Abstract
BACKGROUND
Brugada syndrome is an inherited disease associated with sudden cardiac death. The electrocardiographic pattern associated with Brugada syndrome has been linked to the use of sodium channel blockers, including antiarrhythmics, trycyclics and anesthetics.
OBJECTIVE
We report a case of bupivacaine-induced Brugada syndrome, in which we investigated the genetic, biophysical and path physiological mechanism involved.
METHODS AND RESULTS
The patient developed a Brugada-like electrocardiographic pattern twice under the influence of bupivacaine. The first occurrence was accompanied by ventricular tachycardia (VT) which subsided after withdrawal of the anesthetic. The VT was also observed during co-administration of diltiazem and isosorbide-5-mononitrate, agents thought to facilitate ST segment elevation in the Brugada syndrome. Genetic analysis revealed a missense mutation in the α subunit of the cardiac sodium channel, SCN5A. Biophysical analysis by whole-cell patch-clamping revealed a reduction in sodium current as a result of the mutation. The study of bupivacaine in the wedge model revealed use-dependent changes in conduction, heterogeneous loss of the action potential dome in RV epicardium and phase 2 re-entry when the preparations were pretreated with low concentrations of the calcium channel blocker verapamil.
CONCLUSION
Our findings indicate that bupivacaine may induce the electrocardiographic and arrhythmic manifestations of the Brugada syndrome in silent carriers of SCN5A mutations. The data have important implications in the management of patients who develop ST segment elevation when under the influence of anesthetics such as bupivacaine.
Keywords: Bupivacaine, Ventricular arrhythmias, Brugada Syndrome
Introduction
Bupivacaine is a potent long-acting anesthetic recommended for infiltration, peripheral nerve block, epidural and spinal anesthesia. It blocks the generation and the conduction of nerve impulses by inhibiting sodium channel current in nerve membranes. This results in an increased threshold for electrical excitation, a slower impulse propagation, and reduced rate of rise of the action potential of the nerve membrane.1 Although bupivacaine has few side effects when properly administered, it can produce ventricular arrhythmias and cardiac depression at toxic levels.1
Brugada syndrome is an inherited sudden death syndrome whose electrocardiographic and arrhythmic manifestations can be unmasked or exacerbated in the presence of agents that block the cardiac sodium channel.2 It is characterized by an ST segment elevation on the electrocardiogram (ECG) in the precordial leads V1 to V3, and a right bundle branch block pattern in the absence of structural heart disease.3 The syndrome has in 19984 been linked to mutations in SCN5A, the gene that encodes for the α subunit for the sodium channel and since then over 80 mutations in SCN5A have been identified.
We report a case of Brugada-like ECG associated with unexplained ventricular tachyarrhythmias developing during epidural infusion of bupivacaine. We identified a mutation in SCN5A and delineated the biophysical mechanisms involved in the bupivacaine-induced phenotype by whole-cell patch-clamp technique and in the arterially-perfused wedge preparation.
Methods
Case
A 56-year-old man underwent lung volume reduction surgery for chronic obstructive pulmonary disease. His medical history revealed a transient ischemic attack and lung emphysema for 13 years. The preoperative ECG showed a sinus rhythm with a heart rate of 90 bpm, low voltages, non-specific T-wave changes and atrial dilatation. The echocardiogram showed left ventricular concentric hypertrophy, left atrial and right ventricular (RV) dilatation with normal left and right ventricular function. Routine preoperative blood tests, including kidney function tests and serum electrolytes, were normal.
