Abstract
Objective
Minimally invasive epicardial atrial ablation to cure atrial fibrillation through the use of a percutaneous subxiphoid approach currently has a lack of dedicated technology for intrapericardial navigation around the beating heart. We have developed a novel articulated robotic medical probe and performed preliminary experiments in a porcine preparation.
Methods
In five large, healthy pigs, the teleoperated robotic system was introduced inside the pericardial space through a percutaneous subxiphoid approach. Secondary visualization of the left atrium and left atrial appendage was achieved with the use of a 5-mm scope inserted through a left thoracic port. The operator actively controlled the path of the robot by using a master manipulator. The catheter, with an irrigated radiofrequency tip, was guided through the working port of the robot to achieve epicardial ablation of the left atrium.
Results
Access to the pericardial space and progression around the left atrium was successful in all cases, with no interference with the beating heart such as a fatal arrhythmia, unexpected bleeding, and hypotension. Epicardial ablation was successfully performed in all five cases. No adverse hemodynamic or electrophysiological events were noted during the trials. When the animals were killed, there was no visually detected injury on the surrounding mediastinal structures caused by ablation. Transmural ablation was confirmed by histopathology of the left atrium.
Conclusions
We have developed a dedicated articulated robotic medical probe and successfully performed epicardial left atrial radiofrequency ablation. Based on the feedback from these preliminary experiments, the radius of curvature and proper visualization of the device are being improved in the next generation prototype.
Keywords: Robotic surgery, Cardiac surgery, Minimally invasive, Epicardial ablation
INTRODUCTION
Progression of medical technology has brought dramatic improvements in surgical outcomes and prognosis. Recently, minimally invasive surgery has been emphasized for reducing large invasiveness of traditional surgical techniques. In particular, robotic-assisted surgery is playing an important role in minimally invasive surgery. Minimally invasive robotic surgery has been applied in the cardiothoracic, abdominal, urologic, and gynecologic fields.1 In cardiothoracic surgery, minimally invasive robotic surgery has been developed to minimize surgical trauma and improve cosmetic results in comparison with the conventional full sternotomy. As additional benefits, it includes a decrease in length of hospital stay, less postoperative pain, faster patient recovery, and faster return to normal daily activities.2 The ZEUS (Computer Motion, Goleta, Calif) and the da Vinci (Intuitive Surgical, Mountain View, Calif) are the most advanced and sophisticated commercially available robotic systems today.1,3,4 However, these systems still have some problems, such as prohibitive cost and increased set-up time. In addition, these systems require multiple ports in the left chest, right chest, or both sides and require special anesthetic techniques with double-lumen endotracheal tubes to alternately collapse the left or the right lung.5 Multiple port placements are associated with postoperative pain secondary to muscle and nerve damage and rib spreading.6
Atrial fibrillation (AF), which is the most common sustained cardiac arrhythmia, is a major contributor to cardiovascular morbidity in the global population. Stroke continues to be a fatal complication associated with AF, with an overall incidence of 5% to 7% per annum.7,8 AF is responsible for 20% of all strokes.9 Each year, 60,000 strokes occur among 2.3 million Americans with AF, and both numbers are predicted to more than double in coming decades.10 In terms of medical treatment of AF, rhythm control does not reduce stroke,11 and antithrombotic therapies have to take a risk of bleeding, including intracranial hemorrhage, which occurs at a rate of 0.5% per year.12 As for surgical treatment, the Cox-Maze III operation is the most curative therapy.13 However, because of complexity and morbidity, the procedure is still controversial. Percutaneous catheter ablation for AF has long procedure times and associated complications such as cardiac tamponade, stroke, and pulmonary vein stenosis.14–16 On the other hand, surgical epicardial ablation has been reported with midterm success rate.17 However, a full sternotomy used in the epicardial ablation is relatively invasive, whereas the ablation procedure itself is simple. A dedicated technology for epicardial ablation will potentially enable a surgeon to minimize the invasiveness of surgery.
