mimics exert protective effects in rodent models of arthritis, induced by adjuvant (20, 21) or collagen (22). The mechanisms of joint degradation, which are similar in all animal models of arthritis, have not been fully characterized. Using immunohistochemistry and Western blotting of proteins in aqueous joint extracts, we observed the appearance of nitrotyrosine-positive staining in the inflamed joints, but not in healthy animals (Fig. 4). These findings are in accordance with a recent study in human samples from arthritic patients (23). Nitrotyrosine formation is generally accepted as a specific “footprint” of peroxynitrite (10, 24), although recent studies proposed additional pathways of tyrosine nitration, such as the one related to the myeloperoxidase-dependent conversion of nitrite to NO2Cl and ⋅NO2 (25). Thus, nitrotyrosine may rather serve as a collective indicator of “reactive nitrogen species” (26).
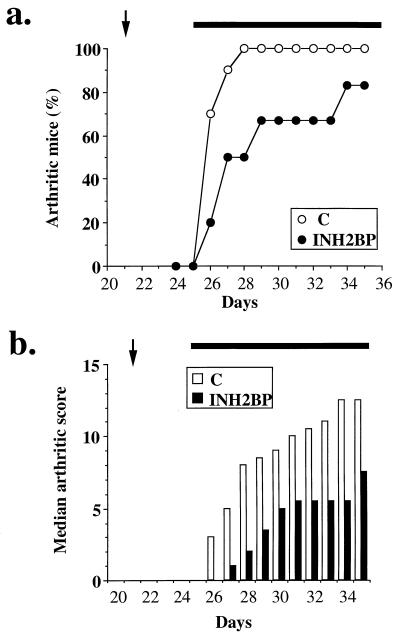
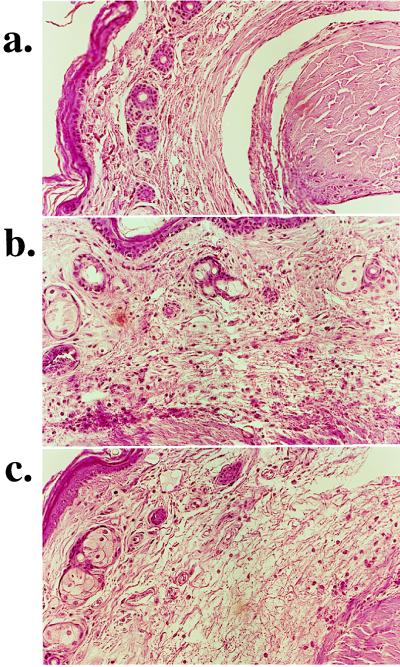
On the basis of our in vitro data indicating the importance of the peroxynitrite-PARS pathway in cell injury, we used INH2BP to define the role of PARS in a mouse model of collagen-induced arthritis. Between days 26 and 35 after the first collagen immunization, animals progressively developed arthritis (Fig. 5). INH2BP reduced the incidence of arthritis until day 33 and reduced the severity of the disease throughout the experimental period. At day 35, histological evaluation of the paws in the vehicle-treated arthritic animals revealed signs of severe suppurative arthritis, with massive mixed (neutrophil, macrophage, and lymphocyte) infiltration. In addition, severe or moderate necrosis, hyperplasia and sloughing of the synovium could be seen, together with the extension of the inflammation into the adjacent musculature with fibrosis and increased mucous production (Fig. 6b). In the INH2BP-treated animals, the degree of arthritis was significantly reduced: a moderate, primarily neutrophil infiltration into several of the larger joints, coupled with mild to moderate necrosis and hyperplasia of the synovium (Fig. 6c).
Figure 5.
