Abstract
During myofibril formation, Z-bodies, small complexes of alpha-actinin and associated proteins, grow in size, fuse and align to produce Z-bands. To determine if there were changes in protein dynamics during the assembly process, Fluorescence Recovery after Photobleaching was used to measure the exchange of Z-body and Z-band proteins with cytoplasmic pools in cultures of quail myotubes. Myotubes were transfected with plasmids encoding Yellow, Green or Cyan Fluorescent Protein linked to the Z-band proteins: actin, alpha-actinin, cypher, FATZ, myotilin, and telethonin. Each Z-band protein showed a characteristic recovery rate and mobility. All except telethonin were localized in both Z-bodies and Z-bands. Proteins that were present both early in development in Z-bodies and later in Z-bands had faster exchange rates in Z-bodies. These results suggest that during myofibrillogenesis, molecular interactions develop between the Z-band proteins that decrease their mobility and increase the stability of the Z-bands. A truncated construct of alpha-actinin, which localized in Z-bands in myotubes, and exhibited a very low rate of exchange, lead to disruption of myofibrils, suggesting the importance of dynamic, intact alpha-actinin molecules for the formation and maintenance of Z-bands. Our experiments reveal the Z-band to be a much more dynamic structure than its appearance in electron micrographs of cross-striated muscle cells might suggest.
Keywords: Myofibrillogenesis, Fluorescence recovery after photobleaching, Z-band proteins, Myofibril, Premyofibril, Nascent myofibril, Mature myofibril, Sarcomere, alpha-actinin
INTRODUCTION
The ordered structure of sarcomeres, the basic units of striated muscle, arises from interactions between several groups of filaments. These interactions produce an alignment of the filaments precise enough that protein components of the sarcomere are localized in discrete bands both in the resting state and during contraction. Titin and the actin/nebulin filaments terminate in one of these bands, the Z-band. The Z-band also serves as the attachment site for desmin intermediate filaments and often, transverse tubules (reviewed in Ervasti, 2003 and in Sanger et al., 2004). Through connections to integrin via the Z-band proteins, alpha-actinin and talin, the sarcomere is linked to the membrane and the extracellular matrix (Danowski et al., 1992; O’Neil et al., 2002; Ervasti, 2003). Alpha-actinin, an abundant Z-band protein, forms anti-parallel homodimers that cross-link actin/nebulin filaments in the myofibrils. Alpha-actinin also binds the amino terminal end of the giant protein, titin, which extends from middle of the A-band to the Z-band. Recently, several new Z-band proteins have been identified that bind to titin or alpha-actinin, including myotilin (Salmikangas et al., 1999), telethonin (Valle et al., 1997; Gregorio et al., 1998; Mues et al., 1998; Mason et al., 1999), FATZ (Faulkner et al., 2000) and cypher/ZASP (Zhou et al., 1999, 2001; Faulkner et al., 1999). Myotilin, FATZ and cypher bind alpha-actinin and telethonin binds titin and FATZ.
The linking of Z-band proteins with one another has important implications for muscle formation, maintenance, and structure. The formation of mature Z-bands requires the addition and organization of Z-band proteins to form a stable unit that can support contractile forces of the muscle. During myofibrillogenesis, Z-bands assemble by the lateral fusion of the Z-bodies of premyofibrils and nascent myofibrils (Sanger et al., 1986; McKenna et al., 1986; Dabiri et al., 1997; Du et al., 2003). In the transition from Z-bodies to Z-bands, the number of proteins in the Z-band increases, and this may lead to increased organization and stability needed for contraction (Rhee et al., 1994; Sanger et al., 2000; Sanger and Sanger, 2001 a; Siebrands et al., 2004). The earliest Z-bodies in premyofibrils are positive for actin, alpha-actinin, filamin, and nebulin (Mittal et al., 1987 a; Rhee et al., 1994; Moncman and Wang, 1995; Sanger et al., 2000, Siebrands et al., 2004). Z-band proteins that also interact with the sarcolemma, such as talin and vinculin become localized after the Z-bodies of nascent myofibrils have fused laterally to form Z-bands (or Z-lines) of the mature myofibrils (Hilenski et al., 1991; Rhee et al., 1994; Sanger et al., 2000). Between the earliest Z-body stage (premyofibrils) and the mature Z-band stage, titin becomes localized in Z-bodies (nascent myofibrils; Rhee et al., 1994). At this time muscle-specific myosin II filaments begin to become aligned along the forming myofibrils (nascent myofibrils) in a process thought to be guided by titin (Wang and Wright, 1988; Whiting et al., 1989; Rhee et al., 1994).
The half-lives of sarcomeric proteins have been reported to be of the order of three to ten days (Zak et al., 1977; Isaacs and Fulton, 1989). Therefore, once formed, the Z-bands must maintain their integrity while accommodating the replacement of older protein molecules with newly synthesized molecules. It is clear from several studies of protein dynamics in live cells that superimposed on the processes of formation and maintenance of macromolecular complexes such as stress fibers and myofibrils there is an active exchange of proteins between organized structures and cytoplasmic pools (Kreis et al., 1979; McKenna et al., 1985 a; Sanger et al. 1986; Mittal et al., 1987 b). The time scale for this dynamic behavior is much shorter than protein half-lives, and occurs without affecting structural integrity.
In this work, we transfected skeletal muscle cells with cyan, green or yellow fluorescent proteins (CFP, GFP or YFP) coupled to Z-band proteins, and used the technique of Fluorescence Recovery After Photobleaching (FRAP) to reveal protein dynamics inside living cells (White and Stekzer, 1999; Bastiaens and Pepperkok, 2000; Lippincott-Schwartz et al., 2001). We have used FRAP to measure the dynamics of proteins present in Z-bodies and Z-bands during myofibrillogenesis to determine if there were differences among the different Z-band proteins, and if there were differences in the dynamic exchange of the same protein when it was localized in (a) Z-bodies of premyofibrils and nascent myofibrils, and (b) in Z-bands of mature myofibrils. Our experiments indicate that the Z-band is a dynamic structure with proteins exchanging out of and into the Z-band from cytoplasmic pools, despite its apparent solid structure observed in electron micrographs of cross-striated muscle cells, and its role in anchoring the filaments of the sarcomere. Experiments using a truncated construct of alpha-actinin also demonstrate the importance of dynamic, intact alpha-actinin molecules in the formation, stability and maintenance of the Z-bands in sarcomeres and myofibrils.
MATERIALS AND METHODS
Cell culture, Transfections and Immunostaining
Skeletal myoblasts were isolated from the breast muscles of 9-day old quail embryos and plated on collagen-coated dishes at a density of 1×105 cells per dish (Dabiri et al., 1999). The cells were transfected after 2 days of culture with plasmids encoding YFP-, GFP- or CFP-fused to Z-band proteins. DNA-liposome complexes were prepared by combining 3 ul FuGene-6 transfection reagent with 1ug plasmid in 100 ul of serum-free medium for each dish. After incubating for 30 minutes, the complexes were added drop wise to the cells that were in dishes with 1.5 ml transfection medium (Ayoob et al., 2001). After 12–24 hours, the cells were washed 3 times and cultured in normal medium. The cells were transferred to HEPES-buffered medium for observations with confocal microscopy. Immunostaining of transfected cells was performed as previously described using an anti-sarcomeric alpha-actinin antibody and a rhodamine-labeled secondary antibody (Dabiri et al., 1999).
Constructs
Since publication of the original paper describing cypher isoforms 1 and 2 (Zhou et al., 1999, 2001), additional cypher isoforms have been identified and characterized (CH and JC, unpublished data). In particular, there are specific isoforms expressed in cardiac and skeletal muscle, which are now denoted as “c” and “s” respectively. To clarify the identity of each isoform, the original cypher1 and cypher 2 are now referred to as cypher1c and cypher2s, respectively. Cypher1s is identical to cypher1c except that amino acids 107–228 of Cypher1c are replaced by amino acids 107–189 of cypher2s. Construction of GFP-fused cypher-2s, cypher-PDZ (amino acids 1 to 84) and cypher-LIM (amino acids 507 to 683) has been previously described (Zhou et al., 1999, 2001). GFP-fused cypher 1s was constructed by PCR amplification of the full-length coding region using Pfu DNA polymerase (Stratagene) followed by cloning into pEGFP-C1 (Clontech). The other constructs used in this study were assembled using techniques previously reported (Ayoob et al., 2000). The 95-kD sarcomeric alpha-actinin molecule can be divided into two main domains as previously described (Palvalko and Burridge, 1991; Huang et al., 2002): the N-terminal region, amino acids 1 to 273, contains the actin-binding region of alpha-actinin, and is termed 27-kD in reference to its size; the C-terminal region, amino acids 274 to 898, contains the spectrin repeats and the integrin-binding domain and is designated 53-kD. The cDNA for skeletal alpha-actinin and 27-kD fragment were cloned into pEYFP-N1 and pECFP-N1 (Clontech). The cDNAs of myotilin, telethonin, FATZ and actin were derived from RT-PCR reactions using the human skeletal muscle total mRNA (Stratagene) and cloned into the YFP plasmid, pEYFP-N1 (Clontech). All constructs were sequenced to ensure that no PCR errors had been introduced. For alpha-actinin, it is essential for YFP and CFP to be fused to the carboxyl terminus of alpha-actinin for the fusion protein to become specifically localized in the transfected cells. This was first discovered in the assembly of the GFP construct for alpha-actinin by Dabiri et al. (1997). For all other constructs, the position of the fluorescent probe had no effect on the localization of the fusion protein or on the fluorescence recovery after photobleaching. Transfection of these full-length constructs had no effect on cell growth or myofibril formation. In several experiments, cells were doubly transfected with CFP-alpha-actinin and YFP-Z-band proteins to determine if the two colored proteins located to the same Z-bands, and had the same type of recoveries after photobleaching as experiments performed with single transfections. The plasmid encoding the photoactivatible GFP was obtained from Patterson and Lippincott-Schwartz (2002).
