Abstract
CMV-specific CD4+ and CD8+ T cell IFNγ expression and proliferation were measured in healthy volunteers by flow cytometry after CMV lysate or CMV pp65 or IE peptide pool stimulation. Cutoff values were set to maximize specificity (i.e., no false positive CMV-seronegatives). Sensitivity (defined as a positive response in CMV-seropositives to at least one of the 3 antigen preparations used) was 100% for CMV-specific CD4+ and CD8+ T cell IFNγ expression and CD4+ T cell proliferation and 95.4% for CMV-specific CD8+ T cell proliferation. All 22 CMV-seropositive individuals had positive responses by at least three of these four measurements. These findings support the concept that a multiplicity of antigen-specific functional immune responses and persistence of robust virus-specific CD4+ T cells are important components of protective immunity in this chronic viral infection.
INTRODUCTION
Human cytomegalovirus (CMV) is a significant cause of morbidity and mortality in immunocompromised patients and neonates (1). In contrast, healthy immunocompetent people infected with CMV develop protective humoral and cell-mediated viral-specific immune responses that control viral replication sufficiently well enough to prevent the development of end organ disease (1). However, CMV does establish life-long latency in such healthy CMV seropositive individuals, and periodic episodes of sub-clinical viral reactivation can occur throughout life (2,3). Nevertheless, immunocompetent CMV seropositive individuals do not develop CMV end-organ disease, and they also appear to have some degree of immune protection in terms of resisting re-infection with exogenous CMV strains (4). The laboratory correlates of this immune protection in healthy CMV seropositive individuals are not fully understood. Although previous studies have suggested that viral-specific CD4+ and CD8+ T cell cytokine expression and proliferation responses are each important in immune protection for CMV disease as well as disease caused by other chronic viral infections (2,5–9), there have been no studies that have examined both types of CMV-specific responses for both CD4+ and CD8+ T cells in the same group of healthy CMV-seropositive individuals. A more complete characterization of the immune response to CMV in normal healthy seropositive individuals could help to define what is lacking in the immune responses of patients with CMV end organ disease. Furthermore, the comparison of responses in healthy CMV-seropositive donors with those in CMV-seronegative donors or patients with CMV disease might help to establish the laboratory correlates of immune protection for the evaluation of potential CMV vaccines.
To this end, we characterized two functional assays of both CMV-specific CD4+ and CD8+ T cells (IFNγ expression and proliferation) in CMV-seropositive and CMV-seronegative donors by ex vivo stimulation using three CMV antigens: a CMV whole virus preparation (viral lysate) that can rapidly engender a CD4+ T cell response and two pools of overlapping 15mer peptides spanning the immunodominant CMV proteins pp65 and IE that can rapidly stimulate a CD8+ T cell response. The large number of epitopes in each of these three antigen preparations were suitable for stimulating CMV-specific immune responses from a group of individuals with a variety of different HLA haplotypes.
MATERIALS AND METHODS
Subjects and specimens.
Samples were collected from subjects who had serum CMV IgG measured at screening for a study of CMV vaccination or as part of a laboratory control study. These studies were approved by the University of California Committee on Human Research, and informed consent was obtained from all subjects. The human experimentation guidelines of the U.S. Department of Health and Human Services and of participating institutions were followed in conducting this research. Fresh whole blood or peripheral blood mononuclear cells (PBMC) freshly-separated from whole blood by Ficoll-Hypaque (Sigma, St. Louis, MO), density gradient centrifugation, were used directly in the assays.
Antigens.
Lysate of whole CMV was obtained from Advanced Biotechnologies (Columbia, MD) and used at a final concentration of 3 μg/mL. Peptide pools of 15 amino acid (aa) long peptides with 11 aa overlaps were used of CMV pp65 matrix protein (138 peptides) and IE protein (120 peptides). Peptides were synthesized by standard solid-phase chemistry, with free N and C termini. Peptide stocks were dissolved in DMSO and kept at −70°C at 0.6–2.1 mg/mL. Final concentrations of 1.75 μg/mL for pp65, and 1 μg/mL for IE were used in these assays. The pp65 peptides were a gift from BD Biosciences (San Jose, CA). IE peptide was obtained from Jerini Peptide Technologies (Berlin, Germany). Staphylococcal enterotoxin B (SEB, Sigma-Aldrich, St. Louis, MO) at 10 μg/mL was used as a positive control. Negative controls included no stimulation (NS) or addition of DMSO only.
Cytokine flow cytometry.
The CFC assay was performed using fresh whole blood (10). One mL of blood was stimulated with either CMV lysate, pp65 peptide pool, IE peptide pool or SEB. Purified anti-CD28/CD49d (1 μg/mL of each, BD Biosciences) was included for co-stimulation. For the negative control, only co-stimulatory antibodies were added. After 2 h at 37¡C, brefeldin A (Sigma-Aldrich) was added at a concentration of 10 μg/mL, and the cells were incubated for an additional 4 h in a programmable water bath then held at 18¡C overnight. The cells were washed, FACSLysing Solution (BD Biosciences) added to remove red blood cells, and cells were permeabilized with FACS Permeabilizing Solution (BD Biosciences). Cells were then stained for flow cytometry with fluorescein isothiocyanate (FITC)-conjugated anti-IFNγ, allophycocyanin (APC)–conjugated anti-CD3, R-phycoerythrin (PE)-conjugated anti-CD69 (BD Biosciences) and Phycoerythrincyanin 5.1 (PC5)–conjugated anti-CD4 (Beckman Coulter, Inc., Fullerton, CA) for 50 min in the dark at room temperature. Following staining, the cells were washed, fixed in 0.5% paraformaldehyde, and collected on a FACSCal-ibur instrument using CellQuest software and an Auto-loader with Worklist Manger software (BD Biosciences). For acquisition, a large lymphocyte gate excluding granulocytes and most monocytes was used, and only events falling within the gate were collected. To acquire sufficient CD4+ T cells, a gate was drawn on CD3+CD4+ events and at least 12,000 events were collected. Data was analyzed post-acquisition using FlowJo software (Tree Star, Ashland, OR). The percentage of CD4+ and CD8+ T cells that expressed IFNγ was measured by gating on CD3+ CD4+ and CD3+CD4− events, respectively, then displaying dot plots of IFNγ versus CD69 expression. IFNγ bright cells were defined by setting the positive gate 3 logs above the IFNγ-negative cells and including CD69 positive events (11). IFNγ bright events were quantified as a percentage of the gated CD4+ and CD8+ populations, respectively. Background signal was subtracted in each experiment.
