Summary
Synaptic activity regulates the postsynaptic accumulation of AMPA receptors over timescales ranging from minutes to days. Indeed, the regulated trafficking and mobility of GluR1 AMPA receptors underlies many forms of synaptic potentiation at glutamatergic synapses throughout the brain. However, the basis for synapse-specific accumulation of GluR1 is unknown. Here we report that synaptic activity locally immobilizes GluR1 AMPA receptors at individual synapses. Using single-molecule tracking together with the silencing of individual presynaptic boutons, we demonstrate that local synaptic activity reduces diffusional exchange of GluR1 between synaptic and extraynaptic domains, resulting in postsynaptic accumulation of GluR1. At neighboring inactive synapses, GluR1 is highly mobile with individual receptors frequently escaping the synapse. Within the synapse, spontaneous activity confines the diffusional movement of GluR1 to restricted subregions of the postsynaptic membrane. Thus, local activity restricts GluR1 mobility on a submicron scale, defining a novel input-specific mechanism for regulating AMPA receptor composition and abundance.
Introduction
Rapid excitatory synaptic transmission at glutamatergic synapses is mediated by AMPA receptors, which are highly mobile at the postsynaptic membrane (Malinow and Malenka, 2002; Bredt and Nicoll, 2003; Cognet et al., 2006). AMPA receptors undergo dynamic intracellular trafficking through endocytosis and recycling (Luscher et al., 1999; Ehlers, 2000; Lee et al., 2004; Park et al., 2004), and can diffuse laterally into and out of the postsynaptic density (Borgdorff and Choquet, 2002; Tardin et al., 2003; Groc et al., 2004; Triller and Choquet, 2005; Ashby et al., 2006). Further, activity-dependent regulation of AMPA receptor mobility and synaptic abundance mediates diverse forms of synaptic plasticity (Malenka and Bear, 2004; Turrigiano and Nelson, 2004). Recent studies have found that synaptic activity regulates the number and subtypes of AMPA receptors present at the postsynaptic membrane over timescales ranging from minutes to days (O'Brien et al., 1998; Turrigiano et al., 1998; Liao et al., 1999; Shi et al., 1999; Harms et al., 2005; Thiagarajan et al., 2005; Sutton et al., 2006). In particular, heterologous overexpression of receptor subunits and C-terminal domains along with analyses of synaptic AMPA receptor currents has shown that AMPA receptors containing the GluR1 subunit accumulate at activated synapses (Shi et al., 2001; Rumpel et al., 2005; Plant et al., 2006).
Electrophysiological and immunocytochemical studies support a highly localized synapse-specific mobilization of GluR1 upon synaptic stimulation (Shi et al., 2001; Matsuzaki et al., 2004; Harms et al., 2005; Plant et al., 2006). Yet glutamate receptors undergo diffusional exchange between synaptic and extrasynaptic compartments (Tovar and Westbrook, 2002; Tardin et al., 2003; Triller and Choquet, 2005; Ashby et al., 2006; Bats et al., 2007), highlighting the need for input-specific control over the movement of AMPA receptors. Despite intense study, the molecular basis for synapse-specific accumulation of GluR1, and the mechanisms by which nearby synapses maintain distinct complements and quantities of AMPA receptors are unknown. Intriguingly, GluR1 knock-out mice have nearly normal synaptic AMPA receptor currents but reduced overall functional AMPA receptors (Zamanillo et al., 1999), suggesting a role for GluR1 in the lateral trafficking of AMPA receptors between extrasynaptic and synaptic membranes.
At the postsynaptic membrane, AMPA receptors are associated with an array of binding partners, whose physical association regulates AMPA receptor movement and location (Malinow and Malenka, 2002; Song and Huganir, 2002; Kim and Sheng, 2004; Cognet et al., 2006; Nicoll et al., 2006; Bats et al., 2007). The number of AMPA receptors present at glutamatergic synapses has been estimated at 50 to 100 by anatomical methods (Racca et al., 2000; Tanaka et al., 2005) and between 60 and 190 using physiological methods (Matsuzaki et al., 2001; Smith et al., 2003). Single molecule tracking and fluorescence photobleaching experiments have revealed that both mobile and immobile receptors are present among this cohort of 50 to 200 synaptic receptors (Tardin et al., 2003; Ashby et al., 2006), suggesting ongoing ‘online’ control of receptor diffusion. Indeed, synaptic activity or glutamate binding regulates the association of AMPA receptors with numerous scaffold and trafficking proteins (Xia et al., 2000; Lee et al., 2002; Mauceri et al., 2004; Tomita et al., 2004; Lu and Ziff, 2005; Palmer et al., 2005), but whether such events are translated into synapse-specific changes in receptor diffusion or synapse dwell time is unclear. Consistent with this notion, global pharmacological manipulations of neuronal activity and glutamate application alter AMPA receptor diffusional properties (Tardin et al., 2003; Groc et al., 2004). Moreover, mathematical simulations have postulated the existence of metastable ‘clusters’ of interacting AMPA receptors based on local changes in receptor movement within a single synapse (Shouval, 2005). Intriguingly, electron microscopy studies indicate that postsynaptic receptors are enriched in specific subcompartments of the postsynaptic membrane (Baude et al., 1993; Kharazia and Weinberg, 1997; Nusser et al., 1998; Tanaka et al., 2005; Perez-Otano et al., 2006), suggesting limited exchange between PSD subregions. In the case of AMPA receptors, intrasynaptic positioning relative to presynaptic release sites is predicted to markedly influence synaptic transmission (Franks et al., 2003; Raghavachari and Lisman, 2004). Such studies suggest tight spatial control over AMPA receptor lateral mobility at the level of single synapses.
Within minutes after initial synaptic contact, AMPA receptors accumulate at the postsynaptic membrane (Friedman et al., 2000) and spontaneous transmitter release becomes detectable (Zona et al., 1994; Bellingham et al., 1998). This spontaneous activity is not required for synapse formation per se (Augustin et al., 1999; Varoqueaux et al., 2002; Harms and Craig, 2005), but instead regulates the subunit composition of postsynaptic AMPA receptors. For example, spontaneous activity is sufficient to deliver GluR4 and GluR2L AMPA receptors to developing hippocampal synapses (Zhu et al., 2000; Kolleker et al., 2003). Similarly, synapse-specific silencing of neurotransmission causes a reduction in GluR1 AMPA receptors relative to nearby active synapses (Harms et al., 2005). These observations are consistent with models of AMPA receptor trafficking whereby receptors containing subunits with long carboxy-terminal domains (i.e, GluR1, GluR2L, GluR4) are mobilized to the postsynaptic membrane by activity (Zhu et al., 2000; Passafaro et al., 2001; Shi et al., 2001; Kolleker et al., 2003). More recent studies have revealed an unexpected role for spontaneous synaptic activity in regulating postsynaptic signaling (Murphy et al., 1994), spine stability (McKinney et al., 1999), synapse refinement (Yu et al., 2004) and dendritic protein synthesis (Sutton et al., 2004; Sutton et al., 2006), suggesting diverse functions for spontaneous synaptic activity which remain poorly understood.
In the present study, we have investigated the effect of local spontaneous synaptic activity on the diffusional behavior of AMPA receptors at individual synapses. We have focused on the lateral mobility of GluR1, the principal receptor subtype implicated in activity-dependent synaptic potentiation. Using a combination of single-molecule tracking together with the selective silencing of individual presynaptic terminals, we demonstrate that spontaneous synaptic activity confines GluR1 AMPA receptors to submicron domains at single synapses. In the vicinity of spontaneously active synapses, diffusional exchange of GluR1 between synaptic and extraynaptic domains is markedly reduced, resulting in the postsynaptic accumulation of GluR1. In contrast, at neighboring inactive synapses GluR1 is highly mobile with individual receptors passing through and frequently escaping the synapse. High resolution analysis revealed that, within the postsynaptic membrane itself, spontaneous activity confines the diffusional movement of GluR1 receptors to a more compact subdomain of the synapse. These results define a novel role for local synaptic activity in limiting GluR1 diffusional mobility and demonstrate an input-specific mechanism for regulating the arrangement and complement of postsynaptic AMPA receptors. Spatially delimited diffusional trapping of GluR1 thereby links localized molecular compartmentalization to synapse-specific signaling.
Results
GluR1 is Less Mobile at Active Synapses
AMPA receptors containing the GluR1 subunit rapidly accumulate at synapses in response to activity (Liao et al., 1999; Shi et al., 1999). Electrophysiological studies support input-specific regulation of GluR1 trafficking by synaptic activity (Shi et al., 2001; Matsuzaki et al., 2004; Bagal et al., 2005; Plant et al., 2006), but methods for directly visualizing receptor trafficking at individually activated or inactivated synapses have been limited. To examine synapse-specific effects of activity on GluR1 trafficking, we employed a genetic strategy to target the expression of the tetanus toxin light chain (TetTx) to a subpopulation of hippocampal neurons in primary culture using lentivirus, while at the same time visualizing the presynaptic boutons of these neurons by co-expression of synaptophysin-GFP (Figure 1A). Co-expression of synaptophysin-GFP and TetTx was ensured by placing the cDNA for TetTx downstream of an internal ribosome entry site (IRES) following synaptophysin-GFP. Expression of TetTx produces an essentially complete block of evoked and spontaneous neurotransmitter release by the proteolytic activity of the toxin against the requisite synaptic vesicle SNARE protein VAMP2 (Harms et al., 2005). Use of synaptophysin-GFP:IRES:TetTx thus allows inactive or silent boutons to be selectively visualized. Together with live labeling of all presynaptic terminals using low concentrations of a rhodamine derivative of a mitochondrial marker (Mitotracker Red) (Tardin et al., 2003; Groc et al., 2004; Groc et al., 2006), nearby active and inactive synapses are readily identified (Supplementary Figure S1). In control experiments, Mitotracker Red labeling exhibited 84 ± 7% colocalization with the presynaptic marker bassoon.
Figure 1. GluR1 is Stabilized at Active Synapses but Rapidly Moves Through Inactive Synapses.