Confocal imaging, photobleaching and data analysis
Cells were observed with a Zeiss LSM 510 confocal microscope, using a 63X water-immersion objective, an excitation wavelength of 488 and a long pass 505 nm emission filter for both GFP and YFP probes. Regions of interest were bleached using the Zeiss software, with the laser at 100% power. Post-bleach images were obtained periodically at time points between 20 or 60 seconds apart with 2% laser intensity. Data were analyzed using MetaMorph software on a PC workstation. Stacks of images in each time series were aligned and the average fluorescence intensity of the bleached Z-band, an unbleached Z-band as a control and a background region were recorded for each time point. The background intensity was subtracted from the bleached and unbleached intensity values, and the ratio was calculated between the resulting corrected intensities. The value from the initial bleach image (t = 0) was then subtracted from each time point and the data were normalized so that the pre-bleach intensity was set equal to 1 and the intensity of the initial bleach image (t = 0) was set to 0. Finally the normalized recovery data from a number of Z-bands (2–5) in one cell were averaged for further quantitative analysis. The curve fitting method was used to extract the mobile fraction, rate and half time of recovery (t1/2) from the FRAP curve as previously reported (Tyska and Mooseker, 2002) with some modifications. KaleidaGraph was used to fit the averaged data to the following one or two exponential expression:
For the data analysis, we fit the data to single- and double-exponential processes and compared these curves with the experimental curve. Almost all of the plots indicated the best fit was with a two step process for the measured rates of recovery after photobleaching. R is the relative recovery of fluorescence intensity of the bleached area at time t. M1 and M2 are the mobile fractions of the two exponential processes with rate constants k1 and k2, respectively. The total mobile fraction (M) was given by M1 + M2. The half time of fluorescence recovery (t1/2) for each process was calculated by t1/2 = ln2/k. The number of samples is 6–12 different cells for each protein for FRAP analysis. Grouped data are presented as the mean ± standard deviation (SD).
RESULTS
Localization of the GFP or YFP fused Z-band proteins in the mature myofibrils and premyofibrils
When skeletal myoblasts, isolated from quail embryonic breast muscle, fuse to form myotubes in culture, the muscle isoform of alpha-actinin is present initially in premyofibrils as small punctate bodies (Z-bodies) that align and fuse to form the Z-bands of mature myofibrils (Sanger et al., 2002; Siebrands et al., 2004; Golson et al., 2004). Premyofibrils and nascent myofibrils continue to form at the sides and ends of the growing myotubes as the central cytoplasm of the myotube fills with mature myofibrils. The Z-band proteins, myotilin, FATZ and cypher, when expressed with GFP or YFP tags, also colocalized with alpha-actinin in the Z-bodies of premyofibrils and nascent myofibrils, as well Z-bands, in transfected muscle cells that had been fixed and stained with a muscle-specific alpha-actinin antibody (Figure 1). Telethonin, on the contrary, did not co-localize with alpha-actinin in Z-bodies, rather it showed a diffuse distribution until mature myofibrils formed, when it localized in Z-bands of the mature myofibrils (Figure 1). In older myotubes, telethonin remained diffusely distributed in the ends where premyofibrils were present. None of the myotubes expressing any of the different full length GFP- or YFP-Z-band proteins were inhibited in their growth, ability to assemble myofibrils, and spontaneous contractions.
Figure 1.
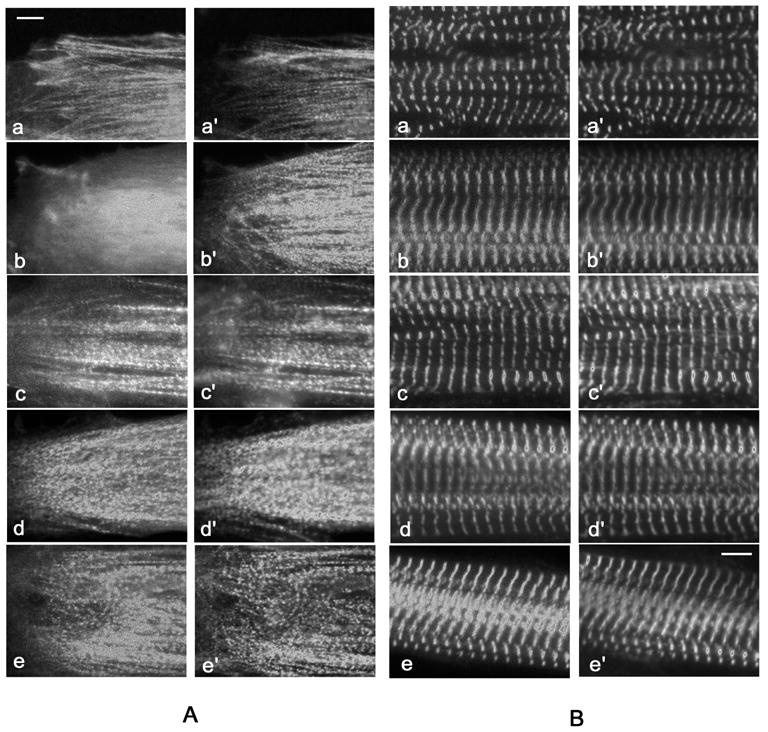
Fluorescence image of myotubes at their (A) spreading ends and in their (B) central regions previously transfected with plasmids encoding different Z-band proteins: (a) myotilin, (b) telethonin, (c) FATZ, (d) cypher-1s, and (e) cypher-2s. The transfected myotubes were fixed and stained with sarcomeric alpha-actinin antibodies (a′–e′). Myotilin, FATZ and cypher are localized in the Z-bodies at the ends of myotubes, but telethonin is not. All the proteins are co-localized with alpha-actinin in the Z-bands of mature myofibrils in the central regions of the myotubes. (Bar = 5 μm.)
Antibodies to mouse cypher also stained the Z-bodies and Z-bands of the quail myotubes yielding images identical to those of the GFP-cypher transfected quail myotubes (data not shown). Antibodies directed against avian FATZ and myotilin are unavailable, and antibodies directed against human telethonin were unreactive in quail myotubes.
FRAP of Z-band proteins in mature myofibrils
To compare the dynamics of these different Z-band component proteins, FRAP technology was used with myotubes that had been transfected with GFP or YFP fusions of the proteins. Figure 2 shows an example of one such trial of fluorescence recovery of YFP-myotilin (Figure 2 A), YFP-alpha-actinin (Figure 2 B), and YFP-telethonin (Figure 2 C) in the Z-bands of mature myofibrils. It is clear from images that myotilin recovers much more completely than either alpha-actinin or telethonin. To determine the fluorescence recovery rates of these and other Z-band proteins, the fluorescence intensity data of each trial were normalized for an initial bleach level of 0% and the recoveries of fluorescence were plotted as a function of time after bleaching (Figure 3 A). The three Z-band proteins highlighted in Figure 2 span the range of recoveries: myotilin fluorescence recovered to 98% of the initial intensity within 10 minutes whereas only 40 % of bleached alpha-actinin and 22% of the photobleached telethonin exchanged in the same time period (Figure 3 A).
Figure 2.
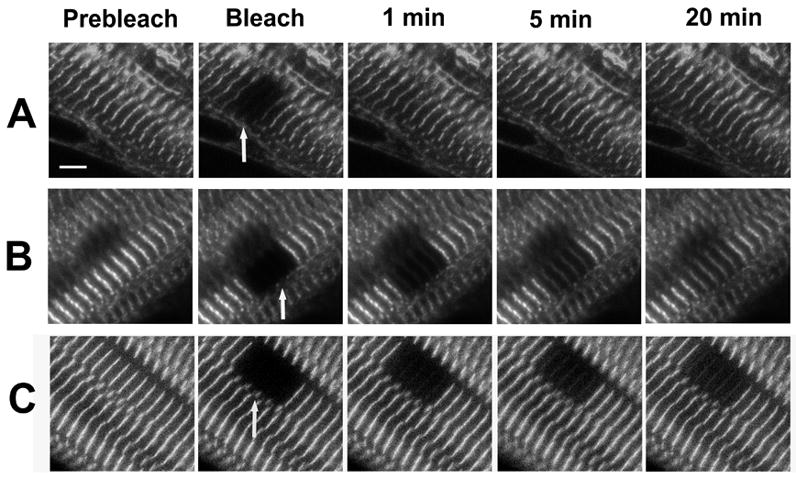
Fluorescence recovery after photobleaching (FRAP) experiments on the Z-bands of mature myofibrils. Myotubes were transfected with YFP-myotilin (A), YFP-alpha-actinin (B) and YFP-telethonin (C). Images shown from left to right are pre-bleach, bleach, 1, 5 and 10 minutes recovery after bleach. The arrows indicate the bleached region in the bleach frame. (Bar = 5 μm.)
Figure 3.