Lymphocyte proliferation flow cytometry.
Carboxy-fluorescein diacetate, succinimidyl ester (CFSE) labeling was modified from the method of Aandahl et al. (12). PBMC were labeled in 1 μM CFSE (Molecular Probes, Eugene, OR) for 5 min at 37¡C/5%CO2, washed and resuspended in AIM-V serum free media with 2.5% HEPES, then plated at a concentration of 5 × 105 cells per well in a 96-well plate containing either CMV lysate, pp65 or IE peptide pools or SEB for 4 days. Harvested cells were stained with anti-CD3-APC, -CD4-PerCP-Cy5.5 and -CD14-PE and analyzed on a FACSCalibur using the Multiwell Autosampler (MAS) and MP3 software (BD Biosciences). Cells were stimulated, stained, and collected in one 96-well u-bottom plate, reducing the loss of cells.
In order to quantitate proliferating (CFSElo) CD4+ and CD8+ T cells we used the following schema illustrated in Figure 1a. Because cells that have proliferated have a high forward scatter, CD14 was used to exclude monocytes from the forward scatter gate and a large lymph/blast gate was then drawn on CD14− PBMC. From this gate CD4+ cells were identified as CD3+CD4+ cells, while CD8+ cells were defined as CD3+CD4−. Antigen-stimulated CD4+ cells included a population of CD4hi cells; many of the proliferating cells were found within this population. Because proliferating cells have high forward scatter (FS), we displayed CFSE against FS and drew a gate on the CFSElo events that excluded the forward scatter low (FSlo) events, as illustrated in Figure 1a. A small number of PBMC fail to become labeled following CFSE incubation and can contaminate the CFSElo population; most of these cells have low forward scatter and can therefore be excluded using this strategy. Experiments in which CFSE labeled cells were stained and run the same day or one day later confirmed that CFSEloFSlo events were not a product of cell division, but are cells that fail to become labeled (data not shown). In addition, CFSEloFSlo events do not vary between antigen-stimulated and non-stimulated samples. Proliferating cells were quantitated as a percentage of the CD4+ and CD8− T cell populations. Cells in the proliferation gate have gone through a number of cell divisions, so the percentage of proliferating cells does not represent the original percentage of cells stimulated by antigen, but rather an amplification of this population. We did not attempt to correct the data for cell division. Typical antigen-specific proliferation responses to CMV lysate, peptide pools and to no stimulation (NS) are shown in Figure 1b for both CD4+ and CD8+ T cells. Since responses in NS and DMSO controls were similar, only NS controls are shown in Figure 1b.
FIG. 1.
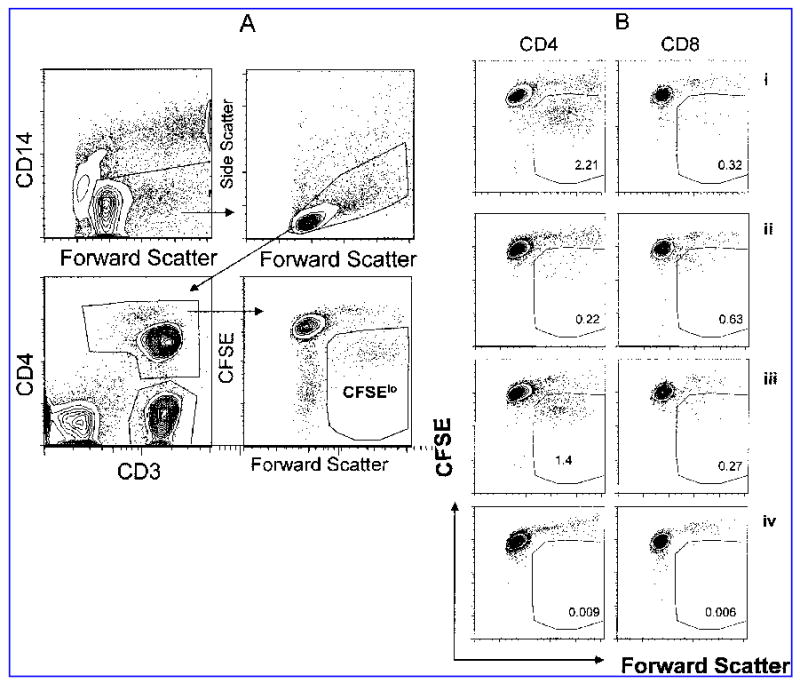
Flow cytometric measurement of antigen-specific proliferation. In the gating strategy to quantify the percentage of proliferating T cells (A), monocytes are excluded (upper left) before a lymphocyte/blast gate is drawn (upper right), and then CD3+CD4+ and CD3+CD4− gates are drawn (lower left). Proliferating cells are CFSElo with high forward scatter (lower right). (B) Typical antigen-specific proliferation patterns in a CMV-seropositive donor are shown. CD4+ T cell responses are shown on the right and CD8 (CD3+ CD4−) are shown on the right in response to stimulation with pp65 peptide pool (i), IE peptide pool (ii), CMV lysate (iii), and unstimulated (iv).
