Abstract
Purpose
Much has been written about conceptual concern for voluntary assent with children and adolescents. However, little empirical data exists examining the frequency with which, or context in which, adolescents and parents disagree on research participation decisions. The purpose of this study was to compare parent and adolescent willingness to participate in minimal and above minimal risk pediatric asthma research protocols.
Method
36 adolescents diagnosed with asthma and one of their parents independently rated their willingness to participate in 9 pediatric asthma research protocol vignettes. The selected protocols were chosen by an expert panel as representative of typical minimal and above minimal risk pediatric asthma studies.
Results
Parents and adolescents were significantly less likely to agree to enroll in above minimal risk studies. However, this was qualified by a finding that adolescents were significantly more willing than parents to enroll in above minimal risk research. Across all 9 studies, parents and adolescents held concordant views on participation decisions approximately 60% of the time. Perception of potential study benefit was the most frequent reason provided for participation decisions by both parents and adolescents.
Conclusion
Parents and their adolescents report a consistent 40% discordance in their views about participating in asthma research across a variety of asthma research protocols, with adolescents more willing than their parents to enroll in above minimal risk studies. These differences of opinion highlight the need to carefully consider the process by which families are offered the option of adolescent research participation.
Keywords: informed consent, assent, adolescent research, biomedical research ethics, research participation decision making
The role of the adolescent in research participation decision-making is ambiguous. While federal regulations give parents legal responsibility for providing permission, consensus standards view adolescent assent as a moral and ethical imperative [1-4], with an adolescent's desire to dissent generally considered binding, particularly for non-therapeutic research.
Several models for adolescent participation in research and treatment decision-making have been proposed [5-8]. Inherent in the formulation of these models are differing views on the best way to protect the rights and interests of children and adolescents. Autonomy focused models [6, 9] propose that adolescents should be approached alone after parental permission has been granted. This procedure highlights concern that adolescents exercise decision-making independently of their parents and treatment providers. Proponents of this model argue there may be competing interests between parents and adolescents in participation decision-making that result in different choices [10-12]. Investigators have found, for example, that parents who volunteer their children for research endorse altruism as a value more than do non-volunteering parents [13].
Others have argued that requiring parental permission for certain types of minimal risk research is unnecessary, especially for mature minors [9] and may result in inappropriate constraints on adolescent research participation. Moreover, minimal risk studies on sensitive topics such as HIV or substance use may be hindered by privacy concerns when parental permission is required [14]. A National Survey of Institutional Review Boards' attitudes finds substantial support for revision of legal requirements that would allow adolescents to independently consent to certain categories of minimal risk research [15], a position also recently endorsed by the Society for Adolescent Medicine [16].
Recent interview studies of parents and adolescents involved in research find that parents see their role as ensuring their child's best interest is upheld and protecting them from harm [6, 17]. Factors that appear salient to parents' attitudes on what constitutes the child's best interest include perceptions of study risks and benefits. However, it is unknown whether adolescents hold views on these factors that would be concordant or discordant with those of their parents.
Thus, while much has been written about conceptual concern for voluntary assent with children and adolescents, little empirical data exists examining the frequency with which, or context in which, adolescents and parents disagree on research participation decisions. A previous study found that 26% of adolescents with asthma and their parents disagreed on the participation decision in a hypothetical protocol involving a randomized clinical trial for an experimental asthma medication [18]. It is difficult to generalize from a single protocol because studies with substantially different risk and benefit profiles or participation incentives may result in different decisions. However, this preliminary evidence suggests that parents and adolescents may disagree with measurable frequency.
The purpose of this study was to examine similarities and differences in attitudes among adolescents with asthma and their parents on research participation decisions across several different research designs. Since preliminary studies find perceptions of risk to be highly salient to parental decision-making, and there is growing interest in allowing adolescents to independently consent to some forms of minimal risk research, we were especially interested in establishing the extent of disagreement across a variety of minimal and above minimal risk studies. We hypothesized that adolescents would tend to be less risk adverse than their parents, and thus would demonstrate greater willingness to participate in research.