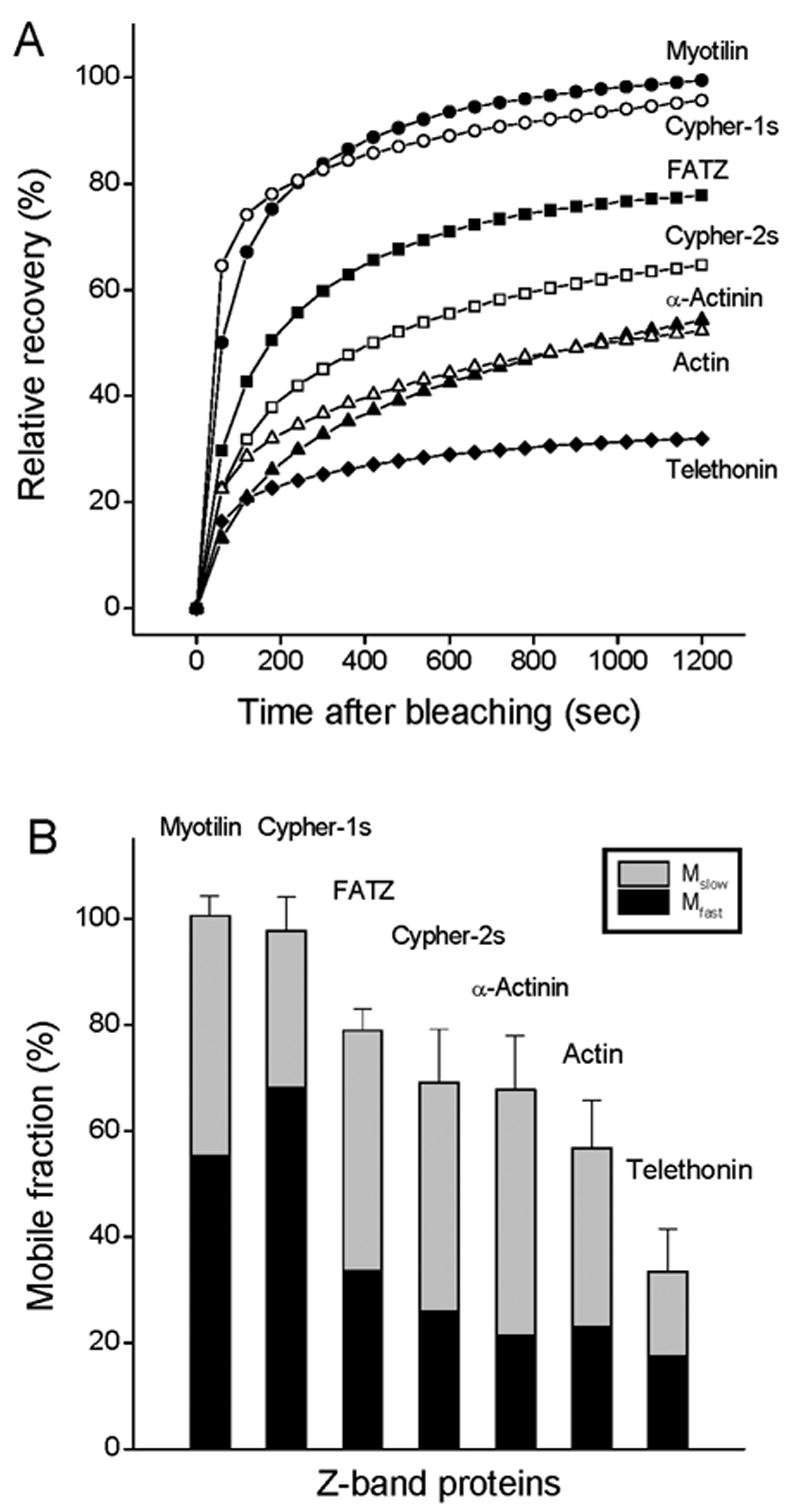
FRAP of different Z-band proteins in mature myofibrils. (A) The average FRAP recovery curves within 20 minutes of myotilin (black circles), cypher-1s (white circles), FATZ (black rectangles), cypher-2s (white rectangles), alpha-actinin (black triangles), actin in the Z-band (white triangles) and telethonin (black diamond). (B) Comparison of the fast (solid black columns) and slow mobile (stippled columns) fractions of different Z-band proteins. Values are the means ± SDs. Note that myotilin and cypher-1s were 100% mobile, and telethonin is the slowest protein recovering from photobleaching.
The FRAP curves (Figure 3 A) of the Z-band proteins indicate that recovery rates are faster initially, and are best fitted with a two exponential process to extract the mobile fraction and recovery rate constants shown in Table 1. Figure 3B compares the mobile fraction of the two exponential processes of each protein. Both myotilin and cypher-1s are fully mobile in the Z-band, but cypher-1s shows higher recovery rate in the early recovery process as it has a higher fast mobile fraction and rate constant (Figure 4 and table 1). All the other Z-band proteins are partly mobile in the Z-bands with different mobile fractions. Alpha-actinin and cypher-2s have similar mobile fractions, but cypher-2s has a higher recovery rate constant than alpha-actinin for both fast and slow process. Telethonin is the least mobile protein among these Z-band proteins.
Table 1.
Summary of FRAP data for different Z-band proteins in mature Z-bands
| Z-band proteins | MW (kD) | M (fast) (%) | K (fast) (s−1) | T1/2 (fast) (s) | M (slow) (%) | K (slow) (s−1) | T1/2 (slow) (s) | M (total) (%) |
|---|---|---|---|---|---|---|---|---|
| Myotilin | 57 | 55 ± 10 | 0.026 ± 0.009 | 30 ± 14 | 45 ± 13 | 0.0036 ± 0.0006 | 197 ± 45 | 100 ± 4 |
| Cypher-1s | 76 | 68 ± 8 | 0.042 ± 0.012 | 18 ± 5 | 30 ± 7 | 0.0022 ± 0.0008 | 342 ± 123 | 98 ± 6 |
| FATZ | 32 | 34 ± 12 | 0.014 ± 0.004 | 51 ± 13 | 45 ± 12 | 0.0025 ± 0.0007 | 298 ± 99 | 79 ± 4 |
| Cypher-2s | 31 | 26 ± 6 | 0.021 ± 0.011 | 39 ± 14 | 43 ± 10 | 0.0021 ± 0.0006 | 357 ± 102 | 69 ± 10 |
| α-Actinin | 104 | 21 ± 8 | 0.012 ± 0.002 | 61 ± 11 | 46 ± 5 | 0.001 ± 0.0002 | 721 ± 127 | 68 ± 10 |
| Actin | 42 | 23 ± 7 | 0.028 ± 0.004 | 25 ± 4 | 34 ± 9 | 0.0016 ± 0.0002 | 453 ± 63 | 57 ± 9 |
| Telethonin | 19 | 18 ± 7 | 0.033 ± 0.008 | 22 ± 6 | 16 ± 3 | 0.0023 ± 0.0006 | 330 ± 96 | 33 ± 8 |
Figure 4.
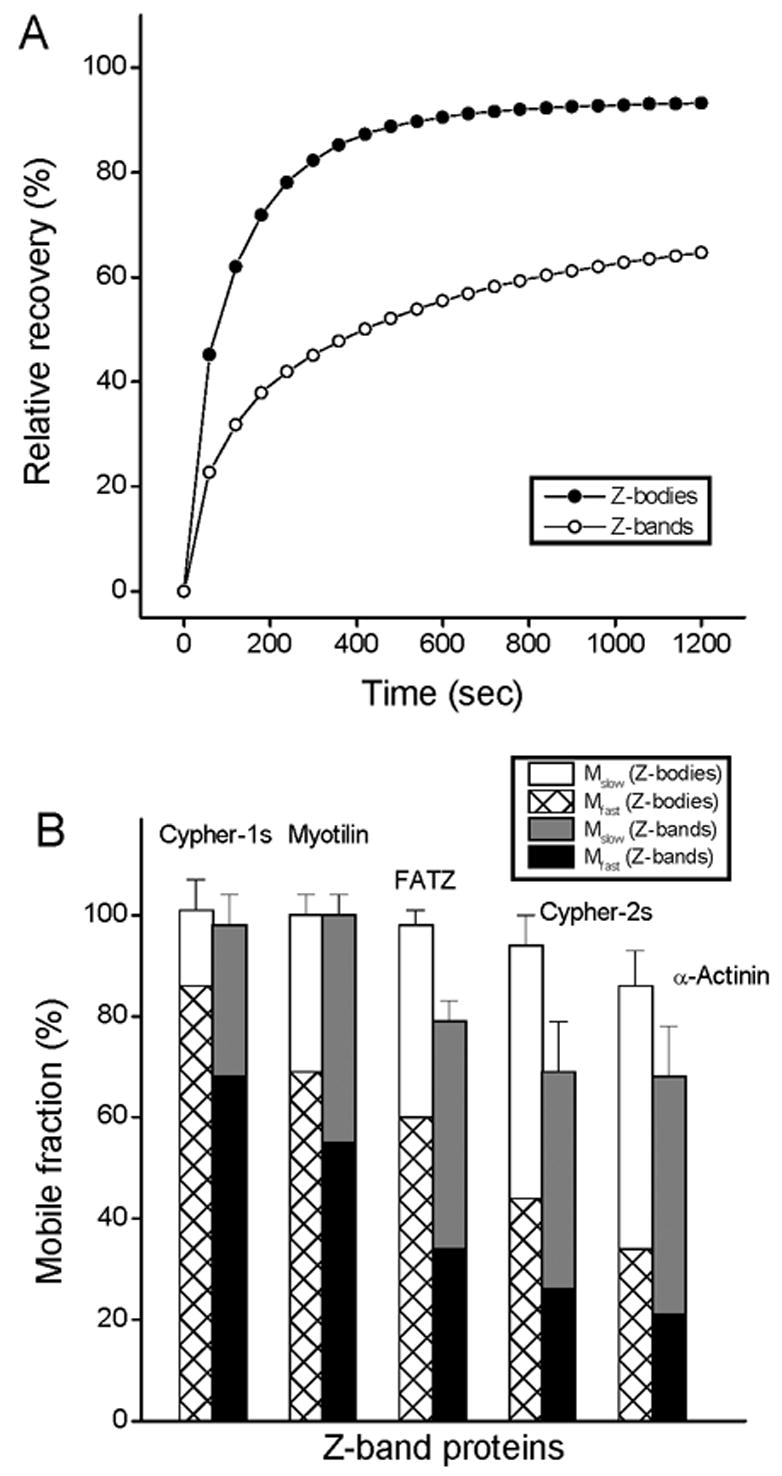
FRAP recovery rates of different Z-band proteins in Z-bodies and in Z-bands. (A) The FRAP curves of cypher-2s in the Z-bodies (black circle) and in the Z-bands (white circle). Note that the recovery rate for cypher is faster in the Z-bodies than in the Z-bands of the mature myofibrils. (B) Comparison of the fast and slow mobile fractions of different proteins in the Z-bodies and in the Z-bands of mature myofibrils. Values are the means ± SDs. Note that the fast mobile fractions for the proteins are always higher in the Z-bodies compared to the Z-bands.
Comparison of the dynamics of the Z-band proteins in mature myofibrils and premyofibrils
To investigate whether the dynamic properties of the Z-band proteins changed during myofibrillogenesis, FRAP analysis was also performed on the proteins localized in Z-bodies of the premyofibrils and compared with the data for the same proteins in Z-bands of the mature myofibrils. The example in Figure 4 A, shows the fluorescence recovery of cypher-2s in premyofibrils occurs at a faster rate and to a greater extent (almost 100% after 20 minutes) than it does in mature myofibrils (about 60% in 20 minutes). The proteins that exhibited the highest fluorescence recovery in mature myofibrils also exhibited the highest recovery in Z-bodies in premyofibrils (Figure 4 B). In each case, Z-band proteins showed differences in recovery between Z-bodies and the Z-bands (Figure 4 B). A comparison of the mobile fractions in Tables 1, 2 and Figure 4 indicates that the fast mobile fraction for each protein was greater when the protein was localized in Z-bodies than that when it was in Z-bands. This suggests that during myofibrillogenesis, as the Z-bodies of premyofibrils align and fuse to form the Z-bands of mature myofibrils, constraints are imposed that restrict each protein’s mobility.