(a) Effect of INH2BP on the onset of collagen-induced arthritis. The percentage of arthritic mice (mice showing clinical scores of arthritis >1) are represented. (b) Effect of INH2BP on the severity of collagen-induced arthritis. Median arthritic score during collagen-induced arthritis. The arrow at 21 days represents the time of the second collagen immunization, the horizontal bar from day 25 represent the time of the start of treatment with INH2BP (n = 6) or vehicle (control; C) (n = 10). There was a significant increase in the arthritic score from day 26 (P < 0.01), and there was a significant suppression of the arthritic score by INH2BP between days 26 and 35 (P < 0.05).
Figure 6.
Representative histology of the paw of a control animal (a), an arthritic animal (b), and an INH2BP-treated arthritic animal (c). Note the reduction in the degree of mononuclear cell infiltration in the paws of the INH2BP-treated arthritic animals. (×32.)
DISCUSSION
Role of PARS in the Peroxynitrite-Induced Acute Cytotoxicity.
The studies presented in the current paper, using a recently developed pharmacological tool (INH2BP) (7, 8) and cells from a genetically engineered animal lacking PARS (9), provided evidence for a role of PARS activation in the peroxynitrite-induced acute suppression of mitochondrial respiration. We have also demonstrated that the protection offered by pharmacological inhibitors of PARS or by the PARS−/− phenotype against the peroxynitrite-induced cytotoxicity diminishes when cells are exposed to high concentrations of peroxynitrite. A likely explanation for this finding is that peroxynitrite triggers PARS-independent, parallel cytotoxic mechanisms. At lower concentrations of peroxynitrite, inhibition of PARS is sufficient to influence the net cellular energetic changes, whereas at higher concentrations of peroxynitrite overwhelming cytotoxicity occurs, which is possibly related to inhibition of membrane pumps, lipid peroxidation and protein oxidation and nitration, and direct inhibition of mitochondrial respiration (10–13, 27). In vivo, under inflammatory conditions, cells are likely to be exposed to lower concentrations of peroxynitrite for prolonged periods of time. Therefore, the exposure to lower concentrations of peroxynitrite is more likely to be comparable to the in vivo situation during inflammation.
Role of PARS in the Process of NO Production in Response to Immunostimulation.
The present studies also provide evidence for diminished NO production by the PARS−/− cells in response to immunostimulation, compared with the response in wild-type controls. Moreover, in immunostimulated wild-type cells, INH2BP reduced the production of NO to a level seen in the immunostimulated PARS−/− cells, but the agent had no effect on NO production in the PARS−/− cells. The production of NO in immunostimulated cells is due to the expression of iNOS (28). Our data are consistent with the view that basal PARS activity is involved in the process of iNOS expression. A similar conclusion has previously been reached with pharmacological inhibitors of PARS, such as nicotinamide and 3-aminobenzamide, and, more recently, also with INH2BP (29–31). Although the specific mechanisms whereby PARS regulates the expression of iNOS (and, possibly, the expression of other genes) remain to be clarified, recent studies have proposed a role for PARS in the process of transcription (31–33). There may be a specific region of the iNOS promoter that is regulated by inhibition of PARS (31). Suppression of iNOS expression may be an additional mechanism whereby inhibition of PARS suppresses the inflammatory response.
Role of PARS in Arthritis Development.
NO (derived from iNOS), oxy radicals, and peroxynitrite are important factors in the pathogenesis of various forms of inflammation, including arthritis (11, 20–22). Collagen-induced arthritis induces significant levels of iNOS expression, to an extent that is lower than the levels induced by adjuvant arthritis (20–22). Nevertheless, recent studies indicate that inhibition of iNOS and peroxynitrite scavenging suppresses the course of collagen-induced arthritis (22). The present data, demonstrating the presence of nitrotyrosine in the inflamed joint, further indicate the importance of the reactive nitrogen species in the pathophysiology of arthritis. The mediators, produced in arthritis, which are known triggers of DNA single-strand breakage are peroxynitrite and hydroxyl radical (1, 2).