Statistical analysis.
Assay results from different subject groups were compared by the Mann-Whitney test. The Spearman test was used to test for correlation between the two antigen-stimulation assays performed on the same specimens. Differences in the proportion of responses by different immunoassays were compared by the Fisher exact test.
RESULTS
CFC and LPFC assays were respectively performed on fresh whole blood and fresh PBMC from 22 CMV-seropositive and 24 CMV-seronegative donors.
IFNγ expression and proliferation in the absence of antigen stimulation.
Because CMV-seropositive donors have been reported to have high levels of spontaneous cytokine production (2), we compared the NS controls of all CMV-seropositive and seronegative donors for IFNγ expression and proliferation. The level of CD8+ T cell spontaneous IFNγ expression was higher in the CMV-seropositives (median 0.085%, IQR 0.020–0.150%) than in the CMV-seronegatives (median 0.019%, IQR 0.003–0.048%, p = 0.0020). Although CD4+ T cells exhibited much lower levels of spontaneous IFNγ expression, CMV-seropositives had higher values than higher than CMV-seronegatives (median 0.009% vs. 0.003%). In the LPFC assay, NS controls did not differ between CMV-seropositive and CMV-seronegative donors (CD4+ T cell median values of 0.033% and 0.021%, respectively; CD8+ T cell median values 0.036% and 0.019%, respectively).
Frequency of antigen-specific IFNγ-expression and proliferation in response to CMV antigens.
No significant differences were found in the SEB responses of CMV-seropositives and CMV-seronegatives with either CFC or LPFC assays. In the CFC assays, the median CD4+ and CD8+ T cell responses to SEB were 6.20% (IQR 2.91–8.35%) and 6.08% (IQR 4.33–7.93%), respectively, in the CMV-seropositives and 4.07% (IQR 3.54–6.34%) and 3.56% (IQR 2.31–5.96%), respectively, in the CMV-seronegatives. Median CD4+ T cell SEB responses in the LPFC assay were 74.4% (IQR 64.3–76.7%) in the CMV-seropositive group and 68.6% (IQR 61.0–71.4%) in the CMV-seronegative group. Median CD8+ T cell proliferation responses were lower than CD4+ T cell proliferation responses in both CMV-seropositives (33.7%, IQR 14.8–40.97%) and CMV-seronegatives (25.1%, IQR 15.7–32.8%).
The frequency of CMV antigen-specific IFNγbright and CFSElo cells detected in the CFC and LPFC assays, after subtraction of the background responses, are shown in Figure 2. The CMV-seropositive group had significantly higher responses than the CMV-seronegative group to all 3 CMV antigens (p < 0.0001 for both IFNγ production and proliferation). Median CD4+ T cell IFNγ responses were highest to stimulation with the CMV lysate (1.67%, range 0.18–10.29%), followed by the pp65 peptide pool (0.28%, range 0.015–2.69%) and lowest to the IE peptide pool (0.06%, range 0.00–0.20%). CD4+ T cell proliferation responses showed the same pattern with median (range) responses of 2.55% (0.065–5.88%), 1.07% (0.01–5.49%) and 0.22% (0.00–7.52%) to lysate, pp65 and IE, respectively. The median CD8+ T cell IFNγ responses to CMV lysate were lower than the CD4+ T cell IFNγ responses (0.25%, range 0.00–1.47 %). The median CD8+ T cell IFNγ responses to pp65 were similar to CD4+ T cell IFNγ responses (0.18%, range 0.00–3.32%) while the CD8+ T cell IFNγ responses to IE were higher (0.14%, range 0.00–7.22%). Median CD8+ T cell proliferation responses were lower than CD4+ T cell proliferation responses to lysate and pp65 peptide (0.48% [0.00–1.32%] and 0.26% [0.01–3.04%] but similar in response to IE peptide pool (0.37% [0.00–17.98%]).
FIG. 2.
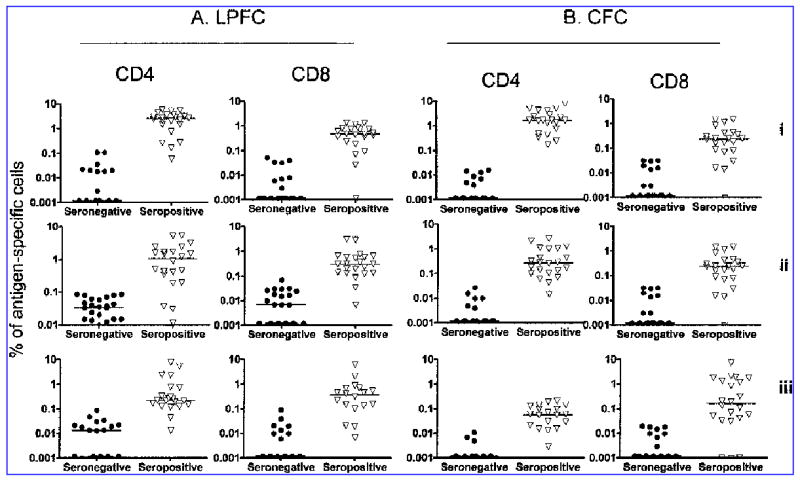
CD4+ and CD8+ T cell responses of CMV-seropositive and CMV-seronegative donors to stimulation with CMV lysate (i), pp65 peptide pool (ii), and IE peptide pool (iii). CD4+ and CD8+ T cell proliferation (A) was significantly higher in the CMV-seropositive donors, compared with the CMV-seronegatives for all three antigens (p < 0.0001 for all comparisons except CD8+ IE response, p = 0.0005). Likewise CD4+ and CD8+ T cell IFNγ expression, measured in the CFC assay (B), was significantly higher in the CMV-seropositives (p < 0.0001). IFNγ expression and proliferation were quantified as a percentage of the total CD4+ (gated on CD3+CD4+) or CD8+ (gated on CD3+CD4−) T cell population.