To determine if inhibition of protein synthesis affected the dynamics of YFP-alpha-actinin in Z-bands, we measured the rates of recovery from photobleaching (FRAP) experiments in the same transfected myotubes, before, during three-hour exposures to 100 micromoles of cycloheximide, and after removal of this inhibitor of protein synthesis. There were no differences in the recovery times and mobile fractions of YFP-actinin during these three different conditions (data not shown). These results suggested that the exchange of proteins in the Z-band, minutes to hours, was independent of protein synthesis and the long half-lives of the sarcomeric proteins (three to ten days determined by radioisotopes; Zak et al., 1977; Isaacs et al., 1989).
We reported previously that the GFP probe had to be placed on the C-terminus of alpha-actinin to obtain Z-band localization in cardiomyocytes (Dabiri et al., 1997). We observed similar results for the Z-bands of skeletal muscle cells. The N-terminus of alpha-actinin contains the actin-binding domain, and it appears to be rendered inactive when the YFP is placed there. The rates of recovery for all the other probes used in this study were the same whether the fluorescent probes were ligated to either the N- or the C-termini of the Z-band proteins (data not shown). The measurements were also the same whether the fluorescent probe was CFP, GFP or YFP (data not shown).
The dynamics of photoactivatable GFP-alpha-actinin in Z-bands
Photoactivatable GFP offers a complementary tool to GFP that is photobleached, allowing protein dynamics to be analyzed by tracking the loss of fluorescence after photactivatable molecules are induced to fluoresce (Patterson and Lippincott-Schwartz, 2002). When Z-bands in myotubes transfected with photoactivatable GFP-alpha-actinin were irradiated with 364 nm laser light, fluorescence was greatly increased in the activated region (Figure 5A b). As expected, the fluorescence decreased over time as the fluorescent alpha-actinin exchanged with non-fluorescent alpha-actinin in the cytoplasmic pool (Figure 5A c, d; 5B). Quantitative analysis based on 5 different transfected myotubes indicated that about 55% of the fluorescence decreased after 20 minutes. These results are comparable to those obtained for GFP-alpha-actinin using FRAP (Figure 3A). Thus two different methods demonstrate that alpha-actinin in Z-bands are in a dynamic exchange with the cytoplasmic pool in the muscle cells.
Figure 5.
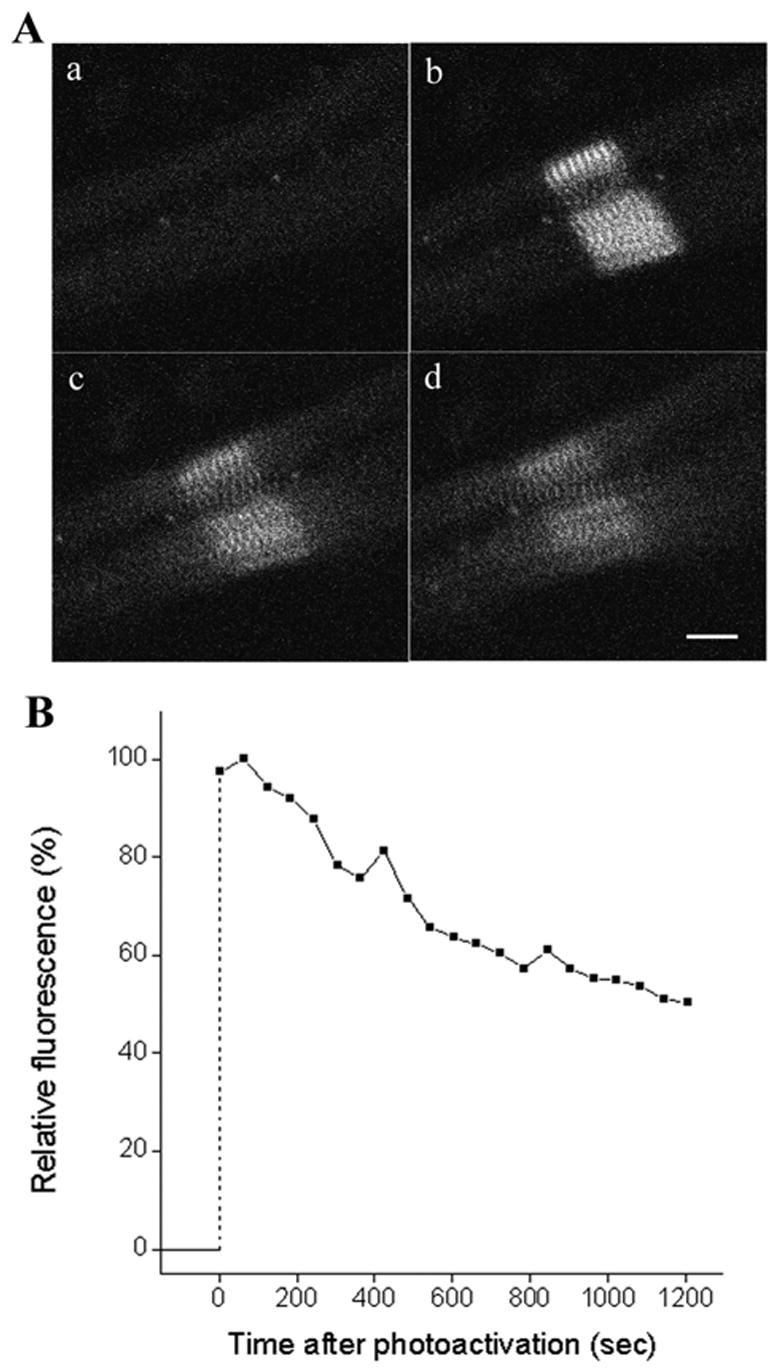
A myotube expressing photoactivatable GFP-alpha-actinin was imaged by using low levels of 488 nm excitation before and after irradiation with high levels of 364 nm laser light. (A) Images (a) to (d) are pre-activation, activation, 10 and 20 minutes after activation. (Bar = 10 μm.) (B) The fluorescence intensity curve in the photoactivated region after irradiation. Note that 50 % of the fluorescence was lost after 20 minutes.
Comparison of the dynamics of alpha-actinin and alpha-actinin (1-273) fragment
When the actin-binding, N-terminal domain of alpha-actinin (aa 1-273) was fused to YFP and expressed in myotubes, it co-localized with alpha-actinin in the Z-bodies of premyofibrils and in the Z-bands of mature myofibrils. FRAP analysis of this truncated alpha-actinin (1-273) in the Z-bodies and Z-bands demonstrated that it had a significantly lower recovery rate than the full-length alpha-actinin (Figure 6). After 20 minutes, when full-length alpha-actinin reached about 80% recovery in Z-bodies and 60% recovery in Z-bands, the recovery of the alpha-actinin (1-273) fragment was only about 10% recovery in both Z-bodies and Z-bands (Figure 6). An analysis of the mobile fractions of the truncated probe indicated the recovery fit a single exponential process (Table 3). In contrast to myotubes transfected with the full construct of YFP-alpha-actinin, the truncated construct induced disruptions of existing myofibrils and inhibited the assembly of new myofibrils in transfected myotubes (Figure 7).
Figure 6.
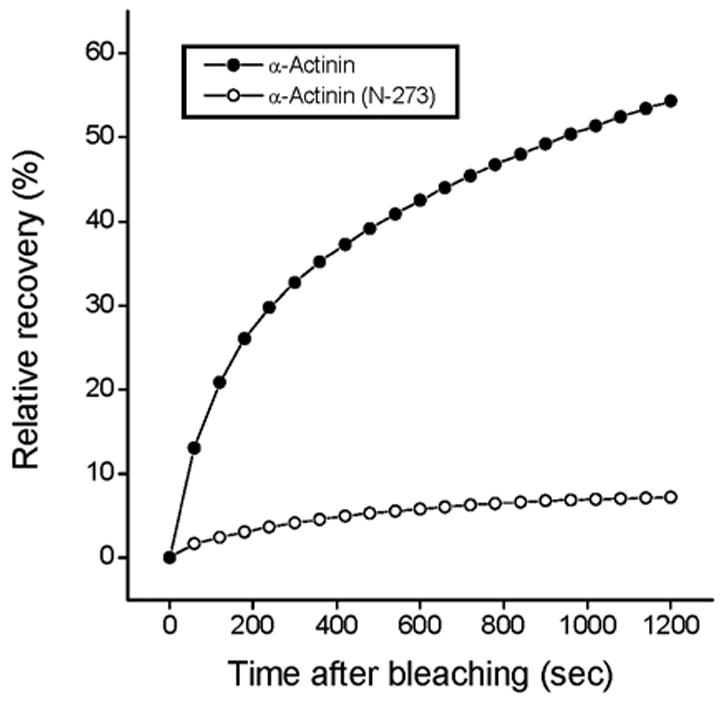
FRAP recovery rates of alpha-actinin (black circle) and alpha-actinin (N-273) fragment (white circle) in Z-band of mature myofibrils.
Figure 7.