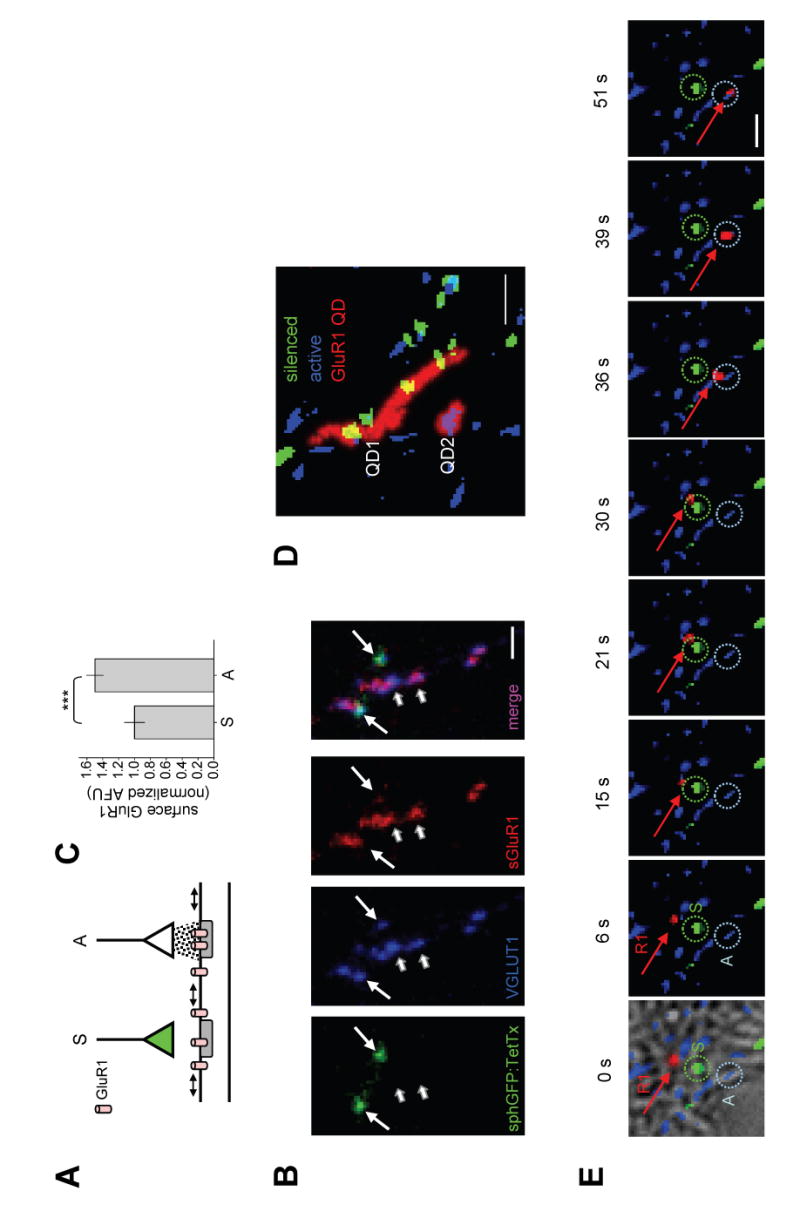
(A) Schematic diagram of experimental approach. GluR1 movement is visualized on a postsynaptic dendrite that receives synaptic contact from a neuron expressing synaptophysin-GFP:IRES:TetTx (green) whose presynaptic boutons are visible as green due to expression of synaptophysin-GFP but do not release glutamate (silenced, S) due to co-expression of tetanus toxin light chain (TetTx). The same dendrite receives nearby input from an untransfected neuron (white) which is spontaneously active (A). All presynaptic boutons are visualized live by Mitotracker Red. See Experimental Procedures for details.
(B) Spontaneous activity recruits GluR1. Hippocampal neurons were infected with lentivirus expressing synaptophysin-GFP:IRES:TetTx. Prior to visualization, neurons were incubated live with a polyclonal antibody directed against the extracellular N-terminal domain of GluR1 to label surface GluR1 (sGluR1). Neurons were then fixed and inactivated synapses visualized by synaptophysin-GFP fluorescence (sphGFP). Glutamatergic terminals were visualized by immunocytochemical detection of the vesicular glutamate transporter VGLUT1. Surface GluR1 was detected by labeling with fluorescent anti-rabbit secondary antibody. Triple overlap appears magenta (short arrows). Lack of sGluR1 appears cyan (long arrows). Note that inactivated synapses expressing synaptophysin-GFP (long arrows) had much less surface GluR1 than nearby active synapses (short arrows). Scale bars, 5 μm.
(C) Data represent means ± SEM of surface anti-GluR1 immunocytochemical labeling at silenced (S) or active (A) synapses. Silenced, n = 46 synapses on 9 neurons from 4 coverslips. Active, n = 82 synapses on 5 neurons from 4 coverslips. AFU, arbitrary fluorescence units. ***p < 0.01; t-test.
(D) Maximum projections of two quantum dot-labeled GluR1 receptors (GluR1-QD, red) near silenced (green) and active (blue) synapses. The total area explored by the two GluR1-QDs (labeled QD1 and QD2) during the 52 sec imaging period is indicated by red traces. Note that GluR1 moves readily through and between inactive synapses (QD1) but remains near an active synapse (QD2). Scale bar,2 μm. See Supplemental Movie S1.
(E) Individual frames from a timelapse showing a single GluR1-QD (R1, red arrow) that moves rapidly through a silenced synapse (S, green dashed circle) before encountering and remaining at a nearby active synapse (A, blue dashed circle). Time in seconds is shown above. Scale bar, 1 μm. See Supplemental Movie S2.
Neurons were infected after seven to eight days in vitro (DIV7-8) and were allowed to express synaptophysin-GFP:IRES:TetTx for 7–8 days prior to imaging to ensure ample time for TetTx expression and VAMP2 proteolysis. Dye loading experiments using FM4-64 confirmed that presynaptic boutons expressing synaptophysin-GFP:IRES:TetTx exhibit no detectable synaptic vesicle recycling (Supplemental Figure S2). Whole cell voltage clamp recordings of hippocampal neurons treated with TetTx (2 nM, 7 days) revealed a complete absence of all large amplitude (>300 pA) spontaneous action potential-mediated currents (3.1 ± 1.0 Hz control vs. none observed TetTx) and a 200-fold reduction in the frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs) (0.86 ± 0.39 Hz control vs. 0.004 ± 0.002 Hz TetTx, p < 0.01, Mann-Whitney test) indicating an essentially complete block of all spontaneous activity whether action potential-mediated or miniature events.
Consistent with previous studies (Harms et al., 2005), individually silenced synapses positive for synaptophysin-GFP contained significantly fewer GluR1 AMPA receptors than nearby active synapses (49.8 ± 10.3% more GluR1 at active synapses; Figure 1B and 1C), but exhibited no changes in PSD-95 family proteins (98.6 ± 8.9% relative to active synapses; n = 39, 86 at silenced and active synapses, respectively; p = 0.44). In addition, silenced synapses displayed no detectable change in presynaptic abundance of either the vesicular glutamate transporter VGLUT1 (110 ± 15.6% relative to active synapses, n = 41, 69 at silenced and active synapses, respectively; p > 0.1) or the active zone protein bassoon (108 ± 11.3% relative to active synapses, n = 28, 58 at silenced and active synapses, respectively; p > 0.1), and no difference in PSD-95, Shank, or bassoon puncta size (data not shown), in keeping with the overall preserved synaptic structure and composition reported upon exposure to tetanus toxin (Harms and Craig, 2005). At both silenced and active synapses, fluorescence intensity of bassoon labeling at individual presynaptic puncta correlated strongly with the fluorescence intensity of PSD-95 at the contacting postsynaptic membrane (r = 0.87), indicating that variation in presynaptic terminal size corresponded with the size of the postsynaptic membrane. Moreover, at the ages in culture examined (DIV14-16), dendritic spines were relatively rare and there was no preference for active or silenced synapses to selectively contact spines. Together, these data indicate that synaptic activity selectively and locally recruits GluR1 without grossly affecting synapse structure or composition.
Given the synapse-specific precision of GluR1 enrichment, we hypothesized that recruitment of GluR1 might arise from selective stabilization or retention of GluR1 at active synapses. To test this possibility, we monitored the surface mobility of endogenous GluR1 receptors on dendrites by rapid timelapse imaging of individual semiconductor quantum dots (QD) coupled to GluR1 antibodies (Dahan et al., 2003). The movement of single GluR1-QDs on hippocampal neuron dendrites was followed near sites of synaptic contact with active and silenced presynaptic boutons (Figure 1A). Individual GluR1-QDs were highly mobile in the extrasynaptic plasma membrane and frequently passed near or through one or more silenced synapses during the course of an experiment (Figure 1D and Supplementary Movie S1). In contrast, GluR1 receptors at active synapses were much less mobile and often remained tightly associated with the synapse (Figure 1D and Supplementary Movie S1). In some instances, individual GluR1-QDs were observed which traversed inactive synapses with little hesitation prior to lodging firmly and remaining at a nearby active synapse (Figure 1E and Supplementary Movie S2). Although GluR1-QDs were observed to exchange from a silenced to a nearby active synapse (Figure 1E and Supplementary Movie S2), rarely did we observe GluR1 receptors move from an active synapse to an inactive synapse (2/1778 trajectories). Control experiments performing acid stripping (pH 5.5, 1 min) removed >95% of GluR1-QDs from dendrites, indicating that imaged GluR1-QDs were at the dendritic surface. Together these results indicate that GluR1 AMPA receptors at the neuronal plasma membrane move rapidly through inactive synapses but becomes physically immobilized at active synapses, suggesting a diffusional basis for local activity-induced accumulation of GluR1.
Synaptic Activity Locally Limits GluR1 Diffusion
To determine whether the observed differential mobility of GluR1 near active and silenced synapses was due to local changes in lateral diffusion, we constructed trajectories of single GluR1-QDs acquired at a rate of 33 Hz and calculated the instantaneous diffusion coefficient (D) (Tardin et al., 2003). Synapses were labeled with Mitotracker Red as above and silenced synapses expressing synaptophysin-GFP:IRES:TetTx were visualized by GFP fluorescence (Supplementary Figure S1). Diffusion coefficients were calculated for all episodes longer than 250 msec at active synapses, neighboring silenced synapses, or in extrasynaptic membranes. Plotted histograms of extrasynaptic GluR1 diffusion coefficients revealed a characteristic rightward tailing distribution (Figure 2A, left). At normal active synapses, GluR1 diffusion was much slower (Figure 2C, left). However, chronic inactivation of transmission at single presynaptic boutons resulted in a marked increase in GluR1 diffusion at synapses (Figure 2B, left). This local activity-dependent diffusional trapping of GluR1 was readily apparent upon examination of individual GluR1-QD trajectories (Figures 2A–C, right). GluR1 diffusion at silenced synapses was intermediate between the very slow diffusion at active synapses and the free diffusion in the extrasynaptic membrane (Figure 2D). This intermediate diffusion extended across the full range of diffusion coefficient values (Figure 2D). The uniform shift in the distribution indicates that the reduced diffusion at active synapses is not due to the selective stabilization of a specific subpopulation of GluR1 receptors, but rather that the diffusion of all GluR1 receptors is coordinately reduced. Moreover, these results demonstrate the presence of both activity-dependent and activity-independent mechanisms for limiting AMPA receptor diffusion at synapses.