Sensitivity of CFC and LPFC assays for the detection of CMV antigen-specific responses.
CMV-seronegative responses were used to define a positive level of response for both CFC and LPFC assays. Cutoff values were set to maximize specificity (no false positives) at 0.03% for CMV-specific CD4+ T cell IFNγ response, 0.07% for CMV-specific CD8+ T cell IFNγ response, 0.2% for CMV-specific CD4+ T cell proliferation, and 0.1% for CMV-specific CD8+ T cell proliferation. The sensitivities of the different antigens in each assay are compared in Table 1. The sensitivity of the LPFC assay was similar to that of the CFC assay, although there was a trend for fewer false negatives (CMV-seropositive individuals lacking a positive response) detected with CFC. The lowest proportion of positive responders were detected with the IE peptide pool in both assays. CMV viral lysate was the most sensitive antigen for detecting CD4+ T cell response in both assays and (surprisingly) for CD8+ T cell responses in the CFC assay. For detection of CD8+ T cell responses in the proliferation assay, pp65 gave the highest sensitivity. Defining sensitivity as a positive response to either of the two peptide pools increased the sensitivity of CMV-specific CD8+ T cell responses in both the CFC and LPFC assays to >85%, while defining sensitivity as a positive response to any of the three antigen preparations used increased the sensitivity of CD4+ and CD8+ T cell CFC and CD4+ T cell LPFC responses each to 100% and of CD8+ T cell LPFC responses to 95.4%.
Table 1.
Sensitivity of LPFC and CFC Assays for Stimulation with One Antigen and for Combined Results of Two or Three Antigens (Percent of CMV-Seropositive Donors (n = 22) with Positive Response)
| CMV lysate | pp65 | IE | pp65/IEa | 3 antigensb | |
|---|---|---|---|---|---|
| CD4+ LPFC | 90.9 | 77.3 | 59.1 | 81.2 | 100.0 |
| CD8+ LPFC | 81.8 | 86.4 | 77.3 | 95.4 | 95.4 |
| CD4+ CFC | 100.0 | 95.4 | 59.1 | 95.4 | 100.0 |
| CD8+ CFC | 81.8 | 72.7 | 63.6 | 86.4 | 100.0 |
Percentage of CMV-seropositive donors that responded to at least one of the two peptide pools.
Percentage of CMV-seropositive donors that responded to at least one of the three CMV antigens.
Distribution of CFC and LPFC antigen-specific responses.
Table 2 shows the antigen-specific responses of each CMV-seropositive subject. The antigen preparation that stimulated the highest response in each assay (i.e., dominant response) is indicated by an asterisk; negative responses (below the cut-off value for the assay) are highlighted in gray. CFC CD4+ T cell responses were highest to CMV lysate stimulation in 100% of subjects while CFC CD8+ T cell responses were highest to this antigen in only 23%. More CFC CD8+ T cell responses than CD4+ T cell responses were highest to pp65 peptide pool (32% vs. 0%) and IE peptide pool (45% vs. 0%) stimulation. In the LPFC assay, the antigen preparations that induced the highest response were similarly distributed for CD4+ and CD8+ T cell proliferation responses. CMV lysate was the antigen to which most donors had the highest response (73% and 45% for CD4+ and CD8+ T cell responses, respectively); followed by pp65 peptide pool (18% and 27%) and IE peptide pool (9% and 23%).
Table 2.
Individual Proliferation and IFNγ Responses of CMV-Seropositive Donors to Stimulation with CMV Lysate and pp65 and IE Peptide Pools
|
CD4+T cell proliferation |
CD8+T cell proliferation |
CD4+T cell
IFN-γ expression |
CD8+T cell IFN-γ expression |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CMV lysate | pp65 | IE | CMV lysate | pp65 | IE | CMV lysate | pp65 | IE | CMV lysate | pp65 | IE | |
| Subject | CFSE low (% of CD4+T cells) | CFSE low (% of CD8+T cells) | IFN-γ bright (% of CD4+T cells) | IFN-γ bright (% of CD8+T cells) | ||||||||
| A9 | 0.065 | 1.438* | 0.188 | 0.071 | 0.131* | 0.101 | 1.299* | 0.999 | 0.055 | 0.184* | 0.070 | 0.042 |
| A11 | 5.600* | 1.620 | 2.340 | 1.322* | 0.570 | 0.220 | 0.440* | 0.180 | 0.061 | 0.000 | 0.079* | 0.000 |
| A12 | 2.660* | 2.410 | 0.720 | 0.490* | 0.310 | 0.000 | 0.490* | 0.130 | 0.000 | 0.080 | 0.090* | 0.033 |
| A13 | 2.430 | 3.190 | 5.190* | 0.890 | 2.920 | 6.230* | 0.350* | 0.099 | 0.096 | 0.070 | 0.000 | 0.319* |
| A22 | 2.018 | 5.487* | 0.127 | 0.236 | 0.313* | 0.020 | 1.660* | 1.040 | 0.016 | 0.249 | 0.749* | 0.000 |
| A24 | 3.094* | 1.749 | 0.249 | 0.733* | 0.203 | 0.000 | 1.690* | 0.340 | 0.000 | 0.256* | 0.000 | 0.000 |