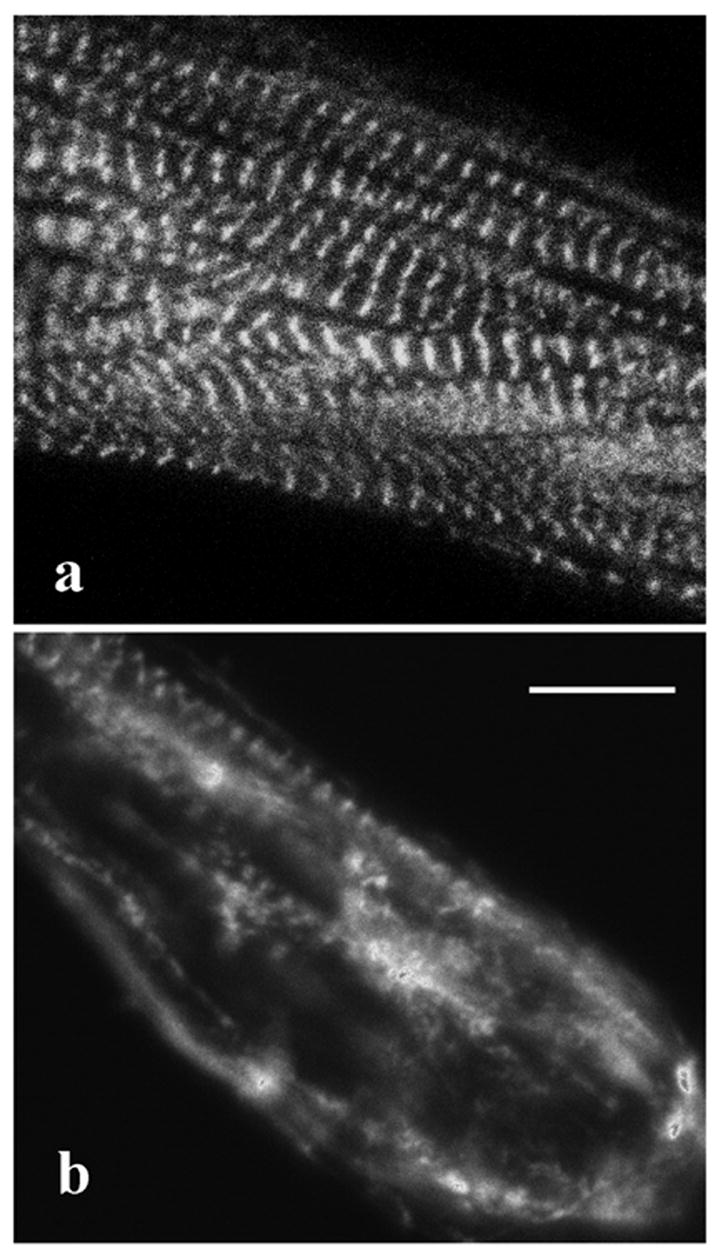
Fluorescence image of a myotube transfected with an alpha-actinin (N-273) fragment. (a) Alpha-actinin (N-273) fragment localized to the Z-bands of mature myofibrils one day post transfections just like the full construct alpha-actinin. (b) In contrast to transfections with the full construct of alpha-actinin, myotubes expressing the alpha-actinin fragment exhibited increasing numbers of disassembled myofibrils after one or more days.
Comparison of the dynamics of myotilin and Thr57Ile mutation
A mutation in the myotilin gene that results in the conversion of residue 57 from threonine to isoleucine has been found in limb girdle muscular dystrophy 1A (LGMD1A) (Hauser et al., 2000). To investigate whether this mutation would perturb the normal localization or dynamic properties of myotilin, we introduced a Thr57lle mutation into myotilin cDNA and linked it to YFP in the YFP expression vector. When transfected into myotubes the mutant myotilin co-localized in the same way as normal myotilin with alpha-actinin in the Z-bodies of premyofibrils and Z-bands of mature myofibrils and had no effect on the formation or stability of myofibrils (data not shown). Photobleaching experiments showed that the mutated protein and the normal protein were equally dynamic as judged by FRAP data in myotubes transfected with YFP fusions of the two proteins (Tables 1 and 3). Furthermore in myotubes doubly transfected with YFP-myotilin-Thr57lle and CFP-alpha-actinin, the presence of the mutated myotilin had no effect on either the localization of CFP-alpha-actinin or on its fluorescence recovery compared to control photobleached cells (data not shown).
Comparison of the dynamics of Cypher-1s, Cypher-2s and two Cypher domains: Cypher-LIM and Cypher-PDZ
Of the two skeletal cypher isoforms, the more dynamic, Cypher-1s, has a PDZ domain and three LIM domains, whereas the smaller cypher-2s has a PDZ domain but no LIM domains (Figure 8A). GFP fusions of PDZ or LIM domains of the two variants expressed in myotubes co-localized with alpha-actinin in the Z-bands with no detectable long-term effects on the structure or contractions of the myofibrils (data not shown). FRAP analyses of Z-bands with these domains showed markedly different rates of fluorescence recovery for the two domains (Figure 8B; Table 3). The cypher-PDZ domain showed 100% recovery in 200 seconds, exhibiting a recovery rate that was significantly higher than the rates for the full-length cypher isoforms and cypher-LIM, as well as for the other Z-band proteins examined in this study (Figure 3B). The FRAP data for the PDZ domain were best fit by a single exponential (Figure 8C; Table 3). The fluorescence recovery of the cypher-LIM domain in Z-bands was lower than that for the full-length cypher-1s, but similar to that for cypher-2s (Figure 8C).
Figure 8.
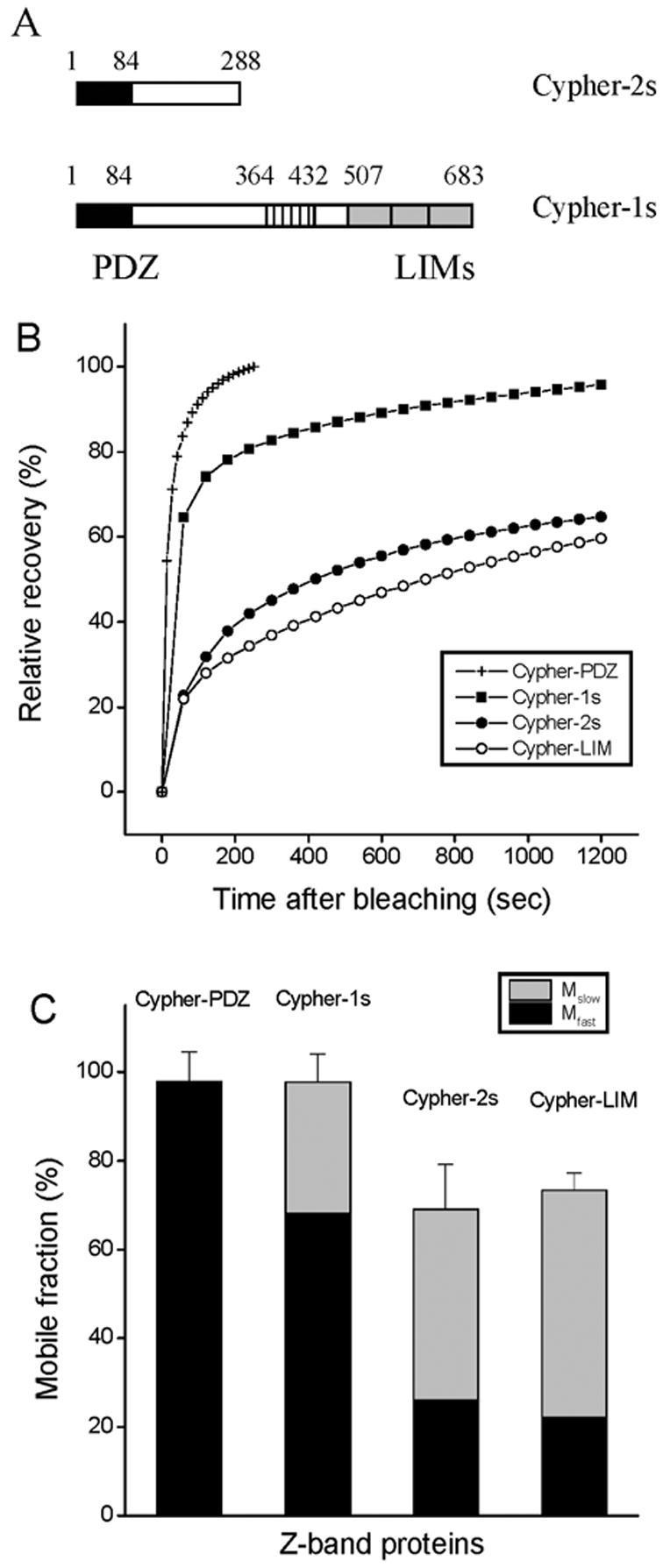
FRAP recovery rates of two cypher skeletal muscle isoforms and their domains in the Z-bands of mature myofibrils. (A) The schematic diagrams of cypher-1s and cypher-2s indicates the shared PDZ domain (amino acids 1-84) present in both isoforms, and the three LIM domains (amino acids 404–472) present only in cypher-1s. (B) The average FRAP recovery curves of cypher-1s (black rectangles), cypher-2s (black circles), cypher-LIM domains (white circles) and cypher-PDZ domain (cross) in the Z-bands of mature myofibrils. The C-terminal region of cypher-2s decreases the dynamics of the cypher-2S PDZ domain, while cypher-1s amino acids 1-364 increase the dynamics of the LIM domains. (C) Comparison of the fast and slow mobile fractions of the cyphers, as well as their LIM and PDZ domains in the Z-bands of mature myofibrils. Values are the means ± SDs.
DISCUSSION
Z-bodies to Z-bands
The mature Z-band is a hub of filaments and proteins whose interconnections, in many cases, have been mapped (reviewed in Gregorio et al., 1999). During the formation of Z-bands, structural complexity develops as the number of component proteins increases. Before they fuse and form Z-bands, Z-bodies in premyofibrils are small amorphous aggregates that contain actin and alpha-actinin (McKenna et al., 1986; Rhee et al., 1994) as well as three proteins that bind to alpha-actinin: myotilin, FATZ and cypher. When premyofibrils, that have Z-bodies and non-muscle myosin IIB but no muscle isoform of myosin II, develop to a nascent myofibril stage, where Z-bodies, non-muscle myosin IIB and a non-striated arrangement of muscle myosin are present, titin appears in the Z-bodies (Rhee et al., 1994). Proteins such as talin and vinculin are in Z-bands, but have not been detected in Z-bodies (Schafer et al., 1993; Rhee et al., 1994; Sanger et al., 2000).