Figure 2. Local Spontaneous Synaptic Activity Reduces GluR1 Diffusion.
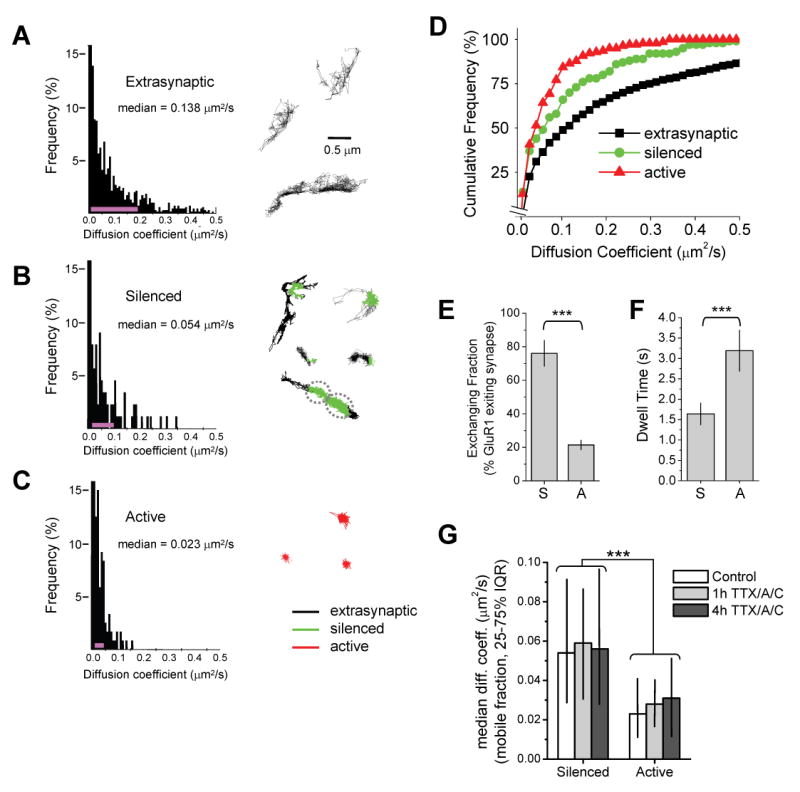
(A) Surface diffusion of extrasynaptic GluR1. Left, histogram of GluR1 diffusion coefficients (D) in the extrasynaptic plasma membrane (n = 1478 trajectories reconstructed from 69 image fields on 13 coverslips, median D value is shown). The pink line indicates the 25–75% interquartile range (IQR). Right, examples of GluR1 trajectories over extrasynaptic dendritic regions.
(B) Diffusion of GluR1 at silenced synapses. Left, histogram of GluR1 diffusion coefficients during episodes spent in inactive synapses (n = 125 trajectories reconstructed from 34 image fields on 13 coverslips, median D value is shown). Pink line, 25–75% IQR. Right, examples of GluR1 trajectories near silenced synapses (green). Trajectory color code as in (C).
(C) Diffusion of GluR1 at active synapses. Left, histogram of GluR1 diffusion coefficients during episodes spent in active synapses (n = 175 trajectories reconstructed from 26 image fields on 11 coverslips, median D value is shown). Pink line, 25–75% IQR. Right, examples of GluR1 trajectories at active synapses (red). The distributions in (A)–(C) are statistically different (p < 0.0001 for each pairwise comparison, Mann-Whitney test).
(D) Cumulative probability plot of GluR1 diffusion coefficients. GluR1 exhibits slower diffusion at active synapses relative to silenced synapses and at all synapses relative to extrasynaptic membrane.
(E) GluR1 receptors frequently exit silenced synapses. Data represent means ± SEM of the percent GluR1-QDs present at silenced (S) or active (A) synapses which leave the synapse during any portion of the 60 sec imaging period. ***p < 0.001, t-test.
(F) Exchanging GluR1 receptors remain for longer periods at active synapses. Data represent means ± SEM of the dwell times of GluR1-QDs at silenced (S) or active (A) synapses. Note that only GluR1-QDs which depart the synapse are included in the analysis. ***p < 0.001, t-test.
(G) Acute activity blockade does not alter GluR1 mobility at previously active or previously silenced synapses. Hippocampal cultures infected with synaptophysin-GFP:IRES:TetTx on DIV7 were incubated with 1 μM TTX, 50 μM D-AP5 (A), and 10 μM CNQX (C) for one or four hours before imaging on DIV15. Data represent median diffusion coefficents. IQR, interquartile range. Note that acute treatment with TTX/A/C did not alter GluR1 diffusion at either chronically silenced synapses expressing synaptophysin-GFP:IRES:TetTx or neighboring active synapses. Control, n = 125, 175 trajectories at silenced and active synapses, respectively. One hour TTX/A/C, n = 13, 11; 4 hours TTX/A/C, n = 15, 19. *** p < 0.001 for all pairwise comparisons between previously active and silenced synapses, Mann-Whitney test.
A change in instantaneous diffusion at the postsynaptic membrane alone is insufficient to account for a net difference in the number of receptor molecules at the synapse (Triller and Choquet, 2005). To determine whether the increased diffusion of GluR1 at inactive synapses corresponds with the loss or escape of receptors by lateral diffusion, we measured the exchange of GluR1 between synaptic and extrasynaptic membrane compartments at active and silenced synapses. At inactive synapses, 76.1 ± 7.6% of GluR1-QDs present at the synapse departed the synapse at some point during the 60 sec imaging period. In contrast, at nearby active synapses, only 21.4 ± 2.7% of GluR1-QDs exited the synapse over the course of 60 sec (Figure 2E). In addition, for those GluR1-QDs that exited the synapse, the average dwell time for any given episode at active synapses was significantly longer (Figure 2F). This latter analysis necessarily underestimates the overall synaptic dwell time since only GluR1-QDs which leave the synapse during the 60 sec imaging period are included in the analysis (see Experimental Procedures for details). Also, given the much smaller fraction of GluR1 receptors that exit active synapses (Figure 2E), the actual difference in dwell times between active and silenced synapses is almost certainly much larger. Nevertheless, these data show that synaptic activity locally limits GluR1 lateral diffusion over spatial dimensions of single synapses and simultaneously reduces the rate of release of GluR1 from the postsynaptic membrane. Moreover, these findings can quantitatively account for the steady-state recruitment of GluR1 at active synapses (Figures 1B and 1C) (Harms et al., 2005).
Local effects of synaptic activity could result from acute release of glutamate or from long-term structural changes. To test whether ongoing transmitter release and activation of postsynaptic glutamate receptors was required for diffusional trapping of GluR1, we acutely blocked basal spontaneous activity by applying tetrodotoxin (TTX, 1 μm) together with the glutamate receptor antagonists D-AP5 (50 μM), and CNQX (10 μM) prior to and during the imaging of GluR1-QDs. Under these conditions, blocking activity for either one hour or four hours had no effect on GluR1 mobility at previously active or silenced synapses (Figure 2G). In other words, those synapses which had been active before the addition of TTX/AP5/CNQX continued to exhibit decreased GluR1 mobility relative to those synapses which had been chronically silenced by tetanus toxin prior to addition of TTX/AP5/CNQX. These results demonstrate that the diffusional trapping of GluR1 at active synapses is not an immediate or acute effect of basal spontaneous activity, but rather reflects a longer term change in synapse organization. The lack of effect of short term activity blockade is somewhat surprising, as activity is well known to trigger rapid trafficking of AMPA receptors to synapses during LTP (Shi et al., 1999; Shi et al., 2001; Bredt and Nicoll, 2003; Park et al., 2004; Kopec et al., 2006), and may reflect a difference between slower accumulation of synaptic receptors induced by spontaneous activity (Zhu et al., 2000) and more rapid GluR1 trafficking by high frequency trains of action potentials or pairing protocols.
Active Synapses Capture GluR1 by Diffusional Exchange
The measured differences in GluR1 lateral mobility at active and inactive synapses (Figure 2) support diffusional capture as a mechanism for augmenting GluR1 synaptic content. Indeed, we were able to directly observe GluR1-QDs which, upon release from a silenced synapse, moved to a neighboring active synapse and were rapidly immobilized for sustained periods (Figure 3A). Notable during these episodes was the repeated dissociation and reassociation of GluR1 with inactive synapses which was apparent in an analysis of instantaneous diffusion coefficient over time as sharp transitions between highly diffusive (D > 0.15 μm2/s) and reduced diffusive (D ≤ 0.05 μm2/s) states (Figure 3B). This rapid dissociation and reassociation is consistent with the larger exchanging fraction and the shorter dwell time of GluR1 at silenced synapses (Figures 2E and 2F). Further, as with the observed population differences in diffusion coefficients (Figures 2B and 2C), the diffusion of single GluR1-QDs was significantly lower within active synapses relative to the diffusion of the same GluR1-QD at inactive synapses (Figures 3B and 3C). These results indicate that changes in GluR1 diffusion are a reflection of the specific synapse and not an enduring property of the specific receptor. In addition, the fact that single receptors exhibit reduced mobility at active synapses indicates that differences in GluR1 diffusion are not due to a distinct composition of receptors at active and silenced synapses. Together, these findings demonstrate that the diffusional behavior of individual GluR1 receptors is locally modified by synaptic activity and rapidly changes upon reaching active synapses.
Figure 3. Active Synapses Capture GluR1 Released from Inactive Synapses by Diffusional Exchange.
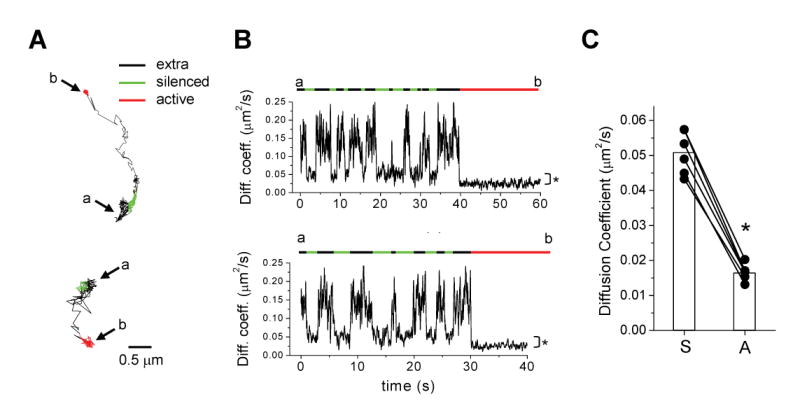
(A) Movement of GluR1 from silenced to active synapses. Shown are example trajectories of GluR1-QDs which begin in a silenced synapse (green), escape the synapse (black, extrasynaptic), and move to an adjacent active synapse (red). The start point (a) and end point (b) of the trajectories are indicated.