Before induction of general anesthesia with propofol (2.5 mg/kg/hr), an epidural catheter was inserted and a combination of 0.25% bupivacaine and 1 mcg/ml sufentanil was started at 7 ml/h. The first day postoperatively the ECG showed a remarkable pattern: sinus rhythm of 90 bpm, right bundle branch block-like pattern, down-sloping ST segment elevation in leads V1 to V3, known as Brugada-like ECG pattern as well as frequent ventricular extrasystoles with a left bundle branch morphology and horizontal axis (Figure 1). The incidence of these extrasystoles increased dramatically resulting in ventricular tachycardia (VT) and electrical storm. Lidocaine and amiodarone were administered in an attempt to convert the arrhythmia. Isosorbide-5-mononitrate and diltiazem were started because of suspected RV overload. Five days after surgery, bupivacaine was withdrawn due to malfunction of the epidural catheter and piritramide perfusion (2 mg/hr IV) was started. Surprisingly, the next day the ventricular arrhythmias were no longer present and the typical Brugada-like ECG disappeared.
Figure 1.
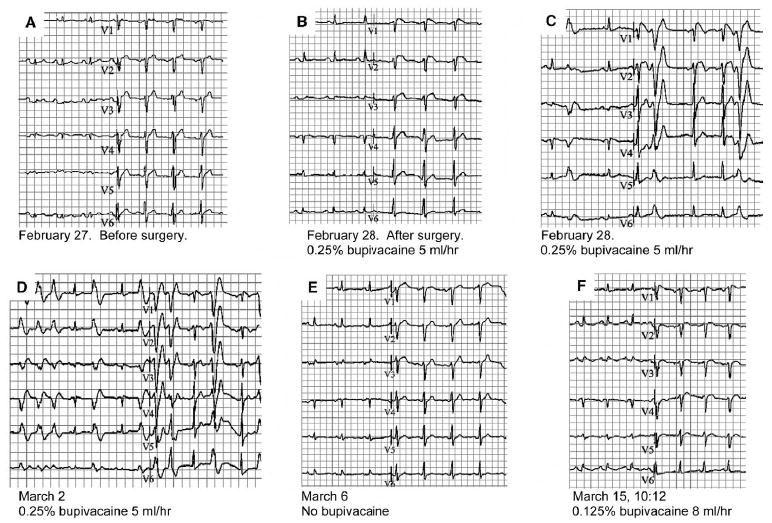
Serial electrocardiograms on the patient, before, after the first surgical procedure (under bupivacaine) and after the second surgical procedure (lower dose of bupivacaine). A: Baseline ECG before surgery shows sinus rhythm and normal conduction times (PQ 190 ms, QRS 100 ms) with somewhat low voltages in extremity leads on the basis patients COPD. B: ECG after surgery and placement of the epidural catheter with bupivacaine (0.25% 5 ml/h). ECG shows sinus rhythm and normal conduction times. However, note the J point elevation in V1-2 with downslope ST segment elevation in V1 known in Brugada syndrome. C: ECG with Brugada-like ECG changes with premature ventricular extra systoles. D: ECG shows non-sustained ventricular tachycardia. E: Brugada-like ECG changes have disappeared and ECG has normalized to baseline after withdrawal of the epidural catheter. F: Slight J point elevation and downslope ST segment elevation in V1 and normal conduction times after second surgery and again insertion of an epidural catheter with bupivacaine (0.125%, 8 ml/h).
Four days later, a new surgical intervention, performed to correct persistent air leakage, necessitated the insertion of an epidural catheter and administration of 0.125% bupivacaine and 1 mcg/ml sufentanil at 8 ml/hr. Surgery was successfully carried out under the same anesthetic conditions as before. On the first day after surgery the ECG once again displayed a Brugada-like pattern. The epidural catheter was withdrawn the following day because of malfunction and the Brugada-like ECG disappeared. The patient did not experience fever during the admission. The patient was discharged after two weeks.
Genetic analysis
After informed consent was obtained, genomic DNA was isolated from peripheral blood leukocytes using a commercial kit (Gentra System, Puregene). The exons of SCN5A were amplified and analyzed by direct sequencing. Polymerase chain reaction products were purified with a commercial reagent (ExoSAP-IT, USB) and were directly sequenced from both directions with the use of ABI PRISM 3100 Automatic DNA Sequencer.