We have developed a novel articulated robotic medical probe (ARM) that is suitable for many minimally invasive epicardial interventions, including ablation. The teleoperated device is designed to be introduced into an intrapericardial space through a subxiphoid approach and to be able to reach around the corner of intrapericardial locations on the beating heart without causing hemodynamic and electrophysiological interference. We performed preliminary tests of epicardial ablation with the ARM by using a radiofrequency ablation catheter that was introduced through the working port of the robot in a porcine preparation.
METHODS
Design of the Robot
The ARM consists of a probe and a feeder. In the current prototype, the probe is 12 mm in diameter and 300 mm in length; the current geometry allows for a minimum radius of curvature of 40 mm; 12 mm was chosen to match available port sizes. Probe construction is composed of rigid cylindrical links articulated by spherical joints that can rotate ±10° relative to the longitudinal axis. Actuation of the ARM is controlled off-board by the feeder, which has all mechanical apparatus to provide behavior of the probe (500 mm length, 170 mm width, 100 mm height) (Fig. 1B). The device is capable of preserving its previous configuration and can create a curve in three-dimensional space (Fig. 1A). In retracting phase, the probe passes through the exact same pathway of the extending phase. The robot has 32 degrees-of-freedom (DOF), 30 for the snake and 2 for the feeder. The surgeon directly controls the heading and direction (3 DOF) of the tip through the use of a joystick (Fig. 1C). Our software then uses a follow-the-leader technique to compute the values of all of the internal DOF to positing the feeder and the robot so as the tip of the robot goes where the surgeon commands it. The average speed of advancing forward or backward is approximately 20 mm/s. Once in position, off-the-shelf catheter devices can be inserted through the 7-mm working port. For this series of experiments, the distal end of the probe was configured to accept a catheter up to 8F (Fig. 1D), a size commonly used in ablation procedures.
FIGURE 1.
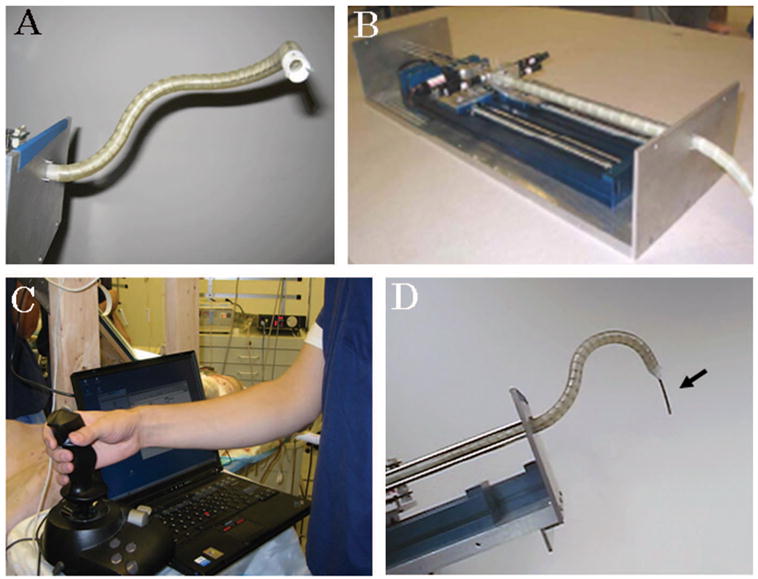
A, Articulated robotic medical probe (ARM); B, feeder; C, joystick controller; D, ablation catheter (Biosense Webster) (arrow) through the working port of the robot.
Epicardial Ablation With a Porcine Preparation
Large, healthy, Yorkshire swine of either sex (n = 5; body weight, 40 to 45 kg) were anesthetized and laid in supine position. A 20-mm skin incision was made at the subxiphoid. The pericardium was then incised in 2-cm length. The ARM advanced into the pericardial space through the pericardial hole, while the test was observed by using a thoracoscope inserted from the left thoracic wall (Fig. 2, A, B, and C). Operations of pathways from the subxiphoid to the left atrium through the anterior wall of the heart and through the oblique sinus were tested several times repeatedly. Once the tip of the robot had reached the left atrium, an 8F catheter with an irrigated radiofrequency tip (Biosense Webster, Diamond Bar, Calif) was guided through the working port of the robot (Fig. 1D). Epicardial ablation (40 W, 15 seconds) was performed three times in each point. Two or three points of ablation were carried out in every animal. The blood pressure and electrocardiogram were continuously monitored to evaluate hemodynamic and electrophysiological influences of the robot and the ablation procedure. All animals were killed after the test, and the heart was excised for triphenyltetrazolium chloride (TTC) stain.
