Abstract
Despite the central role of human epidermal stem cells in tissue homeostasis, wound repair, and neoplasia, remarkably little is known about these cells, largely due to the absence of molecular markers that distinguish them from other proliferative cells within the germinative/basal layer. Epidermal stem cells can be distinguished from other cells in the basal layer by their quiescent nature in vivo and their greater overall proliferative capacity. In this study, we demonstrate enrichment and isolation of a subpopulation of basal epidermal cells from neonatal human foreskin based on cell surface phenotype, which satisfy these criteria. These putative stem cells are distinguished from other basal cells by their characteristic expression of high levels of the adhesion molecule α6, a member of the integrin family (α6bri), and low levels of a proliferation-associated cell surface marker recognized by recently described mAb 10G7 (10G7dim). We conclude that cells with the phenotype α6bri10G7dim represent the epidermal stem cell population based on the demonstration that these cells (i) exhibit the greatest regenerative capacity of any basal cells, (ii) represent a minor subpopulation (≈10%) of immature epidermal cells, which (iii) are quiescent at the time of isolation from the epidermis, as determined by cell cycle analysis.
In common with other rapidly renewing tissues such as the hemopoietic system and the intestinal epithelia, the human epidermis is in a process of constant regeneration. Terminally differentiated cells lost continuously from the skin surface are replaced by an intricate and highly regulated proliferative process within the basal layer of the epidermis. In murine epidermis, this process is achieved by two kinetically distinct subpopulations of basal epidermal cells: (i) keratinocyte stem cells (KSC), which represent a minor subpopulation of relatively quiescent cells, defined by their great proliferative potential and an unlimited capacity for self-renewal, identified as slow-cycling, [3H]Tdr label-retaining cells; and (ii) transit amplifying (TA) cells—the progeny of the stem cells, with a limited proliferative capacity identified as a pool of rapidly proliferating cells that are lost from the basal layer to terminal differentiation within 4–5 days (1–5). In addition, a third subpopulation of basal keratinocytes representing postmitotic differentiating cells in the early stages of keratinization can also be identified (6, 7).
In the hemopoietic system, multilineage reconstituting stem cells can be physically separated from committed progenitor cells (analogous to the TA cells of the epidermis), and their differentiated progeny can be based on differences in their expression of cell surface markers (8–12). Clearly, the availability of appropriate cell surface markers on basal epidermal cells would greatly facilitate the isolation and characterization of human KSCs. However, the cell surface antigenic phenotype of these cells remains relatively poorly defined. One of the best-studied classes of cell surface molecules expressed by keratinocytes are the integrin superfamily of cell adhesion receptors. Integrins are heterodimeric cell surface glycoproteins that primarily mediate the attachment of basal keratinocytes to extracellular matrix proteins found in the basement membrane, but can also mediate intercellular adhesion. In vivo, basal keratinocytes express the β1 integrins α2β1 and α3β1 as well as the integrin α6β4 (13–15). Important evidence for proliferative heterogeneity in human basal keratinocytes has been provided by recent work that used a fluorescence-activated cell sorting (FACS) approach, demonstrating that both cultured and primary human foreskin keratinocytes could be separated into cells with high levels of β1 integrin that had a high plating efficiency assayed after 2 weeks in culture compared with those keratinocytes with low levels of this integrin (16, 17). Furthermore, keratinocytes expressing high levels of β1 integrin were shown to be capable of generating an epithelial sheet when grafted onto mice, suggesting that this fraction of the basal layer contain KSCs (17).
In vivo studies suggest that epidermal stem cells constitute between 1 and 10% of the basal layer, depending on the methodology used (2, 3, 5, 18, 19). Because ≈40% of the basal layer in human foreskin exhibits high levels of β1 integrin in vivo (17), it is highly likely that basal keratinocytes with this phenotype contain both the KSC population and a significant number of TA cells. In the present study we describe a strategy for distinguishing between these two populations of keratinocyte progenitors based on the use of two cell surface antigens. In view of functional data demonstrating the role of integrin α6β4 in mediating adhesion of basal keratinocytes to the basement membrane via hemi-desmosomes (20–23), we reasoned that this integrin may provide a suitable marker for epidermal stem cells because these cells are permanently anchored to the basement membrane. Here, we report that although basal keratinocytes expressing low levels of α6β4 represent a subpopulation of postmitotic, differentiating keratinocytes, this integrin is expressed at high levels on both the KSC and TA cells. Thus this cell surface marker alone cannot be used to separate KSCs from TA cells. However, we provide convincing evidence that enrichment for human KSCs can be successfully achieved on the basis of a second cell surface component whose expression is proliferation-related, and is detected by a mAb (10G7) recently generated in our laboratory (24).