(B) Plots of instantaneous diffusion coefficient versus time for the trajectories shown in (A). Overlying bars indicate episodes within extrasynaptic domains (black), silenced synapses (green), or active synapses (red). The start point (a) and end point (b) of the trajectories are indicated. GluR1 exchanges frequently in and out of inactive synapses but remains fixed and immobilized at an active synapse. Note the difference in diffusion coefficient during episodes in silenced and active synapses (*p < 0.0001, Mann-Whitney test).
(C) Single GluR1-QDs exhibit reduced diffusion at active synapses. Each data point represents a single GluR1-QD which began in a silenced synapse (S) and subsequently moved to an active synapse (A). Bars indicate means. n = 6 trajectories, *p < 0.001, paired t-test.
Spontaneous Activity Confines GluR1 Intrasynaptic Movement
Once at the synapse, electron microscopy studies indicate that AMPA receptors are enriched in selective subcompartments of the postsynaptic membrane (Kharazia and Weinberg, 1997; Nusser et al., 1998; Tanaka et al., 2005) supporting limited mixing. Consistent with this notion, mathematical models posit the existence of metastable clusters of interacting AMPA receptors whose activity-dependent regulation can contribute to long-term stabilization of synaptic strength (Shouval, 2005). Such stabilized receptors could form part of the hypothetical “slot” apparatus postulated to participate in postsynaptic plasticity (Shi et al., 2001; Barry and Ziff, 2002; Lisman, 2003; McCormack et al., 2006). However, little is known of how receptors move within the synapse.
Given the above findings that GluR1 diffusion is reduced at active synapses (Figures 2 and 3), we hypothesized that spontaneous synaptic activity could confine the range of GluR1 movement within the synapse itself. To test this hypothesis, we analyzed the lateral mobility of synapse-associated GluR1 at high resolution. Live imaging of single GluR1-QDs revealed tightly confined movement of GluR1 at active synapses (Figure 4A and Supplementary Movie S3). This confined movement persisted for several seconds and consisted of small restricted displacements over a synaptic subregion (Supplementary Movie S3). In contrast, single GluR1-QDs at nearby silenced synapses moved throughout the synaptic region, often entering and exiting the synapse, and frequently displaying large displacements in the synaptic compartment (Figure 4B and Supplementary Movie S4). The effect of spontaneous activity on GluR1 confinement was restricted to single synapses as indicated by the simultaneously confined and mobile behavior of GluR1 at immediately adjacent active and inactive synapses (Figure 4C).
Figure 4. GluR1 Explores the Interior of Inactive Synapses.
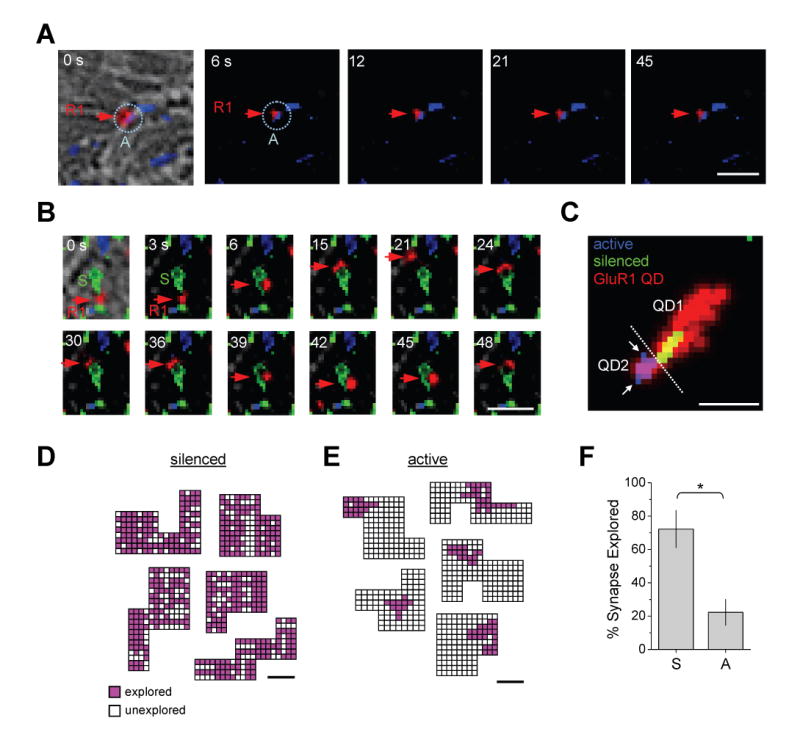
(A) Individual frames from a timelapse showing a single GluR1-QD (R1, red arrow) immobilized at an active synapse (A, blue dashed circle). Time in seconds is shown. Scale bar, 1 μm. See Supplemental Movie S3.
(B) Individual frames from a timelapse showing a single GluR1-QD (R1, red arrow) moving rapidly within and near a silenced synapse (S, green). Time in seconds is shown. Scale bar, 1 μm. See Supplemental Movie S4.
(C) Maximum projections of two quantum dot-labeled GluR1 receptors (GluR1 QD, red) at adjacent silenced (green) and active (blue) synapses. The total area explored by the two receptors (labeled QD1 and QD2) during the 55 s imaging period is indicated by red traces separated by the dashed white line. Note that GluR1 moves readily into and out of inactive synapses with a trajectory that covers the entire synaptic domain (QD1), but remains fixed to a synaptic subregion at the adjacent active synapse (QD2). Arrows indicate unexplored portions of the synapse. Scale bar, 1 μm.
(D) Single GluR1-QDs explore large areas within inactive synapses. Shown are five synaptic regions defined as a set of connected pixels obtained using object segmentation by wavelet transform. Each pixel was divided into 0.0016 μm2 subdomains and coded based on the presence (pink) or absence (white) of the GluR1-QD at any time during the imaging period as defined by the centroid of a two-dimensional Gaussian function fit to the GluR1-QD fluorescent signal (see Experimental Procedures for details). Coded areas at each synaptic region represent the trajectory of one GluR1-QD. Scale bar, 0.2 μm.
(E) GluR1 explores only small subregions within active synapses. Objects, color code, and scale bar as in (D).
(F) Data represent means ± SEM of the percent of the synaptic surface explored by GluR1-QDs at silenced (S) and active (A) synapses. Silenced, 72.2 ± 11.2% of the synapse explored, range from 58.7 – 94.1%, n = 11 synapses on 4 neurons from 3 coverslips. Active, 22.3 ± 7.7% explored, range from 10.0 – 35.0%, n = 13 synapses on 3 neurons from 3 coverslips. *p < 0.01; t-test.
Close inspection indicated that single GluR1-QDs at active synapses seldom appeared to explore the entire synaptic compartment (Figure 4C, see also Supplementary Movie S3). To quantitatively examine the submicron movement of GluR1 within synapses, we took advantage of the fact that single fluorescent objects can be positionally placed with a precision well below the resolution limit of the light microscope (Gelles et al., 1988; Kubitscheck et al., 2000; Cheezum et al., 2001). The fluorescent signal from single GluR1-QDs was fit to a two-dimensional Gaussian function to define the centroid of the object, and the position of the centroid was then mapped onto a registered image of the synapse. Synaptic regions were defined as a set of connected pixels obtained using two-dimensional object segmentation by wavelet transform (Starck et al., 1998; Laine, 2000; Racine et al., 2006). Based on the pointing accuracy of our optical system (45 ± 5 nm), each pixel was divided into 0.0016 μm2 subdomains and these subdomains subjected to a binary code corresponding to the presence or absence of the GluR1-QD at any time point during the time lapse image. We restricted our analysis to large synapses (> 0.13 μm2) to maximize detection of subsynaptic events. To avoid transient events that could represent unconfined diffusion at immediately perisynaptic membranes, only GluR1-QDs with 5 sec or more total time spent in the synaptic compartment during the imaging period were included in the analysis (see Experimental Procedures for further details). Mean total times for all episodes spent in the synaptic compartment during the 60 sec imaging period were 22 ± 6 sec for silenced synapses (n = 11) and 33 ± 8 sec for active synapses (n = 13). Using this approach, we found that, in the absence of activity, single GluR1-QDs moved throughout the synaptic region, exploring the majority of the synapse (Figure 4D). Despite this wide ranging movement, subdomains of the synapse remained unexplored in each case (Figure 4D), suggesting that not all subdomains were equally accessible for GluR1 diffusion. In contrast, single GluR1-QDs explored only a small area within spontaneously active synapses, typically being confined to a subdomain at the edge extending to the interior of the synapse (Figure 4E). Quantitative analysis confirmed a significant reduction in the total synaptic surface explored at active synapses (silenced, 72.2 ± 11.2% of the synapse explored, range from 58.7 – 94.1%, n = 11; active, 22.3 ± 7.7% explored, range from 10.0 – 35.0%, n = 13; Figure 4F).
The reduced fraction of synaptic membrane explored by GluR1 at active synapses suggests diffusional confinement. To more rigorously measure the zone of GluR1 confinement at synapses and to extend our analysis to a larger population of synaptic diffusion events, we calculated the mean square displacement (MSD) of GluR1-QDs over time. For free diffusion, the MSD is a linear increasing function of time, and such behavior was observed for GluR1-QDs moving within the extrasynaptic dendritic membrane (Figure 5A). In contrast, at both active and silenced synapses, the average MSD of GluR1 during synaptic episodes was curved and approached a quasi-maximum value at late time points, indicating that synaptic GluR1 diffused within a confined zone (Figure 5A). At active synapses, the maximum approached value of the MSD curve over time was significantly lower than at silenced synapses (Figure 5A), indicating a smaller confinement zone. Indeed, calculations of the confinement radius based on a fit of the MSD curves to the relation for confined diffusion (Kusumi et al., 1993) showed that GluR1 movement at active synapses is significantly more confined (confinement radius: silenced synapse, 0.151 ± 0.013 μm; active synapse 0.096 ± 0.005 μm, p < 0.01, ANOVA; Figure 5B). Taken together, these findings demonstrate that synapse-specific activity confines the intrasynaptic movement of GluR1. Moreover, these data provide strong evidence that AMPA receptors can segregate into isolated subdomains at synapses, and suggest that local spontaneous activity reorganizes AMPA receptors on a submicron scale.
Figure 5. Spontaneous Activity Confines GluR1 Movement Inside Synapses.