FIGURE 2.
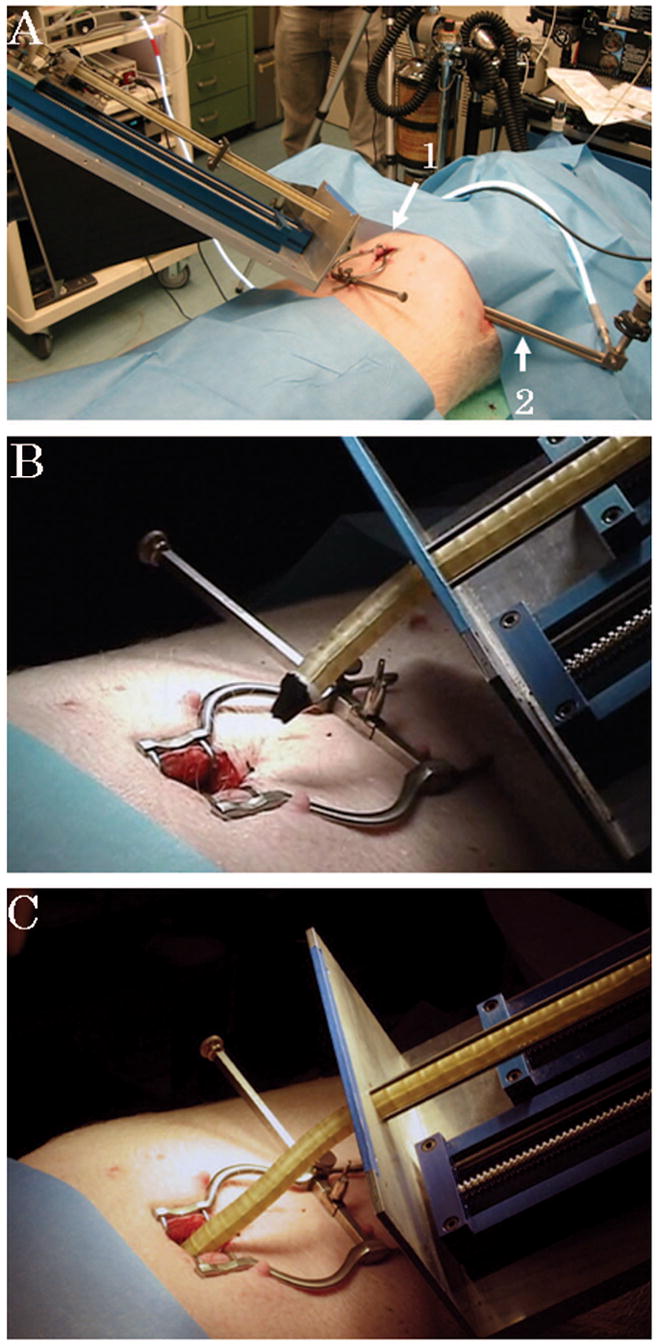
A, The robot is mounted on the table. A small incision is made at the subxiphoid (arrow 1). Visualization is achieved by a thoracoscope inserted from the left thoracic wall (arrow 2). B and C, The robot advances forward into the intrapericardial space through the subxiphoid incision.
The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. All subjects received humane care in compliance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH publication No.85-23, revised 1985).