| A32 | 5.323* | 2.403 | 0.263 | 0.396 | 0.370 | 0.700* | 1.670* | 0.440 | 0.021 | 1.180* | 0.000 | 0.050 |
| A34 | 5.875 | 1.505 | 7.519* | 1.300* | 0.120 | 0.883 | 5.119* | 2.689 | 0.129 | 0.890 | 3.320* | 0.110 |
| A39 | 3.715 | 5.201* | 2.401 | 0.733* | 0.647 | 0.467 | 4.250* | 1.090 | 0.190 | 0.230 | 0.190 | 1.790* |
| A44 | 3.540* | 0.030 | 0.320 | 0.520* | 0.033 | 0.423 | 0.240* | 0.015 | 0.000 | 0.016 | 0.052 | 0.135* |
| A45 | 4.320* | 0.896 | 0.206 | 0.783* | 0.140 | 0.130 | 4.425* | 0.275 | 0.029 | 0.419* | 0.043 | 0.030 |
| A47 | 2.252* | 0.429 | 0.014 | 0.093 | 0.190* | 0.019 | 1.617* | 0.267 | 0.137 | 0.370 | 0.380* | 0.057 |
| A52 | 2.839* | 0.473 | 0.213 | 0.473* | 0.227 | 0.467 | 5.130* | 0.170 | 0.060 | 0.090 | 0.170 | 2.110* |
| A53 | 2.965* | 0.195 | 0.125 | 0.364* | 0.082 | 0.148 | 0.780* | 0.140 | 0.070 | 0.030 | 0.180 | 0.850* |
| A65 | 0.772* | 0.038 | 0.000 | 0.027 | 0.007 | 0.007 | 10.291* | 0.431 | 0.015 | 0.462 | 0.912 | 1.152* |
| CIL69 | 1.479* | 1.243 | 0.223 | 0.183 | 0.300* | 0.150 | 1.660* | 1.280 | 0.200 | 0.014 | 0.187* | 0.070 |
| CIL32 | 0.28* | 0.190 | 0.170 | 0.000 | 0.529 | 2.079* | 2.460* | 0.045 | 0.013 | 0.305 | 1.135 | 1.934* |
| CIL41 | 0.261* | 0.012 | 0.043 | 0.360* | 0.125 | 0.325 | 0.180* | 0.062 | 0.003 | 0.165 | 0.000 | 1.305* |
| CIL43 | 1.432* | 0.335 | 0.755 | 0.730 | 0.580 | 10.460* | 1.377* | 0.107 | 0.137 | 0.170 | 0.130 | 7.220* |
| CIL48 | 2.140* | 0.426 | 0.336 | 0.612 | 0.659 | 17.979* | 2.757* | 0.287 | 0.096 | 1.470 | 1.020 | 1.740* |
| S129 | 3.216* | 1.740 | 0.150 | 0.433 | 0.759* | 0.529 | 7.870* | 2.150 | 0.056 | 0.250 | 0.290* | 0.190 |
| S132 | 0.165 | 0.508* | 0.118 | 1.180 | 3.040* | 0.660 | 2.220* | 0.070 | 0.030 | 1.470* | 0.410 | 0.200 |
| Cut off | 0.2 | 0.2 | 0.2 | 0.100 | 0.100 | 0.100 | 0.030 | 0.030 | 0.030 | 0.70 | 0.70 | 0.70 |
Highest response to the three antigens.
In the CFC assay, all CMV-seropositive subjects had CD4+ and CD8+ T cell IFNγ responses to at least one of the three antigen preparations, and the majority responded to two of three. A CD4+ or CD8+ T cell IFNγ response to all three antigens was present in 59% and 41%, respectively. In the LPFC assay, one subject, A65, did not have a CD8+ T cell response to any of the three antigens. The majority of these CMV seropositive subjects had CD4+ and CD8+ T cell proliferation responses to at least two of three antigens (73% and 91%) or all three antigens (55% and 59%). Of particular note, if a true positive result is defined as a positive response to any one of the three antigen preparations, then all of the CMV-seropositive individuals had true positive responses in at least three of the four measurements (CD4+ CFC, CD8+ CFC, CD4+ LPFC, and CD8+ LPFC) and by the cut-off value definition, none of the CMV-seronegative individuals had a false positive result in any assay with any antigen preparation.
The IE peptide pool was the antigen preparation that most frequently failed to elicit a positive response among CMV-seropositive subjects in both the CFC and LPFC assays. However, a positive response to IE peptide pool was observed in some individuals who did not respond to pp65 peptide pool stimulation (i.e., subjects A13, A44, and CIL41 by CFC and A44 and A53 by LPFC in Table 2). Of note, subject A44 showed no response to pp65 in either assay but had a CD8+ T cell IFNγ response to IE peptide pool stimulation and both CD4+ and CD8+ T cell proliferation responses to IE.
Robust CMV-specific T cell responses in CMV seropositives.
Defining a robust response as >10 times the cutoff value, all 22 CMV seropositives had a robust CMV-specific response by at least one of the four CMV-specific T cell measurements (CD4+ CFC, CD8+ CFC, CD4+ LPFC, or CD8+ LPFC), and 18 (82%) of 22 had a robust response by two or more of these measurements. The dominant CD4+ T cell IFNγ expression response to the three different CMV antigens was robust in 20 (91%) of 22 seropositives, but the dominant CD8+ T cell IFNγ response was robust in only 12 (55%) of 22 (p = 0.016, two-tailed Fisher exact test). A similar difference was observed between dominant CD4+ and CD8+ T cell proliferation responses which were robust in 15 (68%) of 22 for the CD4+ T cell response but in only seven (32%) of 22 for the CD8+ T cell response (p = 0.034, two-tailed Fisher exact test).
Correlation between CFC and LPFC assays.