The present study, demonstrating anti-inflammatory effects of INH2BP in the collagen-induced arthritis, supports the view that PARS is involved in the progression of the inflammatory process and that pharmacological inhibition of PARS is of anti-inflammatory potential. The primary mode of action of INH2BP is most likely related to an interruption of the futile intracellular cascade characterized by DNA injury, PARS activation, poly(ADP-ribosyl)ation, and metabolic suppression in various cell types of the inflamed joints. An additional mode of action may be related to suppression of the process of iNOS expression. Oxidant injury of the synovial fibroblasts and the endothelium promotes the infiltration of mononuclear cells into the inflamed joints (34). The roles of PARS activation in the oxidant-induced endothelial cell injury (35) and neutrophil recruitment into inflammatory sites (17, 36) are consistent with our observation that INH2BP reduces the degree of mononuclear cell infiltration into the joints.
Conclusions.
The present study establishes the role of PARS in cellular oxidant injury and the development of inflammation. PARS inhibition, alone or in combination with other anti-inflammatory agents, represents an additional anti-inflammatory approach. The demonstration of the lack of effect of INH2BP on oxidant injury or NO production in PARS−/− cells underlines the specificity of INH2BP as a cellular PARS inhibitor. This agent may be used in future studies to delineate the role of PARS in various forms of inflammation and oxidant injury.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R29GM54773) to C.S.
ABBREVIATIONS
- IFN-γ
interferon γ
- INH2BP
5-iodo-6-amino-1,2-benzopyrone
- LPS
bacterial lipopolysaccharide
- iNOS
inducible NO synthase
- PARS
poly(ADP-ribose) synthase
References
- 1.Cochrane C G. Mol Aspects Med. 1991;12:137–147. doi: 10.1016/0098-2997(91)90009-b. [DOI] [PubMed] [Google Scholar]
- 2.Szabó C. Free Radical Biol Med. 1996;21:855–869. doi: 10.1016/0891-5849(96)00170-0. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi Y, Shimizu S, Tsujimoto Y. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 4.Wilson G L, Patton N J, McCord J M, Mullins D W, Mossman B T. Diabetologia. 1984;27:587–591. doi: 10.1007/BF00276973. [DOI] [PubMed] [Google Scholar]
- 5.Cantoni O, Sestili P, Spadoni G, Balsamini C, Cucchiarini L, Cattabeni F. Biochem Int. 1987;15:329–337. [PubMed] [Google Scholar]
- 6.Farber J L, Kyle M E, Coleman J B. Lab Invest. 1990;62:670–679. [PubMed] [Google Scholar]
- 7.Bauer P I, Kirsten E, Varadi G, Young L J T, Hakam A, Comstock J A, Kun E. Biochimie. 1995;77:347–377. doi: 10.1016/0300-9084(96)88149-1. [DOI] [PubMed] [Google Scholar]
- 8.Bauer P I, Kirsten E, Young L J T, Varadi G, Csonka E, Buki K G, Mikala G, Hu R, Comstock J A, Mendeleyev J, Hakam A, Kun E. Int J Oncol. 1996;8:239–252. doi: 10.3892/ijo.8.2.239. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z Q, Auer B, Stingl L, Berghammer H, Haidacher D, Schweiger M, Wagner E F. Genes Develop. 1995;9:510–520. doi: 10.1101/gad.9.5.509. [DOI] [PubMed] [Google Scholar]
- 10.Beckman J S, Koppenol W H. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 11.Szabó C. Shock. 1996;6:79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Szabó C, Zingarelli B, O’Connor M, Salzman A L. Proc Natl Acad Sci USA. 1996;93:1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabó C, Zingarelli B, Salzman A L. Circ Res. 1996;78:1051–1063. doi: 10.1161/01.res.78.6.1051. [DOI] [PubMed] [Google Scholar]