RESULTS
All animals survived until elective killing at the end of the trials. The thoracoscope inserted from the left thoracic wall provided adequate visualization of the left aspect of the heart through the pericardium. Two pathways from the subxiphoid to the left atrium through the anterior wall of the heart and through the oblique sinus were successfully accomplished in all cases without interference with the beating heart. The ARM was precisely navigated by the surgeon to preselected remote locations. Series of epicardial ablation were successfully performed under a beating heart with the thoracoscopic view (Fig. 3, A and B). Throughout the trials, hemodynamic status was stable and there were no adverse events such as fatal arrhythmia, including multiple premature ventricular contractions, injury to the heart, and malfunction of the robot in all cases.
FIGURE 3.
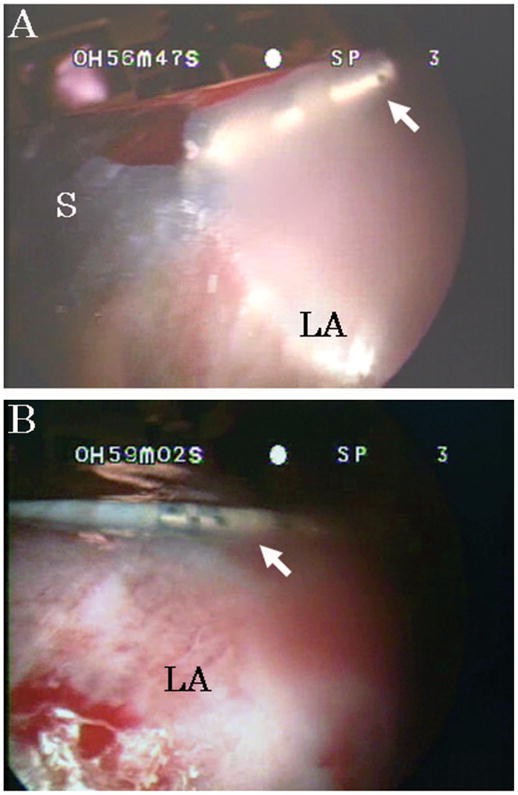
A and B, Tip of the robot and the ablation catheter (arrows) are seen through the pericardium. S indicates tip of the probe; LA, left atrium.
After the epicardial ablation procedures, the animals underwent a full sternotomy for observation. Their diaphragms were not paralyzed under the spontaneous respiration, and no injury to the surrounding mediastinal structures (eg, esophagus, pulmonary artery) and active bleeding caused by the ARM manipulation was noted. At gross examination, there was also no damage of both the heart and surrounding structures such as a pericardium, pulmonary veins, pulmonary arteries, and the aorta (Fig. 4A). Transmural ablation was confirmed by TTC stain in all points of all cases (Fig. 4, B and C).
FIGURE 4.
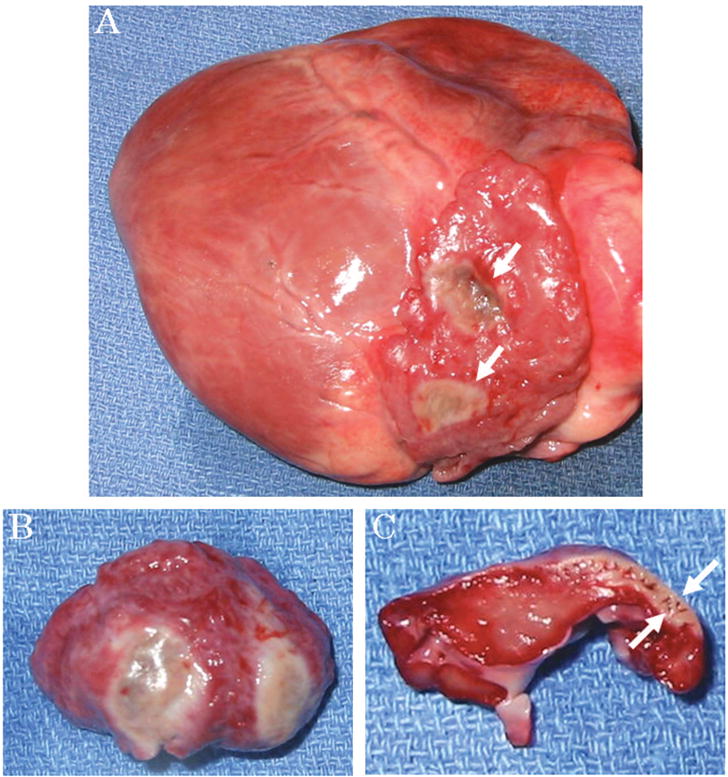
A, The excised heart. No injury is observed on the surface of the heart except ablated points (arrows). B and C, Triphenyltetrazolium chloride stain. Trans-mural ablation is confirmed (arrow).