Method
Participants
Thirty-six adolescents with the diagnosis of asthma and one of their parents (predominantly mothers) participated in this study. Adolescents ranged in age from 11 to 17 (Mean = 13.17) and parent age ranged from 30 to 60 years (Mean = 43.19) years. Ethnicity of the patient sample was representative of the southwestern United States, where data were collected: 36.1% Hispanic, 41.7% Non-Hispanic White, and 22.2% of mixed ethnicity (see Table 1).
Table 1.
Demographic characteristics of adolescent and parent participants (N=36 dyads).
| n | % | |
|---|---|---|
| Relationship with child | ||
| Parent | 34 | 94.4 |
| Other | 2 | 5.6 |
| Child ethnicity | ||
| White | 15 | 41.7 |
| Hispanic | 13 | 36.1 |
| Other (Mixed) | 8 | 22.2 |
| Highest parental educational level | ||
| High School Diploma | 8 | 22.2 |
| Associates/Vocational Degree | 9 | 24.0 |
| Bachelors Degree | 8 | 22.2 |
| Post-graduate degree | 8 | 12.3 |
| Missing | 3 | 8.3 |
| Yearly household income | ||
| < $20,000 | 9 | 25.0 |
| $20,001-$40,000 | 8 | 22.2 |
| $40,001-$60,000 | 3 | 8.3 |
| > $60,001 | 16 | 44.4 |
Sixty-seven percent of adolescents and 61% of parents reported no prior research experience. Parents reported their adolescent typically missed a median of 4 days of school in the past year due to asthma symptoms, with a maximum of 70 missed days due to asthma. Adolescent participants had relatively few visits to the emergency department (83% had no visits) and few hospitalizations (95% had none) in the past year. In the 4 weeks before participation in this study, 44% of participants reported no awakenings due to nocturnal asthma symptoms, whereas 6% reported awakenings every night. Asthma symptom frequency was reported as twice a week or less in 53% of participants, with daily asthma symptoms in 25% of participants.
Stimulus Materials
Nine pediatric asthma research protocols were selected from among a sample of 40 consent forms obtained from pediatric asthma researchers in the United States and England. Protocols were based on previously conducted publicly and privately funded studies. Selections were made by a panel of ethicists and pediatric pulmonary experts, and were designed to establish a representative sample of pediatric asthma studies. The panel unanimously rated 4 protocols as above minimal risk to participants and 5 as representing minimal risk to participants. The minimal risk protocols included procedures such as asthma education and symptom monitoring. Three of the above minimal risk studies involved medication trials. Table 2 provides a summary of the major features of each protocol.
Table 2.
Summary description of research protocols and procedures
| Minimal Risk Studies | ||
|---|---|---|
| PROTOCOL TITLE AND DESCRIPTIONS | REQUIRED PROCEDURES | |
| A | Can some medicine make it harder to learn? | Random assignment to FDA approved medication or placebo |
| 3 weekends of school-like participation over 1 month to evaluate impact of medication on learning ability. | Allergy skin testing | |
| Psychological Testing | ||
|
| ||
| B | What are the characteristics of adolescents with mild to severe asthma? | 8 clinic visits including physical exam, spirometry, and asthma symptom/treatment questionnaires at each visit |
| 3 years participation to evaluate the characteristics of adolescents with mild to severe asthma. | 1 blood test | |
|
| ||
| C | Can a HRCT help in studying asthma? | spirometry test |
| One day participation to examine utility of HRTC (High Resolution Computer Tomography) in studying asthma. | HRTC x-ray | |
|
| ||
| D | How to stay healthy when you have asthma? | Random assignment to 1 of 3 levels of intervention: information only, information and 3 home visits from a health care worker, or information and 3 home visits with discussion about asthma medications |
| 18 month participation to evaluate different methods of helping adolescents control asthma, miss less school, and have fewer health problems | ||
|
| ||
| E | How much cortisol and nitric oxide do I have in my body? | 24 hr urine collection, |
| 36 hour hospital observation to evaluate levels of cortisol and nitric oxide. | 1 spirometry test | |
| 3 peak flow measures | ||
| nitric oxide levels measured every 4 hours. | ||
