Abstract
Neurotransplantation has been used to explore the development of the central nervous system and for repair of diseased tissue in conditions such as Parkinson’s disease. Here, we examine the effects of direct injection into rat brain of human marrow stromal cells (MSCs), a subset of cells from bone marrow that include stem-like precursors for nonhematopoietic tissues. Human MSCs isolated by their adherence to plastic were infused into the corpus striatum. Five to 72 days later, brain sections were examined for the presence of the donor cells. About 20% of the infused cells had engrafted. There was no evidence of an inflammatory response or rejection. The cells had migrated from the injection site along known pathways for migration of neural stem cells to successive layers of the brain. After infusion into the brain, the human MSCs lost their immunoreactivity to antibodies for collagen I. Initially, the human cells continued to stain with antibodies to fibronectin but the region of staining with fibronectin was significantly decreased at 30 and 72 days. The results suggest that MSCs may be useful vehicles for autotransplantation in both cell and gene therapy for a variety of diseases of the central nervous system.
Keywords: neurotransplantation, stem cells, Parkinson’s disease
Over the last few decades, neurotransplantation has been used to explore the development, plasticity, and regeneration of the central nervous system (see ref. 1). Also, neurotransplantation has been used for the repair and functional restoration of diseased and damaged nervous tissue (2–13). In particular, a series of patients with Parkinson’s disease have been treated with mesencephalic cells from human fetuses obtained from 6- to 9-week-old abortuses (4–8). Some of the patients have had significant improvement both in clinical symptoms and in the synthesis of dopamine as assayed by fluorodopa uptake by positron-emission tomography (4–7). However, obtaining the fetal tissue has presented major logistic and ethical barriers (see refs. 9 and 10). Also, only about 5–10% of dopaminergic neurons survive, apparently because of immune reactions (8) and because the fetal tissue is primarily dependent on lipid instead of glycolytic metabolism (9). For these reasons, attempts have been made to develop alternative cells such as fibroblasts (11), fetal astrocytes (12), and sertoli cells (13) for neurotransplantation. Of all the donor cells tested for parkinsonism, only astrocytes were found to both integrate and migrate (12).
For therapy of Parkinson’s disease, donor cells should be (i) easily available; (ii) capable of rapid expansion in culture; (iii) immunologically inert; (iv) capable of long-term survival and integration in the host brain; and (v) amenable to stable transfection and long-term expression of exogenous genes such as tyrosine hydroxylase (2, 3). One possible source of such cells is bone marrow. Several observations (see ref. 14) suggest that monocytes can cross the blood-brain barrier and contribute to the normal turnover of microglia. Recently, whole bone marrow cells from male mice that were systemically infused into irradiated female adult mice were shown to make a small, but detectable, contribution to both microglia and macroglia of the brain (15). However, bone marrow transplantation has been only partially effective in treating central nervous system defects of genetic diseases (see refs. 16 and 17). Therefore, it is unlikely that brain lesions can be effectively treated with either bone marrow transplantation or systemic administration of marrow cells. In addition to hematopoietic stem cells, bone marrow contains stem-like precursors for nonhematopoietic cells such as osteoblasts, chondrocytes, adipocytes, and myoblasts (18–20). The nonhematopoietic precursors have been variously referred to as colony-forming-unit fibroblasts, mesenchymal stem cells, and marrow stromal cells (MSCs). Recently, we observed that systemic infusion of MSCs into irradiated 3-week-old mice was followed by the appearance of progeny of the donor cells in a variety of nonhematopoietic tissues (21, 22), including the brain (22). Here we examined the effects of direct injection of human MSCs into the brains of rats.
MATERIALS AND METHODS
Preparation of Donor MSCs and Astrocytes.