DISCUSSION
Recently, ablation therapies for AF have been well studied, including energy sources, approaches, and their efficacy and safety.18,19 Regarding epicardial ablation therapies on a beating heart, several minimally invasive trials such as robot-assisted or total endoscopic ablation were reported.20–22 However, those studies still required placement of more than two ports. We aim for achieving ablation therapy by using the ARM with minimized port incision, that is, one-port approach.
The ARM has been developed to facilitate therapeutic deliveries to the intrapericardial space for minimally invasive surgery.23,24 The present prototype of the robot is the second generation, which has smaller radius of curvature and increased speed in comparison with the first prototype (radius of curvature, 7.5 cm; speed, 5 mm/s).
The device is designed to be induced through the use of a subxiphoid approach. Zenati et al25,26 reported endoscopic ligation of the left atrium appendage and pacing lead implantation with the subxiphoid videopericardioscopy approach. This minimally invasive approach is appealing because it allows access to the pericardial space through a single subxiphoid port while not requiring general endotracheal anesthesia and lung deflation.
The ARM has several advantages. The first advantage is maintaining its previous shape while moving forward or backward, which is the most different feature between this robot and traditional endoscopic devices. The body of a common endoscope would strike the surrounding structures when manipulated. In this study, the robotic manipulations did not trigger adverse events such as a fatal arrhythmia because the capability of keeping its previous configuration minimized contact of the robot with the heart. Second, the probe of the ARM can be made of any suitable, Food and Drug Administration approved material. The current probe is made of plastic. The probe will be able to be produced with quite low cost as a disposable product. Third, the large working port of the robot has a high utility to use off-the-shelf catheter devices. We have accomplished the left atrial appendage ligation with Endoloop (Ethicon Endo-Surgery Inc., Cincinnati, Ohio) and a pericardial biopsy with an endobiopsy catheter by using the first prototype of the ARM under the open chest porcine preparation (data not shown).
In terms of the epicardial ablation with this robot, we have an immediate goal to accomplish pulmonary vein (PV) isolation. For this purpose, the robot has at least two things to be improved. First, developing a proper visualization is critical. In the present study, although preliminary tests were accomplished with a thoracoscopic view, it could not provide an adequate view to show PVs or behind the heart but only the left aspect of the heart. The present prototype had no built-in visualization, such as an on-board charge coupled device camera. In addition, we will need to know the exact location and the physical relation between the robot and the heart for a precise PV isolation. An electromagnetic tracking system or a three-dimensional electroanatomic mapping system will be able to be incorporated for this purpose.27,28 Specifically, the latter technology has already been applied to an early clinical trial of a catheter-based ablation, and the accuracy of the technology has been validated.28,29 Second, the robot needs to acquire more accuracy operationality of the tip. Although the present ARM could achieve several pathways to reach the left atrium, we believe that tracing lines encircling PVs was technically impossible because of lack of enough small radius of curvature of the probe in the current prototype.
As for future works, we will focus on not only epicardial ablation but also the other epicardial interventions such as cell transplantation, epicardial lead placement, and device-based mitral valve plasty with the use of the Coapsys device (Myocor, Maple Grove, Minn).26,30–32 Furthermore, endoluminal gastric surgery has been recently developed for minimally invasive surgery in the field of abdominal surgery.33 The ARM can also be used in this field. The development of dedicated instruments for intraluminal surgery will enable the next ARM prototype to be inserted from the mouth without any skin incision and to perform surgery in the gastrointestinal tract.