Site-directed mutagenesis
Mutant SCN5A channel cDNAs were prepared by site-directed mutagenesis of the plasmid pcDNA-SCN5A in vector pcDNA3.1 + (Invitrogen, Carlsbad, CA, USA). The SCN5A transcript used contains Q1077. To create the mutant, the “Megaprimer” method of mutagenesis was used, as previously described.5
Electrophysiology
TSA201 cells were co-transfected with pcDNA-SCN5A (wild-type or mutant) and the human sodium channel subunit-β1 (SCN1B) in pc DNA3.1 (pcDNA-Hβ1) construct using the calcium phosphate precipitation method, as previously described.5 The cells were grown on polylysine-coated 35-mm culture dishes and placed in a temperature-controlled chamber for electrophysiologic study (Medical Systems, Greenvale, NY, USA). Standard whole-cell patch-clamp technique was used to measure currents in transfected TSA201 cells as previously described.6
Wedge model
To assess whether the electrocardiographic and arrhythmic manifestations of bupivacaine are due to its direct actions on the ventricular myocardium, we evaluated the effects of the anesthetic in 5 arterially-perfused RV wedge preparations. The detailed methods employed for isolation, perfusion and recording of transmembrane activity have been previously reported.7 Briefly, transmural wedge preparations with dimensions of approximately 20–25 mm by 10–12 mm by 8–10 mm (transmural width) were dissected from the right ventricle of male dog hearts. The preparations were then placed in a small tissue bath and arterially-perfused with Tyrode’s solution at 35–36°C. The preparations were continuously stimulated endocardially at a basic cycle length of 2000 msec. After a stabilization period (~1–2 hours), transmural ECG and transmembrane action potentials were simultaneously recorded from epicardial (Epi) and mid-myocardial (M) regions. The effect of bupivacaine (Sigma, Foster City, CA) was assessed 45 to 60 min after the addition of the drug to the perfusate at concentrations of 10 and 20 ug/ml. In 3 experiments, the calcium channel blocker verapamil was introduced at relatively low concentration (0.1 uM) to sensitize the preparation.
Results
Genetic analysis
Genetic analysis revealed a novel missense mutation (G1743E, SCN5A Genbank accession number AC137587) in the α-sub-unit of the sodium channel, SCN5A (Figure 2). This mutation is located between segments 5 and 6 of domain 4, in a highly conserved amino-acid across species. This mutation was not present in 200 control individuals of the same ethnic background. We did not identify any other variation in the codifying segments of SCN5A.