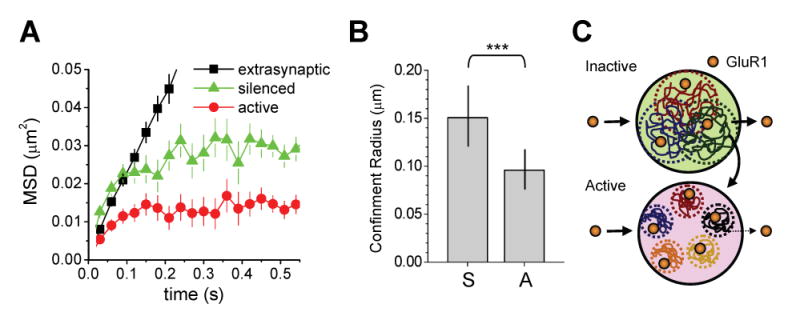
(A) Mean square displacement (MSD) versus time for GluR1-QDs in the indicated compartments. Extrasynaptic GluR1 undergoes free diffusion without confinement as indicated by the linear MSD curve. GluR1 receptors at synapses exhibit confined movement within a zone whose radius is defined by the maximum MSD value approached at the t = ∞ limit. Error bars indicate SD.
(B) GluR1 diffusion is more confined at active synapses. Data represent means ± SD of the confinement radius for GluR1 lateral movement in silenced (S) and active (A) synapses, as determined by the MSD curves in (A). Silenced, n = 125 trajectories reconstructed from 34 image fields on 13 coverslips. Active, n = 175 trajectories reconstructed from 26 image fields on 11 coverslips. ***p < 0.01, ANOVA.
(C) A schematic model for GluR1 lateral diffusion at active and inactive synapses viewed en face. Input-specific spontaneous synaptic activity reduces receptor mobility, limits exchange with the extrasynaptic membrane, and confines GluR1 within small subdomains of the postsynaptic membrane. This diffusional trap leads to GluR1 accumulation at active synapses. See text for details.
Discussion
In the present study, we have demonstrated that spontaneous synaptic activity triggers the accumulation of GluR1 AMPA receptors by input-specific diffusional trapping of GluR1 at the postsynaptic membrane (Figure 5C). In the absence of local synaptic activity, GluR1 AMPA receptors move more rapidly through and within synapses, frequently escaping the postsynaptic membrane. At nearby active synapses, GluR1 is much less mobile, dwells for longer periods, and is confined within a subregion of the synaptic membrane. When in proximity, active synapses can capture GluR1 released from inactive synapses by diffusional exchange, leading to selective accumulation of GluR1 at active synapses.
Activity Regulates AMPA Receptor Lateral Mobility at Single Synapses
It is by now well established that a major determinant of synaptic strength at glutamatergic synapses is the number of postsynaptic AMPA receptors (Malinow and Malenka, 2002; Bredt and Nicoll, 2003; Cognet et al., 2006). AMPA receptors are highly mobile, cycling into and out of the dendritic membrane (Luscher et al., 1999; Ehlers, 2000; Lee et al., 2004; Park et al., 2004) and diffusing laterally into and out of the PSD (Borgdorff and Choquet, 2002; Tardin et al., 2003; Groc et al., 2004; Triller and Choquet, 2005; Ashby et al., 2006; Bats et al., 2007). Such mobility raises the question as to how AMPA receptors are kept at synapses and, more pointedly, how nearby synapses maintain a distinct complement of receptors. Here we have shown that the activity of single synapses locally restricts AMPA receptor diffusion, effectively trapping GluR1-containing receptors by limiting their lateral diffusion away from the synapse.
At first glance, these results differ from current models, as input-specific activity was originally envisioned to promote an active delivery of GluR1 to the synapse (Passafaro et al., 2001; Shi et al., 2001; Park et al., 2004; Kopec et al., 2006). Rather, we have found that GluR1 is less mobile at active synapses and this immobility prevents diffusive loss. These findings can be reconciled in light of recent studies supporting a two-step process of AMPA receptor synaptic delivery, an initial exocytic event distant from the synapse followed by lateral diffusion in the plasma membrane (Schnell et al., 2002; Adesnik et al., 2005; Oh et al., 2006). Indeed, increasing evidence supports the notion that sites of exocytic and endocytic membrane trafficking lie outside synapses (Passafaro et al., 2001; Blanpied et al., 2002; Petralia et al., 2003; Ashby et al., 2004; Racz et al., 2004; Gerges et al., 2006; Park et al., 2006) and that a pool of extrasynaptic surface AMPA receptors contribute to synaptic plasticity (Gardner et al., 2005). In principal, activity-dependent regulation of synaptic AMPA receptors could thus be achieved by regulation of vesicular trafficking, lateral diffusion, or a combination of both, and to date abundant evidence supports the former. Now, consistent with the latter, we have found that local synaptic activity reduces diffusion of GluR1, increases dwell times for individual GluR1 receptors in synapses, and decreases the exchange rate of GluR1 between synaptic and extrasynaptic compartments. Thus, local synaptic activity couples enhanced vesicular trafficking to restricted surface mobility, providing a dual mechanism for mobilizing and maintaining synaptic AMPA receptors.
We have found that short-term blockade of basal spontaneous activity does not lead to changes in GluR1 mobility at chronically active or chronically silenced synapses. At first glance, this seems surprising as a large body of work has described input-specific trafficking of GluR1 receptors to synapses and shown that this trafficking occurs within minutes (Shi et al., 1999; Shi et al., 2001; Park et al., 2004; Cognet et al., 2006; Kopec et al., 2006; Plant et al., 2006). Based on these previous observations, one might have thought that short-term pharmacological block of activity would alter GluR1 synaptic mobility. However, we have shown that the reduced mobility of GluR1 at spontaneously active synapses relative to neighboring silenced synapses expressing tetanus toxin light chain persists after four hours of incubation in TTX/AP5/CNQX. One major difference is that experiments demonstrating rapid GluR1 trafficking have, in general, been conducted using strong stimuli (e.g., high frequency trains of action potentials or pairing protocols) known to elicit long-lasting synaptic potentiation. In other words, for rapid GluR1 trafficking, synapses existing in a ‘resting’ state were subjected to strong activation. In the present study, we have performed the reciprocal manipulation where synapses at rest experiencing basal spontaneous activity were compared to neighboring synapses experiencing no activity. One possibility is that stronger activity manipulations, such as high frequency tetanic stimulation, could have a more acute effect on GluR1 mobility at single synapses. We note that GluR4, an AMPA receptor subunit with a long carboxy-terminal tail similar to GluR1, traffics to synapses in response to spontaneous activity over a period of 36 hours (Zhu et al., 2000), although the precise time course for this spontaneous activity-dependent accumulation of GluR4 is not known. Another possibility is that activity-dependent changes in diffusion represent a slower homeostatic mechanism for synapse modification (Davis, 2006). It will be important for future studes to acutely activate identified synapses with defined patterns of stimulation while monitoring GluR1 mobility. It is also worth noting that the amount of spontaneous activity will vary in cultures of different densities and different ages. Thus, diffusional changes driven by spontaneous activity that may take several hours to happen in DIV14-15 low density cultures may occur faster in more mature and denser networks.
The mobility of receptors in the plasma membrane is determined by the physical properties and geometry of the membrane, protein interactions, and underlying cytoskeletal organization (Kusumi et al., 2005). The postsynaptic membrane provides a rich source of receptor binding partners, actin-based cytoskeletal linkage, and physical barriers which could form the physical basis for the diffusional trapping of GluR1. Here we have shown that the diffusion of GluR1 at inactive synapses is intermediate between the free diffusion in the extrasynaptic membrane and the tightly confined diffusion at active synapses, indicating that glutamatergic synapses contain both activity-dependent and activity-independent mechanisms for restricting GluR1 lateral mobility. Consistently, GluR1 participates in a large array of both activity-dependent and activity-independent protein interactions (Song and Huganir, 2002; Kim and Sheng, 2004; Cognet et al., 2006; Nicoll et al., 2006). Moreover, actin structure and actin dynamics are highly regulated by synaptic activity (Star et al., 2002; Okamoto et al., 2004), and in turn can influence AMPA receptor trafficking and synaptic stability (Allison et al., 1998; Zhou et al., 2001), perhaps by organizing plasma membrane compartments (Morone et al., 2006). Indeed, at the neuromuscular junction in Drosophila, a postsynaptic spectrin-actin lattice organizes synapse microstructure and molecular spacing (Pielage et al., 2006), suggesting an attractive basis for intrasynaptic compartmentalization. Intriguingly, the phosphorylation state of GluR1 is different in synaptic, extrasynaptic, and intracellular compartments (Ehlers, 2000; Oh et al., 2006) and controls synaptic incorporation (Esteban et al., 2003; Boehm et al., 2006), providing a possible link between intracellular signalling and compartment-specific diffusion. Beyond the synapse proper, AMPA receptors traffic in lateral extrasynaptic spine membranes (Ashby et al., 2004), and the geometry of spines themselves can restrict lateral diffusion at the spine neck (Richards et al., 2004; Ashby et al., 2006; Holcman and Triller, 2006). Although spine geometry can have a large effect on diffusion, experiments described here were performed on hippocampal neurons that had few spines (DIV14-16), indicating that activity-dependent diffusional trapping of GluR1 cannot be accounted for by changes in spine morphology alone. Thus, through diverse mechanisms, AMPA receptor lateral mobility can potentially be tightly tuned. Clearly, it will be important for future studies to systematically evaluate the precise role for actin structures, receptor binding proteins, spine geometry, and PSD protein composition in local activity-dependent AMPA receptor diffusion.
A key aspect of the current study is the demonstration that activity regulates GluR1 lateral mobility over spatial scales of single synapses. Such precise spatial control is predicted for any mechanism involved in input-specific synapse modification, but has traditionally been difficult to examine. Selective local inactivation of individual synaptic inputs allows for a comparison of synapse properties at intermingled active and inactive synapses (Harms et al., 2005). Inactivated glutamatergic synapses exhibit a remarkably similar structure and molecular organization compared to active synapses (Harms and Craig, 2005; Harms et al., 2005), which is consistent with the lack of requirement for spontaneous activity in synapse formation (Augustin et al., 1999; Varoqueaux et al., 2002; Harms et al., 2005). Indeed, one of the few detectable postsynaptic changes at single silenced synapses is the lack of GluR1-containing AMPA receptors (Harms et al., 2005) (Figures 1B and 1C), suggesting highly selective effects of local spontaneous activity.