Despite the contributions of this study, several limitations must be addressed. First, because the sample size is small, further studies including chronic cases are needed to prove safety of the device and the procedure. Second, we need to test the robot in a diseased heart model (eg, infarction) as a next step.
In conclusion, we have developed a novel articulated robotic medical probe and successfully performed epicardial left atrial radiofrequency ablation. Based on the feedback from these preliminary experiments, the radius of curvature and proper visualization of the device are being improved in the next prototype.
Acknowledgments
This research was supported by NIH/NHLBI (grant no. 1-R01-HL079940-01 A2 awarded to M.A. Zenati). Drs Choset, Wolf, and Zenati are coinventors of the medical robot (intellectual property assigned to Carnegie Mellon University) and have an equity interest in Innovention Technologies LLC (licensee of the IP).
Footnotes
Presented at the annual meeting of the International Society for Minimally Invasive Cardiothoracic Surgery, San Francisco, CA, June 2006.
References
- 1.Marescaux J, Rubino F. The ZEUS robotic system: experimental and clinical applications. Surg Clin North Am. 2003;83:1305–1315. doi: 10.1016/S0039-6109(03)00169-5. [DOI] [PubMed] [Google Scholar]
- 2.Morgan JA, Peacock JC, Kohmoto T, et al. Robotic techniques improve quality of life in patients undergoing atrial septal defect repair. Ann Thorac Surg. 2004;77:1328–1333. doi: 10.1016/j.athoracsur.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne GH, Moll F. The da Vinci telerobotic surgical system: the virtual operative field and telepresence surgery. Surg Clin North Am. 2003;83:1293–1304. doi: 10.1016/S0039-6109(03)00164-6. [DOI] [PubMed] [Google Scholar]
- 4.Zenati MA. Robotic heart surgery. Cardiol Rev. 2001;9:287–294. doi: 10.1097/00045415-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Falk V, Diegler A, Walther T, et al. Developments in robotic cardiac surgery. Curr Opin Cardiol. 2000;15:378–387. doi: 10.1097/00001573-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Landreneau RJ, Wiechmann RJ, Hazelrigg SR, et al. Effect of minimally invasive thoracic surgical approaches on acute and chronic postoperative pain. Chest Surg Clin N Am. 1998;8:891–906. [PubMed] [Google Scholar]
- 7.Singh BN. Atrial fibrillation: epidemiologic considerations and rationale for conversion and maintenance of sinus rhythm. J Cardiovasc Pharmacol Ther. 2003;8(Suppl 1):S13–S26. doi: 10.1177/107424840300800103. [DOI] [PubMed] [Google Scholar]
- 8.Schwartzman D, Zenati MA. Innovative Management of Atrial Fibrillation. Oxford, UK: Blackwell Publishing; 2005. [Google Scholar]
- 9.Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 11.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(5):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 12.Connolly SJ. Prevention of vascular events in patients with atrial fibrillation: evidence, guidelines and practice. J Cardiovasc Electrophysiol. 2003;14:S52–S55. doi: 10.1046/j.1540-8167.14.s9.2.x. [DOI] [PubMed] [Google Scholar]
- 13.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 14.Pappone C, Oreto G, Lamberti F, et al. Catheter ablation of paroxysmal atrial fibrillation using a 3D mapping system. Circulation. 1999;100(14):1203–1208. doi: 10.1161/01.cir.100.11.1203. [DOI] [PubMed] [Google Scholar]
- 15.Haissaguerre M, Jais P, Shah DC, et al. Right and left atrial radiofrequency catheter therapy of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 1996;7:1132–1144. doi: 10.1111/j.1540-8167.1996.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 16.Robbins IM, Colvin EV, Doyle TP, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation. 1998;98(27):1769–1775. doi: 10.1161/01.cir.98.17.1769. [DOI] [PubMed] [Google Scholar]