We used FRAP analysis to test the idea that as Z-bodies develop into Z-bands, the constituent proteins become less dynamic as judged by their exchange with the cytoplasmic pool of proteins. In this study we compared the dynamics of actin, alpha-actinin, myotilin, FATZ and cypher in Z-bodies and Z-bands. Each of these proteins, when expressed in cultured quail myotubes as fusions with GFP or YFP, exchanged more rapidly when localized in Z-bodies of premyofibrils than when localized in Z-bands of mature myofibrils (Figure 4B). As Z-bodies fuse in nascent and mature myofibrils the addition of sarcomeric proteins like the actin-capping protein, Cap-Z (Schafer et al., 1993), and titin and its capping protein, telethonin (Gregorio et al., 1999), may anchor the proteins already present in the precursor Z-bodies by increasing the binding between proteins in the Z-bands. Most Z-band proteins have two or more binding partners, which makes the Z-band a very complex network of protein interactions (Figure 9). Structural changes in the Z-bands during myofibrillogenesis (Dabiri et al., 1997) may also change the spatial arrangement of proteins and cause a decrease in their mobility. Although Z-band proteins are less dynamic than their Z-body counterparts, nevertheless, they are still capable of exchanging with the cytoplasmic pool. The recovery process from photobleaching of Z-band proteins in this study could not be fitted with just one exponential curve. These analyses suggest that the dynamic processes of proteins in the Z-band are complex due to the multiple interactions of Z-band proteins (Figure 10). Future experimental approaches will be needed to test the role of each Z-band protein in the assembly and dynamics of the Z-band. This task becomes increasingly more difficult as additional Z-band proteins are discovered (Faulkner et al., 2001; Ervasti, 2003; Sanger et al., 2005).
Figure 9.
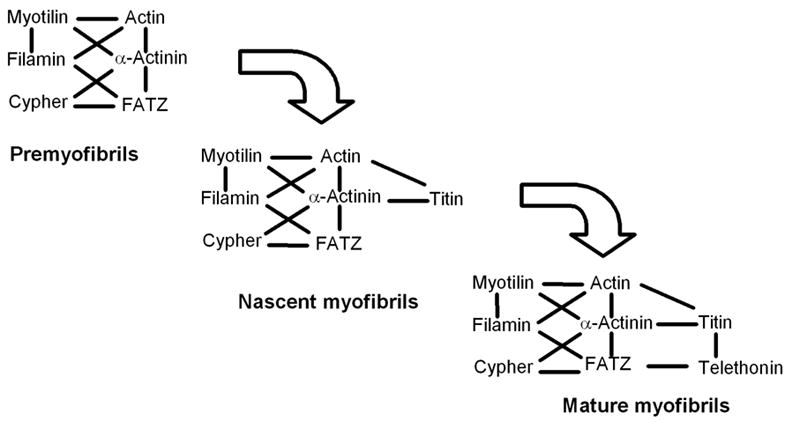
A model of the progression of Z-bodies in premyofibrils and nascent myofibrils to Z-bands of the mature myofibrils. Immunofluorescence experiments indicate that titin, not present in the Z-bodies of premyofibrils, is first detected in the Z-bodies of the nascent myofibrils (Rhee et al., 1994). The exchange of proteins would permit the remodeling of Z-bodies in the transitions needed to form Z-bands during myofibrillogenesis.
Figure 10.
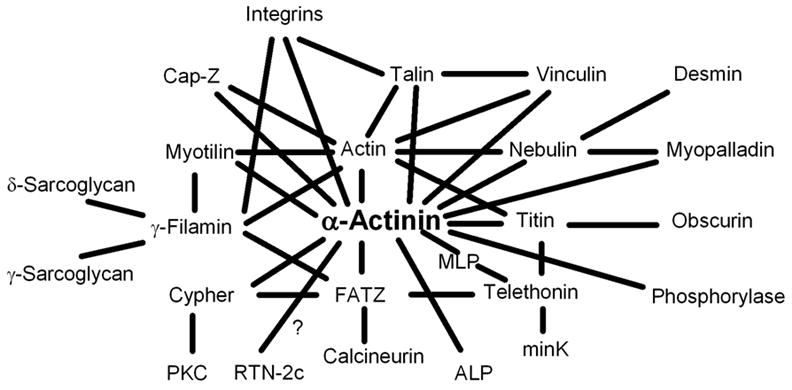
A growing list of Z-band proteins in the mature myofibrils in skeletal muscle cells. The diagram is arranged to indicate the central role of alpha-actinin in the Z-bands that directly and indirectly binds to the major elements of the muscle cell, i.e., thin and thick filaments, intermediate filaments, costameres, and membranous structures like the sarcolemma, transverse tubules and the sarcoplasmic reticulum.
The proteins that bind alpha-actinin exchanged more quickly with the cytoplasmic pools than did alpha-actinin, with the exception of actin and cypher-2s that had recovery profiles similar to alpha-actinin (Figure 3). The fastest recoveries occurred for myotilin and the cypher isoform 1s. Myotilin, a 57-kD protein in heart and skeletal muscle that contains two immunoglobulin domains, similar to those in titin, was found by the yeast two-hybrid method to interact with alpha-actinin, filamin and with itself (Salmikangas et al., 1999; van der Ven et al., 2000). A mutation in human myotilin that changes residue 57 from threonine to isoleucine has been identified in limb girdle muscular dystrophy 1A (LGMD1A), although both the expression level and localization of myotilin is normal in LGMD1A muscle (Hauser et al., 2000). We also found that when we introduced the Thr57Ile substitution into myotilin neither the assembly, localization nor recovery rate after bleaching of myotilin was affected in myofibrils transfected with the YFP-myotilin mutant protein (data not shown). Furthermore, the localization and recovery rate of CFP-alpha-actinin also was not affected when it was co-expressed with the mutated YFP-myotilin in the myotube. The effects of LGMD1A, streaming of Z-bands and disruption of sarcomeres, appear late in the second decade of life (Salmikangas et al., 1999) indicating that prolonged activity is necessary before the effects of the mutated myotilin become visible.
Cyphers
Cyphers (ZASPs) appear to be essential for the integrity of the Z-bands in contracting myofibrils. Myofibrils are assembled normally in non-contracting fetal (E 17.5 days) diaphragm muscles of cypher-null mice (Zhou et al., 2001). However, during the first day of neonatal life when contractions of these muscles begin there is a dramatic disruption of the Z-bands. In the same mice, damage to the Z-bands in cardiac muscles that begin to contract much earlier (E 8), is evident much earlier (E 17.5). Cypher has two mRNA splice variants expressed in skeletal muscle: cypher-1s and cypher-2s (Zhou et al., 1999, 2001, also see Materials and Methods). Both of them contain an amino-terminal PDZ domain through which binding to alpha-actinin occurs (Xia et al., 1997; Zhou et al., 1999). Cypher-1s also binds protein kinase C via three carboxyl-terminal LIM domains that are not present in cypher-2s (Zhou et al., 1999, 2001). It has been suggested that cypher 1s may function as an adapter in striated muscle to couple protein kinase C-mediated signaling to the cytoskeleton.
Although larger in size, cypher-1s exhibits a higher recovery rate than cypher-2s (Figure 8). The PDZ domain, common to the two variants, has a higher recovery rate than either full length cypher-1s or -2s, with fluorescence recovery almost complete less than 2 minutes after Z-band bleaching (Figure 8 B). This suggests that other regions of cypher must stabilize it in the Z-band by binding other proteins or modifying the PDZ domain’s binding to alpha-actinin. Although the LIM domains do not bind to alpha-actinin (Zhou et al., 1999, 2001), it localized in the Z-band (not shown), presumably by binding another Z-band protein. The LIM domains showed a lower recovery rate that was similar to that of cypher-2s. It is not clear why the smaller 2s isoform with no LIM domains should have a lower recovery rate than the 1s isoform with three LIM domains.
Alpha-actinin
Two previous studies using photobleaching to study alpha-actinin dynamics in muscle cells yielded differing results (McKenna et al., 1985; Hasebe-Kishi and Shimada, 2000). In those studies alpha-actinin coupled to rhodamine dye was microinjected into muscle cells. In one study, where skeletal alpha-actinin was injected, there was almost no recovery of alpha-actinin fluorescence in the Z-bands over a 2-hour recovery period after photobleaching (Hasebe-Kishi and Shimada, 2000). Photobleaching of the same alpha-actinin in fibroblasts resulted in almost complete fluorescence recovery indicating that the protein was capable of dynamic exchange. The absence of exchange in Z-bands is surprising in view of the exchange we found with YFP-alpha-actinin. In the other study, the recovery of fluorescence of alpha-actinin bleached in Z-bands was about 23% in 30 minutes and 51% in 6 hours (calculated from data in McKenna et al., 1985). The relative recovery of fluorescence in Z-bodies of premyofibrils (“punctate structures” in McKenna et al., 1985) was faster, reaching about 32% in 30 minutes and 75% in 7 hours (our calculations from data in McKenna et al., 1985). Our results also show a faster recovery after photobleaching of skeletal muscle alpha-actinin-YFP in Z-bodies than in Z-bands, but in each case recovery was much faster than in this earlier study where smooth muscle alpha-actinin was injected. This may reflect differences in the sequences of the two alpha-actinin isoforms (Bretscher et al., 1979) and different binding properties of smooth versus skeletal muscle alpha-actinins in skeletal muscle cells.
When alpha-actinin is proteolytically cleaved into two major fragments of 27- and 53-kD using the enzyme thermolysin (Pavalko and Burridge, 1991), the 27-kD fragment contains an actin-binding site and binds stress fibers when microinjected into non-muscle cells. The 27-kD fragment will eventually induce the loss of stress fibers in injected cells (Pavalko and Burridge, 1991). Our results showed that the YFP-27-kD construct (aa 1-273) localized in the Z-bodies of premyofibrils and Z-bands of mature myofibrils. The low fluorescence recovery rate of the 27-kD construct suggests that it binds more tightly in the Z-band than the full alpha-actinin construct allowing it to compete with the parent molecule and act as a dominant negative inhibitor. The YFP-27-kD construct also caused disruption of stress fibers in transfected fibroblasts (data not shown), and the premyofibrils and myofibrils in transfected muscle cells. Similar disruptions of stress fibers and myofibrils were also found with expression of a truncated alpha-actinin that was much larger (aa 1-728, Schultheiss et al., 1992). Taken together these results suggest that a pool of dynamic alpha-actinin molecules is important for the maintenance and formation of stress fibers, premyofibrils and myofibrils. Figure 9 illustrates the central role alpha-actinin plays in the binding of a number of proteins in the Z-band.