Human MSCs (18–22) were grown from aspirates taken from the iliac crest of normal male and female volunteers 19 to 46 years old. Aspirates are diluted 1:1 with α-MEM/10% fetal bovine serum (FBS) and centrifuged through a density gradient (Ficoll-Paque Plus; 1.077 g/ml; Pharmacia) for 30 min at 1,000 × g. The supernatant and interface were combined, diluted to about 40 ml with α-MEM/10% FBS, and centrifuged. The nucleated cells were suspended at a concentration of 1 × 107/ml in α-MEM/10% FBS and plated at 3 × 106/cm2 in 25 cm2 culture dishes. The cells are incubated for 3 days, and the nonadherent cells were removed by replacing the medium. After the cultures reached confluency, the cells were lifted by incubation with 0.25% trypsin and 1 mM EDTA at 37°C for 3–4 min. They were diluted 1:2 or 1:3 and replated. The procedure was repeated for 3–5 passages. Beginning with the second passage, 5 ng/ml of platelet-derived growth factor αα (PDGF-AA; GIBCO/BRL) was added to the medium. For isolation of rat MSCs, tibias and femurs were dissected from 8- to 12-week-old Lewis/SsNHsd female rats (Harlan–Sprague–Dawley). The ends of the bones were cut, and the marrow was extruded with 5 ml of DMEM (GIBCO/BRL) by using a needle and syringe. Between 100 and 200 × 106 whole marrow cells were plated on 175-cm2 tissue culture flask in DMEM/10% FBS. After 24 hr, the nonadherent cells were removed by replacing the medium. The medium was replaced every 2–3 days as the cells were grown to confluency. The cells were lifted by incubation with 0.25% trypsin and 1 mM EDTA, passed three or four times, and stored frozen.
Primary cultures of astrocytes (23) were obtained from brains of albino Sprague-Dawley adult rats (Harlan–Sprague–Dawley). After decapitation, the brains were removed under aseptic conditions and floated in cold PBS over ice. The meninges and brain stem were removed and discarded. The forebrain was mechanically dissociated into small pieces, and the tissues were incubated at 37°C for 30 min in 2.4 units/ml of dispase (GIBCO/BRL). At 10-min intervals, the tissue was dissociated by flushing through a large bore pipette. The dissociated tissue was centrifuged, and the supernatant was discarded. The residue from each brain was suspended in α-MEM and plated in two 175-cm2 culture flasks in α-MEM/10% FBS. Nonadherent cells and debris were removed by changing the medium after 48 hr, and then every 4 days for about 2 weeks until the cultures were confluent. To remove loosely adherent cells, the confluent cultures were treated with 2.4 units/ml dispase for 15 min at 37°C, and shaken on a rotary shaker at 120 rpm for 2 hr. The detached cells were discarded. Fresh media was added to the adherent cells, and the cultures were reincubated for 24–48 hr. The treatment with dispase was repeated three times over a period of about 1 week. One to 2 hr before implantation, the cultures were washed three times with sterile buffered saline and lifted by incubation with 0.25% trypsin for 1–3 min. Trypsin was neutralized by adding media containing 20% serum, and cells were isolated by centrifugation. The cells were suspended as a slurry of about 10,000 cells per μl in α-MEM without serum.
For antibody staining, the cells were subcultured in chambered slides. To label the nuclei fluorescently, the MSCs and astrocytes were incubated with 1 μg/ml bis-benzamide (Sigma) for 24 hr before implantation.
Neurotransplantation of the Cells.
Adult Sprague–Dawley albino rats (200–300 g) were anesthetized in a sealed chamber using 3% halothane in oxygen. Anesthesia was maintained by intramuscular injection of a mixture of 6 mg/kg of xylozine and 60 mg/kg of ketamine. The animals were transferred to a sterotaxic apparatus in a clean field. A 2- to 5-mm incision was made in the scalp 2 mm lateral to the bregma. A burr hole was made in the bone 3 mm lateral to bregma with a dental drill, and about 10 μl of the cell suspension was slowly injected over 30 min into the striatum at a depth of 4–5 mm from the surface of the brain. The wound was closed with interrupted surgical sutures, and the animals were treated with 0.6 mg/kg of xylozine and 6 mg/kg of ketamine. After 5, 14, 30, and 72 days, rats were sacrificed by intracardiac perfusion under deep anesthesia with xylozine and ketamine. The perfusion was with ice-cold PBS, followed by 3% buffered paraformaldehyde and then by 10% sucrose. The brains were removed, the forebrains were trimmed, and the samples were immediately frozen.
Immunohistochemical Staining.