MATERIALS AND METHODS
Isolation and Culture of Primary Basal Keratinocytes.
Human neonatal foreskins from routine circumcisions were processed within 2 h of collection. Epithelial sheets were obtained after overnight incubation with 4 mg/ml Dispase at 4°C, and basal keratinocytes were isolated by trypsinization for 5 min. Keratinocytes were cultured by using the Rheinwald and Green method (25), on irradiated Swiss 3T3-J2 feeder layers in DMEM containing 10% fetal calf serum, 20 ng/ml epidermal growth factor (Sigma), 0.4 μg/ml hydrocortisone (Sigma), and 10 ng/ml cholera toxin (Calbiochem). Keratinocytes were passaged after removing the feeder cells with 0.02% EDTA.
Antibodies.
mAb 4F10 (IgG2b) to the α6 integrin subunit was used at 20 μg/ml (Serotec); mAb 10G7 (IgG2a) developed in our laboratory (24) against a cell surface antigen overexpressed by a previously described tumorigenic keratinocyte cell line (26) was used as undiluted hybridoma supernatant. Isotype-matched negative control mAbs 1D4.5 (IgG2a), 1B5 (IgG1), and 1A6.11 (IgG2b) were available in house. Anti-mouse IgG2b–fluorescein isothiocyanate (FITC) and IgG2a-phycoerythrin (PE) (Caltag, South San Francisco, CA) were used to detect 4F10 and 10G7 binding, respectively. mAbs LHP2 (IgG1) to K10 and LL001 (IgG2a) to K14 were kindly provided by Irene Leigh (Royal London Hospital, London) and used at 1:10 and 1:1,000, respectively.
Immunofluorescence Staining and FACS of Primary Keratinocytes.
Basal keratinocytes were processed for single (α6-FITC) or double (α6-FITC and 10G7 ag-PE) staining along with appropriate negative controls and single color positive controls to establish compensation settings on the FACS. The cells were resuspended in culture medium at 2–3 × 106/ml, sorted by using the Becton Dickinson FACStarPlus, and collected into culture medium. The viability of the cells after sorting was determined to be >95% by trypan blue exclusion. Double staining for keratins and α6 was performed on cells fixed and permeabilized in 70% ethanol at −20°C for 10 min.
Determination of Total Cell Output of Fractionated Basal Cells as an Indicator of Enrichment for Epidermal Stem Cells.
The KSC population has been defined as a minor subpopulation of the basal layer with greatest proliferative capacity because it must sustain tissue renewal for a lifetime. Based on the assumption that KSCs have a specific cell surface phenotype, as has been demonstrated for bone marrow hemopoietic progenitors, when plating equivalent numbers of cells with the hypothesized KSC phenotype and unfractionated (UF) cells, one should clearly expect greater cell output from the former population because it has been enriched for stem cells. At the start of each long-term culture experiment, cells were fractionated on the basis of their cell surface phenotype. A total of 5,000 keratinocytes from each fraction were plated into 24-well plates containing monolayers of feeder cells. All fractions per experiment were plated in triplicate, carried in parallel, and passaged at the same time. The number of cells produced by each fraction was determined at each passage (with the exception of the first passage, where the number of cells are very small) by harvesting the cells and obtaining cell counts. At first passage, cells from each fraction were pooled and plated equally into three wells of a 6-well plate. At subsequent passages, all fractions were replated at 5 × 104 cells per well of a 6-well plate in triplicate, irrespective of cell yield. The keratinocytes were continually passaged until their growth capacity had been exhausted. The cumulated total cell output of 5,000 cells from each fraction was then determined at the end of each experiment. Because only 5 × 104 cells were replated at each passage, the cell outputs were calculated assuming all the cells from the previous passage had been replated. The duration of each experiment was dependent on the individual keratinocyte cultures, but was generally between 75 and 95 days.
Cell Cycle Analysis.
Fractions of primary basal keratinocytes were collected after processing through the FACStarPlus as described above, and washed twice with PBS before fixation with 70% ethanol (−20°C) for 1 h at 4°C. Cells were washed and resuspended in 250–500 μl of PBS. RNase (40 μg) was added prior to the addition of propidium iodide at a final concentration of 40 μg/ml. The cells were incubated for 1 h at 37°C and subsequently stored at 4°C. DNA content was analyzed by flow cytometry on an EPICS XL flow cytometer (Coulter) within 12 h.