|
| ||
| Above minimal risk studies | ||
|
| ||
| F | Which of these medicines works better? | Medication change every 4 weeks |
| 15 week clinical trial comparing two FDA approved medication using a double blind, double dummy placebo crossover design | 3 overnight hospital stays | |
| 8 spirometry tests | ||
| 8 Nitric Oxide measures | ||
| 6 -24hr urine collections | ||
|
| ||
| G | How often should asthma medicine be taken? | Random assignment to treatment |
| 26 week trial to examine fixed versus as needed dosing schedule for established asthma medication. | Medical history | |
| 12 physical exams | ||
| 12 spirometry tests | ||
| 7 methacholine challenge tests | ||
| 1 allergy skin test | ||
| 1 electrocardiogram | ||
| 1 quality of life questionnaire | ||
| 7 urine pregnancy tests | ||
|
| ||
| H | How effective are these treatments for asthma over time? | Random assignment to treatment |
| 5 year clinical trial comparing 2 investigational medications versus placebo | 6 comprehensive physical exams | |
| 2 allergy skin tests | ||
| 6 Tanner Staging exams | ||
| 6 spirometry tests | ||
| 2 blood draws | ||
| 5 psychological tests | ||
| 6 methacholine challenge tests | ||
| 2 neuropsychological tests | ||
| 6 bone density measurements | ||
|
| ||
| I | Does this test help us to understand asthma? | 5 clinic visits including spirometry |
| 4 months participation to evaluate utility of sputum induction findings for asthma treatment. | 1 allergy skin test | |
| 2 methacholine challenge tests | ||
| 2 sputum inductions | ||
Key information about each of the selected protocols was extracted from the consent form and rewritten into a one page standardized research vignette format. Each included an informative study title, a brief statement of the reason for the study, and details of the length and time required for participation. To facilitate presentation of multiple vignettes, procedures were summarized in bullet format, and a description of study incentives was included at the end. Medication trials also included a description of medications and their known risks/side effects.
Procedures
Consent and assent procedures for this study were reviewed and approved by the Health Sciences Center Human Research Review Committee. Adolescent and parent participants were approached about this study while waiting for a scheduled medical appointment at a pediatric pulmonary clinic. For those agreeing to participate, a separate appointment was made to conduct the research at an office located outside the medical clinic. Two families that had indicated earlier interest later declined to participate in the study.
At the beginning of the interview, parents and adolescents met together with a research assistant to review and sign informed consent and assent documents. Participants completed a 15-item demographic form to discern socioeconomic status and other demographic characteristics, as reported by the parent. An asthma history questionnaire comprised of 33 items developed from the Guidelines for the Diagnosis and Management of Asthma [19] to assess asthma symptomatology was also used. Additional items developed for the current study, included questions pertaining to the names of current asthma medications and parent rating of the effectiveness of the current medication regimen. Parents were asked whether they or their children had participated in other asthma research studies, and if so, how many studies and for what length of time. Finally, seven questions were asked about the frequency of various asthma procedures that had been completed in the preceding 12 months (e.g., spirometry, allergy/skin testing, and venipuncture).
Parents and adolescents were then moved to separate rooms where independent data collection proceeded. Participants were first asked to separately evaluate the risks and benefits associated with common asthma research procedures, such as spirometry, allergy skin testing, and methacholine challenge. These findings are presented elsewhere [20]. The 9 vignettes describing the research protocols were then presented to participants. Alternate presentation orders were established for each parent-adolescent dyad using a standard Latin square design procedure.