Ten micron tissue sections were prepared with a cryostat. The transplant site was located by microscopically identifying the fluorescently labeled cells in the tissue sections. Frozen sections were attached to gelatin-coated slides and were quickly immersed in cold acetone for 5 min and stored at −20°C for further processing. Cells in chambered slides were fixed with acetone for 5 min. Immunocytochemistry was performed at room temperature. Cells and tissue sections were treated with blocking antibodies of 2% goat serum and 5% FBS for 30 min. In experiments requiring the labeling of collagen and vimentin, the cells were further treated with Triton X-100 for 30 min and rinsed in PBS. Primary antibodies were applied (see Table 1) for 1–2 hr in chambered slides and 24 hr for tissue sections. The secondary antibodies were species-specific IgGs coupled to either fluorescein isothiocyanate or rhodamine.
Table 1.
Immunostaining of human MSCs and rat astrocytes
| Antibodies | Dilution | Human MSCs | Rat astrocytes |
|---|---|---|---|
| Fibronectin | 1:400 | +++ | − |
| (Sigma) | |||
| Collagen I | 1:10 | ++ | − |
| (Biodesign) | |||
| HLA-ABC | 1:100 | ++ | − |
| (Pharmingen) | |||
| Vimentin | 1:40 | +/− | +++ |
| (Sigma) | |||
| Glial fibrillary acidic protein | 1:20 | − | ++ |
| (Sigma) | |||
| Galactocerebrosidase C | 1:50 | NA | − |
| (Sigma) | |||
| von Willebrand factor | 1:200 | NA | − |
| (Sigma) |
NA, not assayed.
Fluorescently labeled cells were visualized and photographed using a fluorescent microscope. The number of fluorescently labeled nuclei were counted in 8–10 tissue sections cut from rostral to caudal limits of the striatum. The procedure was repeated on each brain by two individuals who used alternate sections. Only the clearly labeled nuclei were counted. Dead and lysed cells left a bluish hue in the surrounding tissue and no clear nuclear staining. The observed numbers were extrapolated to total number of sections to estimate the number of surviving engrafted cells.
RESULTS
Donor MSCs and Astrocyte Precursors.
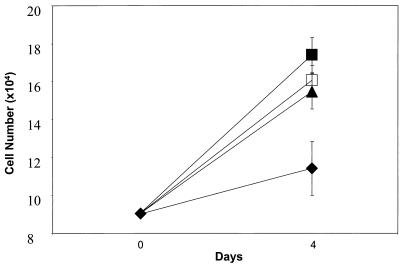
MSCs from human bone marrow were isolated by their adherence to plastic and grown for 3–5 passages. As indicated in Fig. 1, addition of PDGF-AA increased the growth rate of the cells. Therefore PDGF-AA was added to passages 2–5 to obtain adequate numbers of human MSCs for the experiments here. The rat MSCs grew adequately without addition of PDGF-AA (not shown). The astrocytes also were isolated by their tight adherence to tissue culture plastic. The cells were harvested after primary culture for about 3 weeks. As indicated in Table 1, the human MSCs stained heavily for fibronectin, collagen I, and human HLA-ABC. The rat astrocytes stained poorly for fibronectin and were negative for collagen I and human HLA-ABC. In contrast, the human MSCs were stained faintly for vimentin and were negative for glial fibrillary acidic protein whereas the rat astrocytes were positive for both. The rat astrocytes were also negative for von Willebrand factor and galactocerebrosidase C.
Figure 1.
Effect of PDGF-AA on growth of human MSCs. Values are means ± SD (n = 3). ⧫, control; ▴, 1 ng/ml PDGF; □, 5 ng/ml PDGF; ▪, 10 ng/ml PDGF.