RESULTS
Given that stem cells may not maintain their in vivo characteristics in culture after removal from their “niche” or microenvironment (27), we elected to analyze freshly isolated primary epidermal cells. We compared the relative proliferative capacity measured as total cell output, following long-term culture of parallel fractions of basal keratinocytes, and the cycling status of these fractions upon isolation from the foreskin, reasoning that by definition, KSCs would be distinguished from TA cells based on well-accepted differences attributed to these two populations of proliferative cells. That is, the KSC subpopulation defined by its relative quiescence in vivo and the greatest proliferative potential in vitro, compared with the TA cells characterized by their actively cycling status in vivo, reduced proliferative potential and more rapid terminal differentiation in culture (28).
Separation of Basal Keratinocytes into Proliferative Cells and Postmitotic Differentiating Cells Based on Expression of the α6 Integrin.
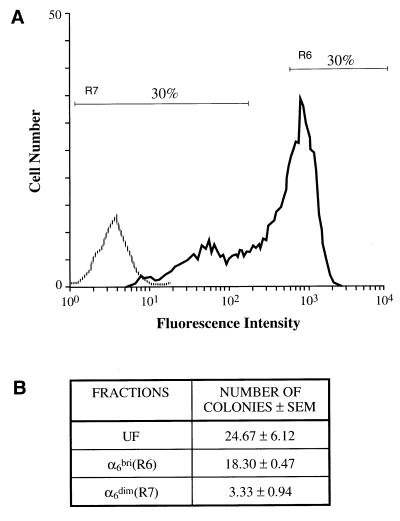
In accord with published studies on the expression of α6β4 in neonatal human foreskin in vivo (15), freshly isolated basal keratinocytes were found to be α6 positive by flow cytometric analysis. However, a bimodal pattern of expression was consistently observed (Fig. 1A, n = 25). Two fractions of cells, the upper 30% population (α6bri) and the lower 30% population (α6dim), together with UF cells, were compared in culture. The colony forming ability of these primary keratinocytes determined at 2 weeks (Fig. 1B), in five separate experiments, showed that the α6bri cells consistently gave rise to greater colony numbers than the α6dim cells (typically 18.3 ± 0.47 versus 3.33 ± 0.94, respectively), but was not significantly different than UF cells (typically 24.67 ± 6.12), suggesting that the majority of proliferating cells were in the α6bri fraction.
Figure 1.
Fractionation and colony-forming ability of neonatal primary human foreskin basal epidermal cells on the basis of α6 integrin expression. (A) Flow cytometric analysis of freshly isolated basal keratinocytes stained with either an anti-α6 mAb (4F10; solid line) or isotype control mAb (1A6.11; broken line) detected by a FITC-conjugated secondary antibody. Two fractions representing the upper 30% (R6) α6bri cells and the lower 30% (R7) α6dim cells were collected by FACS and cultured. (B) Colony numbers obtained from 5,000 UF, α6bri, and α6dim cells. Keratinocyte colony numbers were determined after 2 weeks in culture by staining with toluidine blue after removal of the feeder layers. The α6bri fraction consistently gave rise to greater colony numbers than the α6dim fraction, indicating that the α6bri fraction was enriched for colony forming cells. These results are typical of several replicate experiments (n = 5).
Studies in the hemopoietic system demonstrate that stem cells with marrow repopulating activity do not clone directly in vitro, but will over time in culture give rise, through differentiation, to clonogenic cells (29–31). By analogy, it is possible that the colony-forming ability of keratinocytes measured over a 2-week period may not accurately predict the long-term growth capacity of KSCs. We therefore compared the long-term proliferative capacity of the α6bri and α6dim populations by assaying total cell output following serial passage until all growth potential was exhausted (typically 75–95 days). The data obtained from several separate experiments (n = 5) demonstrated clearly that basal cells with the greatest long-term proliferative capacity reside in the α6bri subpopulation (Fig. 2).
Figure 2.
Long-term growth capacity of α6bri and α6dim cells. (A) Growth curves of UF cells and the α6bri and α6dim fractions in a representative experiment are shown. Each point represents the mean cell output ± SEM of three replicate wells obtained at each passage. The curves show that the α6bri cells consistently grew at a greater rate than the UF cells and the α6dim fraction. (Inset) Growth curves from day 0–50 to illustrate cell proliferation during this period, which is not evident on the main graph due to the scale. These results are typical of several replicate experiments (n = 5). (B) The total cell output (cumulative cell yield) from 5,000 cells of each fraction was determined at the end of the experiment when their ability to proliferate was exhausted. The total cell output of the α6bri fraction was significantly higher than the α6dim fraction and UF cells (P < 0.05), thus confirming that the α6bri population had the greatest long-term proliferative capacity. The numbers above the columns indicate mean cell yields from each fraction. The results shown represent the mean total output ± SEM of three separate experiments.