Figure 2.
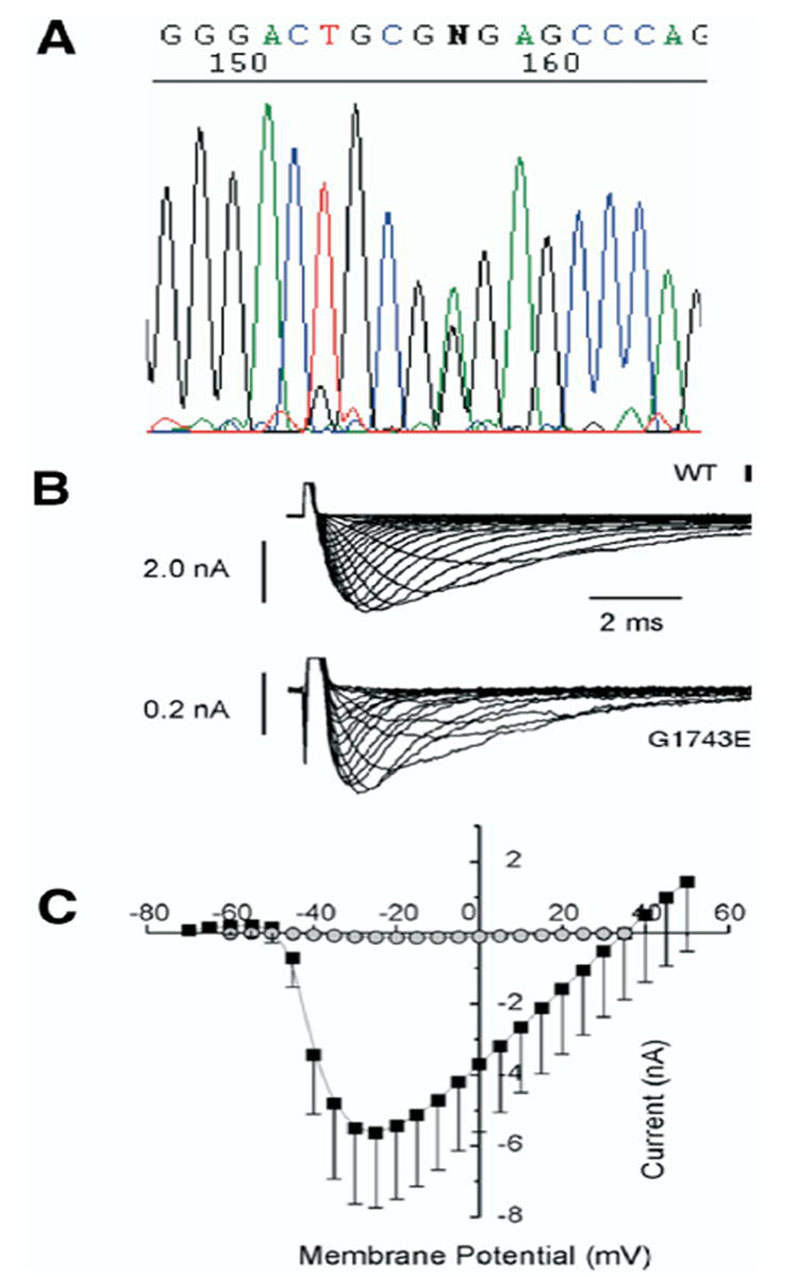
A: Sequencing analysis results of the patient, showing a missense mutation (G1743E) in the α subunit of the cardiac sodium channel. Mutation G1743E in SCN5A reduce expression of NaV1.5. B: Representative current recordings of the unmutated (WT) channel and mutant G1743E elicited by a series of step potentials from − 60 mV to +20 mV in 5 mV increments from a holding membrane potential of − 100 mV. Note the different voltage calibration for WT vs. mutant indicating an important decrease in sodium current in the mutant channel. C: Average peak current-voltage relationship from 7 WT and G1743E cells. (Average ± SEM).
As routine clinical follow-up for a patient with Brugada syndrome, the patient’s family has been approached by the proband regarding participation in clinical and genetic screening at the Maastricht University Hospital for mutations. Only one of the brothers has agreed to participate. He had an ECG positive at baseline and was found to have the same genetic mutation as the proband, therefore proving this to be a familial disease discovered by the use of bupivacaine in one of the genetic carriers with a normal ECG.
Biophysical analysis
In TSA201 cells, expression of the mutant channel G1743E produced a markedly reduced sodium channel current (INa) when compared to wild type (WT), a mechanism known to lead to ST segment elevation and the Brugada syndrome phenotype (Figure 2).5
Wedge model
In all 5 experiments, the sodium channel blocker bupivacaine (10–20 uM) produced use-dependent slowing of conduction, evidenced by a prolongation of the QRS complex of the transmural ECG. Reduced excitability necessitated an increase in stimulus intensity in order to maintain 1:1 activation, particularly at faster rates (basic cycle length ≤ 400 msec). Loss of the action potential dome in the RV epicardium was observed in 3 out of 3 preparations pretreated with verapamil (0.1uM) (Figure 3), but not in the two wedge preparations that were not pretreated with verapamil. Verapamil (0.1uM) alone accentuated the action potential notch but did not lead to loss of the action potential dome in any of the preparations tested (3/3). Heterogeneous loss of the dome permitted the development of phase 2 re-entry due to propagation of the action potential dome from the epicardial sites where it was maintained to sites where it was lost, thus generating a closely coupled extrasystole. Prominent J waves, negative T waves, and T wave alternans, were apparent in the ECG (Figures 3 and 4).