To test for correlation between the two assays, CD4+ and CD8+ T cell IFNγ responses to each of the three antigens were correlated to the corresponding LPFC responses. The CD4+ T cell CFC and LPFC responses to CMV lysate and pp65 were significantly correlated (r = 0.48, p = 0.0165; r = 0.53, p = 0.0120, respectively), while the correlation between CFC and LPFC for CD4+ T cell responses to IE was not significant (r = 0.30). CD8+ T cell responses in the two assays approached significance for the IE peptide pool (r = 0.38, p = 0.0779) but not for the other two antigens (r = −0.004 for CMV lysate and r = 0.098 for pp65).
DISCUSSION
CD4+ and CD8+ T cell IFNγ expression in response to stimulation with CMV lysate, pp65 peptide pool and IE peptide have previously been characterized by flow cytometry (CFC) in healthy CMV-seropositive individuals by several groups (2,10,13–16). We now extend these observations to include simultaneous analysis of CMV-specific CD4+ and CD8+ T cell proliferation in response to these same three antigen preparations using the CFSE proliferation flow cytometry (LPFC) assay, which has been shown to give equivalent results to the standard tri-tiated thymidine uptake assays but is considerably easier to perform and allows phenotypic characterization of proliferating cells (17–20). Using the LPFC assay, our results exceeded the sensitivity previously reported by most groups who have used standard tritiated thymidine uptake assays for measuring CMV-specific T cell proliferation in healthy CMV seropositive individuals (21–24). Combining the results from the CFC and LPFC assays for responses to three different CMV antigen preparations, we observed that all 22 immunocompetent CMV-seropositive volunteers (i.e., individuals with protective immunity against CMV end-organ disease and re-infection with exogenous CMV strains [1,4]) had positive T cell IFNγ and proliferation responses in at least three of the four measurements performed (CD4+ T cell IFNγ expression, CD8+ T cell IFNγ expression, CD4+ T cell proliferation, and CD8+ T cell proliferation). Our observation that correlation between the CFC and LPFC assays for responses to different CMV antigens was variable does suggest that these assays are examining functionally different, though overlapping, populations of CD4+ and CD8+ T cells. Thus, these findings are consistent with the concept that protective immunity in chronic viral infections requires a multiplicity of different antigen-specific immune functions (25).
We also observed that CMV-specific CD4+ T cell responses were more robust than CMV-specific CD8+ T cell responses in healthy CMV seropositives. In contrast, Asanuma, et al, using similar flow cytometry methods found no differences in CMV-specific CD4+ and CD8+ T cell IFNγ or TNF expression in healthy CMV-seropositives (16). Although these investigators used only an infected cell lysate preparation for antigen stimulation, our observation of more a robust CMV-specific CD4+ T cell response was most apparent in the cytokine and proliferation responses to stimulation with a similar lysate antigen preparation. Of note, we recently reported that HIV/CMV co-infected patients who have HIV replication controlled with antiretroviral therapy had CD8+ T cell IFNγ responses to CMV pp65 peptide pool stimulation that were substantially more robust than the CD4+ T cell IFNγ response to this antigen (26). The importance of the persistence of robust virus-specific CD4+ T cell responses to protective immunity in chronic viral infections requires more clarification and may be determinant-specific.
Viral antigen-specific CD4+ T cells have traditionally been detected using whole viral proteins or viral lysates that are processed by the exogenous pathway and presented by MHC II. Viral antigen-specific CD8+ T cells recognize antigens processed by the endogenous pathway and presented by MHC class I. Viral vector constructs that express intracellular proteins, and more recently peptide pools which do not require processing and bind directly to MHC I, are normally used to stimulate such CD8+ T cell responses. Dunn et al., found that CMV lysate was optimal for stimulating IFNγ responses from CD4+ T cells and the pp65 peptide pool was optimal for stimulating CD8+ T cell IFNγ expression (2). In our study, the CD4+ IFNγ response to CMV lysate was considerably higher than the response to either peptide pool and was the most sensitive single measurement (considering CMV IgG seropositivity as the standard for defining CMV protective immunity in immunocompetent volunteers).
The frequencies of CD8+ T cell IFNγ responses to pp65 and IE peptide pools that we observed in healthy CMV-seropositives were lower than those reported by another group who used PBMC preparations with antigen stimulation rather than whole blood as we did (14,15). However, our results are similar to those of Dunn et al., who similarly stimulated whole blood with CMV peptide pool and lysate (2). It is possible that use of whole blood may underestimate the frequency of CMV specific T cells as recently reported by Hoffmeister et al. (27), although others have not observed significant quantitative differences in CMV-responses using these two types of specimens (28). The frequencies of CD8+ T cell IFNγ responses may also be lower in the present study due to the stringency in the cutoffs used.
Surprisingly, for CD8+ T cell IFNγ expression, we observed that response to CMV lysate was a more sensitive measurement than response to either of the CMV peptide pools. Recent evidence suggests that proteins processed by the exogenous pathway can bind to MHC I and be cross-presented to CD8+ T cells (29,30). Although this process is generally considered inefficient, differences in sample processing may explain the higher efficiency of cross-presentation in this study compared to the results of Dunn et al. (2).