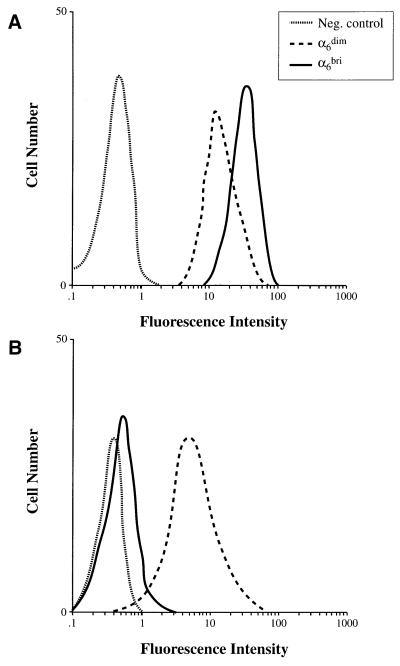
In vivo, epidermal cells exhibit ordered expression of pairs of keratins (K). Thus, K5 and K14 are expressed by basal cells, whereas K1 and K10 are predominantly expressed in the suprabasal differentiating layers of the epidermis (32). However, K10 expression has also been observed in a minor subpopulation of basal cells in murine epidermis (33, 34), suggesting the presence of differentiating cells within the basal layer. Flow cytometric analysis demonstrated that both the α6dim and α6bri fractions were K14 positive (Fig. 3A), although the α6dim cells showed significantly lower levels of K14 than α6bri cells (n = 4). In contrast, although the α6bri keratinocytes were negative for K10, the α6dim fraction expressed this differentiation marker (Fig. 3B). Collectively, these data demonstrate that the α6dim fraction comprise a population of postmitotic differentiating basal cells, whereas the α6bri fraction contains the majority of proliferative basal keratinocytes (i.e., KSCs and TA cells).
Figure 3.
Two-color flow cytometric analysis of α6 and keratins 14 and 10 in neonatal primary human foreskin basal epidermal cells. Freshly isolated keratinocytes were fixed, permeabilized, and double labeled with anti-α6 integrin (mAb 4F10) and either (A) anti-K14 (mAb LL001) or (B) anti-K10 (mAb LHP2). Cells were analyzed for keratin expression after gating into α6bri and α6dim fractions. (A) Both these fractions were positive for the basal keratin K14, but the α6dim cells expressed lower levels of K14 than the α6bri cells. (B) The α6bri fraction was negative for the differentiation-specific keratin, K10, whereas α6dim cells were positive for this marker. Staining with appropriate isotype-matched negative control mAbs (ID4.5 and IB5) is shown (dotted lines) in each panel.
Human Epidermal Stem Cells Can Be Resolved Further Within the α6bri Population on the Basis of 10G7 Antigen Expression.
In vivo cell kinetic studies have established that KSCs are largely quiescent and do not proliferate at high rates, whereas TA cells are actively cycling (1–5). We therefore reasoned that these two populations could be distinguished at the time of initial isolation from the epidermis, on the basis of a second proliferation-associated cell surface marker recognized by mAb 10G7 recently generated in our laboratory (24). mAb 10G7 was raised against a previously described tumorigenic human keratinocyte cell line (26) and recognizes an as-yet-unidentified cell surface component (termed 10G7 Ag) that appears to be highly expressed on actively proliferating keratinocytes and has a classical oncofetal pattern of expression (24). Thus, the 10G7 Ag is readily detected in fetal epithelium including hair follicles, and both the benign and malignant forms of breast and genital carcinoma. Although 10G7 Ag could not be detected in foreskin epithelium in vivo, it was readily detected upon dissociation of the basal epidermal cells from this tissue, suggesting that the epitope identified by mAb 10G7 is somehow masked in normal tissue in vivo. In vitro, subconfluent cultures of keratinocytes express 10G7 Ag, but down-regulate its expression on differentiation. Thus, the expression of the 10G7 Ag is clearly correlated with proliferating keratinocytes (24). We therefore hypothesized that the relatively quiescent KSCs would have low levels of 10G7 Ag, whereas the actively proliferating TA cells would have high levels of this Ag.