Figure 3.
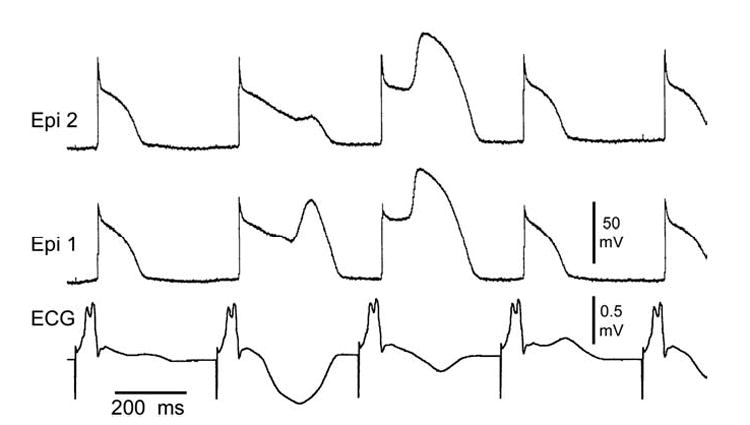
Bupivacaine-induced loss of the action potential dome in right ventricular (RV) epicardium (Epi). Transmembrane action potentials from 2 Epi sites together with a transmural ECG recorded from a canine arterially perfused RV wedge preparation exposed to 10 uM bupivacaine in the presence of 0.1 uM verapamil. Basic cycle length = 400 msec. Prominent J waves, negative T waves and T wave alternans accompany the intermittent loss of the dome in RV epicardium.
Figure 4.
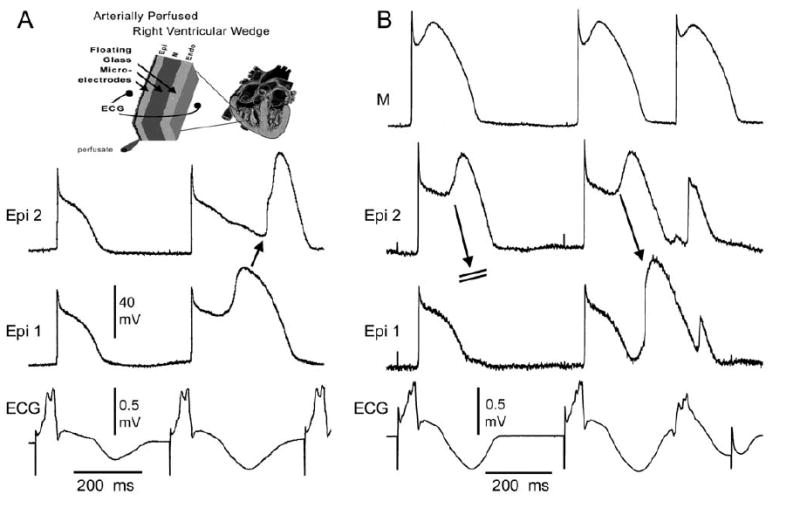
Bupivacaine-induced phase 2 reentry. Shown are transmembrane action potentials from 2 epicardial sites (A), or 2 epicardial sites and an M cell site (B), together with a transmural ECG recorded from a canine arterially-perfused right ventricular wedge preparation exposed to 10 uM bupivacaine in the presence of 0.1 uM verapamil. Basic cycle length = 400 msec. A: Propagation of the action potential dome from epicardial sites at which to dome was maintained to sites at which it was lost gives rise to a concealed form of phase 2 re-entry. The concealed phase 2 reentrant beat accentuates the negative T wave, thus giving rise to marked T wave alternans. B: Propagation of the epicardial action potential dome fails in the first grouping but succeeds in the second, giving rise to a phase 2 reentrant beat that activates the entire preparation, thus generating a closely-coupled extrasystole in the ECG. The reentrant beat further accentuates the negative T wave.