- 17.Benussi S, Nascimbene S, Agricola E, et al. Surgical ablation of atrial fibrillation using the epicardial radiofrequency approach: mid-term results and risk analysis. Ann Thorac Surg. 2002;74:1050–1056. doi: 10.1016/s0003-4975(02)03850-x. [DOI] [PubMed] [Google Scholar]
- 18.Gillinov AM. Surgical ablation of atrial fibrillation. J Interv Card Electrophysiol. 2005;13:115–124. doi: 10.1007/s10840-005-0302-5. [DOI] [PubMed] [Google Scholar]
- 19.Earley MJ, Schilling RJ. Catheter and surgical ablation of atrial fibrillation. Heart. 2006;92:266–274. doi: 10.1136/hrt.2005.067389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JM, Hassan M, Ignacio R. Robot-assisted isolation of the pulmonary veins with microwave energy. J Card Surg. 2006;21:83–88. doi: 10.1111/j.1540-8191.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 21.Poa L. Thoracoscopic ablation for treatment of atrial fibrillation: a 2-port approach. Heart Surg Forum. 2006;9:E590–E591. [PubMed] [Google Scholar]
- 22.Pruitt JC, Lazzara RR, Dworkin GH, et al. Totally endoscopic ablation of lone atrial fibrillation: initial clinical experience. Ann Thorac Surg. 2006;81:1325–1330. doi: 10.1016/j.athoracsur.2005.07.095. [DOI] [PubMed] [Google Scholar]
- 23.Zenati MA, Wolf A, Ota T, et al. High Dexterity Snake Robot for Intrapericardial Interventions. Proceedings, 1st Worldwide Meeting of the Minimally Invasive Robotic Association, December 7 to 10, 2005; Innsbruck, Austria. p. 19. [Google Scholar]
- 24.Degani A, Choset H, Wolf A, et al. Percutaneous Intrapericardial Interventions Using a Highly Articulated Robotic Probe. International Conference on Biomedical Robotics and Biomechatronics (BioRob) February 2006; Pisa, Italy. [Google Scholar]
- 25.Zenati MA, Schwartzman D, Gartner M, et al. Feasibility of a new method for percutaneous occlusion of the left atrial appendage. Circulation. 2002;106:II-619. [Google Scholar]
- 26.Zenati MA, Bonanomi G, Chin AK, et al. Left heart pacing lead implantation using subxiphoid videopericardioscopy. J Cardiovasc Electrophysiol. 2003;14:949–953. doi: 10.1046/j.1540-8167.2003.03255.x. [DOI] [PubMed] [Google Scholar]
- 27.Frantz DD, Wiles AD, Leis SE, et al. Accuracy assessment protocols for electromagnetic tracking systems. Phys Med Biol. 2003;48(21):2241–2251. doi: 10.1088/0031-9155/48/14/314. [DOI] [PubMed] [Google Scholar]
- 28.Tops LF, de Groot NM, Bax JJ, et al. Fusion of electroanatomical activation maps and multislice computed tomography to guide ablation of a focal atrial tachycardia in a Fontan patient. J Cardiovasc Electrophysiol. 2006;17:431–434. doi: 10.1111/j.1540-8167.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- 29.Dickfeld T, Dong J, Solomon SB, et al. Assessment of position error of catheter mapping system (Biosense Carto V8) with CT/MR image integration capabilities. Heart Rhythm. 2005;2:S278. [Google Scholar]
- 30.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra YK, Mittal S, Jaguri P, et al. Coapsys mitral annuloplasty for chronic functional ischemic mitral regurgitation: 1-year results. Ann Thorac Surg. 2006;81:42–46. doi: 10.1016/j.athoracsur.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Grossi EA, Saunders PC, Woo YJ, et al. Intraoperative effects of the coapsys annuloplasty system in a randomized evaluation (RESTOR-MV) of functional ischemic mitral regurgitation. Ann Thorac Surg. 2005;80:1706–1711. doi: 10.1016/j.athoracsur.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 33.Rosen MJ, Heniford BT. Endoluminal gastric surgery: the modern era of minimally invasive surgery. Surg Clin North Am. 2005;85:989–1007. doi: 10.1016/j.suc.2005.05.010. [DOI] [PubMed] [Google Scholar]