Mitogens and large antigen preparations have been the most commonly used stimulants for measuring T cell proliferation by either the tritiated thymidine uptake or CFSE-based flow cytometry assays (7,19) (results have been assumed to be mainly due to CD4+ T cell proliferation in the thymidine uptake assay). We now demonstrate that overlapping CMV peptide pools (previously used to simultaneously detect CMV-specific CD4+ and CD8+ T cell IFNγ expression responses [10]) and CMV whole virus lysate can also be used to simultaneously detect CMV-specific CD4+ and CD8+ T cell proliferation responses. As expected, CMV-specific CD4+ T cell proliferation responses were highest in magnitude and in sensitivity to stimulation with CMV lysate. The magnitude of CMV-specific CD8+ T cell proliferation responses to pp65 peptide pool and CMV lysate stimulation were considerably lower than the CD4+ T cell responses. Surprisingly, CD8+ T cell proliferation responses were generally highest with CMV lysate stimulation; although sensitivity was greatest with pp65 peptide pool stimulation, and sensitivity of combined responses to pp65 or IE peptide pools was considerably greater than that of the response to CMV lysate. Although CD8+ T cells have been shown to undergo limited division in response to a single immunodominant pp65 peptide, this proliferation can be enhanced in the presence of antigens or cytokines that stimulate CD4+ T cell help (31). This may explain the magnitude of the CD8+ T cell proliferation response to CMV lysate that we observed. Additional factors that may affect comparisons of responses to lysate versus peptide pools are that there are many more determinants from additional viral proteins present in lysate preparations and that some proteolytic processing must occur before the peptides in the pools engage T cell receptors.
Spontaneous-cytokine producing cells have been detected by CFC in CMV-seropositive but not seronegative donors in the absence of in vitro antigen stimulation. These cells were found to be largely CMV-specific (2) and thus may be responding to episodes of CMV reactivation. In the present study, spontaneous cytokine production was detected from both CMV-seropositive and seronegative donors, although responses were higher in the seropositive donors. Our larger sample size, or perhaps the different subject pool in this study, may have included donors infected with other viruses that can cause in vivo cytokine production detectable by CFC.
Studies of profoundly immunocompromised patients with advanced HIV disease or undergoing bone marrow transplantation have reported that absence of CMV-specific CD4+ T cells that express IFN-γ or proliferate in response to in vitro CMV stimulation and absence of CMV-specific CTL responses are each associated with increased risk of developing CMV end organ disease (5–8). Reduced HIV-specific CD8+ T cell proliferation responses have been associated with HIV progression [17], but the ability of CD8+ T cells to proliferate in response to ex vivo CMV antigen stimulation has not been examined in the context of clinical CMV immune protection. In our study, CD4+ and CD8+ T cell IFN-γ expression and proliferation responses were all measured at the same time in healthy CMV-seropositive donors. All donors had both CD4+ and CD8+ T cell IFNγ responses to at least one of the three antigen preparations, and most responded to two of three preparations. Only one immunocompetent CMV-seropositive individual lacked a CMV-specific CD8+ T cell proliferation response to all three antigen preparations. By simultaneously testing for both CD4+ and CD8+ T cell CMV-specific responses in the proliferation assay, all 22 healthy CMV-seropositives had a proliferation response, and using just the combination of CMV lysate and pp65 would have been sufficient to detect 100% of the seropositive donors by the LPFC assay.
The evaluation of vaccines to prevent clinically important chronic viral infections would be facilitated by the availability of assays that correlate with protective immunity. Although the relationship between T cell responses and protective immunity has not yet been fully defined, viral antigen-specific T cell proliferation may be a useful immune function to measure. Flow cytometric analysis of CFSE-labeled PBMC that have been stimulated with viral lysates or peptide pools is easy to perform and a sensitive assay for the measurement of both CD4+ and CD8+ T cell proliferation and, when combined with a cytokine expression assay, may give additional information about the viral-specific immune response. More information about virus-specific T cells that may correlate with immune protection in chronic viral infections could be provided by simultaneous measurement of their IL-2 and IFNγ expression (32), by definition of their maturational stage (9,33–35) and by the ability of CD8 cells to degranulate (36). Also, finding true correlates of immunity may require a finer examination of the response specificity (i.e., individual determinants). Measurement of these T cell markers in response to specific viral determinant stimulation in future vaccine evaluation studies may help to determine the laboratory correlates of immune protection for preventing chronic viral infections.
Acknowledgments
This work was presented in part at the International Society for Analytical Cytology (ISAC) International Congress XXII, 22–27 May 2004, Montpellier, France. This work was supported by the following grants from the National Institutes of Health: RO-1 AI49538, P30 AI27763, R01AI47062, and MO1 RR00083. None of the authors have a commercial or other association that might pose a conflict of interest. We also wish to thank Holden Maecker and Stuart Adler for helpful discussions.
References
- 1.Crumpacker, C.S. Cytomegalovirus, pp. 1586–1599. In: Principles and Practice of Infectious Diseases, 5th ed. G.L. Mandell, J.E. Bennett, and R. Dolin (eds.), Churchill Livingstone, Philadelphia.