Telethonin
The least dynamic of the Z-band proteins examined was telethonin, a 19 kD titin binding protein in heart and skeletal muscles (Gregorio et al., 1998; Mues et al., 1998; Mason et al., 1999). Telethonin binding has been mapped to the immunoglobulin domains, Z1 and Z2, at the N-terminus of titin. Over-expression of telethonin in embryonic cardiomyocytes leads to the disassembly of myofibrils in cultures of embryonic heart cells (Gregorio et al., 1998). We found that telethonin-YFP localized only in the Z-bands of mature myofibrils, but not in the premyofibrils (Figure 1). This is consistent with immunofluorescence results from our laboratory that titin first appears localized in nascent myofibrils but not in the Z-bodies of premyofibrils (Rhee et al., 1994). FATZ, however, also binds telethonin (Faulkner et al., 2000), and does localize in Z-bodies when expressed as a YFP-fusion protein (Figure 1). The failure of YFP-telethonin to localize in Z-bodies may indicate that the sites for telethonin-FATZ binding are inaccessible in the Z-bodies.
Exchange of Proteins in the Z-bands of Cross-striated muscles
It is well known that the half-lives of sarcomeric proteins range from several days to almost ten days in embryonic and adult muscle cells (e.g., titin, 3 days; myosin heavy chain, 5.9 days; actin, 7.7 days; light chains, 9 days; Zak et al., 1977; Walker and Strohman, 1978; Isaacs and Fulton, 1989; reviewed in Sanger et al., 2004). These half-lives indicate that within the same myofibril, individual components are replaced at different rates. Thus, the three-day half-life of titin implies that two of the 12 titin filaments associated with a single myosin filament must be replaced every day in embryonic skeletal muscle cells. The turnover rate for actin indicates that 23 of the 370 actin molecules in the one micron long thin filament are replaced daily.
The rates of recovery from our photobleaching experiments are much shorter than the half-lives of sarcomeric proteins, minutes and hours, not days and weeks. Nevertheless, the half-times of recovery after photobleaching are also different for each of the GFP-tagged Z-band proteins, and are not a simple function of molecular size or GFP position on the protein (Figure 3, Table 1). Studies of embryonic muscle cells into which trace amounts of fluorescently dye-labeled sarcomeric proteins, (actin, alpha-actinin, myosin light chains and tropomyosin) were injected demonstrated that the proteins became incorporated into their appropriate sites in the myofibrils in one to six hours post-microinjection (reviewed in Sanger et al., 2004). When the fluorescently dye-labeled actin and alpha-actinin were photobleached, they were replaced by unbleached fluorescent protein at rates that varied for each of the two proteins (McKenna et al., 1985 a, b), but which were much faster than the half-lives of sarcomeric proteins determined by radioisotopes. All of these photobleaching observations suggest that the dynamic equilibrium between myofibrillar and cytoplasmic pools is independent of synthesis of new protein molecules and that a cytoplasmic pool of molecules is always present, as shown for myosin light chains and actin (Horvath and Gaetjens, 1972; Shimizu and Obinata, 1986). These interpretations are further supported by the FRAP experiments we performed in the presence of cycloheximide, an inhibitor of protein synthesis. These observations suggest a dynamic exchange mechanism that permits the continual testing of native sarcomeric molecules, and the rapid entry of newly synthesized molecules. Thus, when a new isoform is synthesized, e.g. in response to developmental or hormonal stimuli, the new protein readily gains access to all of the myofibrils (Bandman, 1985; Bouche et al., 1988).
The photobleaching experiments in this report suggest that the exchange of soluble Z-band proteins with their bound cohorts in Z-bodies and Z-bands is a process that could allow molecules to be constantly tested for their normal binding properties. Clearly the exchange occurs without total disassembly of myofibrils and would also support isoform changes as well as the transition of premyofibrils to nascent myofibrils to mature myofibrils observed in living muscle cells (Figure 9; Dabiri et al., 1997; Sanger et al., 2002; Golson et al., 2004). The exchange process could be inhibited by mutated molecules that bound more tightly. There are a number of muscle myopathies which are characterized by Z-band anomalies (Z-band streaming), and myofibril disarrays (Engel and Banker, 2004). These myofibril mishaps may be due to abnormal exchange processes. The ability of a truncated alpha-actinin to induce myofibril disarrays supports this suggestion.
CONCLUSIONS
In summary, our results demonstrate that proteins in the Z-band are in dynamic exchange with a cytoplasmic pool. This exchange is reduced when compared with that of the same proteins in the Z-bodies that give rise to the Z-bands during myofibrillogenesis. The multiple binding interactions of alpha-actinin with other Z-band proteins may explain why dominant negative effects of a truncated alpha-actinin caused myofibrillar disruption. The experiments reveal the Z-band to be a much more dynamic structure than its appearance in static electron micrographs of cross-striated muscle cells might suggest.
Acknowledgments
We thank Dr. Andrea Stout for her critical reading of this manuscript. This work was supported by grants from the American Heart Association (JMS), Muscular Dystrophy Association (JC; JWS) and NIH (JC; JWS).
Abbreviations footnote
- CFP
Cyan Fluorescent Protein
- GFP
Green Fluorescent Protein
- YFP
Yellow Fluorescent Protein
- FRAP
Fluorescence Recovery After Photobleaching
References
- Ayoob JC, Turnacioglu KK, Mittal B, Sanger JM, Sanger JW. Targeting of cardiac muscle titin fragments to the Z-bands and dense bodies of living muscle and non-muscle cells. Cell Motil Cyotskel. 2000;45:67–82. doi: 10.1002/(SICI)1097-0169(200001)45:1<67::AID-CM7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ayoob JC, Shaner NC, Sanger JW, Sanger JM. Expression of green or red fluorescent protein (GFP or DsRed) linked proteins in nonmuscle and muscle cells. Mol Biotechnology. 2001;17:65–71. doi: 10.1385/MB:17:1:65. [DOI] [PubMed] [Google Scholar]
- Bandman E. Myosin isoenzyme transitions in muscle development, maturation and disease. Int Rev Cytol. 1985;97:97–131. doi: 10.1016/s0074-7696(08)62349-9. [DOI] [PubMed] [Google Scholar]
- Bastiaens PIH, Pepperkok R. Observing proteins in their natural habitat: the living cell. Trends Biochem Sci. 2000;25:631–637. doi: 10.1016/s0968-0004(00)01714-x. [DOI] [PubMed] [Google Scholar]
- Bouche M, Goldfine SM, Fischman DA. Posttranslational incorporation of contractile proteins into myofibrils in a cell-free system. J Cell Biol. 1988;107:587–596. doi: 10.1083/jcb.107.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Vanderkerckhove J, Weber K. Alpha-actinins from chicken skeletal muscle and smooth muscle show considerable chemical and immunological differences. Eur J Biochem. 1979;100:237–243. doi: 10.1111/j.1432-1033.1979.tb02054.x. [DOI] [PubMed] [Google Scholar]
- Chowrashi P, Mittal B, Sanger JM, Sanger JW. Amorphin is phosphorylase, phosphorylase is an alpha-actinin-binding protein. Cell Motil Cytoskeleton. 2002;53:125–135. doi: 10.1002/cm.10059. [DOI] [PubMed] [Google Scholar]
- Dabiri GA, Turnacioglu KK, Sanger JM, Sanger JW. Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proc Natl Acad Sci USA. 1997;94:9493–9498. doi: 10.1073/pnas.94.17.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri GA, Ayoob JC, Turnacioglu KK, Sanger JM, Sanger JW. Use of green fluorescent proteins linked to cytoskeletal proteins to analyze myofibrillogenesis in living cells. Meth Enzymol. 1999;302:171–186. doi: 10.1016/s0076-6879(99)02017-0. [DOI] [PubMed] [Google Scholar]
- Danowski BA, Imanaka-Yoshida K, Sanger JM, Sanger JW. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J Cell Biol. 1992;118:1411–1420. doi: 10.1083/jcb.118.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AP, Sanger JM, Linask KK, Sanger JW. Myofibrillogenesis in the first cardiomyocytes formed from isolated quail precardiac mesoderm. Dev Biol. 2003;257:382–394. doi: 10.1016/s0012-1606(03)00104-0. [DOI] [PubMed] [Google Scholar]
- Engel AG, Banker BQ. Ultrastructural changes in diseased muscle. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3. McGraw-Hill; New York: 2004. 2004. pp. 749–887. [Google Scholar]
- Ervasti JM. Costameres: the achilles’ heel of herculean muscle. J Biol Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- Faulkner G, et al. ZASP: a new Z-band alternatively spliced PDZ-motif protein. J Cell Biol. 1999;146:465–475. doi: 10.1083/jcb.146.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner G, et al. FATZ, a filamin-, actin-, and telethonin-binding protein of the Z-disc of skeletal muscle. J Biol Chem. 2000;275:41234–41242. doi: 10.1074/jbc.M007493200. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Lanfranchi G, Valle G. Telethonin and other new proteins of the Z-disc of skeletal muscle. IUBMB Life. 2001;51:275–282. doi: 10.1080/152165401317190761. [DOI] [PubMed] [Google Scholar]
- Golson M, Sanger JM, Sanger JW. Inhibitors arrest myofibrillogenesis in skeletal muscle cells at early stages of assembly. Cell Motil Cytoskel. 2004;59:1–16. doi: 10.1002/cm.20017. [DOI] [PubMed] [Google Scholar]