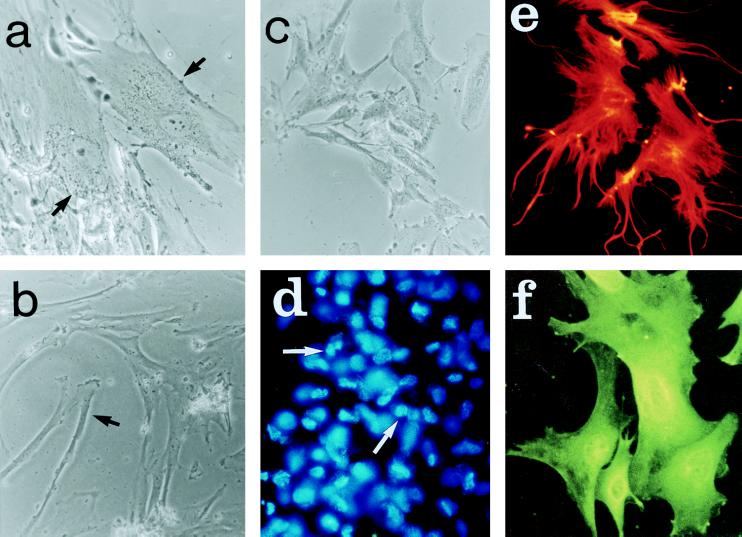
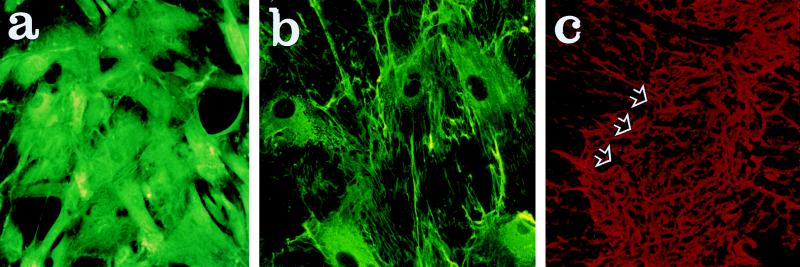
As noted previously (see ref. 24), the human MSCs became relatively homogeneous in appearance as the cells were passed. However, two distinct populations were seen, large flattened cells and relatively elongated or spindle-shaped cells (Fig. 2 a and b). The rat MSCs had a similar morphology (Fig. 2c). The rat brain astrocytes were more dendritic in appearance (Fig. 2 e and f).
Figure 2.
Photomicrographs that demonstrate some of the morphological characteristics of MSCs and astrocytes in culture. a and b demonstrate two types of human MSCs, flat and elongated. c demonstrates the similar morphology of rat MSCs. d shows fluorescent labeling of nuclei of human MSCs immediately before implantation. e and f demonstrate indirect immunofluorescent staining of astrocytes with antibodies against vimentin and glial fibrillary acidic protein, respectively. (Magnification: a, b and c, ×20; d, ×10; e and f, ×40.)
Survival of the Cells After Injection in the Striatum.
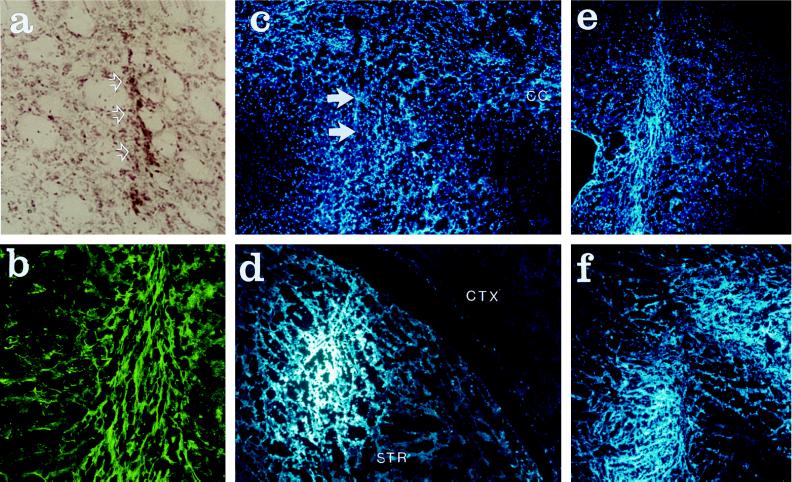
The MSCs and the astrocytes were injected in the corpus striatum of rat brains using minimal perfusion pressure. Five to 72 days later, the rats were killed and the brains were sectioned. Examination of sections stained with hemotoxylin and eosin indicated that there was no significant gliosis or infiltration of leukocytes around the implantation site of either the rat astrocytes (not shown) or the human MSCs (Fig. 3a). Fluorescently labeled cells were readily detected in the brain sections. As noted previously (12, 23, 25), the rat astrocytes readily engrafted (not shown). Similar results were obtained with the rat (Fig. 3f) and human MSCs (Fig. 3 b–e). As indicated in Table 2, from about 20,000 to 42,000 of the human cells were present in the brains. Because the number of cells injected varied from 100,000 to 120,000, about 20% of the infused human MSCs were recovered after 5 to 72 days. There appeared to be a decrease in number recovered between days 30 and 72. With the rat MSCs, 33,000 cells or about 30% were recovered 14 days after the infusion (Fig. 3f).