- 2.Dunn HS, Haney DJ, et al. Dynamics of CD4 and CD8 T cell responses to cytomegalovirus in healthy human donors. J Infect Dis. 2002;186:15–22. doi: 10.1086/341079. [DOI] [PubMed] [Google Scholar]
- 3.Larsson S, Soderberg-Naucler C, Wang FZ, et al. Cytomegalovirus DNA can be detected in peripheral blood mononuclear cells from all seropositive and most seronegative healthy blood donors over time. Transfusion. 1998;38:271– 278. doi: 10.1046/j.1537-2995.1998.38398222871.x. [DOI] [PubMed] [Google Scholar]
- 4.Adler SP, Starr SE, et al. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J Infect Dis. 1995;171:26–32. doi: 10.1093/infdis/171.1.26. [DOI] [PubMed] [Google Scholar]
- 5.Komanduri KV, Viswanathan MN, Weider ED, et al. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med. 1998;4:953–956. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg A, Wohl DA, MaWhinney S, et al. Cytomegalovirus-specific IFN-gamma production is associated with protection against cytomegalovirus reactivation in HIV-infected patients on highly active antiretroviral therapy. AIDS. 2003;17:2445–2450. doi: 10.1097/00002030-200311210-00006. [DOI] [PubMed] [Google Scholar]
- 7.Schrier RD, Freeman WR, Wiley CA, et al. Immune predispositions for cytomegalovirus retinitis in AIDS. The HNRC Group. J Clin Invest. 1995;95:1741–1746. doi: 10.1172/JCI117851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 9.Rufer N, Zippelius A, Batard P, et al. Ex vivo characterization of human CD8+ T subsets with distinct replicative history and partial effector functions. Blood. 2003;102:1779–1787. doi: 10.1182/blood-2003-02-0420. [DOI] [PubMed] [Google Scholar]
- 10.Maecker HT, Dunn HS, Suni MA, et al. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods. 2001;255:27–40. doi: 10.1016/s0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 11.Deeks SA, Martin JN, Sinclair E, et al. Strong cell-mediated immune responses are associated with the maintenance of low-level viremia in antiretroviral treated individuals with drug-resistant human immunodeficiency virus–1. J Infect Dis. 2004;189:312–321. doi: 10.1086/380098. [DOI] [PubMed] [Google Scholar]
- 12.Aandahl EM, Moretto WJ, Haslett PA, et al. Inhibition of antigen specific T cell proliferation and cytokine production by protein kinase A type 1. J Immunol. 2002;169:802–808. doi: 10.4049/jimmunol.169.2.802. [DOI] [PubMed] [Google Scholar]
- 13.Waldrop SL, Pitcher CJ, Peterson DM, et al. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kern F, Faulhaber N, Frommel C, et al. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000;30:1676–1682. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Kern F, Bunde T, Faulhaber N, et al. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J Infect Dis. 2002;185:1709–1716. doi: 10.1086/340637. [DOI] [PubMed] [Google Scholar]
- 16.Asanuma H, Sharp M, Maecker HT, et al. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intra-cellular detection of cytokine expression. J Infect Dis. 2000;181:859–866. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- 17.Migueles SA, Laborico AC, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 18.Mannering SI, Morris JS, Jensen KP, et al. A sensitive method for detecting proliferation of rare auto-antigen-specific human T cells. J Immunol Methods. 2003;283:173–183. doi: 10.1016/j.jim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Angulo R, Fulcher DA. Measurement of Candida-specific blastogenesis: comparison of carboxyfluorescein succinimidyl ester labelling of T cell, thymidine incorporation, and CD69 expression. Cytometry. 1998;34:143–151. [PubMed] [Google Scholar]
- 20.Fulcher DA, Wong SWJ. Carboxyfluorescein succinimidyl ester-based proliferative assays for the assessment of T cell function in the diagnostic laboratory. Immunol Cell Biol. 1999;77:559–564. doi: 10.1046/j.1440-1711.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- 21.Leroux M, Schindler L, Braun R, et al. A whole-blood lymphoproliferation assay for measuring cellular immunity against herpes viruses. J Immunol Methods. 1985;79:251–262. doi: 10.1016/0022-1759(85)90105-x. [DOI] [PubMed] [Google Scholar]
- 22.Omisakin KA, Davidson F, Bevan DH, et al. Lymphocyte responses to human cytomegalovirus in different groups of patients in Britain and in adults from west Africa and the Middle East. J Med Virol. 1996;49:311–318. doi: 10.1002/(SICI)1096-9071(199608)49:4<311::AID-JMV9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Shearer GM, Marincola FM, et al. Discordant cellular and humoral immune responses to cytomegalovirus infection in healthy blood donors: existence of a Th1-type dominant response. Int Immunol. 2001;13:785–790. doi: 10.1093/intimm/13.6.785. [DOI] [PubMed] [Google Scholar]
- 24.Pollard RB, Rand KH, Arvin AM, et al. Cell-mediated immunity of cytomegalovirus infection in normal subjects and cardiac transplant patients. J Infect Dis. 1978;137:541–549. doi: 10.1093/infdis/137.5.541. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi RT, Walker BD. Immunologic control of HIV-1. Annu Rev Med. 2002;53:149–172. doi: 10.1146/annurev.med.53.082901.104011. [DOI] [PubMed] [Google Scholar]
- 26.Martin, J., Sinclair, E., Bredt, B., et al. 2003. Cytomegalovirus-specific CD4+ and CD8+ T-cell responses in HIV-infected patients on virologically suppressive HAART. Presented at the 10th Conference on Retroviruses and Opportunistic Infections. Boston.
- 27.Hoffmeister B, Bunde T, Rudawsky IM, et al. Detection of antigen-specific T cells by cytokine flow cytometry: the use of whole blood may underestimate frequencies. Eur J Immunol. 2003;33:3484–3492. doi: 10.1002/eji.200324223. [DOI] [PubMed] [Google Scholar]
- 28.Suni MA, Picker LJ, Maino VC. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J Immunol Methods. 1998;212:89–98. doi: 10.1016/s0022-1759(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 29.Maecker HT, Ghanekar SA, Suni MA, et al. Factors affecting the efficiency of CD8+ T cell cross-priming with exogenous antigens. J Immunol. 2001;166:7268–7275. doi: 10.4049/jimmunol.166.12.7268. [DOI] [PubMed] [Google Scholar]
- 30.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 31.D. van Leeuwen EM, Gamadia LE, Baars PA, et al. Proliferation requirements of cytomegalovirus-specific, effector-type human CD8+ T cells. J Immunol. 2002;169:5838–5843. doi: 10.4049/jimmunol.169.10.5838. [DOI] [PubMed] [Google Scholar]
- 32.Harari A, Petitpierre S, Vallelian F, et al. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1–infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 2004;103:966–972. doi: 10.1182/blood-2003-04-1203. [DOI] [PubMed] [Google Scholar]
- 33.Ellefsen K, Harari A, Champagne P, et al. Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur J Immunol. 2002;32:3756–3764. doi: 10.1002/1521-4141(200212)32:12<3756::AID-IMMU3756>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 34.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 35.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 36.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]