For each of the nine vignettes, adolescents and parents were asked an identical series of 12 questions. All but one of the questions were evaluated on a 7-point Likert scale. The questions were designed to determine willingness to participate in each of the protocols, perceptions of the other family member's (parent or adolescent) willingness to participate, perceptions of responsibility for the participation decision, influence of others on the decision, and their overall evaluation of the risks, benefits, discomfort, hassle and appropriateness of compensation offered for participation in protocol. This paper reports on a subset of findings comparing parent and adolescent willingness to participate in each the research protocols, and reasons for their decisions.
Results
To compare adolescent and parent views on their willingness to participate in each of the 9 research vignettes, we conducted a 2×2 (minimal vs. above minimal risk and adolescent vs. parent) mixed effects analysis of variance on the 7-point Likert scale question, “Would you agree to enroll in this study?” (The parent form of the question was “Would you agree to enroll your child in this study?”). Anchor points for the scale ranged from “Definitely Not” to “Definitely Yes.”
There was a significant main effect for risk level (F (1, 63) = 277.67, p<.0001; M = 5.09, sd = .13 minimal risk, vs. M = 3.80 sd = .18) above minimal risk, indicating that both adolescents and parents were significantly less likely to agree to enroll in the above minimal risk protocols. However, this finding is qualified by a significant interaction of risk level and respondent (F (1, 63) = 9.16, p < .01), indicating that adolescents were significantly more likely than parents to agree to enroll in the above minimal risk studies.
Concordance of Parent and Adolescent Views
To evaluate how often adolescents agreed their parents, we examined the frequency of agreement/disagreement between each dyad on the 9 vignettes. Responses for this analysis were based on the dichotomous forced choice (yes or no) question asked about their willingness to participate in the protocol (“If you had to say for sure, what would your answer be?”). Overall, collapsing across minimal and above minimal risk studies, parents and adolescents held concordant views on the participation decision approximately 60% of the time (see Figure 1). In the minimal risk studies, parents and adolescents were both willing to participate about 51% of the time and they both declined to participate 10.6% of the time. By contrast, in the above minimal risk studies, parents and adolescents were willing to participate only 25.4% of the time and declined participation 32.6% of the time.
Figure 1.
Adolescent and parent concordance on participation decisions across risk levels.
However, parent and adolescent willingness to participate differed substantially for minimal and above minimal risk studies, with adolescents significantly more willing than their own parents to participate in above minimal risk studies, χ2 = 4.15, p < .04. When parents and adolescents held discordant views on research participation decisions in the minimal risk studies, the adolescent refused while the parent agreed 22.4% of the time and the adolescent agreed while the parent refused 15.9% of the time. The nature of the discordance was reversed for the above minimal risk studies. In these studies, the adolescents said “yes” while their parents said “no” 26.8% of the time and the adolescent said “no” while their parent said “yes” 15.2 % of the time.
Figure 2 details the percent of concordance and discordance in willingness to participate on each of the nine vignettes. Examination of these data reveals protocol specific differences in responses between parents and their adolescents. For example, the highest rate of parent/adolescent concordance was found for Study C, a minimal risk study involving HRCT testing. Almost 80% of parents and adolescents indicated a willingness to participate in this study. By contrast, the greatest amount of discordance overall was found in minimal risk studies B (involving regular clinic visits for 3 years to monitor asthma symptoms) and D (involving an in-home educational intervention), with about half of the parents and adolescents disagreeing with each other concerning willingness to participate.
Figure 2.