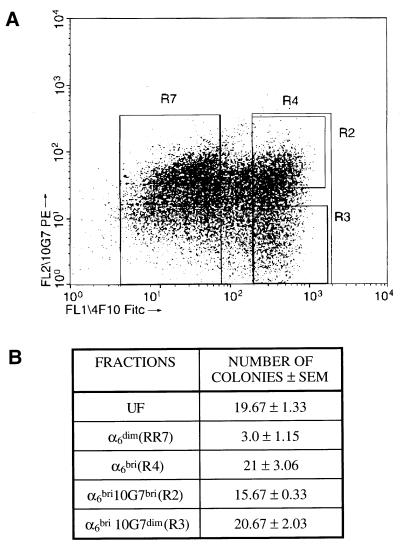
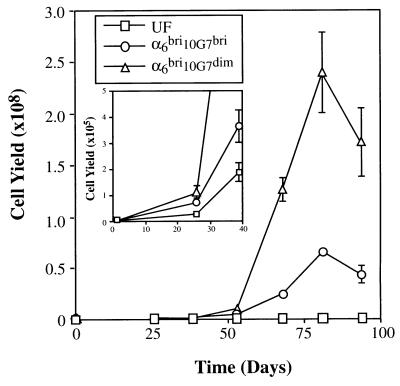
Flow cytometric analysis of freshly isolated human epidermal basal cells double-labeled with mAb 10G7 and anti-α6 integrin antibody consistently showed that the α6bri population demonstrated a broad range of 10G7 Ag expression with the majority exhibiting relatively high levels of expression and the remainder, low levels (Fig. 4A; n = 25). The α6bri population was separated by FACS into the upper 30% (α6bri10G7bri) and the lower 30% (α6bri10G7dim) of 10G7 Ag expressing cells. The short-term proliferative capacity of these fractionated basal keratinocytes was similar (Fig. 4B) as determined by the colony numbers obtained at 2 weeks, typically 15.67 ± 0.33 versus 20.67 ± 2.03 from α6bri10G7bri and α6bri10G7dim, respectively (n = 5). However, these two subpopulations differed markedly in their capacity to sustain long-term generation of keratinocytes. The growth curves from a typical experiment are shown in Fig. 5 and illustrate that the α6bri10G7dim population exhibits a significantly greater proliferative potential than any of the other populations assayed (P < 0.05). In this experiment, total cell outputs from 5,000 UF, α6dim, α6bri10G7bri, or α6bri10G7dim were 7 × 105, 1.9 × 107, 1.4 × 108, and 5.5 × 108, respectively. The absolute number of cells generated by a particular fraction in long-term culture was variable between experiments as shown in Table 1 and can be attributed to variation between skin donors, and the duration of the experiment, dictated by the period for which cells from a particular donor could be maintained in culture. However, importantly the α6bri10G7dim fraction consistently contained basal epidermal cells with the greatest total cell output in several replicate experiments (Table 1, n = 5). Interestingly, the α6bri10G7dim cells exhibited significantly greater rates of growth in culture, particularly between day 50 and 80 compared with the other fractions (Fig. 5), ultimately resulting in the greatest cumulative cell output of any fraction. These data demonstrate that the KSCs can be markedly enriched from the α6bri fraction of basal keratinocytes on the basis of 10G7 Ag expression, and clearly reside in the α6bri10G7dim fraction.
Figure 4.
Fractionation and colony-forming ability of neonatal primary human foreskin basal epidermal cells on the basis of α6 integrin and 10G7 Ag expression. (A) Dot plot showing flow cytometric analysis of freshly isolated basal keratinocytes double-labeled with anti-α6 mAb 4F10 (FITC) and mAb 10G7 (PE) from a representative experiment. This phenotype has been observed in numerous replicate experiments (n = 25). Four fractions of cells were collected in this experiment: the α6dim population (R7 = 45.63%), the α6bri cells (R4 = 30.98%), and the latter cells subdivided on the basis of relatively high (R2 = 13.41%, α6bri10G7bri) or low levels (R3 = 9.79%, α6bri10G7dim) of 10G7 Ag expression. (B) Colony numbers obtained from 5,000 cells from each fraction at 2 weeks in culture. The α6bri10G7dim and α6bri10G7bri fractions consistently gave rise to greater colony numbers than the α6dim fraction. Importantly, no significant difference between colony numbers was obtained from the α6bri10G7dim and α6bri10G7bri fractions. These results are typical of several replicate experiments (n = 5).
Figure 5.
Long-term growth capacity of primary human neonatal foreskin basal epidermal cells fractionated on the basis of α6 and 10G7 Ag expression. Growth curves of UF, α6bri10G7dim, and α6bri10G7bri fractions in a representative experiment (experiment II). The curves show that the α6bri10G7dim cells consistently grew at a greater rate than the UF and α6bri10G7bri cells. (Inset) Cell output at earlier time points (day 0–50) is shown and indicates that all fractions were capable of growth in culture, which is not evident on the main graph due to the scale. Data points represent mean ± SEM of three replicate wells.
Table 1.