Use of tetanus toxin light chain (TetTx) expression abolishes both action potential-mediated transmitter release and miniature synaptic events (Ahnert-Hilger et al., 1996; Martinez-Arca et al., 2001; Harms et al., 2005), producing an essentially complete block of glutamate receptor-mediated signaling. Conditional expression of TetTx in olfactory sensory neurons has been used as an in vivo genetic strategy for examining the role of synaptic release in neural circuit formation and refinement (Yu et al., 2004). Likewise, conditional expression of TetTx has been employed in the granule cells of the cerebellum to suppress glutamatergic transmission in cell populations in vivo (Yamamoto et al., 2003), and in hippocampal neurons in vitro (Harms et al., 2005). In the present study, we have used expression of TetTx to chronically suppress transmitter release at individual boutons. Although this manipulation is typically considered a means to selectively block neurotransmitter release, it is important to note that long-term expression of TetTx could inhibit other VAMP2-dependent exocytotic events. In addition, it is difficult to ascertain the kinetics of TetTx inhibition of glutamate release since this depends on the timing of expression of TetTx, its transport to presynaptic terminals, and its enzymatic activity. Obviously, several days of TetTx expression and associated synaptic blockade is a strong and necessarily nonphysiological manipulation. Ideally, one would like to elicit controlled graded changes in synaptic release while quantitatively monitoring GluR1 diffusion. We have intentionally used prolonged expression of TetTx to binarize postsynaptic receptor mobility into active versus inactive synapses in order to reveal quantitative differences at nearby synapses which might otherwise be elusive. In a physiological context, synaptic receptor mobility likely exists on a continuum ranging from immobile to highly mobile based on fluctuating levels of activity. It will be important for future experiments to acutely and reversibly block synaptic transmission while monitoring postsynaptic receptor diffusion.
Activation of glutamate receptors is known to regulate AMPA receptor trafficking through several mechanisms including direct ligand binding, heterologous activation of NMDA receptors or metabotropic glutamate receptors, and depolarization-induced activation of voltage-gated calcium channels (Carroll et al., 1999; Beattie et al., 2000; Ehlers, 2000; Lin et al., 2000; Snyder et al., 2001). Here we have found that, over hours to days, synaptic glutamate release immobilizes GluR1 at synapses. Importantly, blocking activity for up to four hours had no effect on GluR1 mobility at previously active or silenced synapses. Thus, the diffusional trapping of GluR1 at active synapses is not an immediate or acute effect of activity, but rather reflects a longer term change in synapse organization. In the extrasynaptic membrane, local calcium elevation reduces the mobility of GluR2-containing AMPA receptors (Borgdorff and Choquet, 2002). However, at both silenced and active synapses, the broad range of diffusion coefficients for individual GluR1 receptors argues against a simple binary regulation of receptor mobility. It will be important for future studies to examine the precise pharmacological profile and downstream signaling events which synaptically released glutamate confines GluR1 receptors. Regardless of mechanistic details, local confinement of GluR1 diffusion at single synapses is an attractive mechanism for the input-specific refinement of neural circuits driven by local activity.
Submicron Receptor Dynamics Within the Synapse
At the postsynaptic membrane, PDZ scaffolds and associated proteins are organized in the postsynaptic density (PSD) whose submicron architecture is thought to underlie molecular information storage. Electron microscopy has revealed a distinct three dimensional topography of biochemically extracted PSDs (Petersen et al., 2003) and a laminar steady-state position of scaffold molecules within the PSD (Valtschanoff and Weinberg, 2001) to nanometer scale resolution. Notably, both the molecular content (Ehlers, 2003) and physical structure (Geinisman et al., 1991; Toni et al., 1999; Ostroff et al., 2002) of the PSD are regulated by activity, suggesting submicron reorganization. In the postsynaptic membrane proper, glutamate receptors are not homogenously distributed (Baude et al., 1993; Kharazia and Weinberg, 1997; Nusser et al., 1998; Tanaka et al., 2005; Perez-Otano et al., 2006), suggesting limited exchange between PSD subregions. In particular, AMPA receptors are generally more abundant in the outer edge of the PSD while NMDA receptors occupy the central core (Kharazia and Weinberg, 1997; Nusser et al., 1998). Such studies necessarily provide single snapshots of receptor organization within the synapse and, in general, the submicron dimensions of the synapse have hindered analysis of receptor dynamics directly at the postsynaptic membrane. Here we have shown that spontaneous synaptic activity confines the intrasynaptic movement of GluR1 to small subregions of single synapses.
Taking advantage of the ability to define spatial position of single particles at a resolution below the optical resolution limit, we have mapped the movement of GluR1 receptors within a synaptic domain. At completely silenced synapses incapable of glutamate release, individual GluR1-QDs explore most spatial positions in the synaptic membrane. At active synapses, this intrasynaptic movement of GluR1 is restricted to a small compact portion of the synapse encompassing ∼20% of the synaptic membrane. Using centroid localization, we were able to achieve positional resolutions of 40–50 nm, still several fold lower resolution than the electron microscope, but nearly an order of magnitude higher spatial resolution than standard live imaging methods for receptor movement. This resolution approaches the resolution of single large macromolecular species or protein complexes. Using confinement analysis based on the mean square displacement (MSD) of GluR1 in synapses, we found that spontaneous activity reduces the average confinement radius of GluR1 by ∼50 nm, a significant restriction given the dimension of synapses, and one that could effectively partition or cordon off subsets of AMPA receptors to a distinct PSD subdomain. Given the specific arrangement of glutamate release sites and the biophysical properties of AMPA receptors (e.g., low agonist affinity, strong desensitization), such limited molecular mixing could have significant effects on synaptic transmission. Interestingly, mathematical simulations postulate the existence of activity-dependent metastable clusters of interacting AMPA receptors which contribute to long-lerm stabilization of synaptic strength (Shouval, 2005). One possibility is that small clusters of diffusionally stabilized receptors form part of the hypothetical “slot” apparatus proposed to store molecular information during postsynaptic plasticity (Shi et al., 2001; Barry and Ziff, 2002; Lisman, 2003; McCormack et al., 2006).
In the current study, we have used semiconductor quantum dots as fluorescent reporters which, with a diameter of 10–15 nm, are larger than single organic dye fluorophores and thus may hinder diffusion in the confined domain of the synapses [(Groc et al., 2004) but see (Dahan et al., 2003)]. However, recent in vivo particle tracking experiments indicate that intercellular spaces in the brain in situ are much wider and may be more accessible than measured in aldehyde-fixed tissue (Thorne and Nicholson, 2006). At synaptic sites, we observed GluR1-QDs diffusing into central areas of synapses, indicating that the particles have access to the synapse. The QD method has the advantage of sustained long-term imaging over many minutes permitting extended single particle tracking that is not possible with organic dyes which photobleach within a few hundred milliseconds (Tardin et al., 2003; Groc et al., 2004; Groc et al., 2006). A further current limitation of this method is its restriction to the localization of single fluorescent objects (e.g., single receptors, in this case GluR1) at the synapse. An extension to simultaneous localization of multiple receptors may be possible in the future with recent advances in quantum dot methodologies (Giepmans et al., 2005; Howarth et al., 2005) and nanometer-localized multiple single-molecule fluorescent microscopy (Qu et al., 2004). In this regard, it will be important for future studies to examine whether similar diffusional trapping occurs for other populations of synaptic membrane proteins in conjunction with GluR1.
Synaptic Competition for Local Pools of Diffusible GluR1
Single inactive synapses contain fewer GluR1 receptors, and this local loss of GluR1 occurs only in the presence of nearby active synapses (Harms et al., 2005). These observations suggest synapse-specific competition for a limiting pool of GluR1 based on differing levels of local activity. Here we have shown that local inactivation leads to a decrease in the synaptic dwell time and in increase in the exchanging fraction of GluR1. Individual GluR1 receptors released from silenced synapses can in turn be captured via diffusional exchange by neighboring active synapses. Such diffusional exchange will occur only over distance scales compatible with Brownian movement in two-dimensions. Approximations based on measured diffusion coefficients and dendritic geometries predict a range of receptor movement over dendrite segments of tens to at most a few hundred microns over periods of hours (Adesnik et al., 2005; Triller and Choquet, 2005).
Results presented here indicate that the size and local availability of the surface GluR1 pool will be a function of regional synaptic activity over a small dendritic domain. This notion is supported by the exchange of locally shared pools of cytosolic postsynaptic matrix proteins across synapses (Gray et al., 2006; Tsuriel et al., 2006). Localized diffusional trapping of AMPA receptors may thus provide a basis for restricted or limited synaptic modification within dendritic compartments (Frick and Johnston, 2005), and for graded differences in AMPA receptor synaptic content across dendritic arbors (Magee and Cook, 2000; Nicholson et al., 2006). In this regard, it is interesting to note that whereas prolonged global blockade of activity results in the synaptic accumulation of GluR1 (O'Brien et al., 1998; Thiagarajan et al., 2005), we have shown here that local activity blockade leads to loss of synaptic GluR1 receptors by lateral diffusion, emphasizing distinct modes of global and local activity-dependent synapse adaptation. Moreover, during initial synapse formation, GluR1 receptors accumulate at synapses within 1–2 hours after presynaptic boutons are capable of transmitter release (Friedman et al., 2000) and nascent synapses can switch between AMPA signaling and AMPA silent states (Groc et al., 2006), observations compatible with a diffusion-based mechanism. At glutamatergic synapses, such postsynaptic receptor accumulation may be gradual (Bresler et al., 2004; Adesnik et al., 2005), reminiscent of the initial diffusional incorporation of acetycholine receptors at the neuromuscular synapses (Anderson and Cohen, 1977). Mechanisms of glutamatergic synapse plasticity and development may thus converge on local activity-dependent capture of diffusible AMPA receptors.
Experimental Procedures
DNA Constructs, Antibodies, and Reagents
Synaptophysin-EGFP has kindly provided by George Augustine (Duke University, Durham, NC). Tetanus toxin light chain (TetTxLC) cDNA was a gift from Joseph Gogos (Columbia University, New York, NY). These two cDNAs were cloned in frame before and after the internal ribosome entry site (IRES) of pIRES-EGFP (Clontech). Synaptophysin-EGFP:IRES:TetTxLC was incorporated into a lentiviral expression vector and high titer virus obtained by expression and concentration from cell supernatants (Transzyme, Durham, NC). Viral titers ranged from 0.1 – 1.0 × 109 particles/ml. VGLUT1 and bassoon antibodies were from Synaptic Systems. Rabbit polyclonal N-terminal anti-GluR1 antibody was a gift from Rick Huganir (Johns Hopkins University, Baltimore, MD). Shank polyclonal antibody was a gift from Eunjoon Kim (KAIST, South Korea). PSD-95 antibody was from Chemicon (Temecula, CA). pan-MAGUK antibody was from Affinity BioReagents (Golden, CO). Fluorescent anti-rabbit secondary antibodies (Alexa-488/568/647) (Molecular Probes), goat Fab anti-rabbit IgG-conjugated 605/655 quantum dots (QDs) (Quantum Dot Corporation), MitoTracker Red CMXRos (Molecular Probes), and FM4-64 (Molecular Probes) were used according to manufacturer instructions and as below.