- 14.Zingarelli B, O’Connor M, Wong H, Salzman A L, Szabó C. J Immunol. 1996;156:350–358. [PubMed] [Google Scholar]
- 15.Szabó C, Salzman A L, Ischiropoulos H. FEBS Lett. 1995;372:229–232. doi: 10.1016/0014-5793(95)00984-h. [DOI] [PubMed] [Google Scholar]
- 16.Hughes C, Wolos J A, Giannini E H, Hirsch R. J Immunol. 1994;153:3319–3325. [PubMed] [Google Scholar]
- 17.Cuzzocrea S, Zingarelli B, Costantino G, Szabó A, Salzman A L, Caputi A P, Szabó C. Br J Pharmacol. 1997;121:1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasama T, Strieter R M, Lukacs N W, Lincoln P M, Burdick M D, Kunkel S L. J Clin Invest. 1995;95:2868–2876. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuzzocrea S, Zingarelli B, O’Connor M, Salzman A L, Szabó C. Br J Pharmacol. 1997;122:493–503. doi: 10.1038/sj.bjp.0701387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCartney-Francis N, Allen J N, Mizel D E, Albina J, Xie Q W, Nathan C F, Wahl S M. J Exp Med. 1993;178:749–753. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefanovic-Racic M, Meyers K, Meschter C, Coffey J W, Hoffman R A, Evans C H. Arthritis Rheum. 1994;37:1062–1069. doi: 10.1002/art.1780370712. [DOI] [PubMed] [Google Scholar]
- 22.Brahn E, Banquerigo M L, Firestein G S, Boyle D L, Salzman A L, Szabó C. FASEB J. 1997;11:A530. [PubMed] [Google Scholar]
- 23.Kaur H, Halliwell B. FEBS Lett. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- 24.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin J C, Smith C D, Beckman J S. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 25.Eiserich J P, Hristova M, Cross C E, Jones A D, Freeman B A, Halliwell B, Van der Vliet A. Nature (London) 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 26.Halliwell B. FEBS Lett. 1997;411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- 27.Heller B, Wang Z Q, Wagner E F, Radons J, Burkle A, Fehsel K, Burkart V, Kolb H. J Biol Chem. 1995;270:11176–11180. doi: 10.1074/jbc.270.19.11176. [DOI] [PubMed] [Google Scholar]
- 28.Nathan C. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 29.Pellat-Seceunyk D, Wietzerbin J, Drapier J C. Biochem J. 1994;297:53–58. doi: 10.1042/bj2970053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zingarelli B, O’Connor M, Wong H, Salzman A L, Szabó C. J Immunol. 1996;156:350–358. [PubMed] [Google Scholar]
- 31.Szabó C, Wong H R, Bauer P I, Kirsten E, O’Connor M, Zingarelli B, Mendeleyev J, Hasko G, Vizi E S, Salzman A L, Kun E. Int J Oncol. 1997;10:1093–1101. doi: 10.3892/ijo.10.6.1093. [DOI] [PubMed] [Google Scholar]
- 32.Griffin M J, Kirsten E, Carubelli R, Palakodety R B, McLick J, Kun E. Biochem Biophys Res Commun. 1984;122:770–775. doi: 10.1016/s0006-291x(84)80100-x. [DOI] [PubMed] [Google Scholar]
- 33.Meisterernst M, Stelzer G, Roeder R G. Proc Natl Acad Sci USA. 1997;94:2261–2265. doi: 10.1073/pnas.94.6.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mertens A V, de Clerck L S, Moens M M, Bridts C H, Stevens W J. Res Immunol. 1994;145:101–108. doi: 10.1016/s0923-2494(94)80020-0. [DOI] [PubMed] [Google Scholar]
- 35.Szabó C, Cuzzocrea S, Zingarelli B, O’Connor M, Salzman A L. J Clin Invest. 1997;100:723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabó C, Lim L H, Cuzzocrea S, Getting S J, Zingarelli B, Flower R J, Salzman A L, Perretti M. J Exp Med. 1997;186:1041–1049. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]