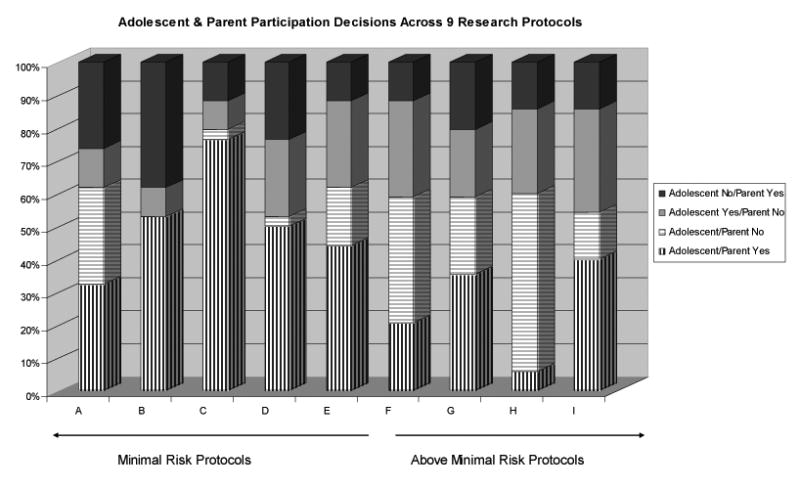
Adolescent and parent participation decisions across 9 research protocols.
Among the above minimal risk studies, the amount of discordance between parents and adolescents was relatively consistent across all 5 above minimal risk vignettes. Variability in dyadic responses across the protocols was primarily due to the form of parent and adolescent concordance (whether to accept or decline enrollment). The greatest amount of concordance between adolescent and parent views in above minimal-risk studies was found in Study H, which involved a 5-year study of investigational medications. In this study, both parents and adolescents most often independently preferred to decline participation. The greatest amount of discordance was found in Study I, which involved sputum induction and methacholine challenge. In this study, adolescents were much more willing to participate than were parents.
Reasons for participation decisions
To explore reasons for participation decisions, adolescent and parent responses to the open ended question “What is the main reason for your decision?” were coded into five possible a priori response categories: Risk, Benefit, Discomfort, Hassle, or Compensation. Negative and positive valence responses were grouped together, i.e. a decision to decline participation due to perceived lack of benefit received the same code as a decision to enroll due to perceived presence of benefit. Coding was conducted by 2 authors (dgs and mlp). A total of 625 responses were provided. (Where multiple responses were given for a single vignette, all responses were coded). Thirteen responses were eliminated for being too vague to code. Inter-rater agreement was calculated at 85%. Disagreements were subsequently discussed and resolved with the assistance of a 3rd author (jlb). For both parents and adolescents, the most frequent reason given for their participation decisions involved perception of research benefit. Over 45% of parent responses and almost 40% of adolescent responses received this code. For adolescents, concern over hassle was the next most important reason, at just under 35%. Parents mentioned risk and hassle each about 25% of the time as reasons for their decisions. By contrast, adolescents mentioned risk only 10% of the time. Financial compensation was also mentioned as a primary reason by adolescents in just under 10% of the responses. Parents almost never mentioned compensation. Discomfort was mentioned by adolescents about 7% of the time and by parents in about 3 % of the responses. A comparison of codes based on participants' enrollment decisions yielded no significant differences.
Discussion
Through the process of permission and assent, parents and adolescents have a shared involvement in research participation decision-making. When family members are in agreement to either accept or decline an offer of research participation, there are no legal or ethical concerns. When differences in opinion between parents and adolescents exist, however, a host of potential problems may transpire in ensuring that the rights and responsibilities of each person are protected and honored. Understanding the frequency of and contexts in which differences of opinion between parent and adolescents are likely to emerge is, therefore, a significant requisite to addressing situations that may challenge the ethical conduct of research with adolescents.
In this study we found surprising consistency in the frequency of concordance/discordance between adolescents and their parents across nine research vignettes, encompassing both minimal and above minimal risk asthma protocols. The overall frequency of discordance was a substantial 40%, with differences in the patterns of concordance/discordance found across the nine studies. Parents were more willing than adolescents to participate in the minimal risk studies, while adolescents were more willing than parents to enroll in above minimal risk research. Thus, the erosion of voluntary assent resulting from a parent's desire to enroll an unwilling adolescent in research appears more likely to occur in minimal risk rather than above minimal risk research.