Primary Neuronal Culture and Viral Transduction
Hippocampal neurons from 18-day old rat embryos were cultured on glass coverslips overlaid on a glial feeder layer using the Banker method as described previously (Borgdorff and Choquet, 2002). Cells were plated at a density of 100 – 200 × 103 cells/ml on poly-D-lysine coated coverslips. Cultures were maintained in serum-free Neurobasal media (Invitrogen) at 37°C in 7.4% CO2 for 14 – 21 days in vitro (DIV) and during this period half of the medium was exchanged weekly. For lentiviral infections, 0.2 – 1.0 × 106 viral particles were added to a 60 mm plate containing 5 coverslips and ∼3 mls of media on DIV7-8. Hippocampal neurons were infected on DIV7-8 with labeling and imaging performed 7–8 days later between DIV14-16.
Immunocytochemistry and Synapse Labeling
For surface GluR1 immunolabeling, live hippocampal neurons were incubated with rabbit anti-GluR1-N (Mammen et al., 1997; Ehlers, 2000) for 20 min at 4°C to label surface AMPA receptors. After washing, neurons were fixed with 4% paraformaldehyde/4% sucrose and then permeabilized with −20°C methanol and 0.1% Triton X-100 in PBS. Cells were washed and incubated with Alexa 568- and Alexa 647-conjugated secondary antibodies (Molecular Probes) for 40 min at room temperature. For surface quantum dot (QD) labeling, live hippocampal neurons were incubated with rabbit anti-GluR1-N (1:200) for 5 min at 37°C prior to incubation in 0.1 nM Fab goat anti-rabbit QD in PBS pre-blocked with casein (Vector Laboratories, Burlingame, CA) for 2 min at RT. Synapse labeling was performed by addition of 5 nM MitoTracker Red for 30 sec in E4 imaging solution containing (in mM): 119 NaCl, 2.5 KCl, 2 MgSO4, 2 CaCl2, 25 HEPES pH 7.4, 30 D-glucose as described (Groc et al., 2004). Under these conditions, MitoTracker Red labeling exhibited 84 ± 7% colocalization with the presynaptic marker bassoon. For FM4-64 labeling, neurons were incubated with 15 μM FM4-64 in high K+ isotonic solution containing (in mM): 78.5 NaCl, 60 KCl, 10 D-glucose, 2 CaCl2, 2 MgSO4, 10 HEPES pH 7.4, 0.01 CNQX, 0.05 D-APV, 0.001 TTX for 60 sec prior to washing in E4 imaging solution containing CNQX, D-APV, and TTX at the same concentration.
Electrophysiology
Whole-cell voltage clamp recordings were performed on DIV 17-20 hippocampal neurons cultured on poly-lysine coated glass coverslips. Neurons were held at −60 mV using a MultiClamp 700A amplifier (Axon Instruments, Foster City, CA) controlled with a Pentium PC running MultiClamp Commander and pClamp (Axon Instruments). The extracellular solution contained (in mM): 150 NaCl, 5 KCl, 10 HEPES, 1 MgCl2, 30 D-Glucose, 2 CaCl2, 0.03 bicuculline (330 mOsm/l, pH 7.4). For mEPSC recordings, 0.001 mM TTX was included. Recording pipettes, with resistances between 3–5 MΩ, were filled with a solution containing (in mM): 30 CsSO4, 70 K2SO4, 25 HEPES, 25 N-methyl-D-glucamine, 0.1 CaCl2, 1 EGTA, 2 Na2ATP, 0.1 leupeptin (300 mOsm/l, pH 7.2). Data were analyzed using MiniAnalysis software (Synaptosoft, Decatur, GA). Detection criteria for mEPSC included amplitude greater than 5 pA and rise times from the onset to the peak of less than 5 msec.
Microscopy and Quantum Dot Imaging
Cells were imaged at 35 – 37°C in an open chamber mounted onto an inverted microscope (Olympus, IX70) equipped with a 100x objective (NA 1.4). QDs, EGFP, Mitotracker Red, and FM4-64 were detected by illumination using a xenon lamp. Excitation and emission wavelength selection was dually controlled by filter wheels containing band-pass filters. For QD imaging, samples were illuminated for 30 ms at rate of 33 Hz. Imaging times on any given field were 60 sec unless otherwise indicated. For EGFP and Mitotracker Red, images were obtained with an integration time of 50 – 100 msec. Emitted fluorescence was detected using a back-illuminated thinned CCD camera (Cascade 512BFT, Roper Scientific). QD-labeled GluR1 receptors were followed on selected dendritic regions across the cover slip containing a mixture of active (MitoTracker only) and inactive (synaptophysin-EGFP:IRES:TetTxLC) synapses for up to 30 min. Active and inactive synaptic regions were defined as a set of connected pixels obtained using two-dimensional object segmentation by wavelet transform (Starck et al., 1998; Laine, 2000; Racine et al., 2006). Image acquisition was performed using Metamorph (Universal Imaging Corp.). Control experiments performing acid stripping (pH 5.5, 1 min) removed >95% of GluR1-QDs from dendrites, indicating that imaged GluR1-QDs were at the dendritic surface. In rare instances, endocytosed GluR1-QDs were observed which underwent rapid directed movement easily distinguishable from diffusional movement of surface receptors. Such cases were excluded from subsequent analysis.
Particle Tracking and Analysis
The spatial distribution of the signals on the CCD originating from individual QDs was fit to a two-dimensional Gaussian surface with a full-width at half-maximum given by the point-spread function of our apparatus. Single QDs were identified by characteristic blinking fluorescent emission and uniform size. Tracking of single QDs was performed with custom software written within MATLAB (The Mathworks Inc., Natick, MA). Subtrajectories of single QD-receptor particles were continuously tracked between QD blinks and reconnected across dark blink periods to produce a complete trajectory based on a maximal allowable displacement of 3 pixels between two frames and a maximal allowable dark period of 25 frames corresponding to 0.76 sec at an acquisition of 33 Hz. Instantaneous diffusion coefficients (D) were calculated by a linear fit of the first 8 points of the mean square displacement (MSD, <r2>) plot versus time according to the relation
| (1) |
where by definition
| (2) |
MSD(t) was calculated according to (2) for reconnected trajectories of more than 100 frames. The pointing accuracy for single QDs was 45 ± 5 nm as determined by the variation in MSD over time for fixed particles.
For extrasynaptic episodes, 1478 trajectories were reconstructed from 69 image fields on 13 coverslips. For silenced synapses, 125 trajectories comprising episodes in 65 synapses from 34 image fields on 13 coverslips were analyzed. For active synapses, 175 trajectories comprising episodes at 161 synapses from 26 image fields on 11 coverslips were analyzed. All imaging episodes were 60 sec in duration.
The exchanging fraction of GluR1-QDs was calculated as the fraction of GluR1-QDs present in a synapse-defined pixel at any time during the imaging period which subsequently moved to any non-synapse-defined pixel at a later time. Synapse dwell time was calculated as the mean duration of each trajectory episode in a synaptic region excluding episodes that ended in a synapse-defined region at the end of the experiment. This latter exclusion minimizes the artificial bias for calculated dwell time to otherwise simply reflect the duration of the imaging experiment for long-dwelling particles, but necessarily causes an underestimate of the actual dwell time.
For intrasynaptic movement, fluorescent signal from single GluR1-QDs was fit to a two-dimensional Gaussian function to define the centroid of the object and the position of the centroid mapped onto a registered image of the synapse. Pixels were assigned to synapse regions defined by image segmentation using a wavelet transform. For our imaging system, pixels were 0.0256 μm2. Synapse pixels (Ps) were subdivided into sixteen equal-sided 0.0016 μm2 subdomains (pn) corresponding to the lower range limit of our pointing accuracy which was determined by measuring the mean square displacement <r2> over time for a nominally fixed object. Synapse-defined regions were transformed into Cartesian coordinates and the (x,y) coordinates of the corresponding GluR1-QD centroid overlaid on this Cartesian space. Although the spatial resolution of an optical system is limited by the Rayleigh criterion (dR = 0.61λ/NA), centroid localization allows determination of single particle position with much better precision than the length scale defined by the Rayleigh criterion (Gelles et al., 1988; Kubitscheck et al., 2000; Cheezum et al., 2001), permitting assignment of subpixel spatial position. Pixel subdomains (pn) were assigned a binary code corresponding to the presence (pn,exp) or absence (pn,abs) of the GluR1-QD centroid at any time during the experiment. Analysis was restricted to large synapses (>0.13 μm2) to maximize detection of subsynaptic events. All events corresponded to the trajectory of a single GluR1-QD at or near one synapse. To minimize the influence of transient trajectory intersections of mobile receptors and to avoid including high frequency ‘flickering’ of QDs present at the edge of the defined synaptic border, only GluR1-QDs with 5 sec or more total time spent in the synaptic compartment during the imaging period were included in the analysis. Mean total times for all episodes spent in the synaptic compartment during the 60 sec imaging period were 22 ± 6 sec for silenced synapses and 33 ± 8 sec for active synapses. The fraction of explored synaptic membrane (Fexp) was calculated as . For measurement of confinement radius within the synaptic compartment, MSD(t) was averaged for the first 500 msec of all intrasynaptic episodes and the data fit to the relationship for confinement movement
| (3) |
where R is the confinement radius, D is the diffusion coefficient, 4D't is free diffusion of the bounded object, and C is an offset constant as described (Kusumi et al., 1993).