Figure 3.
Photomicrographs that demonstrate sites of implantation of bone MSCs in the striatum of adult rats. (a) Site of implantation of human MSCs after 14 days. Arrows indicate needle track. Hemotoxylin and eosin stain. (b) Adjacent section to a stained with antibodies to HLA-ABC. (c) Adjacent section to a examined for nuclear fluorescence of human MSCs. Arrows indicate needle track. (d) Site of implantation of human MSCs after 72 days. Section examined for nuclear fluorescence. (e) Same as d 30 days after implantation. (f) Site of implantation of rat MSCs 14 days after implantation. Examined for nuclear fluorescence. (Magnification: a and b, ×10; c–f, ×4.)
Table 2.
Recovery of fluorescently labeled human MSCs from rat brain
| Days after infusion | Labeled cells per brain |
|---|---|
| 5 | 27,300* |
| 14 | 30,200 |
| 30 | 41,800 |
| 72 | 19,900 |
Total counts on alternative sections by two different observers differed by less than 20%.
Migration of the Implanted Cells.
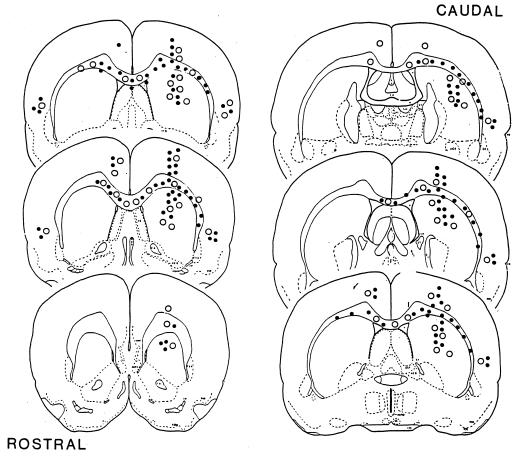
Implanted rat astrocytes migrate through layers of the brain (12, 25) in a manner similar to the migration seen with implanted neural stem cells or with a transformed line of neural stem cells (see ref. 1). Here it was found that MSCs migrated in a similar fashion. The donor cells were found in multiple areas of the brain including the contralateral cortex (Fig. 4). The cells persisted in the sites to which they migrated. The heaviest concentration of cells was found around the rostrocaudal axis in the striatum and along the corpus callosum. There were fewer cells in the cerebral cortex. Clusters of labeled cells consistently were observed in the temporal lobe regions at all time points examined. At day 72, fewer cells were found in the outlying cortical regions, an observation consistent with the apparent decrease in cell number between days 30 and 72 (Table 2).
Figure 4.
Line drawings of rat forebrain demonstrate the extent of migration of human MSCs and astrocytes after implantation in the striatum of the rat at the level of bregma. The drawing is a composite from brains examined at 4, 14, 30, and 72 days after the cell infusions. The pattern of implantation and migration of human MSCs is similar to that of rat astrocytes. The outlying cells, located in the temporal cortex and areas of corpus callosum farthest from the injection sites, were first to disappear. •, clusters of human MSCs; ○, clusters of rat astrocytes.
Immunohistology of the Implanted Cells.
Immunostaining of sections demonstrated that the engrafted human MSCs also were detected with antibodies to HLA-ABC (Fig. 3b). Although the human MSCs stained with antibodies to collagen I before implantation (Fig. 5a), no staining with the same antibodies was seen after implantation (not shown). Staining with antibodies to fibronectin was observed before implantation (Fig. 5b) and 5 days after implantation (Fig. 5c). At 30 and 72 days, the areas staining with the fibronectin antibodies was markedly decreased (not shown).
Figure 5.