Discussion
An ECG pattern characterized by coved-type ST segment elevation in the right precordial leads is observed in the congenital form of the Brugada syndrome, but may also be induced by medications and external factors.8,9 Brugada syndrome affects young individuals with no previous history of cardiac disease. It is characterized by the presence of ST segment elevation in leads V1 to V3, and the occurrence of malignant arrhythmias in structurally normal hearts.3 ST segment elevation in the Brugada syndrome is thought to be due to an accentuation of the RV epicardial action potential notch secondary to rebalancing of the ion channel currents active at the end of phase 1 of the epicardial ventricular action potential.10 An arrhythmogenic substrate can be created under these conditions due to loss of the action potential dome in epicardium but not endocardium, resulting in the development of a marked transmural dispersion of repolarization and refractoriness. The heterogeneous loss of the action potential dome leads to the creation of a vulnerable window across the RV wall during which an extrasystole generated by the same substrate can induce VT and ventricular fibrillation (VF).
Thus, ST segment elevation and electrical instability of the myocardium can result from an outward shift in the balance of currents active at the end of phase 1 of the action potential. Vagotonic agents, IK-ATP activators, and high potassium levels can facilitate this by augmenting outward currents, whereas sodium channel blockers, cocaine, antidepressants, ischemia, acidosis and antihistaminics like terfenadine can accomplish this by reducing inward currents.11 Loss of function mutations in SCN5A, responsible for the Brugada syndrome, shifts the balance of current by reducing sodium channel current. A febrile state has also been shown to facilitate ST segment elevation in the Brugada syndrome owing to its ability to reduce sodium channel current.12 A reduction in inward currents causes ST elevation more readily in the right ventricle because of the prominence of the transient outward current (Ito) in this part of the heart, accounting for its manifestation in the right precordial leads in Brugada syndrome.7
With the use of genetic analysis, we provide the first evidence that bupivacaine can unmask a Brugada syndrome, leading to the development of the substrate for VT/VF. Phillips et al13 previously published a case-report of a 77-year-old man with a characteristic Brugada-like ECG during epidural infusion of bupivacaine. Although no arrhythmias were observed they suggested that the development of Brugada-like ECG changes during bupivacaine warrants immediate discontinuation to prevent potentially fatal arrhythmias.
Like flecainide and ajmaline, sodium channel blockers used to unmask the Brugada syndrome, bupivacaine inhibits the sodium channel current.14 Experimental data from isolated rabbit heart indicate that bupivacaine can facilitate reentrant ventricular arrhythmias because it slows of conduction and prolongs the ventricular effective refractory period.1 The present study demonstrates the action of bupivacaine to create both a transmural and epicardial dispersion of repolarization secondary to its inhibition of INa. This effect of the drug is facilitated by pretreatment with low concentrations of the calcium channel blocker, verapamil. This is consistent with the observation that VT in our patient was observed during co-administration of a calcium channel blocker. The effect of calcium channel block to facilitate sodium channel inhibition-induced Brugada phenotype was recently reported by us in experimental models of the Brugada syndrome.15 It is noteworthy that VT occurrence was also associated with co-administration of isosorbide-5-mononitrate, another agent thought to facilitate ST segment elevation and arrhythmogenicity in the Brugada syndrome.
Conclusion
The data provide the first genetic and biophysical evidence for an effect of epidural anesthesia with bupivacaine to unmask a subclinical form of the Brugada syndrome leading to the development of life-threatening arrhythmias. Thus, bupivacaine may be added to the list of drugs including flecainide, ajmaline and procainamide capable of unmasking the Brugada syndrome. Physicians should be aware that when using anesthetics, the development of ST segment elevation may be a warning sign for electrical instability and that immediate corrective measures are needed.
Acknowledgments
The present study was supported by The Netherlands Organization for Health Research and Development (KV), grant 2000T036 from the Netherlands Heart Foundation (TD) and grant HL47678 and HL 66169 from NHLBI (CA and RB), grants from the American Heart Association (CA and RB) and NYS and Florida Grand Lodges F.& A.M and by the Ramon Brugada Sr. Foundation.