Supplementary Material
Acknowledgments
We thank Marguerita Klein, Irina Lebedeva, Haiwei Zhang, Christelle Breillat, and Delphine Boucheet for excellent technical assistance. We thank Laurent Cognet, Juliet Hernandez, and Sri Ragavachari for critical review of the manuscript. This work was supported by NIH grants MH046478, AG024492, NS047574 and the Fulbright Scholar Program (to M.D.E.), and grants from the Centre National de la Recherche Scientifique, the Conseil Régional d'Aquitaine, the Ministère de la Recherche, the Fondation pour la Recherche Médicale, the Association Française contre les Myopathies and the European Community Grants QLG3-CT-2001-02089 and CT-2005-005320 (to D.C.). M.D.E. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Ahnert-Hilger G, Kutay U, Chahoud I, Rapoport T, Wiedenmann B. Synaptobrevin is essential for secretion but not for the development of synaptic processes. Eur J Cell Biol. 1996;70:1–11. [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ, Cohen MW. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977;268:757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM. Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci. 2004;24:5172–5176. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, Maier SR, Nishimune A, Henley JM. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J Neurosci. 2006;26:7046–7055. doi: 10.1523/JNEUROSCI.1235-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Bagal AA, Kao JP, Tang CM, Thompson SM. Long-term potentiation of exogenous glutamate responses at single dendritic spines. Proc Natl Acad Sci U S A. 2005;102:14434–14439. doi: 10.1073/pnas.0501956102. Epub 12005 Sep 14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D. The Interaction between Stargazin and PSD-95 Regulates AMPA Receptor Surface Trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Lim R, Walmsley B. Developmental changes in EPSC quantal size and quantal content at a central glutamatergic synapse in rat. J Physiol (Lond) 1998;511:861–869. doi: 10.1111/j.1469-7793.1998.861bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Bresler T, Shapira M, Boeckers T, Dresbach T, Futter M, Garner CC, Rosenblum K, Gundelfinger ED, Ziv NE. Postsynaptic density assembly is fundamentally different from presynaptic active zone assembly. J Neurosci. 2004;24:1507–1520. doi: 10.1523/JNEUROSCI.3819-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheezum MK, Walker WF, Guilford WH. Quantitative comparison of algorithms for tracking single fluorescent particles. Biophys J. 2001;81:2378–2388. doi: 10.1016/S0006-3495(01)75884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognet L, Groc L, Lounis B, Choquet D. Multiple routes for glutamate receptor trafficking: surface diffusion and membrane traffic cooperate to bring receptors to synapses. Sci STKE 2006. 2006:pe13. doi: 10.1126/stke.3272006pe13. [DOI] [PubMed] [Google Scholar]
- Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;10:10. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Franks KM, Stevens CF, Sejnowski TJ. Independent sources of quantal variability at single glutamatergic synapses. J Neurosci. 2003;23:3186–3195. doi: 10.1523/JNEUROSCI.23-08-03186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Johnston D. Plasticity of dendritic excitability. J Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res. 1991;566:77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- Gelles J, Schnapp BJ, Sheetz MP. Tracking kinesin-driven movements with nanometre-scale precision. Nature. 1988;331:450–453. doi: 10.1038/331450a0. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Backos DS, Rupasinghe CN, Spaller MR, Esteban JA. Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J. 2006;25:1623–1634. doi: 10.1038/sj.emboj.7601065. Epub 2006 Apr 1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giepmans BN, Deerinck TJ, Smarr BL, Jones YZ, Ellisman MH. Correlated light and electron microscopic imaging of multiple endogenous proteins using Quantum dots. Nat Methods. 2005;2:743–749. doi: 10.1038/nmeth791. [DOI] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 2006;4:e370. doi: 10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Gustafsson B, Hanse E. AMPA signalling in nascent glutamatergic synapses: there and not there! Trends Neurosci. 2006;29:132–139. doi: 10.1016/j.tins.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. Epub 2004 Jun 2020. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. Epub 12006 Nov 18721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- Harms KJ, Tovar KR, Craig AM. Synapse-specific regulation of AMPA receptor subunit composition by activity. J Neurosci. 2005;25:6379–6388. doi: 10.1523/JNEUROSCI.0302-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcman D, Triller A. Modeling synaptic dynamics driven by receptor lateral diffusion. Biophys J. 2006;91:2405–2415. doi: 10.1529/biophysj.106.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth M, Takao K, Hayashi Y, Ting AY. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc Natl Acad Sci U S A. 2005;102:7583–7588. doi: 10.1073/pnas.0503125102. Epub 2005 May 7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ. Tangential synaptic distribution of NMDA and AMPA receptors in rat neocortex. Neurosci Lett. 1997;238:41–44. doi: 10.1016/s0304-3940(97)00846-x. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kolleker A, Zhu JJ, Schupp BJ, Qin Y, Mack V, Borchardt T, Kohr G, Malinow R, Seeburg PH, Osten P. Glutamatergic plasticity by synaptic delivery of GluR-B(long)-containing AMPA receptors. Neuron. 2003;40:1199–1212. doi: 10.1016/s0896-6273(03)00722-0. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitscheck U, Kuckmann O, Kues T, Peters R. Imaging and tracking of single GFP molecules in solution. Biophys J. 2000;78:2170–2179. doi: 10.1016/S0006-3495(00)76764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Sako Y, Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys J. 1993;65:2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine AF. Wavelets in temporal and spatial processing of biomedical images. Annu Rev Biomed Eng. 2000;2:511–550. doi: 10.1146/annurev.bioeng.2.1.511. [DOI] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Lisman J. Long-term potentiation: outstanding questions and attempted synthesis. Philos Trans R Soc Lond B Biol Sci. 2003;358:829–842. doi: 10.1098/rstb.2002.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47:407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci. 2000;3:895–903. doi: 10.1038/78800. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Huganir RL, O'Brien RJ. Redistribution and stabilization of cell surface glutamate receptors during synapse formation. J Neurosci. 1997;17:7351–7358. doi: 10.1523/JNEUROSCI.17-19-07351.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S, Coco S, Mainguy G, Schenk U, Alberts P, Bouille P, Mezzina M, Prochiantz A, Matteoli M, Louvard D, Galli T. A common exocytotic mechanism mediates axonal and dendritic outgrowth. J Neurosci. 2001;21:3830–3838. doi: 10.1523/JNEUROSCI.21-11-03830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. Epub 2004 Jun 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauceri D, Cattabeni F, Di Luca M, Gardoni F. Calcium/calmodulin-dependent protein kinase II phosphorylation drives synapse-associated protein 97 into spines. J Biol Chem. 2004;279:23813–23821. doi: 10.1074/jbc.M402796200. Epub 22004 Mar 23824. [DOI] [PubMed] [Google Scholar]
- McCormack SG, Stornetta RL, Zhu JJ. Synaptic AMPA receptor exchange maintains bidirectional plasticity. Neuron. 2006;50:75–88. doi: 10.1016/j.neuron.2006.02.027. [DOI] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [see comments] [DOI] [PubMed] [Google Scholar]
- Morone N, Fujiwara T, Murase K, Kasai RS, Ike H, Yuasa S, Usukura J, Kusumi A. Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J Cell Biol. 2006;174:851–862. doi: 10.1083/jcb.200606007. Epub 2006 Sep 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Blatter LA, Bhat RV, Fiore RS, Wier WG, Baraban JM. Differential regulation of calcium/calmodulin-dependent protein kinase II and p42 MAP kinase activity by synaptic transmission. J Neurosci. 1994;14:1320–1331. doi: 10.1523/JNEUROSCI.14-03-01320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DA, Trana R, Katz Y, Kath WL, Spruston N, Geinisman Y. Distance-dependent differences in synapse number and AMPA receptor expression in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:431–442. doi: 10.1016/j.neuron.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. Epub 2005 Nov 2004. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. Epub 2004 Sep 1107. [DOI] [PubMed] [Google Scholar]
- Ostroff LE, Fiala JC, Allwardt B, Harris KM. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535–545. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Palmer CL, Lim W, Hastie PG, Toward M, Korolchuk VI, Burbidge SA, Banting G, Collingridge GL, Isaac JT, Henley JM. Hippocalcin functions as a calcium sensor in hippocampal LTD. Neuron. 2005;47:487–494. doi: 10.1016/j.neuron.2005.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Lujan R, Tavalin SJ, Plomann M, Modregger J, Liu XB, Jones EG, Heinemann SF, Lo DC, Ehlers MD. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci. 2006;9:611–621. doi: 10.1038/nn1680. Epub 2006 Apr 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. Internalization at glutamatergic synapses during development. Eur J Neurosci. 2003;18:3207–3217. doi: 10.1111/j.1460-9568.2003.03074.x. [DOI] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. A postsynaptic Spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol. 2006;175:491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. Epub 2006 Apr 2002. [DOI] [PubMed] [Google Scholar]
- Qu X, Wu D, Mets L, Scherer NF. Nanometer-localized multiple single-molecule fluorescence microscopy. Proc Natl Acad Sci U S A. 2004;101:11298–11303. doi: 10.1073/pnas.0402155101. Epub 12004 Jul 11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine V, Hertzog A, Jouanneau J, Salamero J, Kervrann C, Sibarita JB. Multiple-target tracking of 3D fluorescent objects based on simulated annealing. 2006. IEEE ISBI SA-PM-OS1.2. [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. Epub 2004 Aug 2022. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE. Properties of quantal transmission at CA1 synapses. J Neurophysiol. 2004;92:2456–2467. doi: 10.1152/jn.00258.2004. Epub 2004 Apr 2428. [DOI] [PubMed] [Google Scholar]
- Richards DA, De Paola V, Caroni P, Gahwiler BH, McKinney RA. AMPA-receptor activation regulates the diffusion of a membrane marker in parallel with dendritic spine motility in the mouse hippocampus. J Physiol. 2004;558:503–512. doi: 10.1113/jphysiol.2004.062091. Epub 2004 May 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. Epub 2005 Mar 2003. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shouval HZ. Clusters of interacting receptors can stabilize synaptic efficacies. Proc Natl Acad Sci U S A. 2005;102:14440–14445. doi: 10.1073/pnas.0506934102. Epub 12005 Sep 14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ellis-Davies GC, Magee JC. Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2003;548:245–258. doi: 10.1113/jphysiol.2002.036376. Epub 2003 Feb 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5:239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- Starck JL, Murtagh F, Bijaoui A. Image processing and data analysis: the multiscale approach. New York, NY, USA: Cambridge University Press; 1998. [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Matsuzaki M, Tarusawa E, Momiyama A, Molnar E, Kasai H, Shigemoto R. Number and density of AMPA receptors in single synapses in immature cerebellum. J Neurosci. 2005;25:799–807. doi: 10.1523/JNEUROSCI.4256-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 2006;103:5567–5572. doi: 10.1073/pnas.0509425103. Epub 2006 Mar 5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science. 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Tsuriel S, Geva R, Zamorano P, Dresbach T, Boeckers T, Gundelfinger ED, Garner CC, Ziv NE. Local sharing as a predominant determinant of synaptic matrix molecular dynamics. PLoS Biol. 2006;4:e271. doi: 10.1371/journal.pbio.0040271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [see comments] [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. Epub 2002 Jun 9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Wada N, Kitabatake Y, Watanabe D, Anzai M, Yokoyama M, Teranishi Y, Nakanishi S. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J Neurosci. 2003;23:6759–6767. doi: 10.1523/JNEUROSCI.23-17-06759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O'Donnell S, Brown HE, Osborne J, Axel R, Gogos JA. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2001;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: Delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- Zona C, Palma E, Brancati A, Avoli M. Age-dependent appearance of synaptic currents in rat neocortical neurons in culture. Synapse. 1994;18:1–6. doi: 10.1002/syn.890180102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