Total cell output of primary basal keratinocyte fractions in five independent experiments
| Exp. | Fractions | Length of experiment, days | Total cell output |
|---|---|---|---|
| I | UF | 83 | 4.47 × 106† |
| α6dim10G7bri | 3.60 ± 0.02 × 106 | ||
| α6dim10G7dim | 3.74 ± 0.02 × 107 | ||
| α6bri10G7bri | 5.42 ± 0.17 × 107 | ||
| α6bri10G7dim | 1.78 ± 0.16 × 108* | ||
| II | UF | 94 | 7.03 ± 0.02 × 105 |
| α6dim | 1.92 ± 0.01 × 107 | ||
| α6bri10G7bri | 1.37 ± 0.08 × 108 | ||
| α6bri10G7dim | 5.53 ± 0.34 × 108* | ||
| III | UF | 94 | 5.91 ± 0.19 × 108 |
| α6bri10G7bri | 8.02 ± 0.22 × 109 | ||
| α6bri10G7dim | 1.17 ± 0.001 × 1010* | ||
| IV | UF | 90 | 8.34 ± 0.02 × 106 |
| α6bri | 3.84 × 107† | ||
| α6bri10G7bri | 5.16 ± 0.09 × 108 | ||
| α6bri10G7dim | 7.77 ± 0.86 × 109* | ||
| V | α6bri10G7bri | 85 | 5.38 ± 1.00 × 109 |
| α6bri10G7dim | 1.58 ± 0.047 × 1010* |
The total cell output from an initial input of 5,000 cells per fraction ± SEM of three replicates at the end of each experiment is indicated, confirming that the α6bri10G7dim population has the greatest long-term proliferative capacity.
The total cell output of the α6bri10G7dim fraction was significantly higher than the α6bri10G7bri fraction and UF cells (P < 0.05).
Output from a single well.
The Candidate KSC Fraction (α6bri10G7dim) Represents a Quiescent Subpopulation of the Epidermal Basal Layer.
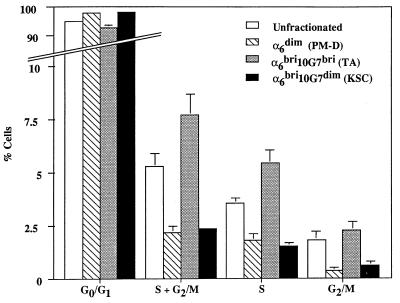
To investigate the in vivo cycling status of the α6bri10G7dim and the α6bri10G7bri subpopulations, freshly isolated basal keratinocytes were fractionated and immediately stained with propidium iodide for flow cytometric analysis. The results obtained from four separate experiments shown in Fig. 6 demonstrate that the majority of actively cycling basal keratinocytes (i.e., cells in S + G2/M phase) reside in the α6bri10G7bri (putative TA) fraction, whereas α6bri10G7dim basal keratinocytes, designated as the putative KSC fraction, contain significantly more quiescent cells (P = 0.0004). Basal keratinocytes designated as the postmitotic differentiating fraction (α6dim) did not contain many cycling cells, as expected. These observations are in close accord with published data demonstrating that in vivo, ≈5% of basal keratinocytes (UF) are engaged in DNA synthesis (7) and confirm that the α6bri10G7dim fraction exhibits predicted stem cell characteristics, representing an initially quiescent subpopulation of basal epidermal cells capable of the greatest regenerative capacity in vitro.
Figure 6.
Cell cycle analysis of primary basal keratinocytes fractionated on the basis of α6 and 10G7 ag expression. The UF cells show that overall, the basal layer contains about 5% of cells progressing through the S phase of the cell cycle. Analysis of fractionated cells clearly demonstrates that the majority of these actively cycling basal cells reside within the candidate TA population (α6bri10G7bri cells), whereas the candidate KSC population (α6bri10G7dim) and the postmitotic differentiating (PM-D) cells (α6dim fractions) comprise mostly quiescent cells, with relatively fewer cells in S or S/G2M phase. The results displayed are the mean ± SEM of four separate experiments.
DISCUSSION
Short-Term Colony Forming Ability Versus Long-Term Total Cell Output as an Assay for KSCs.
Previous work on keratinocyte progenitors has been based on the assumption that cells with greater colony-forming ability determined over 2 weeks are equivalent to clonogenic stem cells (16, 17). Our data clearly indicate that although this assay is a useful tool for distinguishing essentially postmitotic basal keratinocytes from the total proliferative pool of basal keratinocytes, including both the KSCs and TA cells, it does not permit the separation of these two populations of keratinocyte progenitors. Consistent with published data from the hemopoietic system, which indicate that stem cells may not directly form colonies, but will in culture give rise to colony-forming cells (29–31), we have shown that these KSCs can only be distinguished from TA cells by assaying for long-term proliferative potential. This finding is attributed to their ability to sustain a comparatively greater growth rate over the TA fraction during the course of the entire experiment. Thus, our work illustrates the need to more critically evaluate assays for defining the stem cell population.
KSCs Cannot Be Distinguished from TA Cells by the Level of α6 Integrin Expression.