Photomicrographs that demonstrate staining with antibodies to collagen I and fibronectin. (a) Human MSCs in culture stained for collagen I. (b) Same stained for fibronectin. (c) Section of rat brain stained for fibronectin 5 days after implantation of human MSCs. Arrows indicate deposits of fibronectin. (Magnification: a and b, ×20; c, ×4.)
DISCUSSION
The results here demonstrate that human MSCs infused into rat brain can engraft, migrate, and survive in a manner similar to rat astrocytes. Rat astrocytes were shown previously (12, 23, 25) to be similar to neural stem cells in that they migrate along known pathways and are incorporated into successive layers of the brain. Therefore, astrocytes isolated by their adherence to plastic partially mimic stem cells of the neuroepithelium that migrate from the subventricular zone and then differentiate into astrocytes, oligodendrocytes, and laminar-specific neurons (1). The process of migration and differentiation occurs rapidly in the early development of the brain and continues at a much slower rate in the adult brain (26). The results here indicate that at least a subset of the cells that are isolated from bone marrow by their adherence to plastic also can participate in the same pathway. The engraftment and migration of MSCs presents a marked contrast to fibroblasts that continue to produce collagen and undergo gliosis after neural implantation (27).
Human MSCs isolated by their adherence to plastic are not homogeneous but the cells become more homogeneous in appearance (24) and lose hematopoietic markers such as the pan-leukocyte epitope CD45 (28, 29). The relatively large recovery of 20% of the infused cells indicates that either a large fraction of the MSCs survived or a small subset of the MSCs had a high potential to proliferate in the host micro-environment. After injection, the human MSCs lost their immunoreactivity to antibodies of collagen I. Five days after the infusion, the human MSCs continued to stain heavily with antibodies to fibronectin, a protein whose synthesis is frequently increased in cell culture. However, the region of the brain staining for fibronectin was significantly decreased at 30 and 72 days, an observation suggesting that the synthesis decreased and some of the protein may have been degraded. There was no evidence of an inflammatory response or rejection of either the rat or the human MSCs. This observation may be explained by the brain being a partially privileged site for transplantation and by the partially impaired immune status of albino rats. Also, it may in part be explained by the observation that human MSCs as prepared here are negative for HLA class II antigens (G. Kopen and D. Phinney, personal communication).
The results here supported previous suggestions that MSCs may be useful vehicles for both cell and gene therapy for a variety of diseases (19, 21, 22). MSCs have several advantages as cells for neurotransplantation. In particular, they can be readily obtained from bone marrow and expanded in culture. Also, a patient’s own MSCs can be used, thus circumventing the problems of host immunity and graft versus host disease. In addition, the cells can be genetically engineered (30, 31). However, if they are similar to neural stem cells and acquire the phenotype of mature neural cells (1), gene engineering may not be necessary for their use in some diseases.
Acknowledgments
We thank Dr. John Booss for initial guidance. This work was supported in part by a research grant from the March of Dimes/Birth Defects Foundation and Grant AR44210 from the National Institutes of Health and by the Department of Neurology at Allegheny University of the Health Sciences.