References
- 1.de La Coussaye JE, Brugada J, Allessie MA. Electrophysiologic and arrhythmogenic effects of bupivacaine. A study with high-resolution ventricular epicardial mapping in rabbit hearts. Anesthesiology. 1992 July;77(1):132–41. [PubMed] [Google Scholar]
- 2.Brugada R, Brugada J, Antzelevitch C, Kirsch GE, Potenza D, Towbin JA, Brugada P. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000 February 8;101(5):510–5. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 3.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multi-center report. J Am Coll Cardiol. 1992 November 15;20(6):1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O’Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998 March 19;392(6673):293–6. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 5.Dumaine R, Towbin JA, Brugada P, Vatta M, Nesterenko DV, Nesterenko VV, Brugada J, Brugada R, Antzelevitch C. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999 October 29;85(9):803–9. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 6.Hong K, Guerchicoff A, Pollevick GD, Oliva A, Dumaine R, de ZM, Burashnikov E, Wu YS, Brugada J, Brugada P, Brugada R. Cryptic 5′ splice site activation in SCN5A associated with Brugada syndrome. J Mol Cell Cardiol. 2005 April;38(4):555–60. doi: 10.1016/j.yjmcc.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Di Diego JM, Cordeiro JM, Goodrow RJ, Fish JM, Zygmunt AC, Perez GJ, Scornik FS, Antzelevitch C. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation. 2002 October 8;106(15):2004–11. doi: 10.1161/01.cir.0000032002.22105.7a. [DOI] [PubMed] [Google Scholar]
- 8.Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA. Proposed diagnostic criteria for the Brugada syndrome. Eur Heart J. 2002 November;23(21):1648–54. doi: 10.1053/euhj.2002.3382. [DOI] [PubMed] [Google Scholar]
- 9.Darbar D, Yang T, Churchwell K, Wilde AA, Roden DM. Unmasking of brugada syndrome by lithium. Circulation. 2005 September 13;112(11):1527–31. doi: 10.1161/CIRCULATIONAHA.105.548487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antzelevitch C, Brugada P, Brugada J, Brugada R, Shimizu W, Gussak I, Perez Riera AR. Brugada syndrome: a decade of progress. Circ Res. 2002 December 13;91(12):1114–8. doi: 10.1161/01.res.0000046046.53721.90. [DOI] [PubMed] [Google Scholar]
- 11.Antzelevitch C. Brugada syndrome: clinical, genetic, molecular, cellular and ionic aspects. Expert Rev Cardiovasc Ther. 2003 July;1(2):177–85. doi: 10.1586/14779072.1.2.177. [DOI] [PubMed] [Google Scholar]
- 12.Antzelevitch C, Brugada R. Fever and Brugada syndrome. Pacing Clin Electrophysiol. 2002 November;25(11):1537–9. doi: 10.1046/j.1460-9592.2002.01537.x. [DOI] [PubMed] [Google Scholar]
- 13.Phillips N, Priestley M, Denniss AR, Uther JB. Brugada-type electrocardiographic pattern induced by epidural bupivacaine. Anesth Analg. 2003 July;97(1):264–7. doi: 10.1213/01.ane.0000067410.32384.3a. table. [DOI] [PubMed] [Google Scholar]
- 14.Valenzuela C, Snyders DJ, Bennett PB, Tamargo J, Hondeghem LM. Stereoselective block of cardiac sodium channels by bupivacaine in guinea pig ventricular myocytes. Circulation. 1995 November 15;92(10):3014–24. doi: 10.1161/01.cir.92.10.3014. [DOI] [PubMed] [Google Scholar]
- 15.Fish JM, Antzelevitch C. Role of Sodium and Calcium Channel Block in Unmasking the Brugada Syndrome. Heart Rhythm. 2004;1(2):210–217. doi: 10.1016/j.hrthm.2004.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]