Contrary to our expectation that KSCs may exclusively express high levels of α6β4 integrin to maintain tight adhesion to the basement membrane, our data clearly demonstrate that this integrin is highly expressed on both KSCs and TA cells (α6bri cells). However, postmitotic basal cells already exhibiting differentiation characteristics demonstrated lower levels of α6 integrin (α6dim cells), presumably in preparation for migration into the suprabasal layer. Interestingly, these K10 positive basal keratinocytes were able to demonstrate significant proliferative activity in vitro, indicating that their commitment to differentiate in vivo can be reversed by placing them in culture. This finding is similar to the in vivo induction of proliferation in suprabasal cells during wound healing.
Basal Keratinocytes with the Phenotype α6bri10G7dim Have Important Stem Cell Attributes.
Our strategy for enriching for epidermal stem cells on the basis of a proliferation-related cell surface marker has proven to be a valuable approach, allowing the separation of proliferative basal cells into an actively cycling TA compartment with lower proliferative capacity (α6bri10G7bri), with the quiescent KSC compartment demonstrating the greatest regenerative capacity in long-term culture (α6bri10G7dim). A total of 5,000 cells with this phenotype could give rise to ≈1.17 × 1010 cells (mean of experiments III, IV, and V in Table 1). Given the initial colony-forming efficiency of this population of 0.4% (Fig. 4B), and making the assumption that only those cells that give rise to colonies contribute to total cell output, the number of cells generated at the end of the experiment can be attributed to about 20 cells. We therefore calculate that a single candidate KSC with the phenotype α6bri10G7dim can generate ≈5.8 × 108 cells. It is highly likely that the present culture conditions, while promoting very effective growth of the TA population, do not permit optimal cell generation from or self-renewal of the KSC population. Further characterization of the KSCs and the determination of their specific growth factor requirements may allow us to more accurately assess their true regenerative capacity. The ability of our candidate stem cell fraction to reconstitute epithelium in vivo and to exhibit self-renewal are important stem cell characteristics that need to be determined.
Our data also show that the candidate KSC fraction represents an immature and minor subpopulation of ≈10% of the basal layer, consistent with estimates of 1–10% from kinetic studies in murine epidermis (2–5, 18, 19). Given that we have used neonatal human foreskin tissue that is capable of greater proliferation than adult foreskin epithelium (25), it is likely that these KSC numbers are higher than may be found in adult epidermis.
Previous reports suggest that enrichment of KSCs can be achieved by selecting cells expressing high levels of β1 integrin (16, 17). Work in our own laboratory indicates that both the KSC and TA fractions express high levels of β1 and α6 integrins. In addition, we have observed that the selection of α6bri10G7dim cells allows the isolation of greater numbers of putative stem cells than β1bri10G7dim cells (A.L. and P.K., unpublished data). Our data suggests that this can probably be attributed to the fact that although the majority of basal keratinocytes express high levels of both of these integrins, there is a significant subpopulation of β1bri cells that express low levels of α6 (α6dim postmitotic differentiating cells). Thus it appears that both integrins are not down-regulated from the basal cell surface concomitantly and may reflect the adhesive properties of postmitotic differentiating cells within the basal layer, which have decreased their adhesion to the basement membrane via down-regulation of α6 expression, but retain high levels of β1 expression to maintain cell–cell adhesion.
It is noteworthy that the TA compartment remains indistinguishable from the KSC compartment in terms of known molecular markers. Clearly, our ability to recognize this population phenotypically will permit us to investigate the molecular differences between these two populations. This work will provide a basis for the identification of genes with a critical role in epidermal growth and differentiation, and factors regulating self-renewal of KSCs. Further, it has important implications for the study of epidermal carcinogenesis, given that the stem cells are likely to be a target for carcinogens resulting in the development of carcinomas (35). Finally, the accessibility of skin makes human KSCs an ideal vehicle for genetic manipulation and gene therapy for the treatment of both skin disorders and systemic deficiencies. The ability to identify and isolate these cells represents an important prerequisite for the development of these approaches.