ABBREVIATIONS
- MSC
marrow stromal cell
- PDGF-AA
platelet-derived growth factor αα
- FBS
fetal bovine serum
References
- 1.McKay R. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 2.Bjorklund A. Nature (London) 1993;362:414–415. doi: 10.1038/362414a0. [DOI] [PubMed] [Google Scholar]
- 3.Olson L. Nat Med. 1997;3:1329–1335. doi: 10.1038/nm1297-1329. [DOI] [PubMed] [Google Scholar]
- 4.Spencer D D, Robbins R J, Naftolin F, Marek K L, Vollmer T, Leranth C, Roth R H, Price L H, Gjedde A, Bunney B S, et al. N Engl J Med. 1992;327:1541–1548. doi: 10.1056/NEJM199211263272201. [DOI] [PubMed] [Google Scholar]
- 5.Freed C R, Breeze R E, Rosenberg N L, Schneck S A, Kriek E, Oi J X, Lone T, Zhang U B, Snyder J A, Wells T H, et al. N Engl J Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- 6.Kordower J H, Freeman T B, Snow B J, Vingerhoets F J, Mufson E J, Sanberg P R, Hauser R A, Smith D A, Nauert G M, Perl D P, et al. N Engl J Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 7.Defer G L, Geny C, Ricolfi F, Fenelon G, Monfort J C, Remy P, Villafane G, Jeny R, Samson Y, Keravel Y, et al. Brain. 1996;119:41–50. doi: 10.1093/brain/119.1.41. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Lozano J J, Bravo G, Brera B, Dargallo J, Salmean J, Uria J, Insausti J, Martinez R, Sanchez P, de la Torre C, Moreno R. Transplant Proc. 1997;29:977–980. doi: 10.1016/s0041-1345(96)00333-8. [DOI] [PubMed] [Google Scholar]
- 9.Rosenstein J M. Exp Neurol. 1995;133:1–6. doi: 10.1006/exnr.1995.1001. [DOI] [PubMed] [Google Scholar]
- 10.Turner D A, Kearney W. Neurosurgery. 1993;33:1031–1037. doi: 10.1227/00006123-199312000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Kang U J, Fisher L J, Joh T H, O’Malley K L, Gage F H. J Neurosci. 1993;13:5203–5211. doi: 10.1523/JNEUROSCI.13-12-05203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson C, Tytell M, Brunso-Bechtold J. Int J Dev Neurosci. 1993;11:555–568. doi: 10.1016/0736-5748(93)90045-f. [DOI] [PubMed] [Google Scholar]
- 13.Sanberg P R, Borlongan C V, Othberg A I, Saporta S, Freeman T B, Cameron D F. Nat Med. 1997;3:1129–1132. doi: 10.1038/nm1097-1129. [DOI] [PubMed] [Google Scholar]
- 14.Lawson L J, Perry V H, Gordon S. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 15.Eglitis M A, Mezey E. Proc Natl Acad Sci USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neufeld E F, Muenzer J. In: Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 2483–2495. [Google Scholar]
- 17.Peters C, Balthazor M, Shapiro E G, King R J, Kollman C, Hegland J D, Henslee-Downry J, Trigg M E, Cowan M J, Sanders J, et al. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- 18.Owen M E, Friedenstein A J. Cell and Molecular Biology of Vertebrate Hard Tissues, Ciba Foundation Symposium 136. Chichester, UK: Ciba Foundation; 1988. pp. 42–60. [PubMed] [Google Scholar]
- 19.Caplan A I. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 20.Prockop D J. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 21.Pereira R F, Halford K W, O’Hara M D, Leeper D B, Sokolov B P, Pollard M D, Bagasra O, Prockop D J. Proc Natl Acad Sci USA. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira R, O’Hara M D, Laptev A V, Halford K W, Pollard M D, Class R, Simon D, Livzy K, Prockop D J. Proc Natl Acad Sci USA. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azizi S A. Ann Neurol. 1996;40:549a. (abstr.). [Google Scholar]
- 24.Bruder S P, Jaiswal N, Haynesworth SE. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H-F, Lund R D. J Comp Neurol. 1992;317:145–155. doi: 10.1002/cne.903170204. [DOI] [PubMed] [Google Scholar]
- 26.Craig C G, Tropepe V, Morshead C M, Reynolds B A, Weiss S, van der Kooy D. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaja D M, Gage F H. J Comp Neurol. 1992;317:102–116. doi: 10.1002/cne.903170108. [DOI] [PubMed] [Google Scholar]
- 28.Nolta J A, Hanley M B, Kohn D B. Blood. 1994;83:3041–5051. [PubMed] [Google Scholar]
- 29.Rickard D J, Kassem M, Hefferan T E, Sakar G, Spelsberg T C, Riggs B L. J Bone Miner Res. 1996;11:312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 30.Matthews K E, Mills G B, Horsfall W, Hack N, Skorecki K, Keating A. Exp Hematol. 1993;21:697–702. [PubMed] [Google Scholar]
- 31.Allay J A, Dennis J E, Haynesworth S E, Majumdar M K, Clappe D W, Schultz L D, Caplan A I, Gerson S L. Hum Gene Ther. 1997;8:1417–1427. doi: 10.1089/hum.1997.8.12-1417. [DOI] [PubMed] [Google Scholar]