Because adolescents and parents often disagree on research participation decisions, concerns over authority and responsibility for research participation decision-making and the process by which adolescent assent is obtained becomes a highly salient consideration. In the standard informed consent model for children, parents are approached first to obtain permission for child research participation. If parental permission is obtained, child assent is then requested. Our research suggests that for minimal risk research with adolescents, parents are generally more likely to provide permission, allowing adolescents the opportunity to participate, either through assent or dissent, in the decision-making process. However, the extent to which unwilling adolescents might face potentially coercive strategies by parents or others to participate in minimal risk studies is unknown.
In above minimal risk studies, the situation is different because adolescents appear less risk averse than their parents. We found they were more willing than parents to participate in above minimal risk studies and their comments suggested they were much less focused on potential risk when making participation decisions. If parents are approached first to provide permission for their adolescent to participate in above minimal risk research, the findings from this study suggest parents will frequently decline. Thus, utilizing this commonly recommended consent process would result in adolescents having a limited opportunity to participate in decisions concerning research participation in above minimal risk studies.
Integral to the discussion of the ethical nature of any consent process, however, are perspectives surrounding who ought to have the final authority and responsibility for research participation decision-making. A recent extensive discussion of the ethical considerations inherent in allocating greater adolescent decision-making autonomy for nonbeneficial research serves to highlight the existence of a spectrum of views [1-2]. While parents are currently authorized to make such decisions, there is growing advocacy for and legislation authorizing adolescent legal rights to make independent health care decisions in a variety of areas. This is a relevant connection because federal regulations link authority to provide research consent to state laws concerning consent for medical treatment. Moreover, several other potential models for obtaining research consent involving initial adolescent involvement have been proposed. Preliminary qualitative findings reveal that parents and adolescents hold a range of opinions on how consent is most appropriately obtained for adolescent research participation [17]. A systematic examination of views from parents, adolescents, researchers, and policy makers will be important for a thorough consideration of this topic. In the meantime, researchers can be attentive to the possibility that adolescents and parents may hold different views about research participation and provide opportunity for dialogue that both respects adolescents' desire to participate in health care-related decisions and acknowledges parental responsibility for providing permission to enroll in biomedical research.
This study provides a preliminary examination of the independent views parents and adolescents hold concerning participation in nine minimal and above minimal risk asthma research protocols. There are both strengths and limitations to the design that may limit interpretation of the findings. Hypothetical research protocols were presented in summary format with limited discussion of each study. Adolescents and parents were queried independently and did not discuss protocols with one another. Therefore, actual research participation decisions may be different in a more naturalistic setting. While the presentation order of the protocols was systematically varied across participants, reviewing all nine protocols sequentially may have influenced responses in unanticipated ways. However, the use of a spectrum of protocols enhances the generalizability of the findings and provides some important preliminary data on the acceptability of a variety of research designs to the families of adolescent asthma patients. Generalizability was also enhanced through the use of a clinical sample of adolescents with asthma recruited from a medical setting. In summary, our findings suggest that researchers ought to carefully consider the process by which families are offered the option of adolescent research participation.
Acknowledgments
This study was supported by funding from the National Heart Lung and Blood Institute of the National Institutes of Health: R01 HL64677.
Contributor Information
Janet L. Brody, Center for Family and Adolescent Research, Oregon Research Institute
Robert D. Annett, University of New Mexico Health Sciences Center
David G. Scherer, University of New Mexico College of Education
Mandy L. Perryman, University of New Mexico
Keely M. W. Cofrin, Informatix Laboratories Corp
References
- 1.Wendler D, Shah S. Should children decide whether they are enrolled in nonbeneficial research? Am J Bioethics. 2003;3(4):1–7. doi: 10.1162/152651603322614382. [DOI] [PubMed] [Google Scholar]
- 2.McGee G, editor. Children's participation in nonbeneficial research: should they decide? Open peer commentaries. Am J Bioethics. 2003;3(4):8–40. [Google Scholar]
- 3.Department of Health and Human Services. Protection of human subjects (OPRR Reports) Washington DC: U.S. Government Printing Office; 1994. Code of Federal Regulations, Title 45, Part 46. [Google Scholar]
- 4.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Modernization Act 2001; Section 505 Part A.