Acknowledgments
We are grateful to the staff of The Women’s and Children’s Hospital and Ashford Hospital, Adelaide, for providing us with human neonatal foreskins. We thank Judy Haywood and Alan Bishop for their expert help in cell sorting. This work was supported by a grant from the National Health and Medical Research Council of Australia to P.K.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: KSC, keratinocyte stem cell; TA, transit amplifying; UF, unfractionated; FACS, fluorescence-activated cell sorting; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
References
- 1.Potten C S. In: Stem Cells: Their Identification and Characterization. Potten C S, editor. London: Churchill Livingston; 1983. pp. 200–232. [Google Scholar]
- 2.Morris R J, Fischer S M, Slaga T J. J Invest Dermatol. 1985;84:277–281. doi: 10.1111/1523-1747.ep12265358. [DOI] [PubMed] [Google Scholar]
- 3.MacKenzie I C, Bickenbach J R. Cell Tissue Res. 1985;242:551–556. doi: 10.1007/BF00225420. [DOI] [PubMed] [Google Scholar]
- 4.Potten C S. Int J Radiat Biol. 1986;49:257–278. doi: 10.1080/09553008514552541. [DOI] [PubMed] [Google Scholar]
- 5.Bickenbach J R, McCutecheon J, MacKenzie I C. Cell Tissue Kinet. 1986;19:325–333. doi: 10.1111/j.1365-2184.1986.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 6.Christophers E. J Invest Dermatol. 1971;56:165–169. doi: 10.1111/1523-1747.ep12260765. [DOI] [PubMed] [Google Scholar]
- 7.Allen T D, Potten C S. J Cell Sci. 1974;15:291–319. doi: 10.1242/jcs.15.2.291. [DOI] [PubMed] [Google Scholar]
- 8.Civin C L, Strauss L C, Brovall C, Frackler M J, Schwartz J F, Shaper J H. J Immunol. 1984;133:57–165. [PubMed] [Google Scholar]
- 9.Spangrude G J, Heinfeld S, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 10.Berenson R J, Bensinger W I, Hill R S, Andrews R G, Garcia-Lopez J, et al. Blood. 1991;77:1717–1722. [PubMed] [Google Scholar]
- 11.Terstappen L W, Huang S, Safford M, Lansdorp P M, Loken M R. Blood. 1991;77:1218–1227. [PubMed] [Google Scholar]
- 12.Baum C M, Weissman I L, Tsukamoto A S, Buckle A M, Peault B. Proc Natl Acad Sci USA. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peltonen J, Larjava H, Jaakkola S, Grainick H, Akiyama S K, Yamada S S, Yamada K M, Uitto J. J Clin Invest. 1989;84:1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter W G, Wayner E A, Bouchard T S, Kaur P. J Cell Biol. 1990;110:1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter W G, Kaur P, Gil S G, Gahr P J, Wayner E A. J Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones P H, Watt F M. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 17.Jones P H, Harper S, Watt F M. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 18.Potten C S, Hendry J H. Int J Radiat Biol. 1973;24:537–540. doi: 10.1080/09553007314551441. [DOI] [PubMed] [Google Scholar]
- 19.Morris R J, Potten C S. Cell Prolif. 1994;27:279–289. doi: 10.1111/j.1365-2184.1994.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 20.Sonnenberg A, Calafat J, Janssen H, Daams H, Raaij-Helmer L M H, et al. J Cell Biol. 1991;113:907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowling J, Yu Q-C, Fuchs E. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georges-Labouesse E, Messaddeq N, Yehia G, Cadakbert L, Dierich A, Le-Meur M. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 23.Van-der-Neut R, Krimpenfort P, Calafat J, Neissen C M, Sonnenberg A. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 24.Kaur P, Paton S, Furze J, Wrin J, Olsen S, Danks J, Scurry J. J Invest Dermatol. 1997;109:194–199. doi: 10.1111/1523-1747.ep12319332. [DOI] [PubMed] [Google Scholar]
- 25.Rheinwald J G, Green H. Cell. 1975;6:331–344. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 26.Hurlin P J, Kaur P, Smith P P, Perez-Reyes N, Blanton R A, McDougall J K. Proc Natl Acad Sci USA. 1991;88:570–574. doi: 10.1073/pnas.88.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schofield R. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 28.Lajtha L G. Differentiation (Berlin) 1979;14:23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 29.Sutherland H J, Lansdorp P M, Henkelman D H, Eaves A C, Eaves C J. Proc Natl Acad Sci USA. 1990;87:3584–3588. doi: 10.1073/pnas.87.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haylock D N, To L B, Dowse T L, Juttner C A, Simmons P J. Blood. 1992;80:1405–1412. [PubMed] [Google Scholar]
- 31.Haylock D N, Horsfall M J, Dowse T L, Ramshaw H S, Niutta S, et al. Blood. 1997;90:2260–2272. [PubMed] [Google Scholar]
- 32.Fuchs E, Green H. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- 33.Schweizer J, Kinjo M, Furstenberger G, Winter H. Cell. 1984;37:159–170. doi: 10.1016/0092-8674(84)90311-8. [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie I C, Mackenzie S L, Rittman G A. Differentiation (Berlin) 1989;41:127–138. doi: 10.1111/j.1432-0436.1989.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 35.Morris R J, Fischer S M, Slaga T J. Cancer Res. 1986;46:3061–3066. [PubMed] [Google Scholar]