- Gregorio CC, et al. The NH2 terminus of titin spans the Z-disc: its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity. J Cell Biol. 1998;143:1013–1027. doi: 10.1083/jcb.143.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio CC, Granzier H, Sorimachi H, Labeit S. Muscle assembly: a titanic achievement. Curr Opin Cell Biol. 1999;11:18–25. doi: 10.1016/s0955-0674(99)80003-9. [DOI] [PubMed] [Google Scholar]
- Hasebe-Kishi F, Shimada Y. Dynamics of actin and alpha-actinin in nascent myofibrils and stress fibers. J Muscle Res Cell Motil. 2000;21:717–724. doi: 10.1023/a:1010374424143. [DOI] [PubMed] [Google Scholar]
- Hauser MA, et al. Myotilin mutated in limb girdle muscular dystrophy 1A. Hum Mol Gen. 2000;9:2141–2147. doi: 10.1093/hmg/9.14.2141. [DOI] [PubMed] [Google Scholar]
- Hilenski LL, Terracio L, Borg TK. Myofibrillar and cytoskeletal assembly in neonatal rat cardiac myocytes cultured on laminin and collagen. Cell Tiss Res. 1991;264:577–587. doi: 10.1007/BF00319047. [DOI] [PubMed] [Google Scholar]
- Huang L, Mittal B, Sanger JW, Sanger JM. Host focal adhesion protein domains that bind to the translocated intimin receptor (Tir) of Enteropathogenic Escherichia coli (EPEC) Cell Motil Cyotskel. 2002;52:255–265. doi: 10.1002/cm.10050. [DOI] [PubMed] [Google Scholar]
- Horvath BZ, Gaetjens E. Immunochemical studies on the light chain from skeletal muscle myosin. Biochim Biophys Acta. 1972;263:779–793. doi: 10.1016/0005-2795(72)90062-1. [DOI] [PubMed] [Google Scholar]
- Isaacs WB, Kim IS, Fulton AB. Biosynthesis of titin in cultured skeletal muscle cells. J Cell Biol. 1989;109:2189–2195. doi: 10.1083/jcb.109.5.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE, Winterhalter KH, Birchmeier W. In vivo distribution and turnover of fluorescently labeled actin microinjected into human fibroblasts. Proc Natl Acad Sci USA. 1979;76:3814–3818. doi: 10.1073/pnas.76.8.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Snapp E, Kenworthy A. Studying protein dynamics in living cells. Nature Rev Mol Cell Biol. 2001;2:444–456. doi: 10.1038/35073068. [DOI] [PubMed] [Google Scholar]
- Mason P, Bayol S, Loughna PT. The novel sarcomeric protein telethonin exhibits developmental and functional regulation. Biochem Biophys Res Com. 1999;257:699–703. doi: 10.1006/bbrc.1999.0531. [DOI] [PubMed] [Google Scholar]
- McKenna NM, Meigs JB, Wang YL. Identical distribution of fluorescently labeled brain and muscle actins in living fibroblasts and myocytes. J Cell Biol. 1985a;100:292–296. doi: 10.1083/jcb.100.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NM, Meigs JB, Wang YL. Exchangeability of alpha-actinin in living cardiac fibroblasts and muscle cells. J Cell Biol. 1985b;101:2223–2232. doi: 10.1083/jcb.101.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NM, Johnson CS, Wang YL. Formation and alignment of Z lines in living chick myotubes microinjected with rhodamine-labeled alpha-actinin. J Cell Biol. 1986;103:2163–2171. doi: 10.1083/jcb.103.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal BJM, Sanger JM, Sanger JW. Binding and distribution of fluorescently labeled filamin in permeabilized and living cells. Cell Motil Cyotskel. 1987a;8:345–359. doi: 10.1002/cm.970080407. [DOI] [PubMed] [Google Scholar]
- Mittal B, Sanger JM JM, Sanger JW. Visualization of myosin in living cells. J Cell Biol. 1987b;1987;105:1753–1760. doi: 10.1083/jcb.105.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mues A, van der Ven PFM, Yong P, Furst DO, Gautel M. Two immunoglobulin-like domains of the Z-disc portion of titin interact in a conformation-dependent way with telethonin. FEBS Lett. 1998;428:111–114. doi: 10.1016/s0014-5793(98)00501-8. [DOI] [PubMed] [Google Scholar]
- O’Neill A, Williams MW, Resneck WG, Milner DJ, Capetanaki Y, Bloch RJ. Sarcolemmal organization in skeletal muscle lacking desmin: evidence for cytokeratins associated with the membrane skeleton at costameres. Mol Biol Cell. 2002;13:2347–2359. doi: 10.1091/mbc.01-12-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GH, Lippincott-Schwartz J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science. 2002;297:1873–1877. doi: 10.1126/science.1074952. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Burridge K. Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of alpha-actinin. J Cell Biol. 1991;114:481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee D, Sanger JM, Sanger JW. The premyofibrils: evidence for its role in myofibrillogenesis. Cell Motil Cyotskel. 1994;28:1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- Salmikangas P, Mykkanen OM, Gronholm M, Heiska L, Kere J, Carpen O. Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy. Hum Mol Gen. 1999;8:1329–1336. doi: 10.1093/hmg/8.7.1329. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Ayoob JC, Chowrashi P, Zurawski D, Sanger JM. Assembly of myofibrils in cardiac muscle cells. Advances Exper Med Biol. 2000;481:89–102. doi: 10.1007/978-1-4615-4267-4_6. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM. Fishing out proteins that bind to titin. J Cell Biol. 2001a;154:21–24. doi: 10.1083/jcb.200106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM. Green fluorescent proteins improve myofibril research. Biophotonics International. 2001b;8:44–46. [Google Scholar]
- Sanger JW, Chowrashi P, Shaner NC, Spalthoff S, Wang J, Freeman N, Sanger JM. Myofibrillogenesis in skeletal muscle cells. Clin Orthop Related Res. 2002;403S:S153–S162. doi: 10.1097/00003086-200210001-00018. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM, Franzini-Armstrong C. Assembly of the skeletal muscle cell. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3. McGraw-Hill; New York: 2004. pp. 45–65. [Google Scholar]
- Sanger JW, Kang S, Siebrands C, Freeman N, Du A, Wang J, Stout A, Sanger JM. How to build a myofibril. J Muscle Res Cell Motility. 2005 doi: 10.1007/s10974-005-9016-7. In Press. [DOI] [PubMed] [Google Scholar]
- Schafer DA, Waddle JA, Cooper JA. Localization of CapZ during myofibrillogenesis in cultured chicken muscle. Cell Motil Cytoskel. 1993;25:317–335. doi: 10.1002/cm.970250403. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Obinata T. Actin concentration and monomer-polymer ratio in developing chicken skeletal muscle. J Biochem (Tokyo) 1986;99:751–759. doi: 10.1093/oxfordjournals.jbchem.a135534. [DOI] [PubMed] [Google Scholar]
- Siebrands CC, Sanger JM, Sanger JW. Myofibrillogenesis in the presence of taxol. Cell Motil Cytoskel. 2004;58:39–52. doi: 10.1002/cm.10177. [DOI] [PubMed] [Google Scholar]
- Schultheiss T, Choi J, Lin ZX, DiLullo C, Cohen-Gould L, Fischman D, Holtzer H. A sarcomeric alpha-actinin truncated at the carboxyl end induces the breakdown of stress fibers in PtK2 cells and the formation of nemaline-like bodies and breakdown of myofibrils in myotubes. Proc Natl Acad Sci USA. 1992;89:9282–9286. doi: 10.1073/pnas.89.19.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnacioglu KK, Mittal B, Dabiri G, Sanger JM, Sanger JW. An N-terminal fragment of titin coupled to green fluorescent protein localizes to the Z-bands in living muscle cells: overexpression leads to myofibril disassembly. Mol Biol Cell. 1997;8:705–717. doi: 10.1091/mbc.8.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyska MJ, Mooseker MS. MYO1A (Brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys J. 2002;82:1869–1883. doi: 10.1016/S0006-3495(02)75537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle G, Faulkner G, De Antoni A, Pacchioni B, Pallavicini A, Pandolfo D, Tiso N, Toppo S, Trevisan S, Lanfranchi G. Telethonin, a novel sarcomeric protein of heart and skeletal muscle. FEBS Lett. 1997;415:163–168. doi: 10.1016/s0014-5793(97)01108-3. [DOI] [PubMed] [Google Scholar]
- Van der Ven PFM, Wiesner S, Salmikangas P, Auerbach D, Himmel M, Kempa S, Hayess K, Pacholsky D, Taivainen A, Schroder R, Carpen O, Furst DO. Indications for a novel muscular dystrophy pathway, gamma-filamin, the muscle-specific filamin isoform, interacts with myotilin. J Cell Biol. 2000;151:235–248. doi: 10.1083/jcb.151.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CR, Strohman RC. Myosin turnover in cultured muscle fibers relaxed by tetrodotoxin. Exp Cell Res. 1978;116:341–348. doi: 10.1016/0014-4827(78)90457-3. [DOI] [PubMed] [Google Scholar]
- Wang K, Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J Cell Biol. 1988;107:2199–2212. doi: 10.1083/jcb.107.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Stekzer E. Photobleaching GFP reveals protein dynamics inside live cells. Trends Cell Biol. 1999;9:61–65. doi: 10.1016/s0962-8924(98)01433-0. [DOI] [PubMed] [Google Scholar]
- Whiting A, Wardale J, Trinick J. Does titin regulate the length of muscle thick filaments? J Mol Biol. 1989;205:263–268. doi: 10.1016/0022-2836(89)90381-1. [DOI] [PubMed] [Google Scholar]
- Xia H, Winokur ST, Kuo W, Altherr MR, Bredt DS. Actinin-associated LIM protein: identification of a domain interaction between PDZ and spectrin-like motifs. J Cell Biol. 1997;139:507–515. doi: 10.1083/jcb.139.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak R, Martin AF, Prior G, Rabinowitz Comparison of turnover of several myofibrillar proteins and critical evaluation of double isotope method. J Biol Chem. 1977;252:3430–3435. [PubMed] [Google Scholar]
- Zhou Q, Ruiz Lozano P, Martone ME, Chen J. Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to _-actinin-2 and protein kinase C. J Biol Chem. 1999;274:19807–19813. doi: 10.1074/jbc.274.28.19807. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Chu PH, Huang C, Cheng CF, Martone ME, Knoll G, Diane Shelton G, Evans S, Chen J. Ablation of Cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J Cell Biol. 2001;155:605–612. doi: 10.1083/jcb.200107092. [DOI] [PMC free article] [PubMed] [Google Scholar]