- 5.McCabe MA, Rushton CH, Glover J, et al. Implications of the patient self-determination act: Guidelines for involving adolescents in medical decision making. J Adolesc Health. 1996;19:319–324. doi: 10.1016/S1054-139X(96)00160-7. [DOI] [PubMed] [Google Scholar]
- 6.Broome ME. Consent (assent) for research with pediatric patients. Semin Oncol Nurs. 1999;15(2):96–103. doi: 10.1016/s0749-2081(99)80067-9. [DOI] [PubMed] [Google Scholar]
- 7.Fisher CB. Handbook of applied developmental science. Vol. 4. Thousand Oaks, CA: Sage Publications; 2003. Participant consultation: Ethical insights into parental permission and confidentiality procedures for policy-relevant research with youth; pp. 371–396. [Google Scholar]
- 8.Federman DD, Hanna KE, Rodriquez LL, editors. Responsible research: A systems approach to protecting research participants. Washington, DC: Institute of Medicine; 2003. [PubMed] [Google Scholar]
- 9.Holder AR. Can teenagers participate in research without parental consent? IRB: A Review of Human Subjects Research. 1981;3(2):5–7. [PubMed] [Google Scholar]
- 10.Melton GB. Children's rights: Where are the children? Am J Orthopsychiatry. 1982;52(3):530–538. doi: 10.1111/j.1939-0025.1982.tb01439.x. [DOI] [PubMed] [Google Scholar]
- 11.Brooks-Gunn J, Rotheram-Borus MJ. Rights to privacy in research: Adolescents versus parents. Ethics & Behav. 1994;4(2):109–121. doi: 10.1207/s15327019eb0402_4. [DOI] [PubMed] [Google Scholar]
- 12.Gordon VM, Bonkovsky FO. Family dynamics and children in medical research. J Clin Ethics. 1996;7(4):349–354. [PubMed] [Google Scholar]
- 13.Harth SC, Johnstone RR, Thong YH. The psychological profile of parents who volunteer their children for clinical research: a controlled study. J Med Ethics. 1992;18:86–93. doi: 10.1136/jme.18.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine RJ. Adolescents as research subjects without permission of their parents or guardians: Ethical considerations. J Adolesc Health. 1995;17:287–297. doi: 10.1016/1054-139x(95)00175-r. [DOI] [PubMed] [Google Scholar]
- 15.Mammel KA, Kaplan DW. Research consent by adolescent minors and institutional review boards. J Adolesc Health. 1995;17:323–330. doi: 10.1016/1054-139x(95)00176-s. [DOI] [PubMed] [Google Scholar]
- 16.Society for Adolescent Medicine. Guidelines for adolescent health research. J Adol Health. 2003;33:396–409. [PubMed] [Google Scholar]
- 17.Geller G, Tambor ES, Bernhardt BA, et al. Informed consent for enrolling minors in genetic susceptibility research: A qualitative study of at-risk children's and parents' views about children's role in decision-making. J Adolesc Health. 2003;32:260–271. doi: 10.1016/s1054-139x(02)00459-7. [DOI] [PubMed] [Google Scholar]
- 18.Brody JL, Scherer DG, Annett RD, et al. Voluntary assent in biomedical research with adolescents: A comparison of parent and adolescent views. Ethics & Behav. 2003;13(1):79–95. doi: 10.1207/S15327019EB1301_10. [DOI] [PubMed] [Google Scholar]
- 19.Guidelines for the diagnosis and management of asthma: Expert panel report, National Heart, Lung, and Blood Institute, U.S. Department of Health and Human Services, Publication No. 91-3042, 1991.
- 20.Annett RD, Brody JL, Scherer DG, et al. Perceived risk associated with asthma research procedures among children, parents, and pediatricians. doi: 10.1016/j.jaci.2004.07.058. In review. [DOI] [PubMed] [Google Scholar]



